Introduction
The liver is one of the most common sites for tumor
metastases in different types of cancer, including colorectal
cancer, lung carcinoma, ovarian cancer, melanoma, breast carcinoma
and esophageal and urogenital tumors (1,2). This
organ is a site of metastasis in 25% of metastatic cancers
(3). In Western countries, metastases
are the most common type of malignant neoplasms in the liver
(3). The analysis of the
postoperative course in patients following resection of colorectal
cancer reveals that liver metastases occur in 40–60% of patients
(2). The local treatment of secondary
liver cancer is based on surgical interventions. Surgery is the
main curative treatment for both primary and secondary liver
cancer, however, it is feasible in only 20–30% of cases (4). Local non-surgical methods of liver
cancer treatment include radiofrequency ablation, transarterial
radioembolization and chemoembolization, cryotherapy
(cryoablation), electric pulses (electroporation), laser therapy
and various radiotherapy methods (5,6). Radiation
therapy is primarily administered as stereotactic teleradiotherapy
and three-dimensional conformational radiotherapy (7). In previous years, image-guided
brachytherapy has become increasingly popular (8,9). This is
due to the possibility of administering high radiotherapy doses to
the tumor region, while maintaining a low dose in the remaining
healthy liver tissue. This allows for an escalation of the
radiation dose above the mean tolerance dose for the whole liver,
whilst maintaining local control of the irradiated metastases.
The present study describes the initial results of
treatment with the use of liver brachytherapy in the St John's
Cancer Center of Lublin (Lublin, Poland), and involved a
preliminary retrospective analysis. The aim of the present study
was to evaluate the local recurrence-free survival (LRFS),
disease-free survival (DFS) and overall survival (OS) rates of the
patients. Safety, tolerability, adverse events and the technical
feasibility of performing an interstitial liver brachytherapy were
evaluated.
Materials and methods
Group characteristics
The study included all 61 patients with liver
metastases undergoing brachytherapy at the St. John's Cancer Center
of Lublin between April 2014 and December 2016. The patient
population comprised 34 males and 27 females, and the median age
was 68±8,14 years (range, 36–84). All patients had previously
undergone at least one palliative course of chemotherapy; in
patients with colon cancer, two lines of chemotherapy were
administered. In digestive tract cancer; 2–4 cycles of LF
[fluorouracil, 400 and 600 mg/m2 intravenous (IV), d
(day). 1 and 2; calcium folinate, 200 mg/m2 IV, d. 1 and
2]; 4–12 cycles of FOLFIRI (fluorouracil, 400 and 600
mg/m2 IV, d. 1 and 2; calcium folinate, 200
mg/m2 IV, d. 1 and 2; irinotecan, 180 mg/m2
IV, d. 1) or 4–12 cycles of FOLFOX (fluorouracil, 400 and 600
mg/m2 IV, d. 1 and 2; calcium folinate, 200
mg/m2 IV, d. 1 and 2; oxaliplatin, 85 mg/m2
IV, d. 1) were administered. In breast cancer: 4–6 cycles of AT
(doxorubicin, 50 mg/m2 IV, d. 1; docetaxel, 75
mg/m2 IV, d. 1) or capecitabine (2,500 mg/m2
per os, d. 1–14) were administered. In lung cancer 4 cycles of PN
(cisplatin 75 mg/m2 IV, d. 1 and vinorelbine 30
mg/m2 IV, d. 1 and 8.) were administered. In endometrial
cancer 6 cycles of TK (paclitaxel 175 mg/m2 IV, d. 1 and
carboplatin AUC (Area Under the Curve) 6 IV, d. 1) were
administered. In laryngeal cancer 4 cycles of PF (cisplatin 100
mg/m2 IV, d. 1 and fluorouracil 1,000 mg/m2
IV, d. 1–4.) were administered. In melanoma 6 cycles of dacarbazine
(200 mg/m2 IV, d. 1–5.) were administered. All patients
exhibited tumor progression to the liver according to the Response
Evaluation Criteria in Solid Tumors (RECIST) scale ver. 1.1
following chemotherapy (10). All
liver tumors were inoperable. In 24 patients (39%), chemotherapy
following brachytherapy was used [2–12 cycles of FOLFOX, 4–6 cycles
of FOLFLIRI, 6 cycles of cetuximab (400 mg/m2 IV, d. 1
in the first cycle and 250 mg/m2 IV, d. 1 in subsequent
cycles)]. None of the patients had received radiotherapy for
metastases in the liver. No other methods were used to treat local
liver metastases. Group characteristics are summarized in Table I. The present study was approved by
the Ethics Committee of the Lublin Medical Chamber (Lublin, Poland)
(approval no., LIL-KB-20/2014). Written informed consent was
obtained from all participants.
 | Table I.Characteristics of the patient
cohort. |
Table I.
Characteristics of the patient
cohort.
