Introduction
Nasopharyngeal carcinoma (NPC) is a cancer pattern
that often develops in epithelial cells of nasopharynx (1). The tumorigenesis of NPC has a number of
causes, including genetic alternation, environmental factors and
virus infection (2). Most NPCs are
associated with Epstein-Barr virus (EBV) infection, and EBV genome
can be detected in approximately 90% of NPC tissues (3). NPC is the 24th most common cancer type
worldwide. It is estimated that there were approximately 84,400
newly diagnosed cases and 51,600 deaths during 2008 (4). NPCs are categorized into three main
types according to the differentiation degree based on the World
Health Organization classification. The three main types are
keratinizing, non-keratinizing and Basaloid carcinoma (5). Therefore, the development of new
therapeutic interventions for NPCs is imperative.
MicroRNAs (miRNAs) are small non-coding RNAs, which
are 20–22 nucleotides long (6).
miRNAs regulate the expression of target genes by binding to 3′-UTR
at the post-transcriptional level (7,8). At
present, a number of miRNAs have been identified with a
dysfunctional role in various types of cancer. Such miRNAs function
as a key inducer in tumor initiation and development (9). miR-100 is a miRNA that has been
identified in a number of cancers. Henson et al (10) reported that miR-100 markedly inhibited
the cell migration and invasion of oral squamous cell carcinoma
(OSCC). miR-100 also regulates gene expression, including genes
involved in radioresistant OSCC (10). miR-100 is involved in the modulation
of G1/S transition and inhibits the terminal differentiation of
acute myeloid leukemia (11). RBSP3
often functions as a tumor suppressor (12). miR-100 promotes the cell proliferation
of acute myeloid leukemia by targeting RBSP3 (11). However, the potential mechanism of
miR-100 in NPC is unclear.
The insulin-like growth factor pathway plays an
important role in cell differentiation and apoptosis (13). Insulin-like growth factor 1 receptor
(IGF1R) is one component of this signaling pathway (14). IGF1R is a transmembrane receptor,
which is a tyrosine kinase. It is comprised of two β subunits and
two α subunits (15). IGF1R is
involved in a number of diseases. IGF1R expression is highly
increased in non-small cell lung cancer cells compared with
corresponding normal cells. In addition, IGF1R expression is
associated with EGFR expression (16). Yuen et al reported that IGF1R
expression is significantly higher in clear cell renal cell cancer
(CCRCC) than normal kidney cells and is negatively correlated with
von Hippel-Lindau (VHL) expression (17). Nevertheless, the function of IGF1R in
NPC remains unclear.
In the present study, we investigated the expression
of miR-100 and IGF1R and their function in NPC. We identified that
miR-100 expression was significantly reduced in NPC cells compared
with corresponding non-cancerous cells, detected by reverse
transcription-quantitative PCR (RT-qPCR). Overexpression of miR-100
significantly suppressed the migration and invasion of NPC cells.
IGF1R was a downstream target of miR-100, as confirmed by
luciferase reporter assay. IGF1R expression was highly increased in
NPC cells compared with non-tumorous cells. Knockdown of IGF1R by
siRNA significantly inhibited NPC cell proliferation, which was
confirmed by western blot and MTT assays.
Materials and methods
Cell lines and patient samples
A total of 115 pairs of NPC tissues and their
corresponding non-cancerous tissues were collected from patients
who underwent NPC surgery between January 2015 and March 2017 in
the Weifang People's Hospital (Weifang, China). The collected
tissues were preserved in liquid nitrogen. None of the patients had
received radiotherapeutic treatment before tissues were collected.
Patients provided informed consent for the use of tissues in this
study. The study was approved by the Ethics Committee of Weifang
People's Hospital.
NPC cell lines C666-1 and SUNE1 were purchased from
the American Type Culture Collection (Manassas, VA, USA). An NP460
normal nasopharyngeal epithelium cell line was used as the control.
Cell lines were cultured in RPMI-1640 medium, which contained fetal
bovine serum.
Plasmid construction and cell
transfection
miR-100 mimic was synthesized and purchased from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). miR-100 mimic
was used to overexpress miR-100. siRNA for IGF1R was synthesized to
knock down IGF1R gene.
The vectors (including miR-100 mimic and siRNA-IGF1R
associated, which served as the negative control) were transfected
into the cell lines using Lipofectamine 2000 Reagent (Tiangen
Biotech Co., Ltd., Beijing, China) following the manufacturers
protocol.
