Introduction
Gastric cancer has high mortality, ranks second only
to lung cancer and the incidence is fourth with 400,000 individuals
suffering gastric cancer annually in China, according to a global
statistical report published by the World Health Organization (WHO)
in 2016. Patients with gastric cancer were generally diagnosed at
an advanced stage with poor prognosis and recurrence (1–4).
Therefore, identifying tumor molecular markers for early diagnosis
is essential for patients with gastric carcinoma.
MicroRNAs (miRNAs/miRs) are a group of small
non-coding regulatory molecules with 22–28 nucleotides sequence in
length (5). miRNAs through base
pairing with the 3′-untranslated regions (3′UTR) of target
messenger RNAs (mRNAs) or directly cleaving the mRNA at the
post-transcriptional cause mRNA degradation and/or translational
repression (6–9). miR-133a, a kind of miRNA, has been
reported to be aberrantly expressed in several carcinomas,
including osteosarcoma, ovarian carcinoma, lung cancer, esophageal
carcinoma and even in gastric carcinoma and other types of cancer
(10–13). In oral squamous cell carcinoma,
miR-133a inhibits cell proliferation and invasion by suppressing
COL1A1 (14). Furthermore, in
colorectal cancer, miR-133a acts as a tumor suppressor to inhibit
cell growth and migration by targeting elF4A1 in vitro
(15). In addition, miR-133a had a
low expression and inhibited cell proliferation, invasion and
induced apoptosis in gastric carcinoma cells (12,16,17). It
could also bind to a variety of genes, including COL1A1,
FSCN1 and ubiquitin specific protease 39 (USP39)
(14,16,18). It
has been reported that miR-133a inhibits cell progression by
targeting USP39 in pancreatic cancer (18).
USP39, one of the deubiquitinating enzymes (DUBs)
without ubiquitin protease activity, is involved in RNA splicing
and was necessary to maintain a spindle checkpoint (19–22). USP39
has been demonstrated to act as an oncogenic factor in many tumors,
such as colorectal cancer, lung cancer, melanoma, pancreatic cancer
and osteosarcoma cancer (18,23–26). In
osteosarcoma cells, a decreased USP39 expression inhibited cell
growth and induced apoptosis (26).
In melanoma, knockdown USP39 inhibited cell growth and induced cell
cycle arrest and apoptosis (25).
Thus, to determine the molecular mechanism of miR-133a and USP39 on
cell proliferation and prognosis is critical for planning
therapeutic strategies, assessing prognosis, and monitoring
response to therapy. Therefore, in the present study, we aim to
test the functional role that miR-133a plays in tumor progression
and the correlation with USP39. Furthermore, we also measured the
overall survival (OS) and disease-free survival (DFS) according to
the expression of miR-133a and USP39.
Materials and methods
Patients and clinical samples
According to WHO classification, we obtained 53
paired gastric cancer tissues and paracancerous tissues (PT) from
patients presenting for treatment in Jining First People's Hospital
(Jining, China) from January 2014 to December 2016. Fresh resected
samples were immediately cut and snap-frozen in liquid nitrogen and
stored in a freezer at −80°C. The patients had no treatment before
surgery. The complete clinicopathological characteristics of
patients, including age, sex, tumor grade and TNM stage are
described in Table I. Informed
consent to use the specimens in this study was obtained from the
patients. The study was approved by the Ethics Committee of Jining
First People's Hospital (Jining, China).
 | Table I.miR-133a expression and
clinicopathological characteristics in 53 paired gastric
cancer. |
Table I.
miR-133a expression and
clinicopathological characteristics in 53 paired gastric
cancer.
|
|
| miR-133a
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | Cases (n=53) | High (%) | Low (%) |
P-valuea |
|---|
| Sex |
|
|
|
|
|
Male | 30 | 14 (46.7) | 16 (53.3) | 0.511 |
|
Female | 23 | 13 (56.5) | 12 (43.5) |
|
| Age (years) |
|
|
|
|
|
≤60 | 21 | 13 (61.9) | 8
(38.1) | 0.082 |
|
>60 | 32 | 12 (37.5) | 20 (62.5) |
|
| Tumor size
(mm) |
|
|
|
|
|
≤5.0 | 23 | 15 (65.2) | 8
(34.8) | 0.021a |
|
>5.0 | 30 | 10 (33.3) | 20 (66.7) |
|
| TNM stage |
|
|
|
|
|
I–II | 24 | 15 (62.5) | 9
(37.5) | 0.042a |
|
III–IV | 29 | 10 (34.5) | 19 (65.5) |
|
| Local invasion |
|
|
|
|
|
T1-T2 | 25 | 15 (60.0) | 10 (40.0) | 0.077 |
|
T3-T4 | 28 | 10 (35.7) | 18 (64.3) |
|
| Lymph-node
metastasis |
|
|
|
|
|
0-2 | 29 | 18 (62.1) | 11 (37.9) | 0.017a |
|
>2 | 24 | 7
(29.2) | 17 (70.8) |
|
| Ki-67 |
|
|
|
|
|
<14% | 15 | 10 (66.7) | 5
(33.3) | 0.074 |
|
≥14% | 38 | 15 (39.5) | 23 (60.5) |
|
| USP39 |
|
|
|
|
|
Negative | 27 | 17 (63.0) | 10 (37.0) | 0.019a |
|
Positive | 26 | 8
(30.8) | 18 (69.2) |
|
Cell lines and culture condition
Two gastric cancer cell lines (HGC-27 and MGC-803)
and one normal gastric cell GES-1 were purchased from the American
Type Culture Collection (Rockville, MD, USA). The cells were
maintained in RPMI-1640 medium (Gibco, Carlsbad, CA, USA) add in
15% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA) cultured
at 37°C in a fully humidified atmosphere containing 5%
CO2.
