Introduction
Despite its adverse side effects, chemotherapy has
made an essential contribution to cancer treatment in recent
decades (1,2). Anthracyclines, a class of
chemotherapeutic drug that have been widely used to treat several
types of cancer, are among the leading causes of cardiotoxicity in
cancer survivors (3). In particular,
the anthracycline, doxorubicin (DOXO), demonstrates great
therapeutic potential in a range of types of cancer. However,
cardiotoxicity is a major limiting factor of DOXO therapy, with a
spectrum of short- and long-term cardiotoxic effects induced by
this compound, ranging from cardiomyocyte senescence and
subclinical ventricular dysfunction to severe cardiomyopathy and
heart failure that may result in cardiac transplantation or
mortality (4,5). Therefore, there is an urgent requirement
to identify an efficient way to ameliorate DOXO-induced toxicity in
order to prevent future cardiac complications.
Cell-based therapies have huge potential in the
treatment of cardiovascular diseases, particularly bone marrow
derived-mesenchymal stem cells (BM-MSCs), due to their regenerative
properties and known bio-safety (6).
In a rat model of DOXO-induced dilated cardiomyopathy, intravenous
administration of BM-MSCs not only improved cardiac contractility,
but also reduced myocardium fibrosis and left ventricular diameter
(7). Additionally, it has been
reported that the local administration of BM-MSCs after 2 weeks of
DOXO treatment generated a marked improvement in left ventricular
ejection fraction (8). Therefore,
MSCs possess numerous characteristics that make them a promising
tool for preventive or regenerative myocardium treatment, in an
effort to prevent DOXO-induced cardiomyopathy. However, the
mechanisms through which MSCs reduce DOXO-induced cardiomyocyte
damage remain unclear.
MicroRNAs (miRs) are small noncoding RNA molecules
that are critical for a large array of cellular processes,
including survival and growth in both pathological and
non-pathological conditions (9,10). Among
them, the miR-34 family is associated with the cardiovascular
system. Indeed, miR-34 family members (miR-34a, −34b and −34c) are
upregulated in the heart in response to stress, including
myocardial infarction (11), and they
regulate cardiac ageing and function (12). In particular, miR-34a modulates
several important target proteins involved in cell cycle,
apoptosis, senescence and differentiation (13). Additionally, the expression of miR-34a
increases with age and is involved in the regulation of telomere
maintenance and DNA damage responses, providing a potential
mechanism through which telomeres erode during ageing (12). Furthermore, pharmacological inhibition
of miR-34a improves cardiac regeneration and function in
experimental models of myocardial infarction and in aged mice with
heart dysfunction (11,12). Additionally, previous studies have
suggested that miR-34a expression was increased in DOXO-treated rat
cardiac cells culture medium compared with untreated rat cardiac
cells (14). Therefore, modulation of
this microRNA may present a potential therapeutic option for
cardiac protection during DOXO treatment.
SIRT1 is one of the targets of miR-34a and is a
member of the sirtuin family of class III histone deacetylases,
which is known to be widely involved in the regulation of cellular
gene expression, cell senescence, differentiation and survival
(15,16). Previous reports indicate that SIRT1
serves a role in the prevention of various age-related diseases by
deacetylating diverse targets, including histones or non-histone
substrates (e.g., p53 and fork head proteins) (17,18).
Additionally, SIRT1has been reported, in several models of
cardiovascular disease, to be involved in the development and
progression of heart failure through the regulation of cell
senescence-related signaling, with compelling evidence suggesting
that molecules that activate SIRT1 cause an anti-senescence effect,
leading to cardioprotection (11,19).
Additionally, SIRT1 may have the potential to prevent DOXO-induced
cardiotoxicity as SIRT1 activation was demonstrated to protect
DOXO-exposed cardiac progenitor cells and to re-establish their
normal function (20). Consequently,
activation of SIRT1 may have great potential in the treatment of
DOXO-induced cardiotoxicity. The present study investigated the
interaction between MSCs and miR-34a expression, and the
involvement of SIRT1 in DOXO-induced cardiotoxicity.
Materials and methods
Animals
Male Sprague Dawley (SD) rats weighing 60–80 g were
cared for in accordance with the published guidelines from the
United States National Institutes of Health (21). All animal procedures were approved by
the Wenzhou Medical University Institutional Animal Care and Use
Committee (Wenzhou, China).
Reagents
The miR-34a mimic andmiR-34a inhibitor were obtained
from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
The Transcriptor First Strand cDNA Synthesis kit, X-treme GENE HP
DNA transfection reagent, Telo TAGGG Telomerase PCR ELISA PLUS kit
and Fast Start Universal SYBR Master (ROX) were obtained from Roche
Diagnostics GmbH (Mannheim, Germany). Rabbit monoclonal antibody
against SIRT1 (1:1,000; cat. no. 9475) was purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Mouse polyclonal
β-actin antibody was purchased from OriGene Technologies, Inc.
(cat. no. TA-09; Rockville, MD, USA). SIRT1 small interfering
(si)RNA was obtained from Thermo Fisher Scientific, Inc). MTT and
dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich; Merck
KGaA (Darmstadt, Germany).
Cell culture
Rat embryonic H9c2 myoblasts were obtained from
American Type Culture Collection (Manassas, VA, USA). The cells
were cultured in Dulbecco's modified Eagle's medium (DMEM),
supplemented with 10% fetal bovine serum (FBS). Both were purchased
from Hyclone (GE Healthcare, Chicago, IL, USA).
BM-MSCs were isolated from the femur and tibia of SD
rats, as described previously (22).
Briefly, bone marrow cells were flushed out from the femur and
tibia using 5 ml DMEM/F-12 (Hyclone; GE Healthcare). Next, the red
blood cells were lysed in blood cell lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China) and discarded, and the
remaining cells (5×105) were plated into a 25
cm2 flask in 6 ml DMEM/F-12 supplemented with 10% FBS
and 1% penicillin/streptomycin. The cells were cultured at 37°C and
5% CO2. After 3 days in culture, the non-adherent cells
were washed away, while the adherent MSCs were maintained in
culture. The culture medium was replaced every 3 days. Once the
culture reached 80–90% confluence, the cells were trypsinized and
passaged at 2:3 or at a dilution of 1:2. All cells used in
subsequent assays were passaged 3 to 5 times.
Transwell co-cultures of MSCs-H9c2 and
cell treatment
A Transwell system was used to prevent the MSCs from
directly contacting the H9c2 cells. MSCs and H9c2 cells were placed
into the upper and lower chambers of the Transwell system (density,
1×106 cells/well), respectively. The H9c2 cells were
pre-treated with DOXO (0.5 µM; cat. no. D1515; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) at 37°C for 1 h, as previously described
(14). Prior to co-culture, the H9c2
cells were washed with phosphate-buffered saline. The untreated
H9c2 cells were used as a control.