Western blot assay
Tissue proteins were extracted using ProteinExt
Mammalian Total Protein Extraction kit (TransGen Biotech Co., Ltd.,
Beijing, China). Protein concentration was detected using the
Bradford assay. Proteins were separated on 12% SDS-PAGE gel using
Bio-Rad Mini-PROTEAN Tetra instrument (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Then, proteins were electrotransferred onto
polyvinylidene difluoride membranes. The membranes were incubated
with primary antibodies against IGF1R and GAPDH (Sigma-Aldrich, St.
Louis, MO, USA). Signals were visualized on Bio-Rad Gel Doc XR
instrument (Bio-Rad Laboratories, Inc.).
RT-qPCR
Total RNAs of tissues were extracted using the
EasyPure RNA kit (TransGen Biotech Co., Ltd.). RNAs were confirmed
to be eligible using agarose gel electrophoresis. Then RNAs were
inverse transcribed to single-strand cDNA using EasyScript
First-Strand cDNA Synthesis SuperMix (TransGen Biotech Co., Ltd.).
miR-100 expression was normalized by the U6 expression level and
GAPDH expression was used as an internal control for IGF1R.
Experiments were performed three times.
Luciferase reporter assay
Target Scan online tool (http://www.targetscan.org/vert_71/) was used to search
the potential target of miR-100 in human. IGF1R was demonstrated as
a downstream target of miR-100 and binds to 5602–5609 bp at 3′-UTR
of IGF1R. In order to confirm this prediction, Psicheck™-2 vector
was used in this study. The 3′-UTR of IGF1R was cloned and inserted
into Psicheck™-2 plasmid (Psicheck™-2-WT). The binding site was
also mutated and inserted into Psicheck™-2 plasmid
(Psicheck™-2-MT). The Dual-Glo Luciferase Assay System (Promega
Corporation, Madison, WI, USA) was used to measure luciferase
activity.
Migration and invasion assay
Transwell assay was used to measure cell migration
and invasion. Cells (3×104) were placed onto the top
component of one chamber (Corning Life Sciences, Manassas, VA, USA)
(pore size, 8 µm). The lower chamber was filled with serum-free
medium. The chamber was cultured for 48 h using migration assay.
Extracellular matrix gel was used for the cell invasion assay.
Finally, migration and invasion cells were stained with crystal
violet. Results were visualized using a light microscope (Olympus
Corporation, Tokyo, Japan).
Proliferation assay
The 3-[4,
5-di-methylthi-azol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT)
assay was used to detect cell proliferation. Cell suspensions of
100 µl were added to each well. The 96-well plate was incubated at
5% CO2 and 37°C for 48 h. Then, 10 µl MTT solution (5
mg/ml) was added to each well and cultured for 4 h. The crystals
were dissolved in 150 µl DMSO and absorbance at 490 nm was measured
using an Eppendorf BioSpectrometer® kinetic instrument
(Eppendorf, Hamburg, Germany).
Statistical analysis
Experiments in the present study were performed at
least three times. Experimental results were presented as mean ±
SD. The SPSS 16.0 (SPSS, Inc, Chicago, IL, USA) software was used
for data analysis comparisons were made using Student's t-test and
one-way ANOVA post hoc test. A P<0.05 was considered to indicate
a statistically significant difference.