RNA isolation and RT-qPCR
Total miRNAs or mRNAs were isolated and purified by
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) or miRcute and
Separation of miRNAs kit (Tiangen Biotech Co., Ltd., Beijing,
China). Initially, reverse transcription was employed to produce
the first cDNA chain used PrimeScript™ II 1st Strand cDNA Synthesis
kit (Takara Biotechnology Co., Ltd., Dalian, China). Quantitative
PCR was then carried out using SYBR Prime Script miRNA RT-PCR kit
or SYBR Premix kit (both purchased from Takara Bio, Inc., Otsu,
Japan) according to the manufacturer's protocol. The primer
sequences used were: miR-133a forward,
5′-ATAAGAATGCGGCCGCATTCCAAACTAGCAGCACTA-3′ and reverse,
5′-AGCTTTGTTTAAACTTAACCATTCTAGCTTTTCC-3′; USP39 forward,
5′-GCTGATGATGATTGATGCT-3′ and reverse, 5′-GCTCCAAGAATCCCAAGGCT-3′;
GAPDH forward, 5′-CCACTCCTCCACCTTTGAC-3′ and reverse,
5′-ACCCTGTTGCTGTAGCCA-3′; and U6 forward, 5′-CTTCGGCAGCACATATACT-3′
and reverse, 5′-AAAATATGGAACGCTTCACG-3′. The thermocycling
parameters were as follows: 95°C for 3 min and 40 cycles of 95°C
for 15 sec followed by 60°C for 30 sec. The normalization of miRNA
and mRNA was U6 and GAPDH, respectively. miRNA and mRNA expression
levels were subsequently calculated using the 2−ΔΔCq
method. All the RT-qPCRs were run in triplicate.
Transfection
For the miRNA, due to its downregulation of miR-133a
in gastric cancer cells HGC-27 and MGC-803, miR-133a was
overexpressed through transfected miR-133a mimic. In addition,
USP39 was intervened used small interfering RNA (siRNA) to measure
the efficiency of USP39 in gastric cancer cells.
Suitable cells were inoculated into 6-well plates
and cultivated overnight at 37°C. And then we transfected the
special vector utilized Lipofectamine 3000 Reagent
(Invitrogen).
Protein extraction and western blot
analysis
Total proteins were lysed with RIPA lysis buffer
with proteinase inhibitor (both from Beyotime, Shanghai, China)
extracted from cancer cells. Proteins with the same quality were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred onto a PVDF membrane
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membrane were
incubated overnight at 4°C with rabbit anti-USP39 polyclonal
antibody (dilution, 1:1,000, cat. no. U0385; Sigma-Aldrich), with
anti-GAPDH mouse monoclonal antibody (dilution 1:2,000, cat. no.
G8795; Sigma-Aldrich) as internal control. After washed the extra
antibody with TBST, the membranes were incubated with rabbit
secondary antibody (dilution 1:5,000, cat. no. sc-362280; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) containing horseradish
peroxidase-conjugated for 2 h at room temperature. ECL Western
Blotting Detection System (BestBio, Beijing, China) was applying to
perform the interest proteins (CCNG1 and GAPDH) and visualized on
Bio-Rad Gel Doc XR instrument (Bio-Rad Laboratories, Inc.).
Cell proliferation assay
Cells were seeded in 96-well plates and
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT; Santa Cruz Biotechnology, Inc.) was dissolved in
phosphate-buffered saline (Biotech, Jiangsu, China) before
experiment. MTT and dimethyl sulfoxide (DMSO) solutions were
utilized to detect cell proliferative activity. After the cells
were cultured 1, 2, 3 or 4 days, 10 µl MTT solution was added into
each well. Then, 150 µl DMSO was added to destroy the cells after
incubation at 37°C for approximately 4 h. Finally, after agitation
for 10 min, absorbance was measured at 490 nm on a microplate
reader (BioTek Instruments, Inc., Winooski, VT, USA).
Plasmid construction and luciferase
reporter assay
TargetScan (http://www.targetscan.org/vert_71/) was used to
predict potential target genes of miR-133a and identified USP39 as
a potential target. Firstly, we cloned miR-133a mimic into pmirGlo
vector and inserted the target sequences on USP39 into pcDNA3.1
plasmid vector (pcDNA3.1-USP39-WT). Secondly, after mutation
(QuikChange Multi Site-Directed Mutagenesis kit; Santa Clara, CA,
USA) the target sequences used were from 5′-…A CCAGCA…-3′ to
5′-…UAUCGCA…-3′, and the mutation fragment was inserted into
pcDNA3.1 plasmid vector (pcDNA3.1-USP39-MUT).
Luciferase reporter activity was measured after
co-transfection with miR-133a mimic or negative control and
pcDNA3.1-USP39-WT or pcDNA3.1o-USP39-MUT into gastric cancer cells
HGC-27 and MGC-803. The experiment kit used was the
Dual-Luciferase® Reporter Assay System (Promega,
Madison, WI, USA) and Renilla luciferase was used as
normalization.
Statistical analysis
Statistical analyses were presented as the mean ±
standard deviation using SPSS19.0 software (SPSS, Inc., Chicago,
IL, USA). The Student's t-test or ANOVA and Scheffe test were used
to perform the statistical analysis. Pearson's χ2 test
was used to test the correlation between miR-133a and USP39 and
clinicopathologic characteristics of gastric carcinoma. In
addition, the Kaplan-Meier method with log-rank test was used for
analyzing survival. P<0.05 was considered to indicate a
statistically significant difference.