| Parameter | Patients,
na |
|---|
| Sex |
|
|
Female | 27 |
| Male | 34 |
| Localization of
primary focus |
|
| Digestive
tract | 46 |
|
Breast | 7 |
| Lung | 5 |
|
Melanoma | 1 |
|
Larynx | 1 |
|
Endometrium | 1 |
| Number of metastases
(lesions in the liver) |
|
| 1 | 51 |
| 2 | 8 |
| 3 | 2 |
| Other metastases
outside the liver | 10 |
| Chemotherapy after
brachytherapy | 24 |
| Number of
applicators, mean (range) | 2.7 (1–7) |
Inclusion criteria
The following inclusion criteria were used to
identify patients qualifying for the brachytherapy treatment:
Metastatic lesions with a diameter <8 cm; total lesion diameter,
<12 cm; number of metastatic lesions, <4; a lack of direct
proximity of large vessels to the lesions; age, 18–85 years;
histologically diagnosed cancer from primary lesions or metastatic
lesions; lack of possibility of surgical treatment; lack of
efficacy of chemotherapy (first and second line chemotherapy);
failure and/or intolerance of chemotherapy; lack of patient consent
for chemotherapy; the performance status of the patient based on
World Health Organization scale (11), <2 or Karnofski performance status
(11) >60%; serum creatinine
level, ≤2 mg/dl; hemoglobin level, >8 g/dl-grams per decilitere;
white blood cell count, >2,000/mm3; neutrophil count,
>1,500/mm3; platelets, >50,000/mm3;
prothrombin time and partial thromboplastin time, and ≤1.5 times
the normal International Normalized Ratio (0.8–1.2), respectively;
bilirubin level, <2 mg/dl [30 mol/l;1.5 times below the upper
limit of the laboratory norm (0–1.3 mg/dl)]; and aminotransferases
level, <2.5 times of the upper limit of the laboratory norm
(ALT, alanine aminotransferase <45 U/l, AST aspartate
aminotransferase <40 U/l). The qualification process was based
on a multi-disciplinary assessment of the patient by a surgeon, a
radiologist, a clinical oncologist and a radiotherapist.
Course of application process
The application was performed under local
anesthesia, subsequent to administering 0.5% Bupivacaine into the
VIII–XI intercostal spaces and sedation with Midazolam (2–5 mg IV).
The puncture of the metastatic lesion was performed with the
patient in the supine position with an 18-gage biopsy needle (Chiba
Biopsy Needle; Cook Medical LLC, Bloomington, ID, USA), followed by
a rigid angiography steel guide wire (Ref. No. INT6F; Balton SP.
o.o, Warsaw, Poland). Following this, an angiographic sheath with
dilatator and a hemostatic valve was used (INT6F; Balton SP. o.o),
according to the manufacturer's protocol. The radiotherapy was
administered with the control of a multi-row computerized
tomography (CT) scanner, equipped with the option for fluoroscopic
examination (SOMATOM Sensation Open; Siemens Healthineers,
Erlangen, Germany). A 16-gage angiographic sheath (Ref. no. INT6F;
Balton SP. o.o) was introduced into the tumor area for interstitial
brachytherapy. Catheters (size 1.84×350 mm; Varian Medical Systems,
Inc., Palo Alto, CA, USA) were placed at 1–3 cm intervals, if
possible according to the Paris System rules (12). Depending on the size of the tumor, an
adequate number of catheters were inserted so that the entire
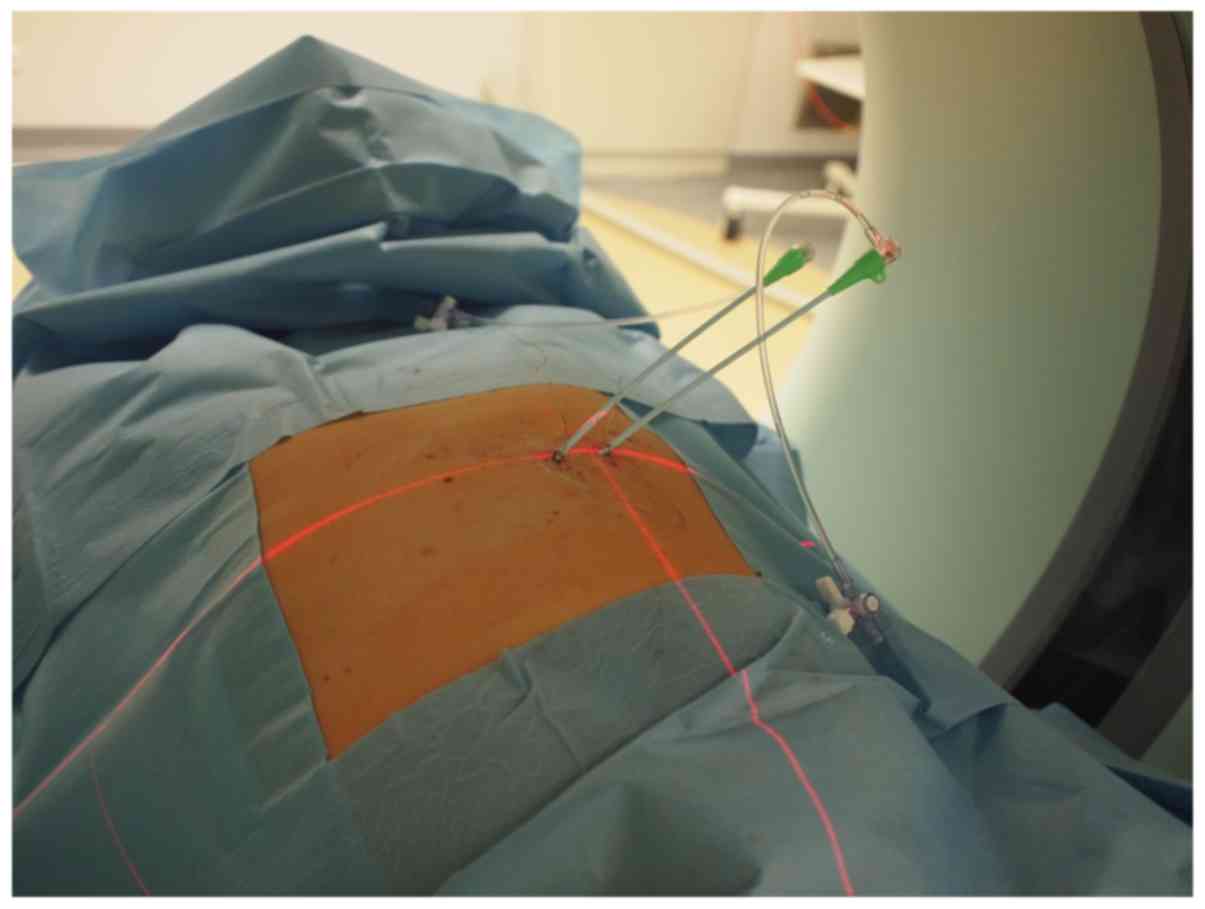
volume of the tumor was covered during brachytherapy (Fig. 1). The positioning of the catheter was
performed with CT scans with the simultaneous administration of a
single-dose intravenous iodinated non-ionizing intravenous contrast
agent (Ultravist 370, Iopromidum 768, 86 mg/ml). Treatment planning
was performed using the Brachyvision (version 10; Varian Medical
Systems, Inc.) treatment planning system. An Iridium-192 source
with a diameter of 0.6 mm and an average activity of 10 Ci was
used. The treatment was performed with a 24-channel
GammaMedPlus™ iX (Varian Medical Systems, Inc.).