Cell transfection
Prior to transfection with Lipofectamine®
(Invitrogen; Thermo Fisher Scientific, Inc.), MSCs were seeded onto
6-well plates at a density of 1×106 cells per well and
were incubated at 37°C overnight. For overexpression or inhibition
of miR-34a, the cells were transfected with amiR-34a mimic or a
miR-34a inhibitor (100 nM), and transfection efficiency was
analyzed using reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) analysis. The interval between transfection and
subsequent experimentation was 6 h.
Cell proliferation assay
The rate of cell proliferation was estimated using
the Cell Counting Kit-8 (CCK-8) assay, according to the
manufacturer's protocol (22).
Briefly, 1×105 cells were grown on a 96-well plate and
were incubated with the CCK-8 solution for 1 h at 37°C.
Subsequently, the absorbance at 450 nm was recorded, and a total of
three repeats were performed.
MTT assay
The MTT assay was used to determine cell viability
as previously described (23).
Briefly, 300 µl MTT reagent was added to each well 2 h prior to
harvesting. The supernatant was then removed and incubated with 400
µl DMSO for 10 min. The absorbance at 540 nm was recorded using an
ELISA plate reader. A total of three repeats were performed.
RT-qPCR
The expression levels of several genes were analyzed
using RT-qPCR. Briefly, total RNA was extracted from MSCs using
TRIzol® reagent (Thermo Fisher Scientific, Inc.) and
reverse transcribed using the Transcriptor First Stand cDNA
Synthesis kit, according to the manufacturer's protocol. The qPCR
was performed using the Fast Start Universal SYBR Master (Roche
Diagnostics GmbH), and fluorescence qPCR system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The samples were subjected
to 40 cycles of amplification at 95°C for 15 sec followed by 64°C
for 20 sec and 72°C for 25 sec using specific primers. The
threshold number of cycles (Cq) was set within the exponential
phase of the PCR reaction. The ∆Cq value for each target gene was
calculated by subtracting the Cq value of GAPDH (internal control)
from the target gene, while relative gene expression levels were
calculated by comparing the 2−ΔΔCq values between the
control and experimental conditions for each target PCR using the
following equation: Relative gene expression=2−(∆Cq sample-∆Cq
control) (24). The primer
sequences used to detect the mRNA levels of target genes are listed
in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Primer | Sequence
(5′-3′) |
|---|
| p16 |
|
|
Forward |
GGTCACCGACAGGCATAACTTC' |
|
Reverse |
AAAGGAGGGCTGAGGCCTAA |
| p53 |
|
|
Forward |
TCTGTCATCTTCCGTCCCTTCTC |
|
Reverse |
CCGTGCACATAACAGACTTGGCT |
| miR-34a |
|
|
Forward |
AAGGCCACGGATAGGTCCATA |
|
Reverse |
CGCTTTGGTGGTTCTGAAAGG |
| SIRT1 |
|
|
Forward |
AAGGCCACGGATAGGTCCATA |
|
Reverse |
CGCTTTGGTGGTTCTGAAAGG |
| Telomere
length |
|
|
Forward |
GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGA |
|
Reverse |
TCCCGACTATCCCTATCCCTATCCCTATCCCTATCC |
| GAPDH |
|
|
Forward |
GGCTCTCTGCTCCTCCCTGTT |
|
Reverse |
GGCTCTCTGCTCCTCCCTGTT |
| miR-34a mimic |
UGGCAGUGUCUUAGCUGGUUGUUCAACCAGCUAAGACACUGCCAUU |
| miR-34a
inhibitor |
UGGCAGUGUCUUAGCUGGUUGUU |
| siRNA-SIRT1 |
|
|
Forward |
GCACCGAUCCUCGAACAAUTT |
|
Reverse |
AUUGUUCGAGGAUCGGUGCTT |
| siRNA-NT |
|
|
Forward |
UUCUCCGAACGUGUCACGUTT |
|
Reverse |
ACGUGACACGUUCGGAGAATT |
Western blot analysis
Western blot analysis was performed as previously
described (23). Briefly, the cells
were ruptured using cell lysis buffer (Beyotime Institute of
Biotechnology). The lysates were centrifuged (12,000 × g) for 5 min
at 4°C, and the supernatant containing cellular protein was used.
For each sample, 20 µg total protein was resolved by SDS-PAGE (10%
gel) and transferred onto polyvinylidene difluoride membranes. The
membranes were incubated overnight at 4°C with primary antibodies,
SIRT1 and β-actin (cat no. 4970; Cell Signaling Technology, Inc.)
at a dilution of 1:1,000. The following day, membranes were
incubated for 1 h at 37°C with the horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:2,000;
cat no. 7074; Cell Signaling Technology, Inc.) and were developed
using chemiluminescent substrates (BeyoECL Plus; Beyotime Institute
of Biotechnology). The stained protein bands were visualizedusing
Bio-Rad ChemiDoc XRS equipment and were analyzed using the Quantity
One software (both Bio-Rad Laboratories, Inc.).
ELISA
The concentration of secreted vascular endothelial
growth factor (VEGF) in the cell culture medium was measured using
an ELISA kit (cat. no. RAB0512; Sigma-Aldrich; Merck KGaA). The
assays were performed in 96-well microplates according to the
manufacturer's protocol. A total of three repeats were
performed.
siRNA knockdown
The cells were transfected using X-tremeGENE HP DNA
transfection reagent (Roche Diagnostics GmbH), according to the
manufacturer's protocol. H9c2 cells were plated onto a 6-well plate
at a density of 1×106 cells/well and were treated with
X-tremeGENE HP DNA Transfection reagent in a 3:1 ratio of reagent
volume to siRNA mass for 20 min. The cells were then transfected
and mixed with 100 nM SIRT1 siRNA, the primer seque nces were as
follows: siRNA-SIRT1 GCACCGAUCCUCGAACAAUTTAUUGUUCGAGGAUCGGUGCTT;
siRNA-non-targeting (NT) UUCUCCGAACGUGUCACGUTTACGUGACACGUUCGGAGAATT
(Thermo Fisher Scientific, Inc.). This mixture was incubated in 2
ml DMEM (aforementioned) medium for 48 h at 37°C. Scrambled siRNA
(siRNA-NT) was used as the control. The transfection efficiency of
siRNA-SIRT1 was assessed by RT-qPCR compared with siRNA-NT. The
time period between transfection and subsequent experimentation was
6 h.
Relative telomere length
measurement
The quantification of the relative telomere length
in H9c2 cells was performed using qPCR based on a previously
established method (25), and GAPDH
was used to normalize the expression of target genes. The sequences
of the primers used to detect the length of telomeres are listed in
Table I.