Results
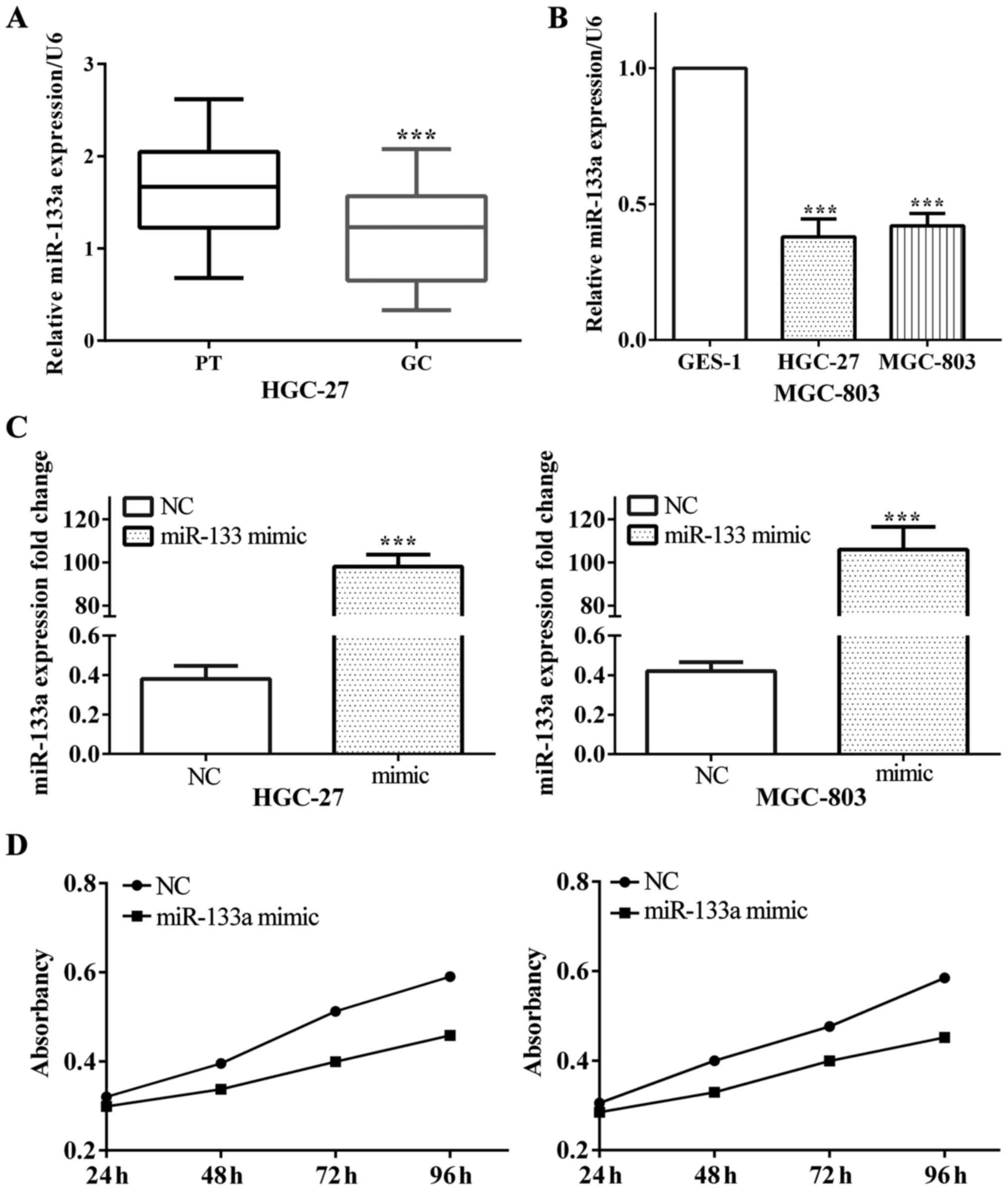
Low expression of miR-133a in gastric
cancer and suppressed cell proliferation
The expression of miR-133a in gastric cancer and
corresponding paracancerous tissues were measured by RT-PCR.
Results showed that, the average expression level of miR-133a was
downregulated in gastric cancer compared with paracancerous tissues
(P<0.001) (Fig. 1A). Further
analysis revealed that miR-133a expression level was downregulated
in gastric cancer cells HGC-27 and MGC-803 and normal cell GES-1 on
contrast to normal gastric cell (both P<0.001) (Fig. 1B).
On the other hand, we assumed that miR-133a played
an important role on the proliferation of glioma, thus the effect
of miR-133a on glioma proliferation was detected. For the sake of
testing the impact of miR-133a on proliferation, we utilized
miR-133a mimic to overexpress miR-133a in gastric cancer cell lines
HGC-27 and MGC-803 (both P<0.001), as shown in Fig. 1C. Subsequently, we measured cell
proliferative ability and found that overexpressed miR-133a
resulted in decreased proliferation ability both in HGC-27
(P<0.001) and MGC-803 (P<0.001) (Fig. 1D).
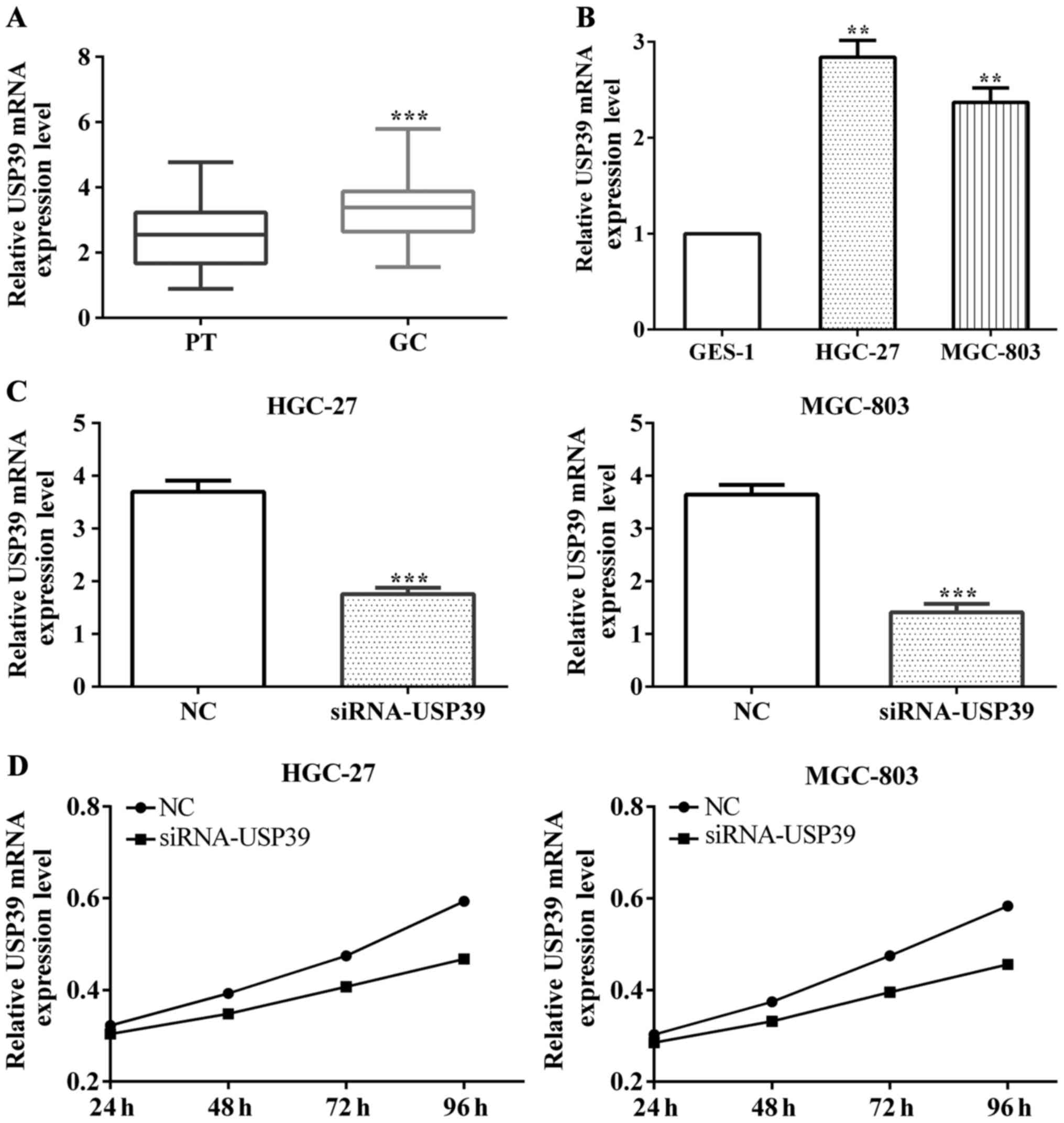
USP39 was overexpressed and promoted
cell proliferation in gastric cancer
The expression of USP39 in gastric cancer and