Treatment planning and dosimetry
analysis
The clinical target volume included all metastatic
changes visualized by CT examination with contrast on the day of
application or images of metastasis resulting from fusion CT with
contrast and magnetic resonance imaging (MRI) with contrast. The
reference dose ranged from 15–25 Gy, depending on the ability of
the patient to accept a treatment plan for tolerance of the organ
at risk, determined based on published literature previous
experience (13). The primary
critical organ was the remaining healthy liver tissue. The limit
was set to not exceed a 5 Gy dose in 2/3 of healthy liver tissue
(D2/3 <5 Gy) (Fig.
2).
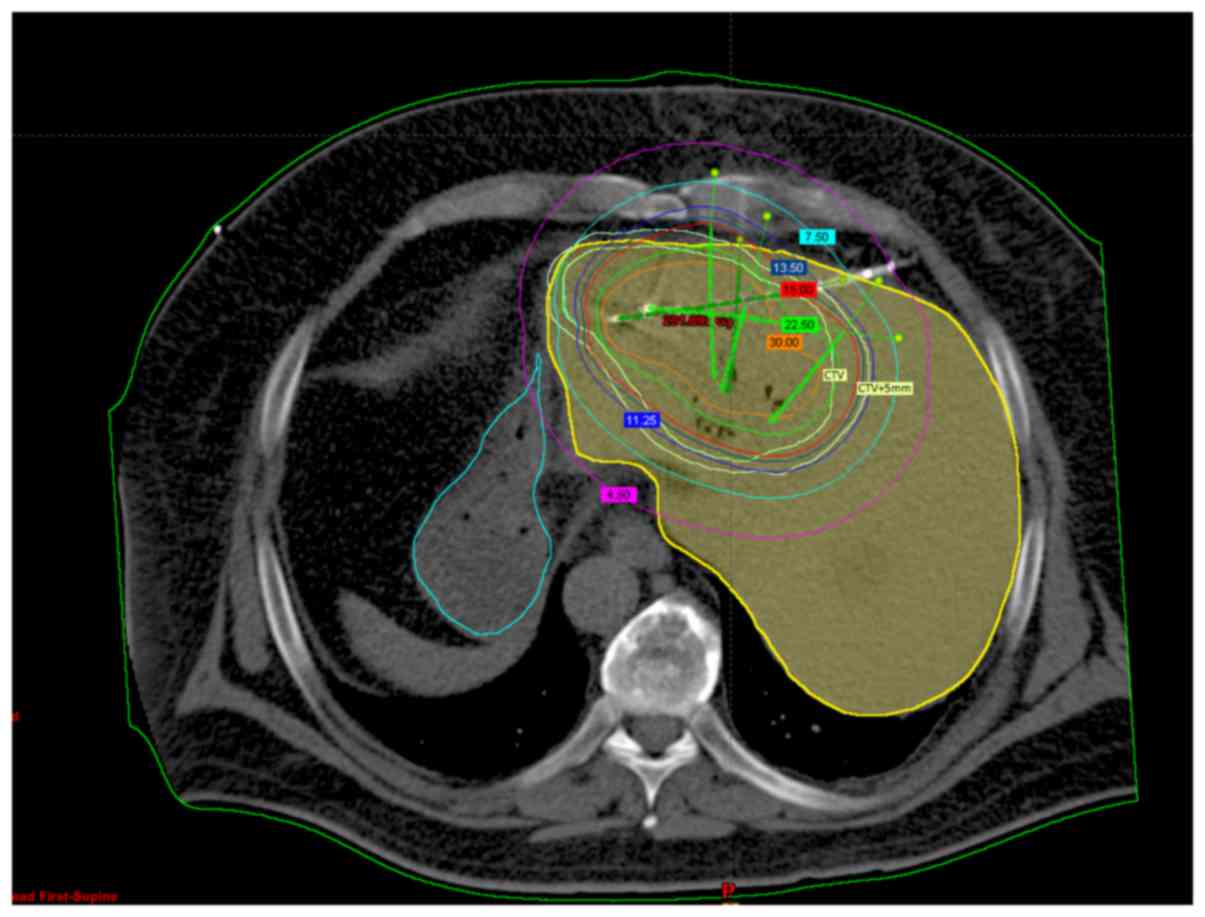 | Figure 2.Treatment planning. Female, 55 years
old, computed tomography scan of the liver. Visible tumor in
segment 4 with applicators. Numbers in colored boxes indicate the
isodose, The purple line indicates isodose 4,5 Gy, the light blue
line indicates isodose 7,5 Gy, the dark blue line indicate isodose
13,5 Gy, the red line indicates isodose 15 Gy, the green line
indicate isodose 22,5 and the orange line indicates isodose 30 Gy.