Relative telomerase activity
measurement (RTA)
The telomerase activity of H9c2 cells was examined
using the Telo TAGGG Telomerase PCR ELISA PLUS kit (Sigma-Aldrich;
Merck KGaA) according to the manufacturer's protocol. Cell lysates
were centrifuged (12,000 × g) for 20 min at 4°C, and 3 µl cell
extract was used for each telomeric repeat PCR amplification
reaction and 3 µl inactivated cell lysate was used for Telomeric
Repeat Amplification Protocol (TRAP) reactions according to the
manufacturer's protocol. Each TRAP reaction was performed with
amplification of an internal control from the kit to validate the
absence of a PCR inhibitor. Using the ELISA method, the amplified
products were immobilized on streptavidin-coated microtiter plates
via a biotin-streptavidin interaction. Next, the amplifications
were detected using peroxidase-conjugated anti-digoxigenin
antibodies provided by the kit. Following the addition of the
peroxidase substrate (3,3′,5,5′-tetramethylbenzidine), the quantity
of TRAP products was determined by measuring absorbance at 450 nm
using a microplate reader.
Statistical analysis
The data are expressed as the mean ± standard
deviation. The differences among multiple groups were determined
using one-way analysis of variance followed by Tukeys s-b(K) test,
and comparisons between two groups were evaluated by Student's
t-test using SPSS software (version 19.0; IBM Corp., Armonk, NY,
USA). Cell proliferation data are presented as the mean ± standard
error of the mean. P<0.05 was considered to indicate a
statistically significant difference.
Results
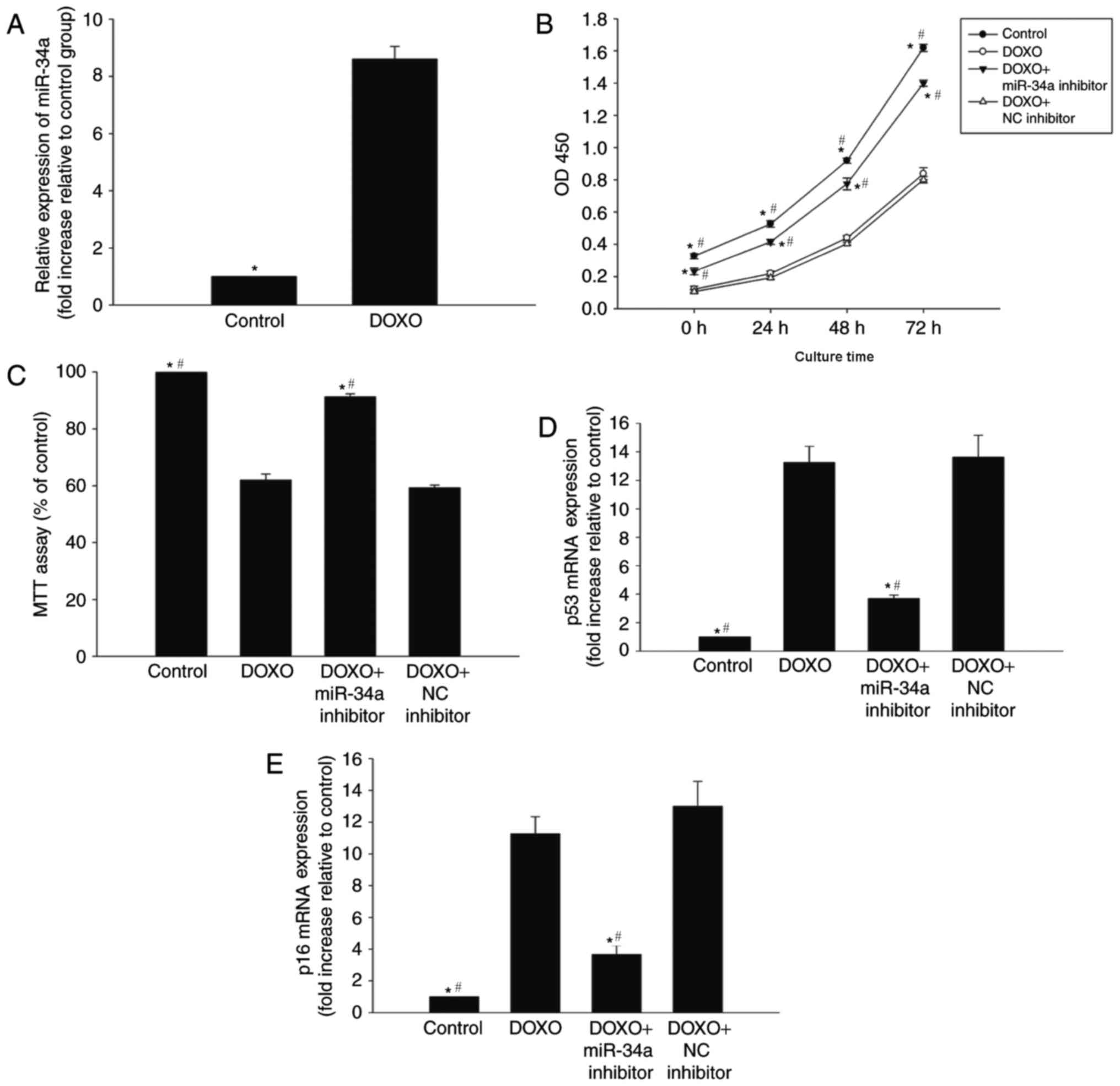
miR-34a is involved in DOXO-induced
changes in senescence in H9c2 cells
To determine whether miR-34a is involved in
DOXO-induced cardiac senescence, the expression of miR-34a was
evaluated in H9c2 cells that were exposed to 0.5 µM DOXO for 24 h.
qPCR analysis revealed a significant upregulation of miR-34a
expression in H9c2 cells following DOXO treatment compared with the
control (Fig. 1A).
In order to further investigate the role of miR-34a
in DOXO-induced senescence in H9c2 cells, the viability and
proliferation of H9c2 cells were analyzed. The proliferation and
percentage of viable cells were decreased following DOXO treatment
compared with the control (Fig. 1B and
C). Furthermore, the expression of senescence-associated genes,
p53 and p16, was markedly increased in the DOXO treatment group
compared with the control (Fig. 1D and
E). To further investigate the role of miR-34a in this process,
it was indicated that the treatment of cells with a miR-34a
inhibitor in the presence of DOXO was able to reverse the
senescence signature induced by DOXO, while treatment with the NC
inhibitor was not able to reverse the senescence signature induced
by DOXO. Additionally, the inhibition of miR-34a resulted in
increased proliferation and viability, and was able to reverse the
DOXO-induced alterations to senescence-associated genes p53 and p16
(Fig. 1B-E).