paracancerous tissues were also measured by RT-PCR. The result was
USP39 upregulated in gastric cancer on contrast to paracancerous
tissues (P<0.001) (Fig. 2A).
Furthermore, we detected that USP39 expression in gastric cancer
cell lines HGC-27 and MGC-803 and normal gastric cell GES-1
respectively. It was highly expressed in gastric cancer cells
compared with normal cells (P<0.001 both in HGC-27 and MGC-803)
(Fig. 2B).
To test the effect of USP39 on proliferation, we
used siRNA-USP39 to interfere with USP39 expression and the results
(P<0.001) were measured using RT-PCR, as shown in Fig. 2C. Following, we measured cell
proliferative ability and found that the proliferative ability was
increased both in HGC-27 (P=0.0002) and MGC-803 (P<0.001) when
interfered with USP39 (Fig. 2D).
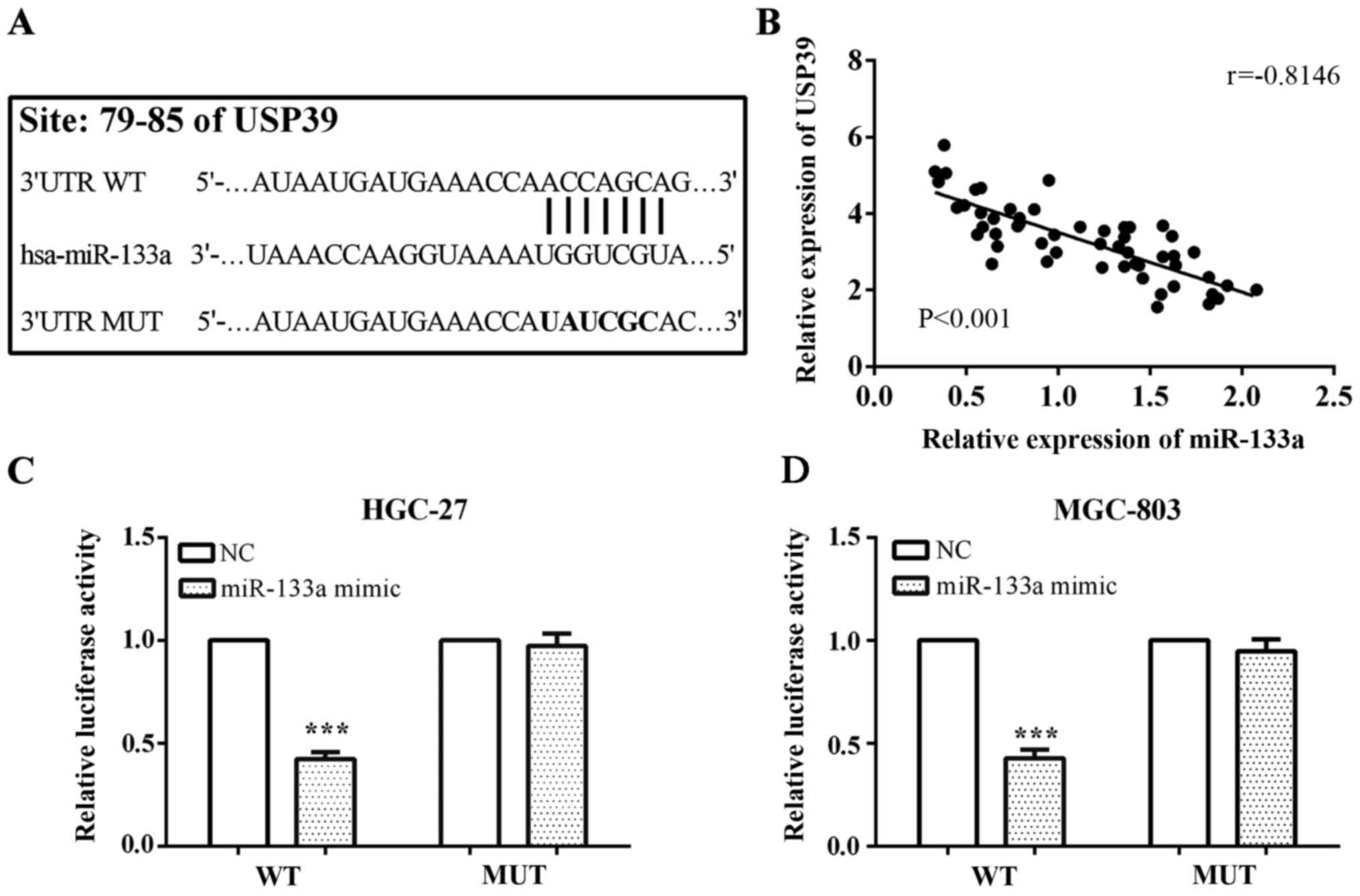
USP39 is a direct target of miR-133a
and partially reversed function of miR-133a
We predicted that USP39 was a potential downstream
target of miR-133a using online software TargetScan with binding
site of USP39 was at its 3′UTR located at 79 to 85. The mutated
target sequences from 5′-…AUAAUGAUGAAACCAACCAGCAG…-3′ to 5′-…AU
AAUGAUGAAACCAUAUCGCAC…-3′, and inserted the wild-type
(pcDNA3.1-USP39-WT) and mutant type (pcDNA3.1-USP39-MUT) fragment
into pcDNA3.1 plasmid vector in prior to the performance of the
luciferase reporter assay (Fig. 3A).