The inner light yellow line defines the CTV, and the outer light
yellow line, defines CTV+5 mm. The yellow structure is the liver
and the blue structure is the stomach. |
Follow-up treatment
In the post-treatment period, patients underwent
periodic imaging studies including CT or MRI scans (4–6
times/year). To evaluate treatment response, RECIST 1.0 criteria
were used. In certain patients, due to difficulties in
interpretation of the CT image, MRI was also performed. Patients
were also evaluated for early treatment toxicity using the Common
Terminology Criteria for Adverse Events (CTCA) scale ver. 4.0
(14). Due to the short duration of
the study, no late toxicity was assessed.
Statistical analysis
Dosimetry data are presented in mean ± standard
deviation and median (full range). Survival analysis was performed
using a Kaplan-Meier survival analysis. Cox's proportional
regression analysis and χ2 were used to analyze
prognostic factors (dose in 90 and 100% isodoses, the effects of
chemotherapy and the location of the primary tumor) with local
relapse free survival (LRFS), progression free survival (PFS) and
overall survival (OS) rates as endpoints. P<0.05 was considered
to indicate a statistically significant difference. Statistical
analysis was performed in MedCalc Statistical Software version
17.9.7 (MedCalc Software bvba, Ostend, Belgium).
Results
Dosage and treatment planning
Within the group of 61 patients undergoing
brachytherapy, 73 metastases were treated. Doses of ≥20 Gy were
administered to a group of 37 patients (61%); a lower dose (15 Gy)
was administered to the rest. The dose was selected based upon the