Results
miR-100 expression is significantly
decreased in NPC tissues
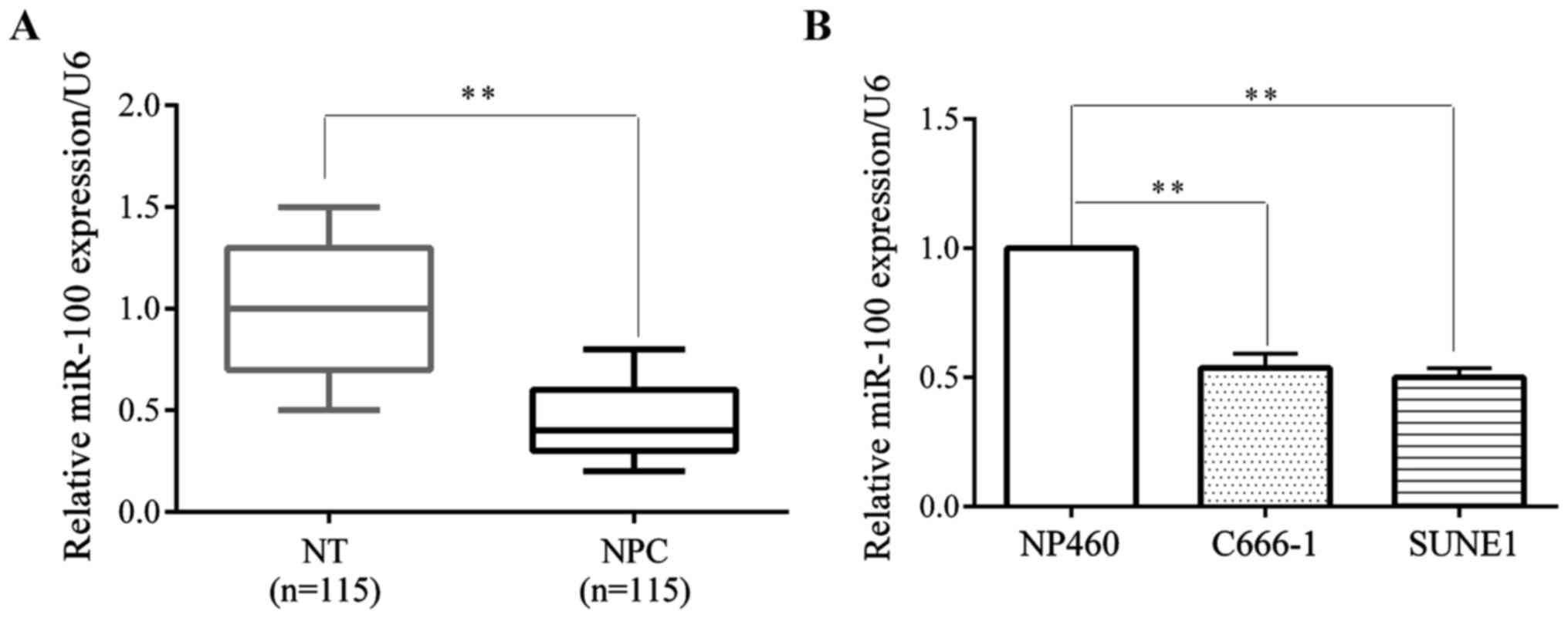
In the present study, we investigated the miR-100
expression level in NPC and normal cells. miR-100 expression level
was significantly decreased in 115 pairs of NPC tissues compared
with non-cancerous tissues by RT-qPCR (Fig. 1A). We also investigated the miR-100
expression levels of NPC C666-1 and SUNE1 cell lines and the normal
NP460 nasopharyngeal epithelium cell line. The data showed that the
miR-100 expression level was significantly suppressed in NPC cell
lines compared with, the NP460 normal nasopharyngeal cell line
(Fig. 1B).
Overexpression of miR-100
significantly inhibits cell migration and invasion in vitro
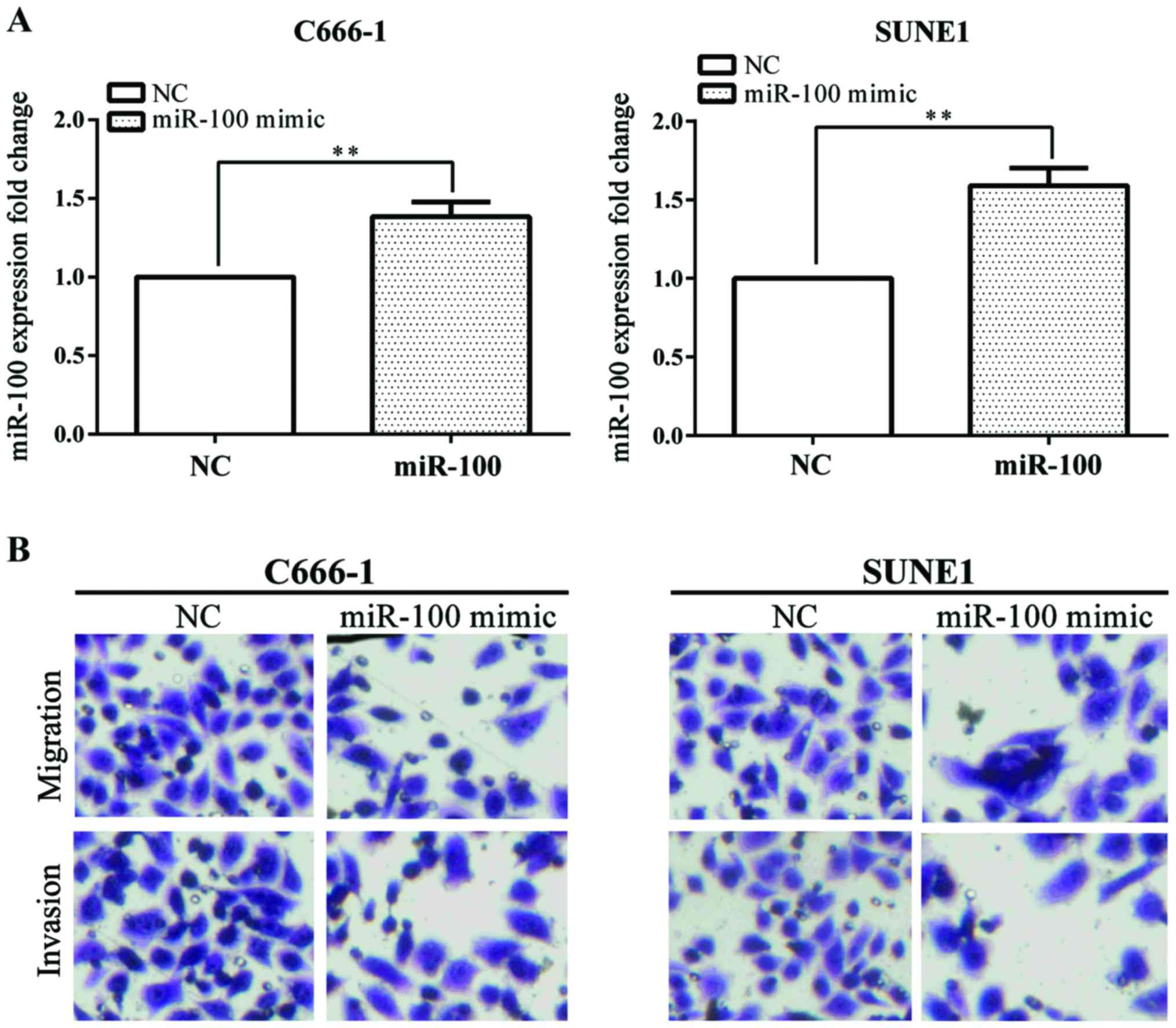
Transwell assay was used to investigate the effect
of miR-100 on cell migration and invasion. miR-100 mimic was used
to overexpress miR-100 in C666-1 and SUNE1 cell lines. The
overexpression of miR-100 was confirmed by RT-qPCR (Fig. 2A).