In addition, we compared the expression of miR-133a and USP39, and
found a negative correlation between miR-133a and USP39 with
r=−0.8146 and P<0.001 (Fig.
3B).
To confirm USP39 was directly suppressed by
miR-133a, we carried out a luciferase reporter assay. Following the
protocol, we co-transfected pmirGlo-miR-133a mimic or negative
control and pcDNA3.1-USP39-WT or pcDNA3.1-USP39-MUT into the
gastric cancer cell lines HGC-27 and MGC-803, respectively, and
measured the luciferase reporter activity. Co-transfection
experiments in HGC-27 and MGC-803 cells showed that miR-133a
significantly decreased the luciferase activity of
pcDNA3.1-USP39-WT 3′UTR (both P<0.001); however, this was not
observed in pcDNA3.1-USP39-MUT 3′UTR (P=0.486 and P=0.190,
respectively) (Fig. 3C).
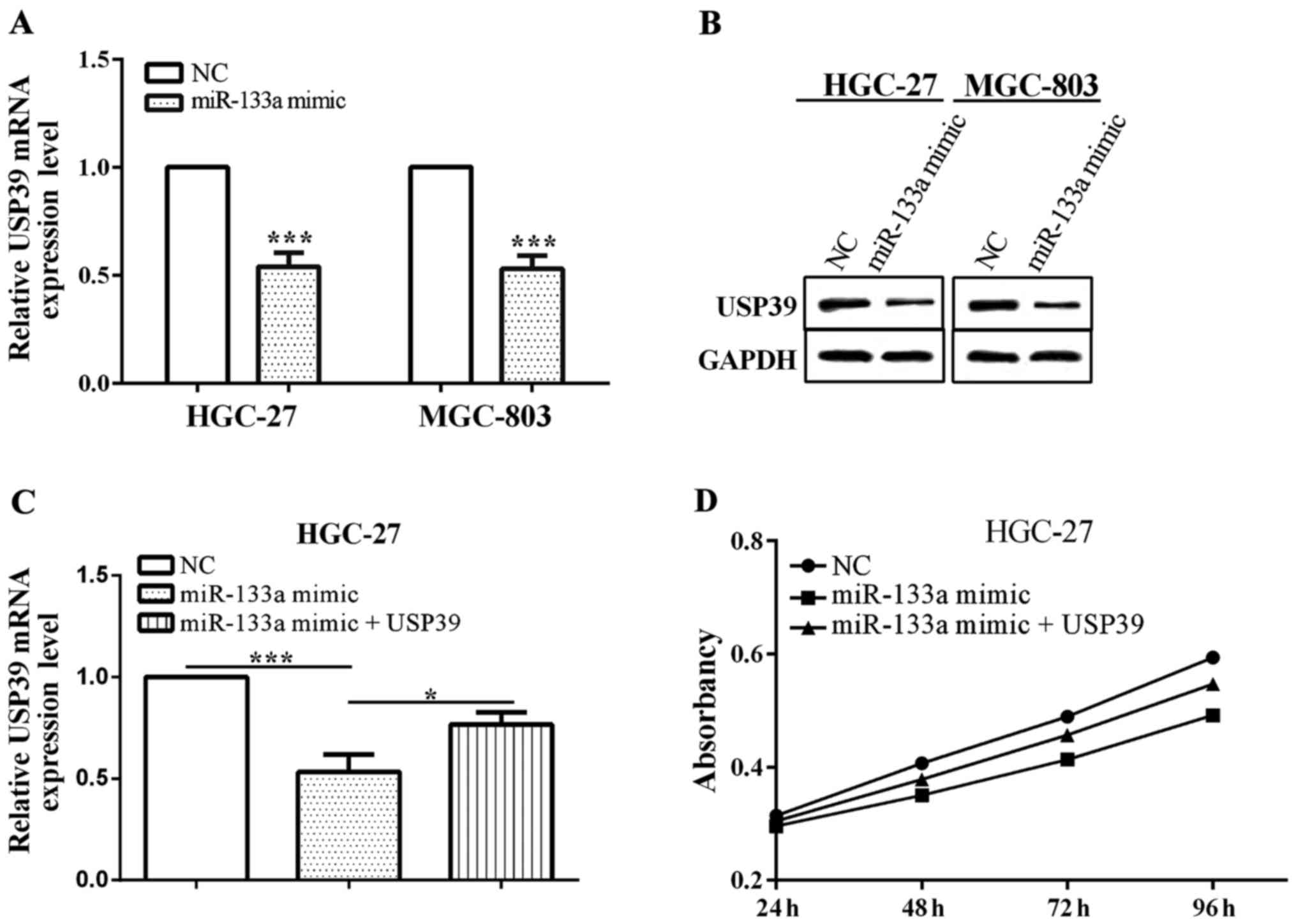
Furthermore, when miR-133a was overexpressed by
transfected miR-133a mimic, the expression of USP39 was decreased
in HGC-27 (P=0.0003) and MGC-803 (P=0.0002) (Fig. 4A). The expression of USP39 was reduced
when transfected with miR-133a mimic (P=0.0007), which was
re-expressed via overexpressed USP39 (P=0.0179). USP39
overexpression deprived miR-133a inhibitor-mediated suppression of
cell proliferation, suggesting that USP39 is involved in
miR-133a-mediated biological role in gastric cancer cell HGC-27
(P<0.0001) (Fig. 4C). Therefore,
depletion of USP39 reversed the partial function of miR-133a.