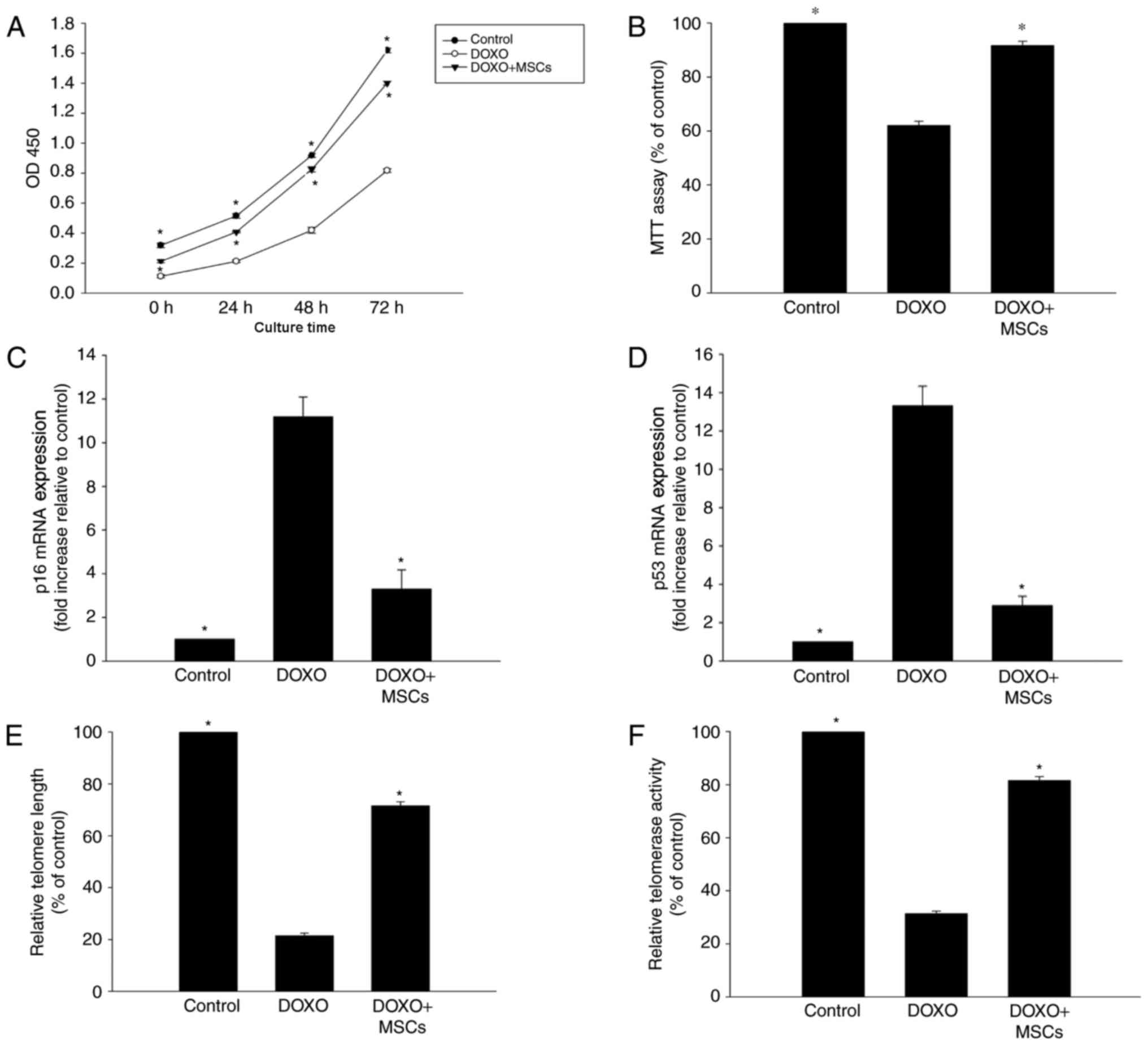
MSCs inhibit DOXO-induced senescence
changes in H9c2 cells
To investigate the anti-senescence effect of MSCs on
DOXO-treated H9c2 cells, a Transwell co-culture system was
employed. As expected, the viability and proliferation of the cells
that were treated with DOXO and co-cultured with MSCs were
significantly higher compared with cells only treated with DOXO
(Fig. 2A and B). Additionally, the
expression of p53 and p16 was significantly decreased in cells that
were exposed to DOXO and co-cultured with MSCs compared with cells
that were only treated with DOXO (Fig. 2C
and D). Furthermore, it was observed that DOXO treatment
induced shortening of telomeres, which was also associated with a
decrease in telomerase activity. However, the addition of MSCs to
the co-culture system was able to partly reverse these DOXO-induced
alterations (Fig. 2E and F).
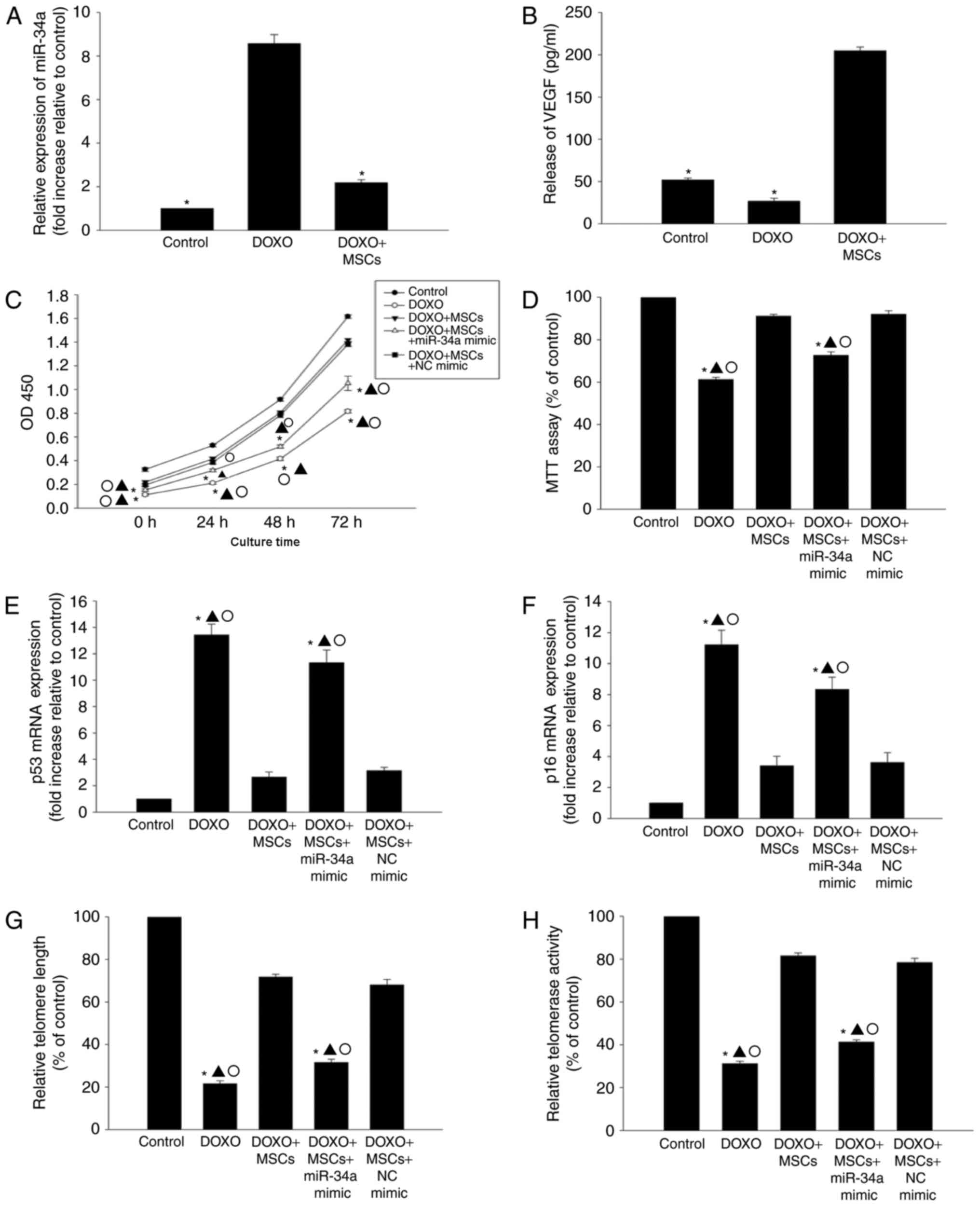
MSCs induce anti-senescence by
inhibiting miR-34a expression
In order to determine whether or not MSCs induce an
anti-senescence effect by inhibiting the expression of miR-34a, the
expression of miR-34a in cells that were co-cultured with MSCs was
assessed. As indicated in Fig. 3A, in
the presence of DOXO, co-culture with MSCs was able to
significantly decrease the expression of miR-34a. Additionally,
co-culture with MSCs was able to markedly increased the release of
VEGF when compared with DOXO treatment only (Fig. 3B).