tolerance of the critical organs. The fractional dose was within
the range of the 90% (D90) and 100% (D100)
isodoses. The mean D90 was 20.2±4.5 Gy. The median
D90 was 20 Gy (13–29 Gy). The mean D100 was
13.2±3.1 Gy, and the median D100 was 13 Gy (7–20 Gy).
The mean volume of the irradiated lesions was 59.1±49.7
cm3. The median volume of the irradiated lesions was
42.9 cm3 (2.7–174.9 cm3). The mean volume
that received 150% of the dose (V150%) was 31.3±24.6
cm3. The median V150% was 26.2 cm3
(1.8–94.5 cm3). The mean volume that received 200% of
the dose (V200%) was 21.4±16.7 cm3. The
median V200% was 18.6 cm3 (1.5–64.1
cm3).
The primary critical organ was the remaining healthy
liver tissue. The dose in 2/3 of normal liver volume
(D2/3) was measured. The mean D2/3 was
1.9±1.2 Gy, and the median D2/3 was 1.5 Gy (0.3–4.9
Gy).
Follow-up
In all patients, the mean follow-up time was
12.6±5.4 months, and the median follow-up time was 11 months (3–25
months). During the whole period of observation, progression of the
treated cancer lesions was observed in 18 patients (29%). It
occurred in individual patients, on mean, after 10 (4–23) months
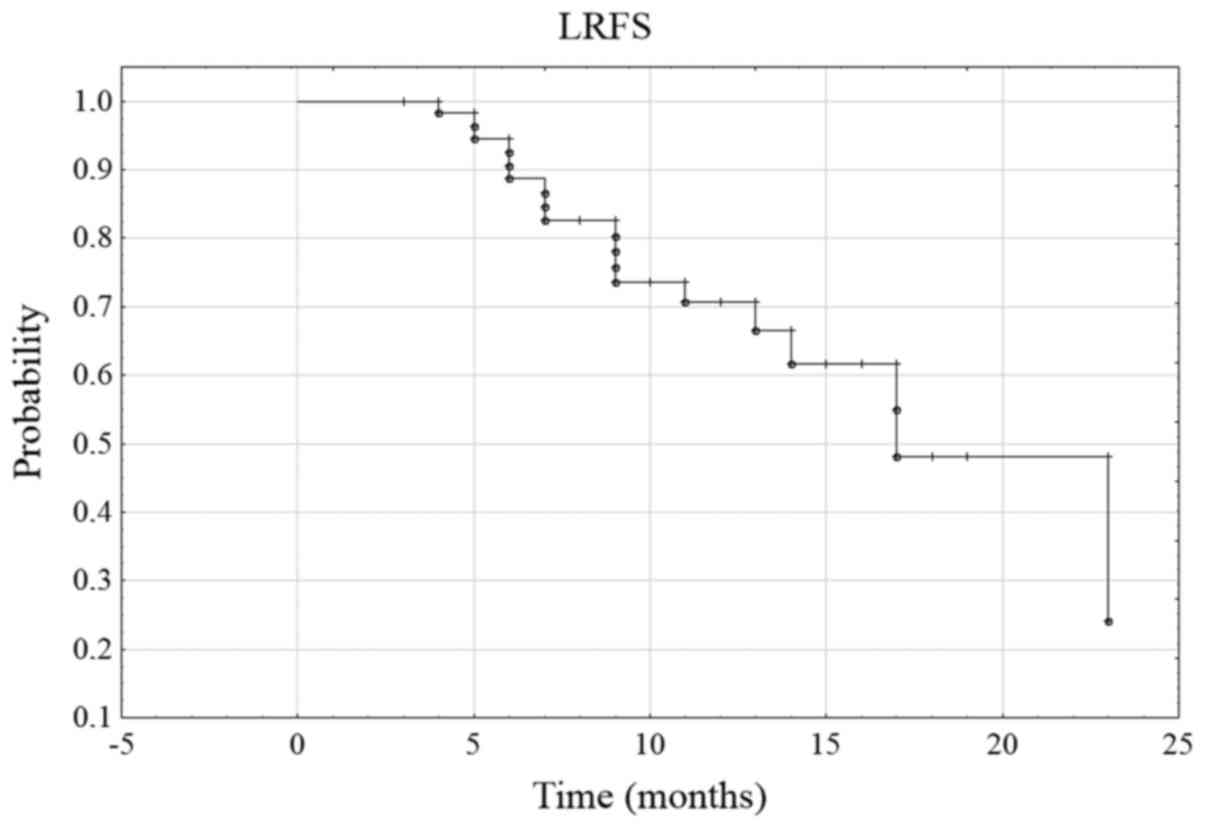
of observation. The probability of 6-month LRFS was 88.7% in the
whole group, and the 12-month LRFS was 70.7% (Fig. 3). During the follow-up period, disease
progression was observed in 35 patients (57%), and was defined as
progression of the treated lesion, or the progression or appearance
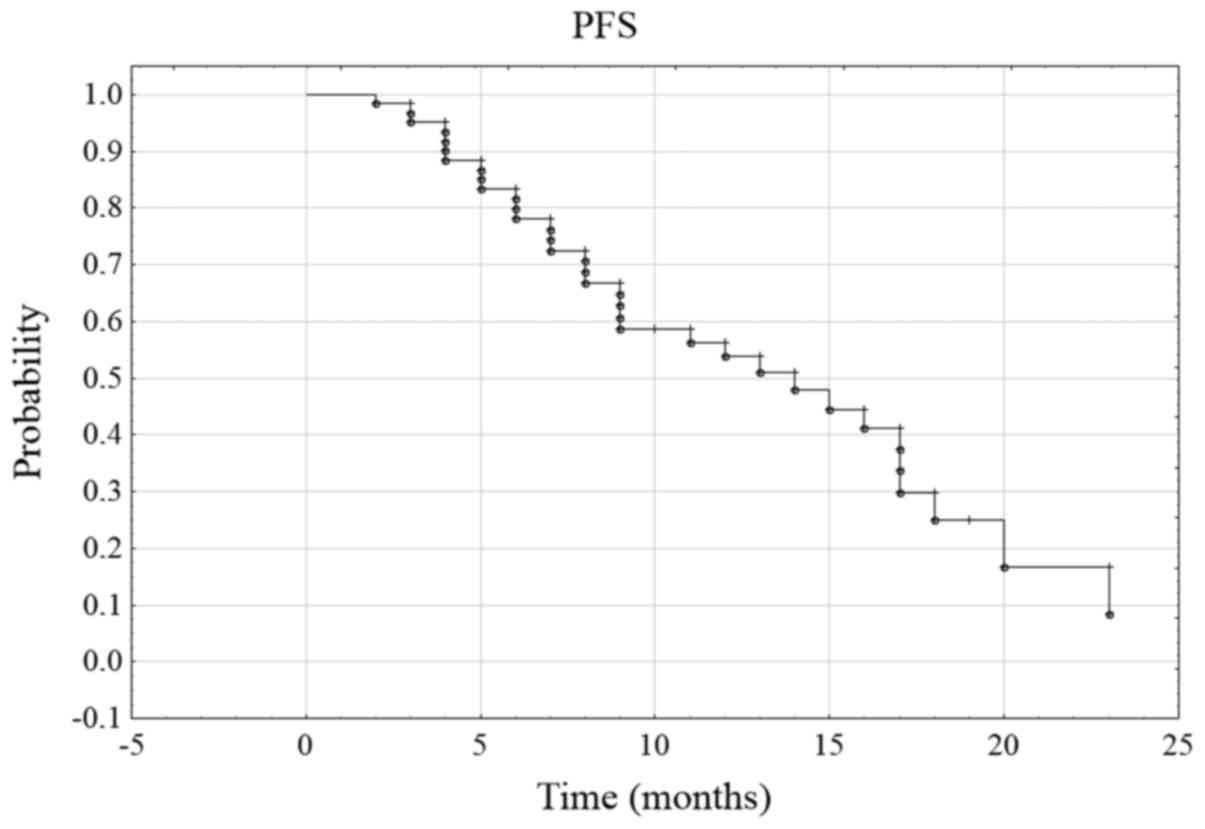
of other metastatic lesions. The probability of 6-month PFS was
78.1% and 12-month PFS was 53.8% (Fig.
