Introduction
Ovarian cancer is one of the three most common types
of malignant tumors of the female reproductive system (1). It accounts for ~25% of female genital
system neoplasms (2), with the
highest mortality rate. In addition, according to official
statistics, the incidence of ovarian cancer is increased in 2012 in
China (1). The majority of patients
with ovarian cancer are diagnosed with the disease at an advanced
stage (3) and therefore, are not
suitable for treatment by surgical resection due to pelvic cavity,
intraperitoneal and lymphatic metastasis, and adjacent organ
infiltration (4). In cases such as
these, disease progression can only be controlled by relying on
chemotherapeutics: however, the results are not ideal, as the
survival rate is low (4). Conversely,
patients diagnosed at an early stage of ovarian cancer can be
treated with a cytoreductive surgery and combined chemotherapy
regimen based on uranium and paclitaxel, which is associated with a
relatively improved survival rate (3). Therefore, early diagnosis is a critical
factor for the effective treatment of ovarian cancer.
The vascular endothelial growth factor receptor
(VEGFR)2/VEGF signaling pathway serves a vital function in the
angiogenesis of ovarian cancer (5)
and is the primary target for antitumor angiogenesis therapy. VEGF
has been demonstrated to be a pro-angiogenic factor (6). It promotes angiogenesis by inducing the
growth and migration of endothelial cells, promoting the formation
of vascular endothelial cells and increasing the permeability of
blood vessels (7).
The downstream effects of EGFR activation primarily
comprises two major signal transduction pathways:
Ras/Raf/mitogen-activated protein kinase kinase (MEK)/extracellular
signal-regulated kinase (ERK) and phosphoinositide 3-kinase
(PI3K)/AKT, which are being increasingly studied (8). Previous studies have demonstrated that
EGFR signaling primarily induces tumor cell proliferation and
differentiation through the Ras/Raf/MEK/ERK signaling pathway and
promotes the survival of tumor cells through the PI3K/AKT signal
transduction pathway (9). Raf-1 and
AKT are two important kinases in the two aforementioned pathways
(10), with previous studies
identifying that they are hubs for signal transduction and
potentially effective targets for the treatment of ovarian cancer
(10,11).
Mitogen-activated protein kinase (MAPK) is an
important intracellular signal transduction system for the transfer
extracellular information to the cell nucleus, thus participating
in and affecting a variety of physiology and pathological processes
(12). p38MAPK and ERK1/2 are
essential members of these signal transduction pathways (13). It has been demonstrated that the
p38MAPK and ERK1/2 signaling pathways serve essential functions in
tumor incidence, drug resistance and metastasis regulation
(14). The p38MAPK signal
transduction pathway is associated with drug resistance in patients
with ovarian cancer (14) and may
also be associated with drug resistance in gastric cancer cells
(13).