miR-133a lowly expressed or USP39
overexpressed predicted poor prognosis
We divided 53 gastric cancer patients into the high
[miR-133a(+)] and low [miR-133a(−)] expression groups according to
miR-133a expression level, with 25 and 28 patients, respectively.
Furthemore, 53 patients were separated into two groups on the basis
of sex, age, tumor size, TNM stage, local invasion, lymph-node
metastasis, Ki-67 and USP39, respectively, and the detailed
grouping is shown in Table I. The
expression of miR-133a had a negative correlation with tumor size
(P=0.021), TNM stage (P=0.042), lymph-node metastasis (P=0.017) and
USP39 (P=0.019). However, there was a tendency for the expression
to be associated with age (P=0.082), local invasion (P=0.077) and
Ki-67 (P=0.074). No association was identified between miR-133a
with sex (P=0.511) (Table I).
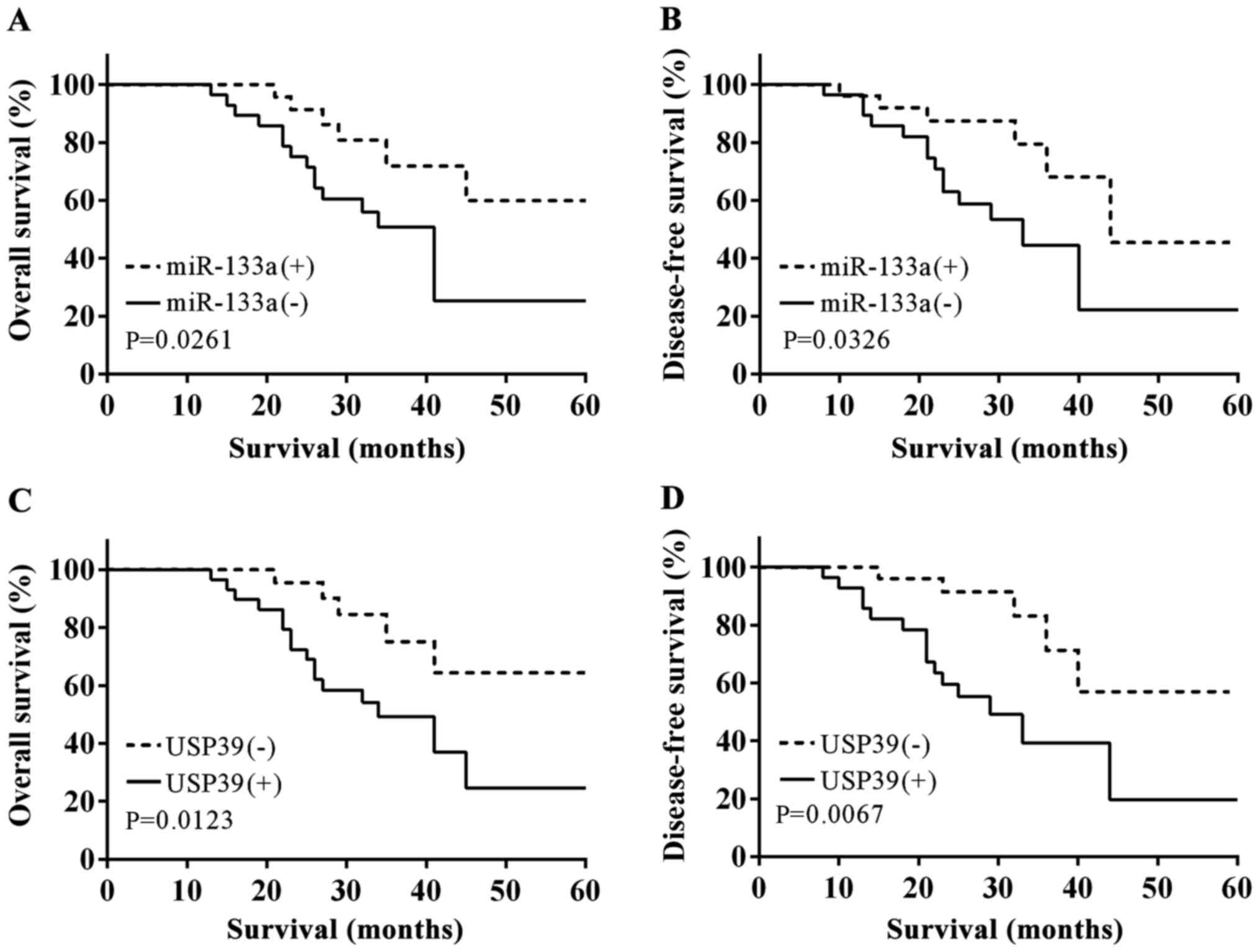
We calculated the OS and DFS with the aid of
Kaplan-Meier in accordance with the expression of miR-133a, and
found that the OS and DFS in the miR-133a(+) group was
significantly higher than that in the miR-133a(−) group (log-rank
test, P=0.0261 and P=0.0326) (Fig. 5A and
B). In addition, we measured OS and DFS according to USP39
expression, and the opposite results were obtained, i.e., OS and
DFS were lower when USP39 overexpression compared with a low
expression (log-rank test, P=0.0123 and P=0.0067) (Fig. 5C and D).