Overexpression of miR-100 stably suppressed C666-1
and SUNE1 cell migration and invasion by Transwell assay (Fig. 2B). These results showed that the
overexpression of miR-100 inhibited NPC cell invasion and
migration.
IGF1R is a downstream target of
miR-100 and is downregulated by miR-100
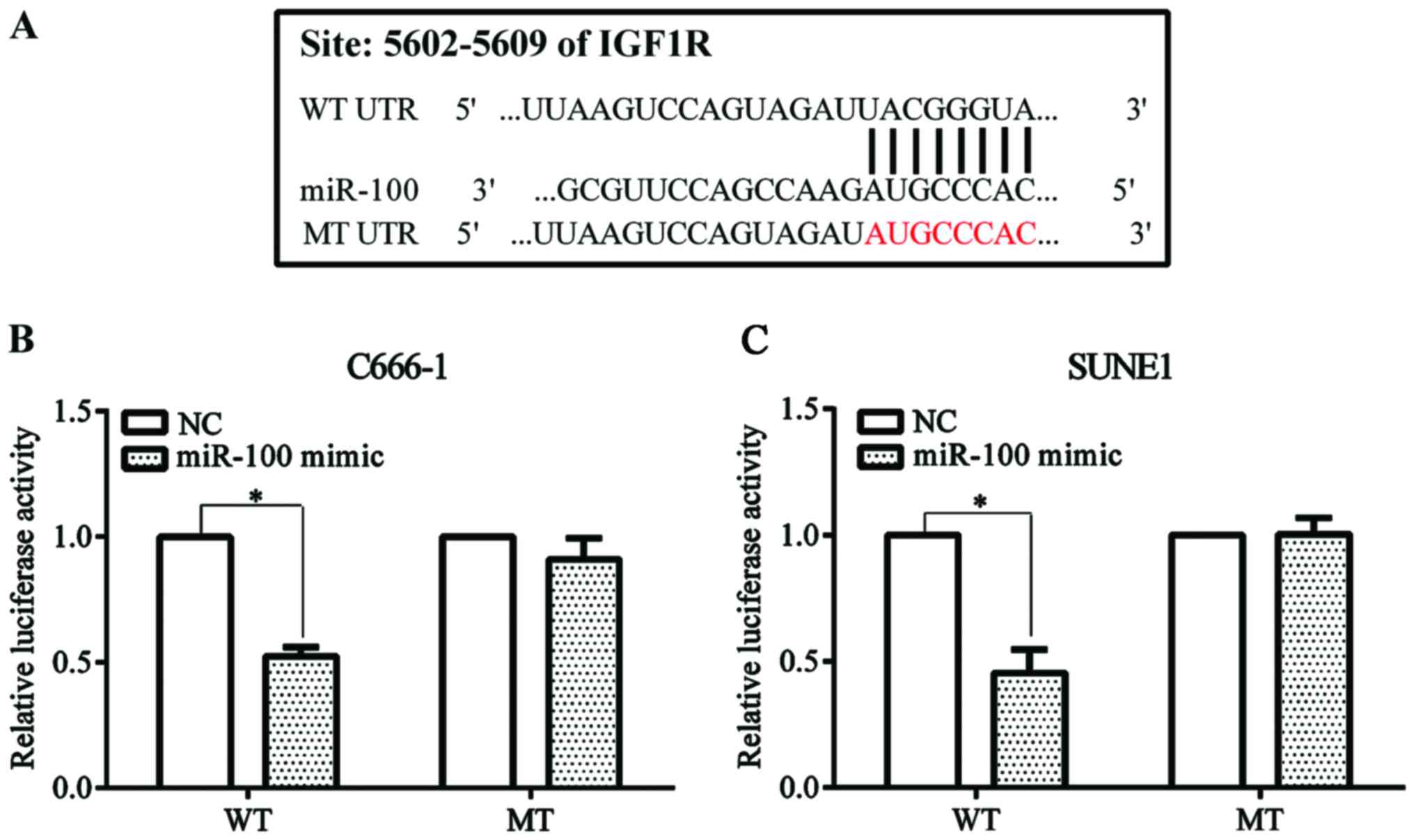
IGF1R was predicted as a potential target of miR-100
by the online Target Scan tool (http://www.targetscan. org/vert_71/) (Fig. 3A). We constructed Psicheck™-2-WT and
Psicheck™-2-MT plasmid. NPC C666-1 and SUNE1 cells were
co-transfected with miR-100 mimic or the negative control and
Psicheck™-2-WT or Psicheck™-2-MT plasmid. Luciferase activity was
significantly decreased when the cell line was co-transfected with
miR-100 mimic and Psicheck™-2-WT plasmid compared with the negative
control. The reduced effect disappeared when the cell line was
co-transfected with miR-100 mimic and Psicheck™-2-MT plasmid
(Fig. 3B).
Knockdown of IGF1R inhibits cell
proliferation of NPC cell lines
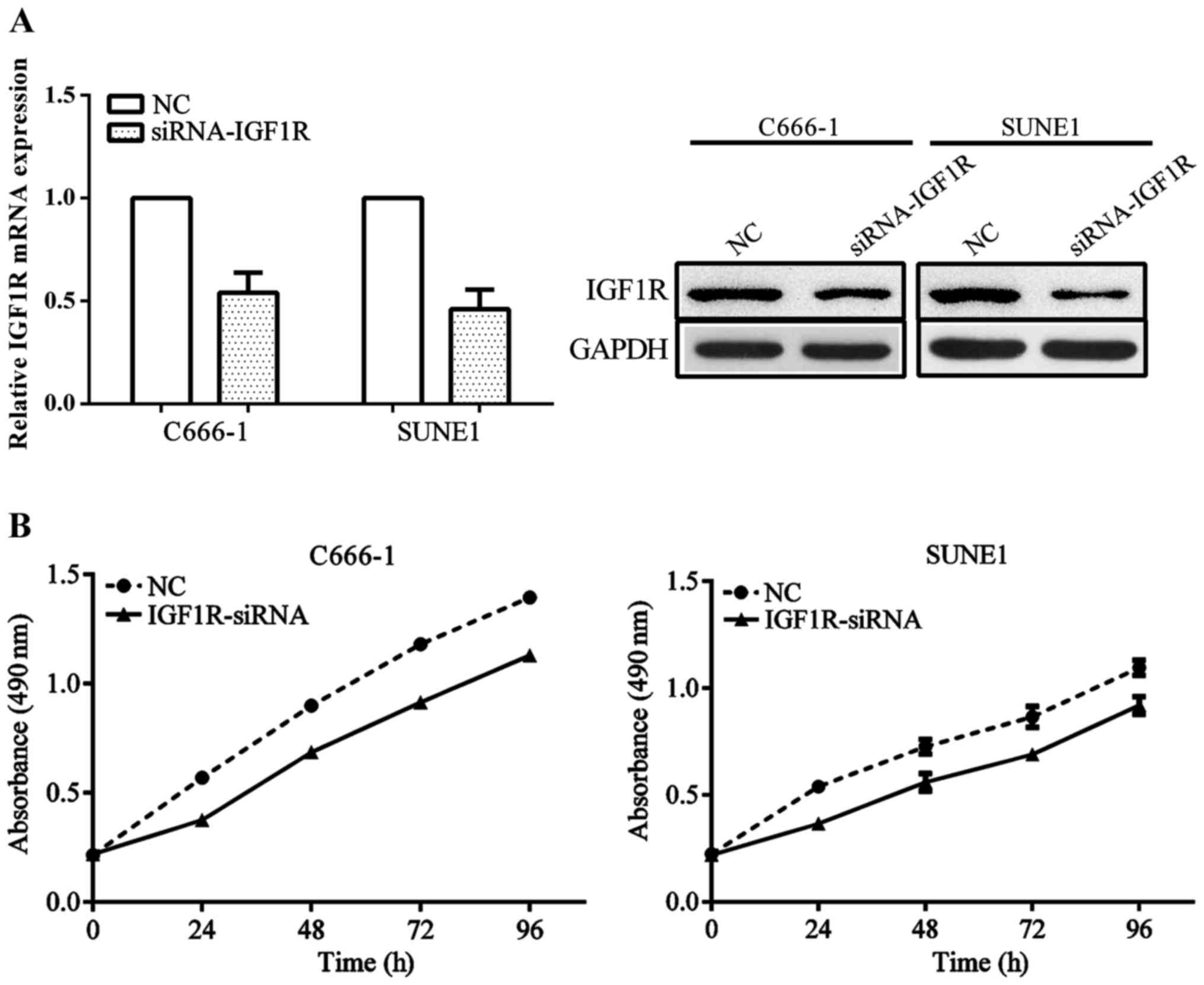