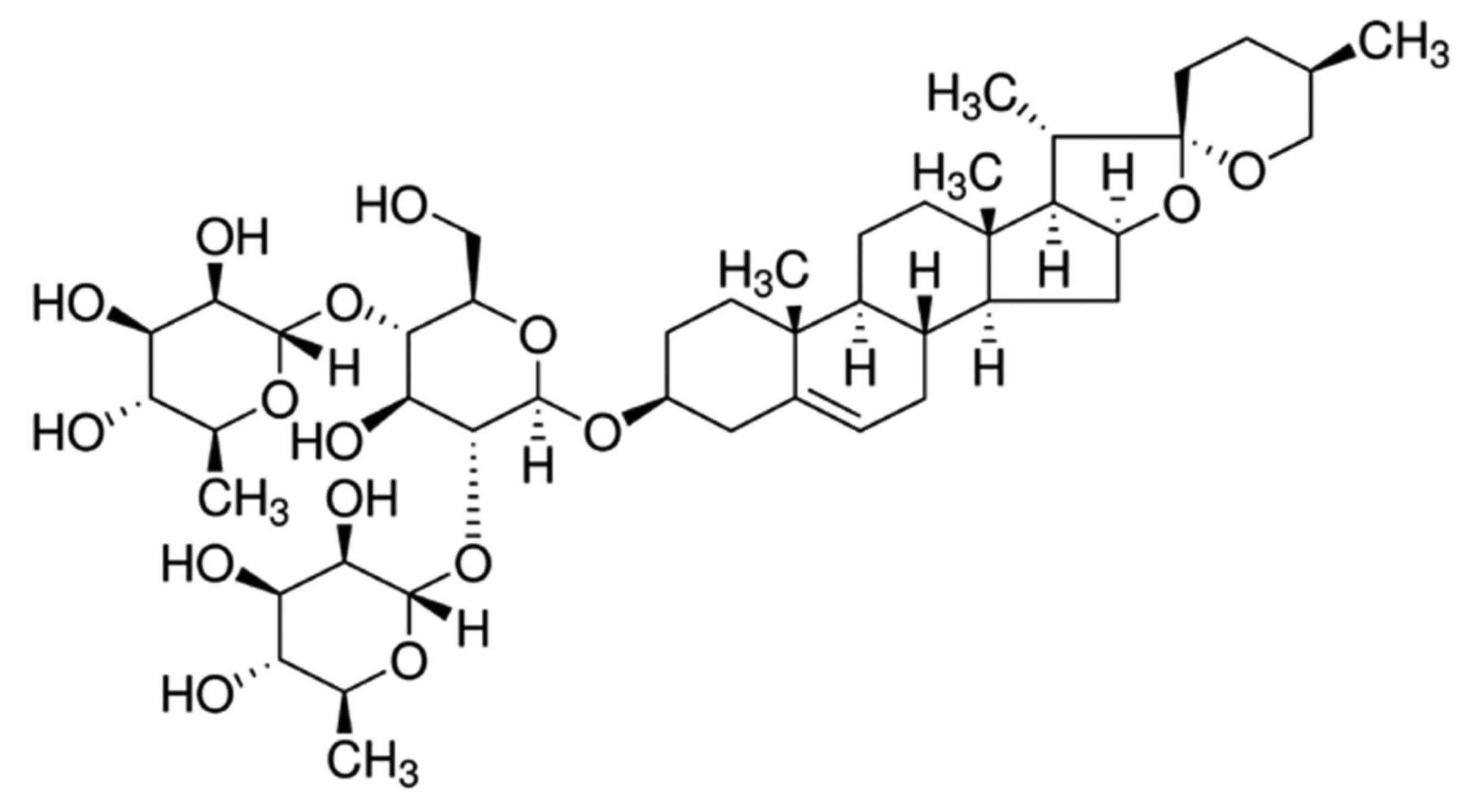
Diosgenin, commonly known as saponin, is a natural
and synthetic steroid sapogenin (Fig.
1) (15). The relative molecular
mass of diosgenin is 414.63 g/mol. Diosgenin is present at high
levels in leguminosae and dioscoreaceae plants. It is an essential
raw material for the synthesis of steroid hormone drugs and
steroidal contraceptives (15). In
the past few decades, in-depth studies on the pharmacological
actions of diosgenin have been performed (16). Diosgenin has exhibited antitumor,
blood lipid-regulating, anti-platelet aggregation and bilifaction
promotion effects (16). It can be
used as a drug for the treatment of cardiovascular disease,
encephalitis, dermatosis and tumors (17). The present study aimed to investigate
the effect of dioscin treatment on ovarian cancer cell growth and
the mechanisms for apoptosis induction by dioscin in ovarian cancer
cells.
Materials and methods
Cell line and cell culture
The ovarian cancer cell line SKOV3 was obtained from
the Experiment Center of Shandong University (Shandong, China) and
was cultured in minimum essential medium α (Promega Corporation,
Madison, WI, USA) containing 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and
100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) at 5% CO2 and 37°C.