In the presence of DOXO, co-culture with MSCs was
able to improve the proliferation and viability of H9c2 cells
compared with DOXO treatment only (Fig.
3C and D), and reduce the expression of p53 and p16 (Fig. 3E and F). Additionally, DOXO-induced
alterations in telomere length and activity were ameliorated when
co-cultured with MSCs (Fig. 3G and
H). To assess if the anti-senescence effect was mediated by the
inhibition of miR-34a, transfection of a miR-34a mimic was
performed to induce the overexpression of miR-34a (data not shown).
The overexpression of miR-34a was able to diminish the
anti-senescence efficiency of MSCs, resulting in decreased cell
viability and proliferation, an increase in expression of p53 and
p16, and shortened telomere length, which corresponded with a
decrease in telomerase activity (Fig.
3C-H).
miR-34a-SIRT1 axis serves a role in
the anti-senescence effect of MSCs on DOXO-treated H9c2 cells
SIRT1, one of several miR-34a targets, is a member
of the sirtuin family of class III histone deacetylases (26). In the presence of DOXO, the expression
of SIRT1 decreased in H9c2 cells, whilst co-culture with MSCs
increased the expression of SIRT1. However, the overexpression of
miR-34a diminished the effect of MSCs on SIRT1 expression (Fig. 4A).
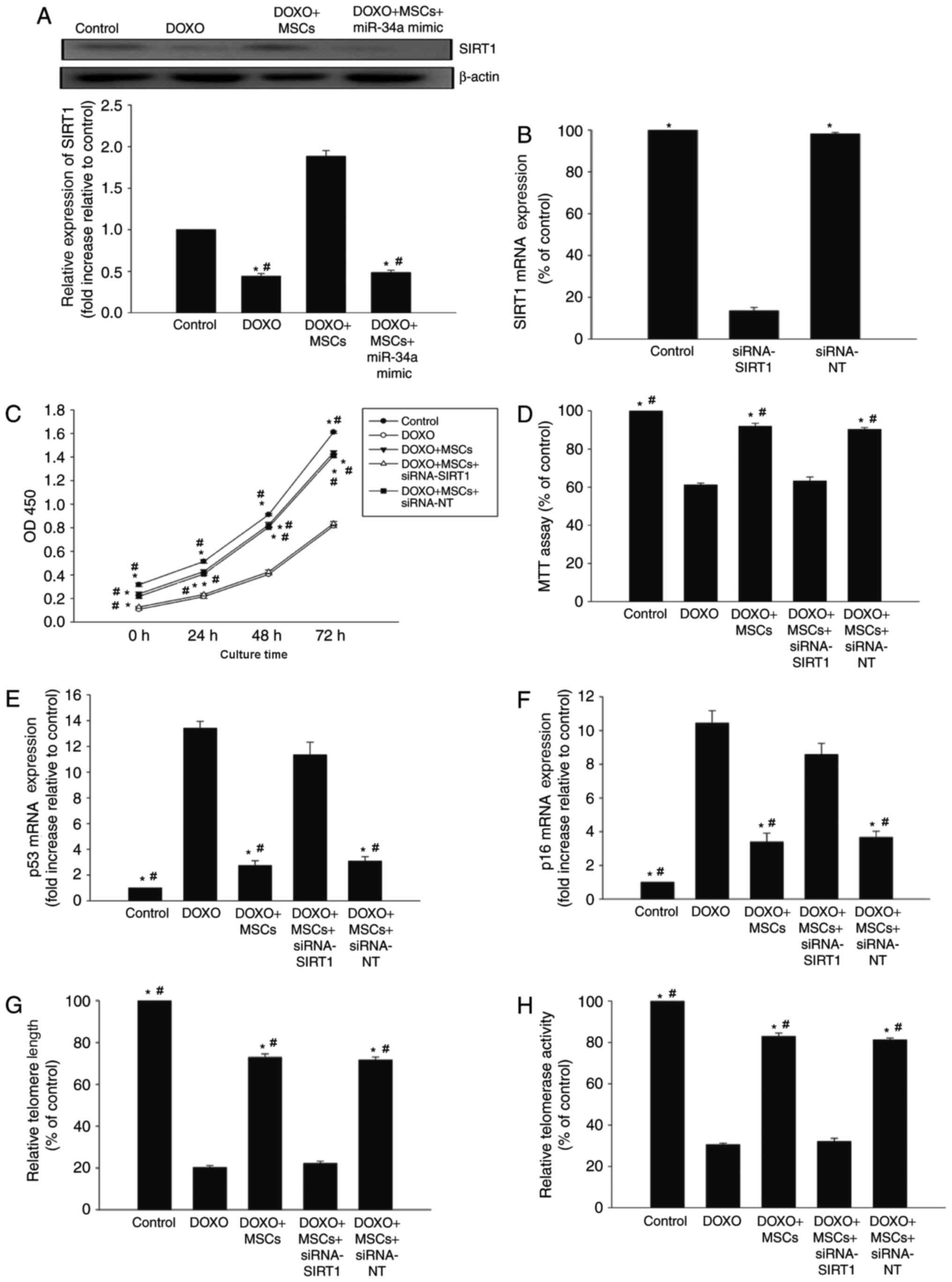 | Figure 4.miR-34a-SIRT1 axis serves a role in
MSC-mediated anti-senescence effect on DOXO-treated H9c2 cells. (A)
The expression of SIRT1 was analyzed by western blot analysis in
H9c2 cells, in the presence of DOXO, MSCs or a miR-34a mimic. Each
column represents the mean ± standard deviation of three
independent experiments. *P<0.05 vs. control;
#P<0.05 vs. DOXO+MSCs. (B) RT-qPCR analysis of SIRT1
expression in non-transfected H9c2 cells and in H9c2 cells
transfected with siRNA-SIRT1 or siRNA-NT. Each column represents
the mean ± standard deviation of three independent experiments.