4). During the follow-up period, 15 patients succumbed to the
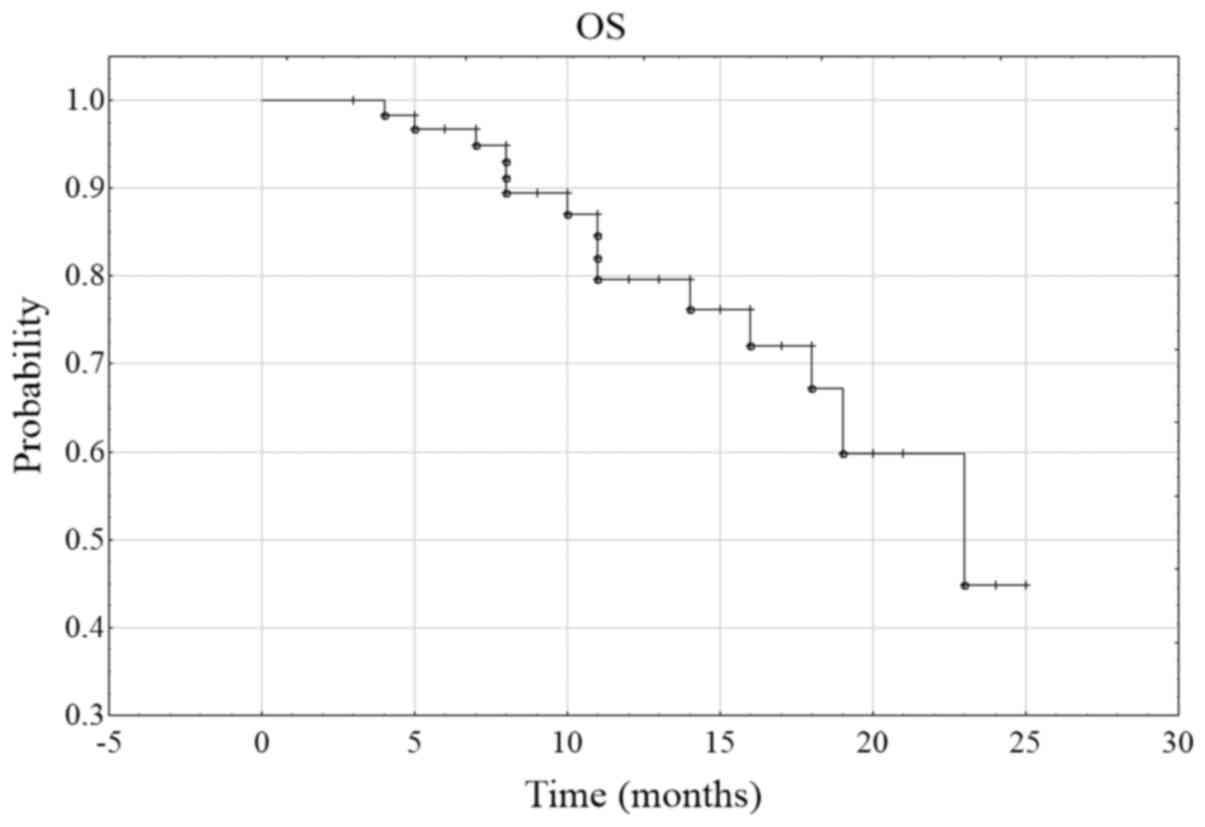
cancer (24.6%). The rate of 6-month OS (6-month overall survival)
was 96.7%, and of 12-month OS was 79.6% (Fig. 5).
In the Cox regression analysis, D100 was
a statistically significant predictor of LRFS and PFS, but it was
not significant in OS. D90 was a statistically
significant predictor of LRFS but it was not significant in PFS and
OS. Lower doses (D90 lower than 20 Gy and
D100 lower than 15 Gy) caused a deterioration of LRFS.
Chemotherapy and localization of cancer were not significant
predictors of outcome (Table
II).
 | Table II.Cox regression analysis. |
Table II.
Cox regression analysis.
|
Characteristics | χ2 | HR | 95% CI | P-value |
|---|
|
D100 |
|
|
|
|
|
LRFS | 8.38 | 0.77 | 0.63–0.94 | 0.01 |
|
PFS | 6.55 | 0.85 | 0.75–0.97 | 0.02 |
| OS | 3.80 | 0.82 | 0.66–1.02 | 0.07 |
| D90 |
|
|
|
|
|
LRFS | 6.24 | 0.85 | 0.73–0.98 | 0.03 |
|
PFS | 1.97 | 0.94 | 0.85–1.03 | 0.17 |
| OS | 1.45 | 0.92 | 0.78–1.06 | 0.25 |
| CHT |
|
|
|
|
|
LRFS | 3.08 | 2.26 | 0.89–5.76 | 0.09 |
|
PFS | 2.33 | 1.68 | 0.86–3.27 | 0.13 |
| OS | 0.01 | 0.16 | 0.38–2.95 | 0.91 |
| Localisation (colon
cancer vs. other neoplasms) |
|
|
|
|
|
LRFS | 1.59 | 2.09 | 0.60–7.25 | 0.24 |
|
PFS | 2.31 | 1.60 | 0.80–4.30 | 0.15 |
| OS | 0.04 | 1.12 | 0.35–3.57 | 0.84 |
Early toxicity of treatment
Complications following brachytherapy of liver
metastases may result from the application of the treatment itself,
and the effects of radiation on liver function (15). In all the patients, no serious
complications (>grade 2 CTCA) were observed. Complications
associated with the application process are presented in Table III. There were no adverse effects of
radiation on liver function in the form of clinical symptoms or
worsening of liver biochemical parameters, including levels of
alanine transaminase, aspartate transaminase or bilirubin.
 | Table III.Early toxicity of treatment. |
Table III.
Early toxicity of treatment.
| Types of
toxicity | CTCAE Grade 1, n
(%) | CTCAE Grade 2, n
(%) | CTCAE Grade 3–5, n
(%) |
|---|
| Pain | 14 (23) | 4 (7) | 0 |
| Nausea or
vomiting | 30 (49) | 0 | 0 |
| Subscapular
Bleeding | 2 (3) | 0 | 0 |
| Pneumothorax | 1 (2) | 0 | 0 |
Discussion
Brachytherapy under CT control has been described as
a safe and effective method for the treatment of primary and
secondary liver lesions (13,16–17). In
the present study, brachytherapy under CT as a palliative method of
treating liver metastases was demonstrated to be an effective and
safe method for the patient and a technically feasible procedure.