Discussion
Gastric cancer mortality is ranked second only to
lung cancer, and generally diagnosed at an advance stage. The
disease is prone to metastasis and recurrence. Although cancer
treatments have been improved in recent years, the outcomes of
patients with GC remain unsatisfactory (1,2). Thus,
identifying new biomarkers for GC effective therapeutics is urgent.
miRNAs typically bind to the mRNA 3′-UTR of its target gene or
directly cut the mRNA at a post-transcriptional level, thereby
inhibiting the expression of the target gene (5,6). miR-133a
has been reported to be an antioncogene, which was correlated with
cell development, proliferation and prognosis in colon cancer, oral
squamous cell carcinoma and gastric cancer (14,16,27). In
the present study, we verified that USP39 was a direct target of
miR-133a in gastric cancer cell lines GC-27 and MGC-803. A high
expression of USP39 was also identified in gastric cancer tissues
and cell lines, while miR-133a was lowly expressed compared with
paracancerous tissues and normal cells. Considering these results,
we strongly believe that the impact of miR-133a on proliferation
may be through direct inhibition of USP39. Gong et al
indicated that miR-133a suppressed gastric cancer cell growth,
migration and invasion and induced cell apoptosis in vitro
and inhibited gastric cancer growth in vivo, thus obtaining
the result that miR-133a was a tumor suppressor in vitro and
in vivo (17). Other findings
have shown that miR-133a inhibited gastric cancer cell
proliferative and invasive ability and promoted apoptosis (16). Although miR-133a is considered a tumor
suppressor factor in gastric cancer, we suggest miR-133a directly
targets USP39 and affects proliferation by regulating USP39. In
addition, to the best of our knowledge, this is the first study to
focus on the impact of miR-133a and USP39 on the survival of
gastric cancer patients.
The effect of miR-133a on cell proliferation,
invasion and cell cycle have been studied previously, although via
different cell lines and biomarkers (12,16,17,28).
Li et al have shown that miR-133a inhibits gastric cancer
cell proliferation by downregulating ERBB2 expression (12). Similar findings were reported by Lai
et al and Gong et al, who showed that miR-133a
inhibited proliferation, migration and invasion, and induced
apoptosis in gastric cancer cells (16,17). Our
findings were consistent with previous findings (12,16,17), as we
have shown that overexpression of miR-133a may inhibit
proliferation by directly targeting USP39 in gastric cancer cell
lines HGC-27 and MGC-803. In addition, we identified that miR-133a
downregulation and/or USP39 upregulation predict poor
prognosis.
In the present study, it was identified that USP39
was involved in influencing miR-133a on HGC-27 and MGC-803 cell
proliferation. USP39 was a kind of DUBs without ubiquitin protease
activity, which played an important role in RNA splicing and
necessary to maintain spindle checkpoint (19–22). USP39
is essential for mitotic spindle checkpoint integrity, and a
decrease of USP39 could suppress cell division (22). Furthermore, USP39 overexpression has
been reported in colorectal cancer, lung cancer, melanoma,
pancreatic cancer and osteosarcoma cancer (18,23–26). In
addition, it has been reported that overexpression of MGC-803 cells
and knockdown of USP39 may inhibit MGC-803 cell proliferation and
induce cell cycle arrest (29).
Furthermore, Wen et al reported that USP39 overexpression
reduced patient survival times in prostate cancer (30). In the present study, USP39 was
over0expressed in gastric cancer tissues and cell lines HGC-27 and
MGC-803 compared with paracancerous tissues and normal gastric
cells. USP39 overexpression promotes cell proliferation and
predicts poor prognosis, which is consistent with present research
(29,30). Therefore, the transfection of miR-133a
mimic inhibits USP39 expression, thereby causing cell
proliferation. Thus, miR-133a and USP39 were associated with the
prognosis of patients. Due to the limitation of experimental
conditions, no flow cytometry was carried out in our laboratory.
Additionally, we did not conduct more experiments to verify the
effects of miR-133a and USP39 on cell proliferation.
In addition, the expression of USP39 has been
reported to be downregulated by miR-133a and was negatively
correlated with miR-133a expression in pancreatic cancer (18). Similarly, in this study, we
demonstrated that USP39 was a direct target of miR-133a and
mediated by miR-133a in gastric cancer cell lines. However, there
was no further mechanism explaining how miR-133a affected GC
proliferation through USP39. This should be considered as a
limitation of our study. The role of USP39 in the molecular
mechanisms involving in migration and invasion of GC will be
studied in further research.
In conclusion, we have indicated that miR-133a acts
as a tumor suppressor in GC by inhibiting cancer proliferation.