In order to better understand the mechanism of IGF1R
on NPC development, siRNA for IGF1R was used to knockdown the
expression of IGF1R. The NPC C666-1 and SUNE1 cell lines were
transfected with siRNA-IGF1R or negative control. The IGF1R mRNA
and protein level was significantly decreased in cell lines
transfected with siRNA compared with the negative control (Fig. 4A). In addition, the proliferation of
cell lines transfected with siRNA were significantly reduced
compared with the cell lines transfected with the negative control
(Fig. 4B).
Discussion
NPC is a rare cancer type compared with other high
incidence cancers. However, in specific areas, including
southeastern Asia, Singapore, Vietnam, and Philippines, NPC has a
markedly high incidence (18,19). NPC tumorigenesis is complicated and
remains unclear.
In the present study, we investigated the mechanism
of miR-100 and the effect of IGF1R on NPC. We identified that
miR-100 was highly downregulated in NPC cell lines compared with
the normal cell lines and NPC tissues compared with non-tumorous
tissues. In addition, the overexpression of miR-100 following
transfection of the mimic into the two cell lines significantly
inhibited migration and invasion. Many miRNAs have been reported to
play an important role in the development of NPC. miR-BART22 is a
newly identified EBV miRNA and can be detected in NPC.
Overexpression of miR-BART22 may promote tumor cell invasion and
proliferation. MAP3K5 is a target of miR-BART22 and is
downregulated by miR-BART22 (20). In
cisplatin-resistant NPC cells, miR-10b expression is increased in
HNE1 cell lines. Overexpression of miR-10b promotes
epithelial-mesenchymal transition (21). Cheung et al reported that
miR-183 and miR-86 expression were decreased in NPC spheroids
(22). In addition, transfection of
miR-183 into cell lines suppressed tumor growth (21,22).
miRNAs have diverse effects on NPC development. Some miRNAs inhibit
NPC cell growth; on the other hand, some miRNAs promote NPC cell
growth. miR-15a demonstrates the suppressive effect on NPC cells.