In an initial study, Ricke et al (13) achieved 6-month local control at a rate
of 87% using 10–20 Gy doses. Subsequent studies also indicated good
local control: In a phase II clinical study including patients with
metastatic breast cancer (18), rates
of local control were 97, 93.5 and 93.5% for 6, 12 and 18 months,
respectively. In these patients, the 6-, 12- and 18-month PFS rates
were 53, 40 and 27%, respectively, and the 6-, 12- and 18-month OS
rates were 97, 79 and 60%, respectively (18). Analysis of metastases in other primary
sites also generated excellent results. Wieners et al
(19), who studied metastatic cancer
of the pancreas, identified local recurrence in only 10% of
patients. Schippers et al (20), who analyzed neurogenic neoplasm
metastases, only 11% of local recurrence was observed. Similarly,
Bretschneider et al (21), who
examined melanoma metastasis, obtained a local control rate of 90%.
In the present study, the 6- and 12-month LRFS rates were 90 and
64%, respectively. These results were similar to the initial
results in German centers, including the Radiology and Nuclear
Medicine Clinic (Magdeburg) that have extensive experience in this
type of treatment (13,6–17,22). In the present study, the 6- and
12-month OS rate was high (97 and 80%, respectively). It should be
considered, however, that chemotherapy, which was applied following
brachytherapy in 39% of patients, may have affected the OS
rates.
Literature analysis indicated that good local
control could be achieved at doses in the range of 15–25 Gy;
however, in a number of previous studies there were no precise
dosimetric data, and it is unclear which isodose was used (16–21). The
majority of studies indicated that there was a marked dose
dependence and no local recurrence after D100 20.4 Gy
(22). Dose dependence in the range
of 15–25 Gy was not identified in cases of hepatocellular
carcinoma: Local control in these cases was high, at 94% (24). The analysis of the data from the Cox
regression model of the present study indicated that local control
of the tumor following brachytherapy depends on the dose in the
D90 and D100 range. The treatment efficiency
increases with increasing doses.
In the analyzed group, the majority of patients were
those with the primary cancer lesion located in the
gastrointestinal tract; only 15 patients had other primary tumors.
There were no statistically significant differences in prognosis
between these groups, but this may be due to the small number of
patients in the groups. The data from previous studies indicate a
similar prognosis for primary cancer of the pancreas, stomach or
kidney, or in melanoma or neuroendocrine tumors (19–21,24,25).
However, these results should be treated with caution as they are
based on retrospective data, and not on a large number of
patients.
The data from the literature also indicated a good
tolerance and low toxicity of treatment. Ricke and Wust (22) described pain and nausea in patients,
mostly at grade 1 and 2 on the CTCA scale, with <1% at grade 3.
The authors identified pneumothorax in 10% of cases, CTCA grade 2
hemorrhage in 3% of cases, gastric and duodenal ulcers in 1% of
cases and liver abscesses in 1% of cases. In the phase II study
conducted by Wieners et al (18), only 1.5% of patients exhibited severe
hemorrhagic complications. Similarly, in the Indian study, no
significant complications were identified.
Similar results were identified in the patient
cohort. The toxicity of treatment was low, with the majority at
grade 1. None of the patients exceeded the dose of 5 Gy in 2/3
healthy liver tissue, and the dose in 1 cm3 of the
stomach or duodenum was <15 Gy, which is consistent with data
from the literature (21–22,26). No
biochemical evidence of liver toxicity was identified in the
analyzed group, in the form of elevated liver enzymes, which is
consistent with previous analysis (15). Lack of toxicity in grade 3 indicates
good tolerance of treatment, similar to stereotactic radiotherapy
(27).
One limitation of the treatment technique is the
size and location of the lesion. The patients with lesions <8 cm
were eligible for treatment, similar to the criterion used by Ricke
et al (13). Owing to the
potential risk of bleeding, patients whose metastatic lesions were
located in the proximity of large vessels were not eligible. On the
basis of the analyzed group of patients, it could be concluded that
the liver brachytherapy technique is relatively easy to administer,
taking into account compliance with the qualification criteria.
Another limitation of this study was the analysis of singular
groups in which the number of patients was small.
Brachytherapy of liver metastases is an effective
method for local metastatic treatment. The effectiveness of the
treatment is probably dose-dependent, and increases with increasing
dosage. This treatment is well-tolerated and the toxicity of
brachytherapy is negligible.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Turdean S, Gurzu S, Turcu M, Voidăzan S
and Sin A: Liver metastases: Incidence and clinicopathological
data. Acta Medica Marisiensis. 58:254–258. 2012.
|
|
2
|
Tarasik A, Jaroszewicz J and Januszkiewicz
M: Surgical treatment of liver tumors - own experience and
literature review. Clin Exp Hepatol. 3:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Swaid F, Downs D and Rosemurgy AS: A
practical approach to liver metastasis from unknown primary cancer:
What surgeons need to know. Cancer Genet. 209:559–566. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hijazi H, Campeau MP, Roberge D, Donath D,
Lapointe R, Vanderbroucke-Menu F, Taussky D, Boudam K, Chan G,
Bujold A and Delouya G: Stereotactic body radiotherapy for
inoperable liver tumors: Results of a single institutional
experience. Cureus. 8:e9352016.PubMed/NCBI
|
|
5
|
de Baere T, Tselikas L, Yevich S, Boige V,
Deschamps F, Ducreux M, Goere MD, Nguyen F and Malka D: The role of
image-guided therapy in the management of colorectal cancer
metastatic disease. Eur J Cancer. 75:231–242. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kennedy AS: Radiation oncology approaches
in liver malignancies. Am Soc Clin Oncol Educ Book. e150–e155.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dawood O, Mahadevan A and Goodman KA:
Stereotactic body radiation therapy for liver metastases. Eur J
Cancer. 45:2947–2959. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brethsneider T, Ricke J, Gebauer B and
Streitparth F: Image-guided high dose rate brachytherapy of
malignances in various organs-technique, indications and
perspectives. J Contemp Brachytherapy. 8:251–261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kordzińska-Cisek I, Brzozowska A, Cisek P,
Kijek J and Mazurkiewicz M: The role of radiotherapy in the
treatment of hepatic neoplasms. Oncol Radiother. 36:53–60.