*P<0.05 vs. siRNA-SIRT1; (C) Proliferation growth curves were
determined using Cell Counting Kit-8 assay in DOXO-treated H9c2
cells, H9c2 cells co-cultured with MSCs in the presence of DOXO,
and H9c2 cells co-cultured with MSCs transfected with siRNA-SIRT1
or siRNA-NT in the presence of DOXO. (D) Cell viability was
analyzed using MTT assay. Each column represents the mean ±
standard deviation of three independent experiments. RT-qPCR
analysis of (E) p53, (F) p16 mRNA levels and (G) telomere length.
(H) Relative telomerase activity was analyzed. Each column
represents the mean ± standard deviation from three independent
experiments. *P<0.05 vs. DOXO; #P<0.05 vs.
DOXO+MSCs+siRNA-SIRT1. miR, microRNA; MSCs, mesenchymal stem cells;
DOXO, doxorubicin; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; siRNA, small interfering RNA; NT,
non-transfected; siRNA, small interfering RNA. |
Next, siRNA-SIRT1 was used to silence the expression
of SIRT1 (Fig. 4B). The knockdown of
SIRT1 was able to decrease the proliferation of H9c2 cells starting
from 24 h of treatment, and this effect continued for ≥72 h. The
knockdown of SIRT1 was able to decrease cell viability compared
with siRNA-NT controls (Fig. 4C and
D). Additionally, abolishing SIRT1 rescued the previously
observed decrease in p53 and p16 caused by co-culturing with MSCs
(Fig. 4E and F), and abolished the
anti-senescence effects of MSCs on telomere length and activity
(Fig. 4G and H).
Discussion
The survival rate of patients with cancer continues
to increase. However, there are new concerns over the long-term
effects of anticancer treatments and measures to reduce the
negative effects of such treatments are an emerging area of
investigation (6).
DOXO, which belongs to the anthracycline family, has
been demonstrated to be effective in numerous types of
tissue-derived cancer, including tumors of the breast, lung,
stomach, bladder and skin (27,28).
Despite the antitumor properties of DOXO, its cardiotoxic effects
have severely restricted its clinical use (5). Pharmacokinetic studies have demonstrated
that DOXO induces cardiac toxicity though multiple pathways
(1,14). Inhibition of the DNA replication
process, leading to the generation of oxidative stress, which
progressively causes cardiac cell senescence, is one potential
mechanism for DOXO-induced cardiac toxicity (4,29). Current
efforts to prevent cardiac toxicity in patients with cancer cannot
guarantee permanent cardiac protection.
One of the main limitations in the prevention of
cancer therapy-induced cardiac stress is the inability to
ameliorate endogenous cardiac cell senescence (6,30). The
results of the present study suggest that co-culturing with MSCs
may effectively rescue H9c2 cells treated with DOXO. Furthermore,
it was demonstrated that this function is mediated through
inhibition of miR-34a and the subsequent activation of SIRT1,
leading to increased cell proliferation and viability, decreased
expression of senescence-associated genes p53 and p16, and an
increase in telomere length and telomerase activity. Taken
together, the results of the present study suggest that MSCs may be
a promising candidate for the treatment of DOXO-induced
cardiomyopathy.
MSC-based therapies have huge potential in the
treatment of cardiovascular diseases (31), and MSCs possess a number of
characteristics that make them a suitable tool for the treatment of
DOXO-induced cardiomyopathy (6). MSCs
secrete paracrine factors, including VEGF, insulin-like growth
factor and basic fibroblast growth factor, with proliferative and
anti-apoptotic properties, and are able to modulate various
senescence-associated signaling pathways involved in the cardiac
regenerative process (32). A
previous study demonstrated that MSCs have a cytoprotective effect
on the anoxia-induced apoptosis of H9C2 cells. The underlying
mechanisms may be associated with increased levels of VEGF mRNA and
protein (33). The same results were
observed in the present study, with an increase in VEGF expression
in H9c2 cells following co-culture with MSCs and DOXO when compared
with control and H9c2 cells treated with only DOXO. Furthermore,
MSCs are known to have anti-inflammatory properties, with a
previous study confirming that, through modulation of the
inflammatory microenvironment, MSCs manage elevated tissue
inflammation stress by suppressing the immune response and
modifying the inflammatory micro environment (34). In line with this, by co-culturing H9c2
cells with MSCs, the present study confirmed that inhibiting
cellular senescence may be an important target in DOXO-induced
cardiomyopathy.
The therapeutic effects of miR-34a in the heart have
been previously examined in various models of cardiac disease,
including myocardial infarction (11). In keeping with the findings of the
present study, miR-34a was reported to be highly expressed in
damaged heart tissue, and the loss of miR-34a was revealed to
improve cardiac function and reduce cell death in ageing hearts
(12). The results of the present
study suggest that DOXO-induced elevated expression of miR-34a is
associated with the senescent state of H9c2 cells. Previous studies
have reported that miR-34a may also confer part of its biological
activity through conditioned medium from cell culture, as well as
the presence in body fluids (35).
The present study used a Transwell co-culture system. A decrease in
miR-34a expression in H9c2 cells and ameliorated cellular
senescence induced by treatment with DOXO was observed. However,
these effects were diminished when a miR-34a mimic was used to
overexpress miR-34a, confirming that MSCs modulate the
anti-senescence effect through inhibition of miR-34a in
DOXO-induced cardiac toxicity.
SIRT1, an NAD-dependent deacetylase, has been
implicated in improving metabolism and the prevention of various
age-related diseases (36). As an
important direct target of miR-34a, SIRT1 has previously been
associated with cellular survival, apoptosis and senescence
(37). In line with this, the
miR-34a-SIRT axis has been implicated in age-related heart
diseases. Previous studies have suggested that activation of the
miR-34a-SIRT axis may be a critical regulator of cardiac repair and
regeneration post myocardial infarction in the aged heart and thus,
modulation of SIRT1 may be harnessed for cardiac repair in the aged
myocardium (11,26). The present study demonstrated that, in
the presence of DOXO, expression of SIRT1 decreases, while
co-culturing with MSCs rescues this effect. To further confirm this
finding, siRNA-SIRT1 was used to silence SIRT1, which was revealed
to abolish the anti-senescence effect of MSCs.