Overexpression of miR-15a significantly inhibits cell growth and
triggers cell apoptosis (23). Few
reports are available on miR-100. miR-100 has been investigated in
oral cancer (10), acute myeloid
leukemia (11) and endometrioid
endometrial carcinoma (24).
IGF1R is important in the functional transformation
of many oncogenes (25). In the
present study, we identified that IGF1R was upregulated in the NPC
C666-1 cell line using a western blot assay and downregulated by
miR-100, which was confirmed by luciferase assay. Knockdown of
IGF1R by siRNA inhibited NPC cell proliferation. IGF1R-targeted
therapy significantly suppresses pancreatic cell growth and induces
apoptosis of pancreatic cancer cells (26). Consistent with our study, in ALK
fusion-positive lung cancer, suppression of IGF1R expression
reduced cell growth and promotes apoptosis (27). IGF1R demonstrates different functions
in different cancer types. In addition, RBSP3 has been identified
as a downstream target of miR-100 (11).
miR-100 may have many downstream targets and these
potential targets remain to be identified. In future research, we
aim to identify other targets to obtain a better understanding of
NPC. Additionally, IGF1R may have other potential downstream genes
and further studies should be conducted.
In summary, we identified that the overexpression of
miR-100 may significantly inhibit the migration and invasion of NPC
cells. IGF1R is a target of miR-100. The newly identified
miR-100/IGF1R axis provides a new biomarker for developing the
treatment of NPC and deepens our understanding of the mechanism of
NPC tumorigenesis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors contributions
YC contributed to the conception of the study. XS
contributed significantly to perform the experiment and helped to
write the manuscript. XL wrote the manuscript and helped to perform
the experiment. YW performed the data analyses. SY and TY helped
perform the analysis with constructive discussions. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Weifang People's Hospital (Weifang, China). Patients provided
informed consent for this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
miRNAs
|
microRNAs
|
|
NPC
|
nasopharyngeal carcinoma
|
|
EBV
|
Epstein-Barr virus
|
|
OSCC
|
oral squamous cell carcinoma
|
|
MTT
|
3-[4,
5-di-methylthi-azol-2-yl]-2,5-diphenyl-tetrazolium bromide
|
|
IGF1R
|
insulin-like growth factor 1
receptor
|
|
CCRCC
|
clear cell renal cell cancer
|
|
VHL
|
von Hippel-Lindau
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
References
|
1
|
Brennan B: Nasopharyngeal carcinoma.
Orphanet J Rare Dis. 1:23–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang ET and Adami HO: The enigmatic
epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol
Biomarkers Prev. 15:1765–1777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruce JP, Yip K, Bratman SV, Ito E and Liu
FF: Nasopharyngeal cancer: Molecular landscape. J Clin Oncol.
33:3346–3355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thompson L: World Health Organization
classification of tumours: Pathology and genetics of head and neck
tumours. Ear Nose Throat J. 85:742006.PubMed/NCBI
|
|
6
|
Hutvágner G and Zamore PD: A microRNA in a
multiple-turnover RNAi enzyme complex. Science. 297:2056–2060.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y and Kowdley KV: MicroRNAs in common
human diseases. Genomics Proteomics Bioinformatics. 10:246–253.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
OConnell RM, Rao DS and Baltimore D:
microRNA regulation of inflammatory responses. Annu Rev Immunol.
30:295–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Henson BJ, Bhattacharjee S, ODee DM,
Feingold E and Gollin SM: Decreased expression of miR-125b and
miR-100 in oral cancer cells contributes to malignancy. Genes
Chromosomes Cancer. 48:569–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng YS, Zhang H, Zhang XJ, Feng DD, Luo
XQ, Zeng CW, Lin KY, Zhou H, Qu LH, Zhang P, et al: MiR-100
regulates cell differentiation and survival by targeting RBSP3, a
phosphatase-like tumor suppressor in acute myeloid leukemia.