Cell viability and apoptosis
assays
SKOV3 cells were seeded into a 96-well-plate
(1×103 cells/well) and treated with 0, 1.25, 2.5 and 5
µM of dioscin for 24, 48 and 72 h. Following dioscin stimulation,
MTT was added to each well (0.5 mg/ml final concentration) and
incubated for 4 h at 37°C. Then, old medium was removed and 150 µl
dimethyl sulfoxide (99.99%) was added for the dissolution of
formazan crystals. The absorbance was measured at 492 nm by an
iMark™ microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
To investigate apoptosis, SKOV3 cells were seeded
into a 6-well-plate (1×106 cells/well) and treated with
0, 1.25, 2.5 and 5 µM of dioscin for 48 h. Subsequently, cells were
washed with PBS and stained with Annexin V-FITC/PI (5 µl/well;
Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) for 15 min in
darkness at room temperature. The rate of apoptosis was measured
using a FACSCalibur™ machine with Image Lab (version 3.0; Bio-Rad
Laboratories, Inc.) and analyzed with ModFIT software (Verity
Software House, Inc., Topsham, ME, USA).
Caspase-3 and caspase-9 activity
SKOV3 cells were seeded into a 6-well-plate
(1×106 cells/well) and treated with 0, 1.25, 2.5 and 5
µM of dioscin for 48 h. Subsequently, cells were washed with PBS
and lysed in radioimmunoprecipitation assay (RIPA) buffer (Beyotime
Institute of Biotechnology, Haimen, China). Following 30 min of
incubation at 37°C, the lysed cells were then centrifuged at 3,000
× g for 10 min at 4°C to pellet large cellular debris. A total of 5
µg of the pellet was incubated with caspase-3 and caspase-9
activity kits (Beyotime Institute of Biotechnology) for 1.5 h at
37°C, according to the manufacturer's protocol. The absorbance was
measured using an iMark™ microplate reader at 405
nm.
Western blot analysis
SKOV3 cells were seeded into a 6-well-plate
(1×106 cells/well) and treated with 0, 1.25, 2.5 and 5
µM of dioscin for 48 h. Subsequently, cells were washed with PBS
and lysed in RIPA buffer (Beyotime Institute of Biotechnology).
Following 30 min of incubation, the lysed cells were centrifuged at
3,000 × g for 10 min at 4°C to pellet large cellular debris. Total
protein extracted was quantified with a BCA Protein Assay kit
(Beyotime Institute of Biotechnology). The samples (50 µg/per lane)
were heated at 100°C for 5 min, loaded onto 6–12% SDS-PAGE gels for
electrophoresis and then transferred onto a polyvinylidene
difluoride membrane (Bio-Rad Laboratories, Inc.). Membrane was
blocked with 5% non-fat milk in TBST for 1 h at 37°C. Following
incubation with the primary antibodies, including anti-Bax (cat.
no. sc-6236; 1:500), anti-cleaved poly(ADP-ribose) polymerase
(PARP; cat. no. sc-1562; 1:500; both Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), anti-VEGFR2 (cat. no. 9698; 1:2,000; Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-PI3K (cat. no.
sc-7174; 1:500), anti-p-AKT (cat. no. sc-7985-R; 1:500),
anti-phosphorylated p38MAPK (cat. no. sc-17852-R; 1:500) and
anti-GAPDH (cat. no. sc-25778; 1:5,000; all Santa Cruz
Biotechnology, Inc.) overnight at 4°C, the membranes were washed
with TBST and incubated with horseradish peroxidase-conjugated goat
anti-rabbit IgG secondary antibody (1:5,000; cat. no. BA1054; Wuhan
Boster Biological Technology, Ltd. Wuhan, China) for 1 h at 37°C.