As an important protein associated with age, SIRT1
functions by deacetylating diverse target histones, H1, H3 and H4,
as well as a large number of non-histone substrates, including p53
and fork head proteins (18,38,39). The
most commonly accepted miR34a-SIRT1-p53 axis induced by miR-34a in
cell senescence is in a positive feedback loop, where miR-34a
inhibits SIRT1, which activates p53, which in turn activates
miR-34a (40). Exogenous stress
activates miR-34a, which inhibits SIRT1 expression and, in turn,
activates p53 (18). It is well
established that cellular senescence is the main process that leads
to the functional failure of stem cells contributing to the onset
and progression of diseases. A recent study suggested that the
activation of the miR-34a/SIRT1/p53 signaling pathway contributes
to age-related hearing loss (41).
Furthermore, the results of the present study suggest that MSCs
inhibit the increase in expression of p53 induced by DOXO and that
the overexpression of miR-34a or silencing of SIRT1 may weaken the
effect of MSCs on p53 expression. Future studies by the authors
will investigate whether the DOXO-induced reduction in H9c2
viability was due to an increase in apoptosis or cell cycle arrest,
and whether MSC co-culture may modulate these processes.
Telomeres are specialized nucleoprotein structures
that protect the ends of eukaryotic chromosomes from unscheduled
DNA repair (42). In vertebrates,
telomeres are constituted by TTAGGG tandem repeats, which serve a
pivotal role in the regulation of telomere length and protection of
chromosome ends (43). As an
important mark of senescence, telomeres are also closely associated
with heart disease. A previous study demonstrated that shortened
telomeres are associated with heart failure (44). Additionally, a previous study
confirmed that decreased telomere length was associated with
vascular aging, leading to hypertension (45). Furthermore, it has been reported that
SIRT1 attenuates telomere attrition in vivo and was
recruited at telomeres in induced pluripotent stem cells (46). The present study revealed that
treatment with DOXO induced telomere shortening and decreased
telomerase activity, while MSCs alleviated these alterations and
silencing SIRT1 reversed the MSC-induced changes.
The present study demonstrated that MSCs may
effectively rejuvenate senescent H9c2 cells. The results of the
present study suggest that MSCs protect H9c2 cells from
DOXO-induced senescence via inhibition of miR-34a, leading to the
activation of SIRT1, which elongates telomere length and increases
telomerase activity. The findings presented here suggest that
exogenous interventions with MSCs leading to activation of
associated signaling pathways in the senescent heart may be useful
in reducing DOXO-induced cardiac damage in patients receiving
chemotherapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81500261), the
Science and Technology Planning Project of Wenzhou (grant no.
Y20160125) and the Medical Science and Technology Project of
Zhejiang Province (grant no. 2018236627).
Availability of data and materials
All data generated or analyzed during the present
study are included in the published article.
Authors' contributions
WZX made substantial contributions to the
acquisition of data, analysis and interpretation of data; and MH
was involved in conception and design, drafting the manuscript and
revising it critically for important intellectual content.
Ethics approval and consent to
participate
All animal procedures were approved by the Wenzhou
Medical University Institutional Animal Care and Use Committee
(Wenzhou, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Rochette L, Guenancia C, Gudjoncik A,
Hachet O, Zeller M, Cottin Y and Vergely C:
Anthracyclines/trastuzumab: New aspects of cardiotoxicity and
molecular mechanisms. Trends Pharmacol Sci. 36:326–348. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stone JB and DeAngelis LM:
Cancer-treatment-induced neurotoxicity-focus on newer treatments.
Nat Rev Clin Oncol. 13:92–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suter TM and Ewer MS: Cancer drugs and the
heart: Importance and management. Eur Heart J. 34:1102–1111. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Franco VI, Henkel JM, Miller TL and
Lipshultz SE: Cardiovascular effects in childhood cancer survivors
treated with anthracyclines. Cardiol Res Pract. 2011:1346792011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Volkova M and Russell R III: Anthracycline
cardiotoxicity: Prevalence, pathogenesis and treatment. Curr
Cardiol Rev. 7:214–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ezquer F, Gutiérrez J, Ezquer M, Caglevic
C, Salgado HC and Calligaris SD: Mesenchymal stem cell therapy for
doxorubicin cardiomyopathy: Hopes and fears. Stem Cell Res Ther.
6:1162015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu Q, Li Q, Na R, Li X, Liu B, Meng L,
Liutong H, Fang W, Zhu N and Zheng X: Impact of repeated
intravenous bone marrow mesenchymal stem cells infusion on
myocardial collagen network remodeling in a rat model of
doxorubicin-induced dilated cardiomyopathy. Mol Cell Biochem.
387:279–285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garbade J, Dhein S, Lipinski C, Aupperle
H, Arsalan M, Borger MA, Barten MJ, Lehmann S, Walther T and Mohr
FW: Bone marrow-derived stem cells attenuate impaired contractility
and enhance capillary density in a rabbit model of
Doxorubicin-induced failing hearts. J Card Surg. 24:591–599. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eulalio A, Mano M, Dal Ferro M, Zentilin
L, Sinagra G, Zacchigna S and Giacca M: Functional screening
identifies miRNAs inducing cardiac regeneration. Nature.
492:376–381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seeger FH, Zeiher AM and Dimmeler S:
MicroRNAs in stem cell function and regenerative therapy of the
heart. Arterioscler Thromb Vasc Biol. 33:1739–1746. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Y, Cheng HW, Qiu Y, Dupee D, Noonan
M, Lin YD, Fisch S, Unno K, Sereti KI and Liao R: MicroRNA-34a
plays a key role in cardiac repair and regeneration following
myocardial infarction. Circ Res. 117:450–459. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boon RA, Iekushi K, Lechner S, Seeger T,
Fischer A, Heydt S, Kaluza D, Tréguer K, Carmona G, Bonauer A, et
al: MicroRNA-34a regulates cardiac ageing and function. Nature.