2016.
|
|
10
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Picot J, Cooper K, Bryant J and Clegg AJ:
The clinical effectiveness and cost-effectiveness of Bortezomib and
thalidomide in combination regimens with an alkylating agent and a
corticosteroid for the first-line treatment of multiple myeloma: A
systematic review and economic evaluation. Health Technol Assess.
15:1–204. 2011. View
Article : Google Scholar
|
|
12
|
Marinello G and Pierquin B: The Paris
system, optimization of dose, and calculation of treatment timeA
practical manual of Brachytherapy. Medical Physics Publishing;
Madison, WI: pp. 53–68. 1997
|
|
13
|
Ricke J, Wust P, Wieners G, Beck A, Cho C,
Seidensicker M, Pech M, Werk M, Rosner C, Hänninen EL, et al: Liver
malignancies: CT-guided interstitial brachytherapy in patients with
unfavorable lesions for thermal ablation. J Vasc Interv Radiol.
15:1279–1286. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
U.S. Department Of Health And Human
Services, . National Institutes of Health, National Cancer
Institute: Common Terminology Criteria for Adverse Events (CTCAE).
Version v4.03. http://www.oncology.tv/SymptomManagement/NationalCancerInstituteUpdatesCTCAEtov403.aspxJune
14–2010
|
|
15
|
Cisek P, Kordzińska-Cisek I, Charkot Ł,
Korona P, Kieszko D, Bilski M, Brzozowska A, Janiszewski M and
Grzybowska-Szatkowska L: Biochemical liver function markers after
CT-guided brachytherapy for liver metastases. Oncol Radiotherap.
39:67–75. 2017.
|
|
16
|
Ricke J, Mohnike K, Pech M, Seidensticker
M, Rühl R, Wieners G, Gaffke G, Kropf S, Felix R and Wust P: Local
response and impact on survival after local ablation of liver
metastases from colorectal carcinoma by computed tomography-guided
high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys.
78:479–485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mohnike K, Wieners G, Pech M,
Seidensticker M, Rühl R, Lopez-Haenninen E and Ricke J:
Image-guided interstitial high-dose-rate brachytherapy in
hepatocellular carcinoma. Dig Dis. 27:170–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wieners G, Mohnike K, Peters N, Bischoff
J, Kleine-Tebbe A, Seidensticker R, Seidensticker M, Gademann G,
Wust P, Pech M and Ricke J: Treatment of hepatic metastases of
breast cancer with CT-guided interstitial brachytherapy-a phase
II-study. Radiother Oncol. 100:314–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wieners G, Schippers AC, Collettini F,
Schnapauff D, Hamm B and Gebauer B: CT-guided high-dose-rate
brachytherapy in the interdisciplinary treatment of patients with
liver metastases of pancreatic cancer. Hepatobiliary Pancreat Dis
Int. 14:530–538. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schippers AC, Collettini F, Steffen IG,
Wieners G, Denecke T, Pavel M, Wust P and Gebauer B: Initial
experience with CT-guided high-dose-rate brachytherapy in the
multimodality treatment of neuroendocrine tumor liver metastases. J
Vasc Interv Radiol. 28:672–682. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bretschneider T, Mohnike K, Hass P,
Seidensticker R, Göppner D, Dudeck O, Streipath F and Ricke J:
Efficacy and safety of image-guided interstitial single fraction
high-dose-rate brachytherapy in the management of metastatic
malignant melanoma. J Contemp Brachytherapy. 7:154–160. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ricke J and Wust P: Computed
tomography-guided brachytherapy for liver cancer. Semin Radiat
Oncol. 24:287–293. 2011. View Article : Google Scholar
|
|
23
|
Mohnike K, Wieners G, Schwartz F,
Seidensticker M, Pech M, Ruehl R, Wust P, Lopez-Hänninen E,
Gademann G, Peters N, et al: Computed tomography-guided
high-dose-rate brachytherapy in hepatocellular carcinoma: Safety,
efficacy, and effect on survival. Int J Radiat Oncol Biol Phys.
78:172–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Geisel D, Denecke T, Collettini F, Grieser
C, Wust P, Thuss-Patence P, Hamm B and Gebaueret B: Treatment of
hepatic metastases from gastric or gastroesophageal adenocarcinoma
with computed tomography-guided high-dose-rate brachytherapy
(CT-HDRBT). Anticancer Res. 32:5453–5458. 2012.PubMed/NCBI
|
|
25
|
Geisel D, Collettini F, Denecke T, Grieser
C, Flörcken A, Wust P, Hamm B and Gebaueret B: Treatment for liver
metastasis from renal cell carcinoma with
computed-tomography-guided high-dose-rate brachytherapy (CT-HDRBT):
A case series. World J Urol. 31:1525–1530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sharma DN, Thulkar S, Sharma S, Gandhi AK,
Haresh KP, Gupta S, Rath GK and Julka PK: High-dose-rate
interstitial brachytherapy for liver metastases: First study from
India. J Contemp Brachytherapy. 5:70–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Joo JH, Park JH, Kim JC, Yu CS, Lim SB,
Park IJ, Kim TW, Hong YS, Kim KP and Yoon SM: Local control
outcomes using stereotactic body radiation therapy for liver
metastases from colorectal cancer. Int J Radiat Oncol Biol Phys.
99:876–883. 2017. View Article : Google Scholar : PubMed/NCBI
|