Furthermore, we demonstrated that miR-133a has an inverse
correlation with USP39 and directly targets it by binding to its
3′UTR. This novelty of miR-133a may offer a promising therapeutic
target for the treatment of GC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XD and HS contributed equally to this study and
share first authorship. FJ as the third author analysed and
prepared the manuscrit. HL and GS as the fourth and fifth authors
analysed the data and wrote the manuscript; LF as the corresponding
author contributed to the conception of the study. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Informed consent to use the specimens in this study
was obtained from the patients. The study was approved by the
Ethics Committee of Jining First People's Hospital (Jining,
China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Piazuelo MB and Correa P: Gastric cancer:
Overview. Colomb Med (Cali). 44:192–201. 2013.PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Y, Lin WS, Zhu WF, Lin J, Zhou ZF,
Huang CZ, Chen G, Shi Y, Guo ZQ and Ye YB: Tumor MICA status
predicts the efficacy of immunotherapy with cytokine-induced killer
cells for patients with gastric cancer. Immunol Res. 64:251–259.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi YY, Noh SH and Cheong JH: Evolution
of gastric cancer treatment: From the golden age of surgery to an
era of precision medicine. Yonsei Med J. 56:1177–1185. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Christodoulatos GS and Dalamaga M:
Micro-RNAs as clinical biomarkers and therapeutic targets in breast
cancer: Quo vadis? World J Clin Oncol. 5:71–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lauressergues D, Couzigou JM, Clemente HS,
Martinez Y, Dunand C, Bécard G and Combier JP: Primary transcripts
of microRNAs encode regulatory peptides. Nature. 520:90–93. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Voinnet O: Origin, biogenesis, and
activity of plant microRNAs. Cell. 136:669–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di Giacomo G, Koss M, Capellini TD,
Brendolan A, Pöpperl H and Selleri L: Spatio-temporal expression of
Pbx3 during mouse organogenesis. Gene Expr Patterns. 6:747–757.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lichtenauer UD, Duchniewicz M, Kolanczyk
M, Hoeflich A, Hahner S, Else T, Bicknell AB, Zemojtel T, Stallings
NR, Schulte DM, et al: Pre-B-cell transcription factor 1 and
steroidogenic factor 1 synergistically regulate adrenocortical
growth and steroidogenesis. Endocrinology. 148:693–704. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujiwara T, Katsuda T, Hagiwara K, Kosaka
N, Yoshioka Y, Takahashi RU, Takeshita F, Kubota D, Kondo T,
Ichikawa H, et al: Clinical relevance and therapeutic significance
of microRNA-133a expression profiles and functions in malignant
osteosarcoma-initiating cells. Stem Cells. 32:959–973. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo J, Xia B, Meng F and Lou G: miR-133a
suppresses ovarian cancer cell proliferation by directly targeting
insulin-like growth factor 1 receptor. Tumour Biol. 35:1557–1564.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li C, Li X, Gao S, Li C and Ma L:
MicroRNA-133a inhibits proliferation of gastric cancer cells by
downregulating ERBB2 expression. Oncol Res. 25:1169–1176. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang LK, Hsiao TH, Hong TM, Chen HY, Kao
SH, Wang WL, Yu SL, Lin CW and Yang PC: MicroRNA-133a suppresses
multiple oncogenic membrane receptors and cell invasion in
non-small cell lung carcinoma. PLoS One. 9:e967652014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He B, Lin X, Tian F, Yu W and Qiao B:
MiR-133a-3p inhibits oral squamous cell carcinoma (OSCC)
proliferation and invasion by suppressing COL1A1. J Cell Biochem.
119:338–346. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li W, Chen A, Xiong L, Chen T, Tao F, Lu
Y, He Q, Zhao L, Ou R and Xu Y: miR-133a acts as a tumor suppressor
in colorectal cancer by targeting eIF4A1. Tumour Biol.
39:10104283176983892017.PubMed/NCBI
|
|
16
|
Lai C, Chen Z and Li R: MicroRNA-133a
inhibits proliferation and invasion, and induces apoptosis in
gastric carcinoma cells via targeting fascin actin-bundling protein
1. Mol Med Rep. 12:1473–1478. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gong Y, Ren J, Liu K and Tang LM: Tumor
suppressor role of miR-133a in gastric cancer by repressing IGF1R.
World J Gastroenterol. 21:2949–2958. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai J, Liu T, Huang P, Yan W, Guo C, Xiong
L and Liu A: USP39, a direct target of microRNA-133a, promotes
progression of pancreatic cancer via the AKT pathway. Biochem
Biophys Res Commun. 486:184–190. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reyes-Turcu FE, Ventii KH and Wilkinson
KD: Regulation and cellular roles of ubiquitin-specific
deubiquitinating enzymes. Annu Rev Biochem. 78:363–397. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lygerou Z, Christophides G and Séraphin B:
A novel genetic screen for snRNP assembly factors in yeast
identifies a conserved protein, Sad1p, also required for pre-mRNA
splicing. Mol Cell Biol. 19:2008–2020. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clague MJ, Barsukov I, Coulson JM, Liu H,
Rigden DJ and Urbé S: Deubiquitylases from genes to organism.
Physiol Rev. 93:1289–1315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Leuken RJ, Luna-Vargas MP, Sixma TK,
Wolthuis RM and Medema RH: Usp39 is essential for mitotic spindle
checkpoint integrity and controls mRNA-levels of aurora B. Cell
Cycle. 7:2710–2719. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan X, Sun X, Shi X, Wang H, Wu G, Jiang
C, Yu D, Zhang W, Xue B and Ding Y: USP39 promotes colorectal
cancer growth and metastasis through the Wnt/β-catenin pathway.
Oncol Rep. 37:2398–2404. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin Z, Xiong L and Lin Q:
Ubiquitin-specific protease 39 is overexpressed in human lung
cancer and promotes tumor cell proliferation in vitro. Mol Cell
Biochem. 422:97–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao Y, Zhang B, Lei Y, Sun J, Zhang Y,
Yang S and Zhang X: Knockdown of USP39 induces cell cycle arrest
and apoptosis in melanoma. Tumour Biol. 37:13167–13176. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gan Z, Han K, Lin S, Hu H, Shen Z and Min
D: Knockdown of ubiquitin-specific peptidase 39 inhibited the
growth of osteosarcoma cells and induced apoptosis in vitro. Biol
Res. 50:152017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li W, Chen A, Xiong L, Chen T, Tao F, Lu
Y, He Q, Zhao L, Ou R and Xu Y: miR-133a acts as a tumor suppressor
in colorectal cancer by targeting eIF4A1. Tumour Biol.
39:10104283176983892017.PubMed/NCBI
|
|
28
|
Qiu T, Zhou X, Wang J, Du Y, Xu J, Huang
Z, Zhu W, Shu Y and Liu P: MiR-145, miR-133a and miR-133b inhibit
proliferation, migration, invasion and cell cycle progression via
targeting transcription factor Sp1 in gastric cancer. FEBS Lett.
588:1168–1177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Yu Q, Huang L and Yu P:
Lentivirus-mediated inhibition of USP39 suppresses the growth of
gastric cancer cells via PARP activation. Mol Med Rep. 14:301–306.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wen D, Xu Z, Xia L, Liu X, Tu Y, Lei H,
Wang W, Wang T, Song L, Ma C, et al: Important role of SUMOylation
of Spliceosome factors in prostate cancer cells. J Proteome Res.
13:3571–3582. 2014. View Article : Google Scholar : PubMed/NCBI
|