495:107–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen F and Hu SJ: Effect of microRNA-34a
in cell cycle, differentiation, and apoptosis: A review. J Biochem
Mol Toxicol. 26:79–86. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Piegari E, Russo R, Cappetta D, Esposito
G, Urbanek K, Dell'Aversana C, Altucci L, Berrino L, Rossi F and De
Angelis A: MicroRNA-34a regulates doxorubicin-induced
cardiotoxicity in rat. Oncotarget. 7:62312–62326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haigis MC and Sinclair DA: Mammalian
sirtuins: Biological insights and disease relevance. Annu Rev
Pathol. 5:253–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morris BJ: Seven sirtuins for seven deadly
diseases of aging. Free Radic Biol Med. 56:133–171. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng CX: SIRT1, is it a tumor promoter or
tumor suppressor? Int J Biol Sci. 5:147–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo J, Nikolaev AY, Imai S, Chen D, Su F,
Shiloh A, Guarente L and Gu W: Negative control of p53 by Sir2alpha
promotes cell survival under stress. Cell. 107:137–148. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang G, Zhang X, Weng X, Liang P, Dai X,
Zeng S, Xu H, Huan H, Fang M, Li Y, et al: SUV39H1 mediated SIRT1
trans-repression contributes to cardiac ischemia-reperfusion
injury. Basic Res Cardiol. 112:222017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Angelis A, Piegari E, Cappetta D, Russo
R, Esposito G, Ciuffreda LP, Ferraiolo FA, Frati C, Fagnoni F,
Berrino L, et al: SIRT1 activation rescues doxorubicin-induced loss
of functional competence of human cardiac progenitor cells. Int J
Cardiol. 189:30–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu WH, Liu JJ, Wu J, Zhang LL, Liu F, Yin
L, Zhang MM and Yu B: Novel mechanism of inhibition of dendritic
cells maturation by mesenchymal stem cells via interleukin-10 and
the JAK1/STAT3 signaling pathway. PLoS One. 8:e554872013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xia W, Zhang F, Xie C, Jiang M and Hou M:
Macrophage migration inhibitory factor confers resistance to
senescence through CD74-dependent AMPK-FOXO3a signaling in
mesenchymal stem cells. Stem Cell Res Ther. 6:822015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xia W, Xie C, Jiang M and Hou M: Improved
survival of mesenchymal stem cells by macrophage migration
inhibitory factor. Mol Cell Biochem. 404:11–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Crepin T, Carron C, Roubiou C, Gaugler B,
Gaiffe E, Simula-Faivre D, Ferrand C, Tiberghien P, Chalopin JM,
Moulin B, et al: ATG-induced accelerated immune senescence:
Clinical implications in renal transplant recipients. Am J
Transplant. 15:1028–1038. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang F, Cui J, Liu X, Lv B, Liu X, Xie Z
and Yu B: Roles of microRNA-34a targeting SIRT1 in mesenchymal stem
cells. Stem Cell Res Ther. 6:1952015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bao Y, Yin M, Hu X, Zhuang X, Sun Y, Guo
Y, Tan S and Zhang Z: A safe, simple and efficient doxorubicin
prodrug hybrid micelle for overcoming tumor multidrug resistance
and targeting delivery. J Control Release. 235:182–194. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tanaka M, Koul D, Davies MA, Liebert M,
Steck PA and Grossman HB: MMAC1/PTEN inhibits cell growth and
induces chemosensitivity to doxorubicin in human bladder cancer
cells. Oncogene. 19:5406–5412. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu J, Mao W, Ding B and Liang CS:
ERKs/p53 signal transduction pathway is involved in
doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am
J Physiol Heart Circ Physiol. 295:H1956–H1965. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Burridge PW, Li YF, Matsa E, Wu H, Ong SG,
Sharma A, Holmström A, Chang AC, Coronado MJ, Ebert AD, et al:
Human induced pluripotent stem cell-derived cardiomyocytes
recapitulate the predilection of breast cancer patients to
doxorubicin-induced cardiotoxicity. Nat Med. 22:547–556. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Golpanian S, Wolf A, Hatzistergos KE and
Hare JM: Rebuilding the damaged heart: Mesenchymal stem cells,
cell-based therapy, and engineered heart tissue. Physiol Rev.
96:1127–1168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Samper E, Diez-Juan A, Montero JA and
Sepúlveda P: Cardiac cell therapy: Boosting mesenchymal stem cells
effects. Stem Cell Rev. 9:266–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao SL, Zhang YJ, Li MH, Zhang XL and
Chen SL: Mesenchymal stem cells with overexpression of midkine
enhance cell survival and attenuate cardiac dysfunction in a rat
model of myocardial infarction. Stem Cell Res Ther. 5:372014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Chen X, Cao W and Shi Y:
Plasticity of mesenchymal stem cells in immunomodulation:
Pathological and therapeutic implications. Nat Immunol.
15:1009–1016. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Creemers EE, Tijsen AJ and Pinto YM:
Circulating microRNAs: Novel biomarkers and extracellular
communicators in cardiovascular disease? Circ Res. 110:483–495.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang H, Han L, Zhao G, Shen H, Wang P, Sun
Z, Xu C, Su Y, Li G, Tong T and Chen J: hnRNP A1 antagonizes
cellular senescence and senescence-associated secretory phenotype
via regulation of SIRT1 mRNA stability. Aging cell. Sep
9–2016.(Epub ahead of print). View Article : Google Scholar
|
|
37
|
McCubbrey AL, Nelson JD, Stolberg VR,
Blakely PK, McCloskey L, Janssen WJ, Freeman CM and Curtis JL:
MicroRNA-34a negatively regulates efferocytosis by tissue
macrophages in part via SIRT1. 196:1366–1375. 2016.
|
|
38
|
Brunet A, Sweeney LB, Sturgill JF, Chua
KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et
al: Stress-dependent regulation of FOXO transcription factors by
the SIRT1 deacetylase. Science. 303:2011–2015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Motta MC, Divecha N, Lemieux M, Kamel C,
Chen D, Gu W, Bultsma Y, McBurney M and Guarente L: Mammalian SIRT1
represses forkhead transcription factors. Cell. 116:551–563. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao T, Li J and Chen AF: MicroRNA-34a
induces endothelial progenitor cell senescence and impedes its
angiogenesis via suppressing silent information regulator 1. Am J
Physiol Endocrinol Metab. 299:E110–E116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xiong H, Pang J, Yang H, Dai M, Liu Y, Ou
Y, Huang Q, Chen S, Zhang Z, Xu Y, et al: Activation of
miR-34a/SIRT1/p53 signaling contributes to cochlear hair cell
apoptosis: Implications for age-related hearing loss. Neurobiol
Aging. 36:1692–1701. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chan SR and Blackburn EH: Telomeres and
telomerase. Philos Trans R Soc Lond B Biol Sci. 359:109–121. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
de Lange T: Shelterin: The protein complex
that shapes and safeguards human telomeres. Genes Dev.
19:2100–2110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mourkioti F, Kustan J, Kraft P, Day JW,
Zhao MM, Kost-Alimova M, Protopopov A, DePinho RA, Bernstein D,
Meeker AK and Blau HM: Role of telomere dysfunction in cardiac
failure in Duchenne muscular dystrophy. Nat Cell Biol. 15:895–904.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Boe AE, Eren M, Murphy SB, Kamide CE,
Ichimura A, Terry D, McAnally D, Smith LH, Miyata T and Vaughan DE:
Plasminogen activator inhibitor-1 antagonist TM5441 attenuates
Nomega-nitro-L-arginine methyl ester-induced hypertension and
vascular senescence. Circulation. 128:2318–2324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
De Bonis ML, Ortega S and Blasco MA: SIRT1
is necessary for proficient telomere elongation and genomic
stability of induced pluripotent stem cells. Stem Cell Reports.
2:690–706. 2014. View Article : Google Scholar : PubMed/NCBI
|