Oncogene. 31:80–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kashuba VI, Li J, Wang F, Senchenko VN,
Protopopov A, Malyukova A, Kutsenko AS, Kadyrova E, Zabarovska VI,
Muravenko OV, et al: RBSP3 (HYA22) is a tumor suppressor gene
implicated in major epithelial malignancies. Proc Natl Acad Sci
USA. 101:4906–4911. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Samani AA, Yakar S, LeRoith D and Brodt P:
The role of the IGF system in cancer growth and metastasis:
Overview and recent insights. Endocr Rev. 28:20–47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Casa AJ, Dearth RK, Litzenburger BC, Lee
AV and Cui X: The type I insulin-like growth factor receptor
pathway: a key player in cancer therapeutic resistance. Front
Biosci. 13:3273–3287. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Steele-Perkins G, Turner J, Edman JC, Hari
J, Pierce SB, Stover C, Rutter WJ and Roth RA: Expression and
characterization of a functional human insulin-like growth factor I
receptor. J Biol Chem. 263:11486–11492. 1988.PubMed/NCBI
|
|
16
|
Dziadziuszko R, Merrick DT, Witta SE,
Mendoza AD, Szostakiewicz B, Szymanowska A, Rzyman W, Dziadziuszko
K, Jassem J, Bunn PA Jr, et al: Insulin-like growth factor receptor
1 (IGF1R) gene copy number is associated with survival in operable
non-small-cell lung cancer: A comparison between IGF1R fluorescent
in situ hybridization, protein expression, and mRNA expression. J
Clin Oncol. 28:2174–2180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuen JS, Cockman ME, Sullivan M, Protheroe
A, Turner GD, Roberts IS, Pugh CW, Werner H and Macaulay VM: The
VHL tumor suppressor inhibits expression of the IGF1R and its loss
induces IGF1R upregulation in human clear cell renal carcinoma.
Oncogene. 26:6499–6508. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Katanoda K and Marugame T: Comparison of
time trends in cancer incidence (1973-1997) in East Asia, Europe
and USA, from Cancer Incidence in Five Continents Vol. IV–VIII. Jpn
J Clin Oncol. 37:157–159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jia WH, Huang QH, Liao J, Ye W, Shugart
YY, Liu Q, Chen LZ, Li YH, Lin X, Wen FL, et al: Trends in
incidence and mortality of nasopharyngeal carcinoma over a 20–25
year period (1978/1983-2002) in Sihui and Cangwu counties in
southern China. BMC Cancer. 6:1782006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen R, Zhang M, Li Q, Xiong H, Liu S,
Fang W, Zhang Q, Liu Z, Xu X and Jiang Q: The Epstein-Barr
Virus-encoded miR-BART22 targets MAP3K5 to promote host cell
proliferative and invasive abilities in nasopharyngeal carcinoma. J
Cancer. 8:305–313. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang P, Hong H, Sun X, Jiang H, Ma S,
Zhao S, Zhang M, Wang Z, Jiang C and Liu H: MicroRNA-10b regulates
epithelial-mesenchymal transition by modulating
KLF4/Notch1/E-cadherin in cisplatin-resistant nasopharyngeal
carcinoma cells. Am J Cancer Res. 6:141–156. 2016.PubMed/NCBI
|
|
22
|
Cheung CC, Lun SW, Chung GT, Chow C, Lo C,
Choy KW and Lo KW: MicroRNA-183 suppresses cancer stem-like cell
properties in EBV-associated nasopharyngeal carcinoma. BMC Cancer.
16:4952016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu K, He Y, Xia C, Yan J, Hou J, Kong D,
Yang Y and Zheng G: MicroRNA-15a inhibits proliferation and induces
apoptosis in CNE1 nasopharyngeal carcinoma cells. Oncol Res.
24:145–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Torres A, Torres K, Pesci A, Ceccaroni M,
Paszkowski T, Cassandrini P, Zamboni G and Maciejewski R:
Deregulation of miR-100, miR-99a and miR-199b in tissues and plasma
coexists with increased expression of mTOR kinase in endometrioid
endometrial carcinoma. BMC Cancer. 12:3692012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sell C, Rubini M, Rubin R, Liu JP,
Efstratiadis A and Baserga R: Simian virus 40 large tumor antigen
is unable to transform mouse embryonic fibroblasts lacking type 1
insulin-like growth factor receptor. Proc Natl Acad Sci USA.
90:11217–11221. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou H, Qian W, Uckun FM, Wang L, Wang YA,
Chen H, Kooby D, Yu Q, Lipowska M, Staley CA, et al: IGF1 receptor
targeted theranostic nanoparticles for targeted and image-guided
therapy of pancreatic cancer. ACS Nano. 9:7976–7991. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
IGF1R signaling is a therapeutic target in
ALK fusion-positive lung cancer. Cancer Discov. 4:12512014.
View Article : Google Scholar
|