Protein bands were detected by an enhanced chemiluminescence kit
(Beyotime Institute of Biotechnology) and quantified by LabWorks
software (version 4.0; Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical significance throughout the present
study was analyzed using one-way analysis of variance and Tukey's
post hoc test. Values are presented as the mean ± standard
deviation from three replicates using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Dioscin treatment decreased the
viability of SKOV3 cells
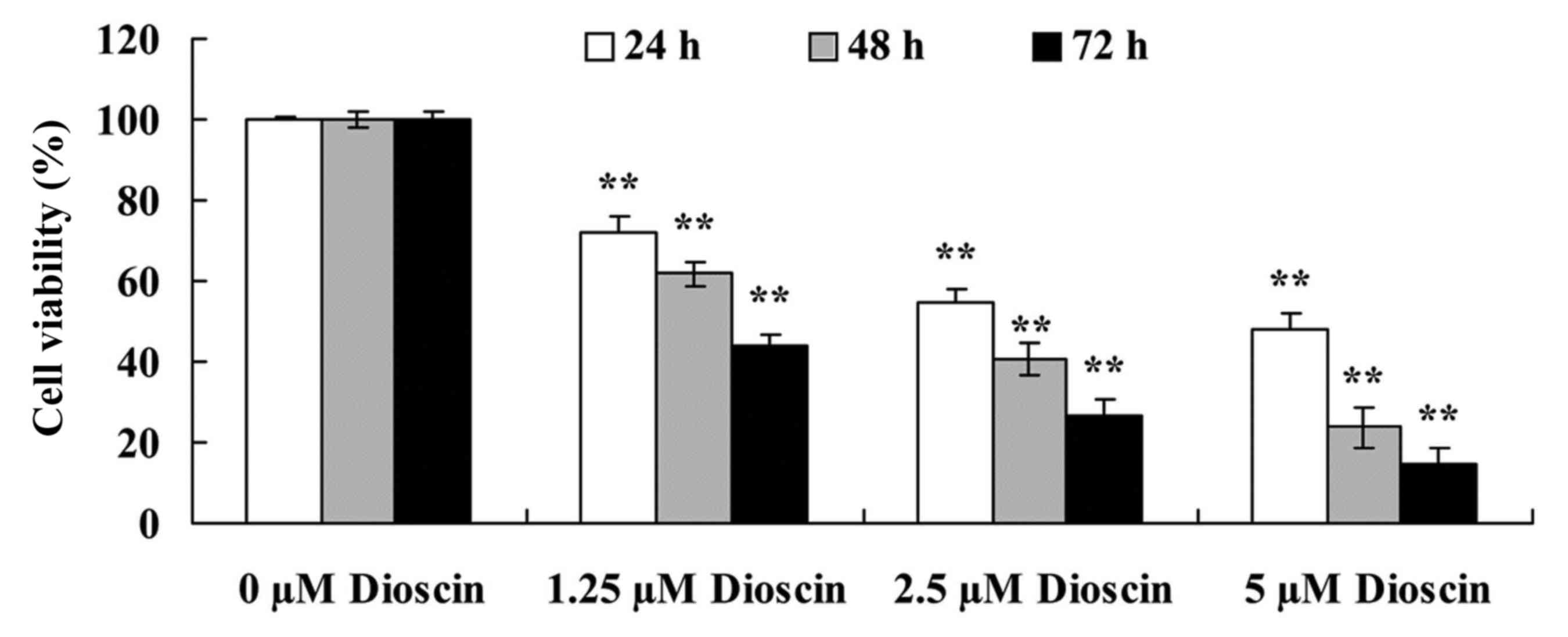
The effects of different doses (0, 1.25, 2.5 or 5 µM
for 24, 48 and 72 h) of dioscin on the viability of human ovarian
cancer SKOV3 cells was investigated using an MTT assay. As
presented in Fig. 2, dioscin
treatment significantly decreased reduced the viability of human
ovarian cancer SKOV3 cell in a dose- and time-dependent manner
compared with the untreated control (P<0.01).
Dioscin treatment induced apoptosis in
SKOV3 cells
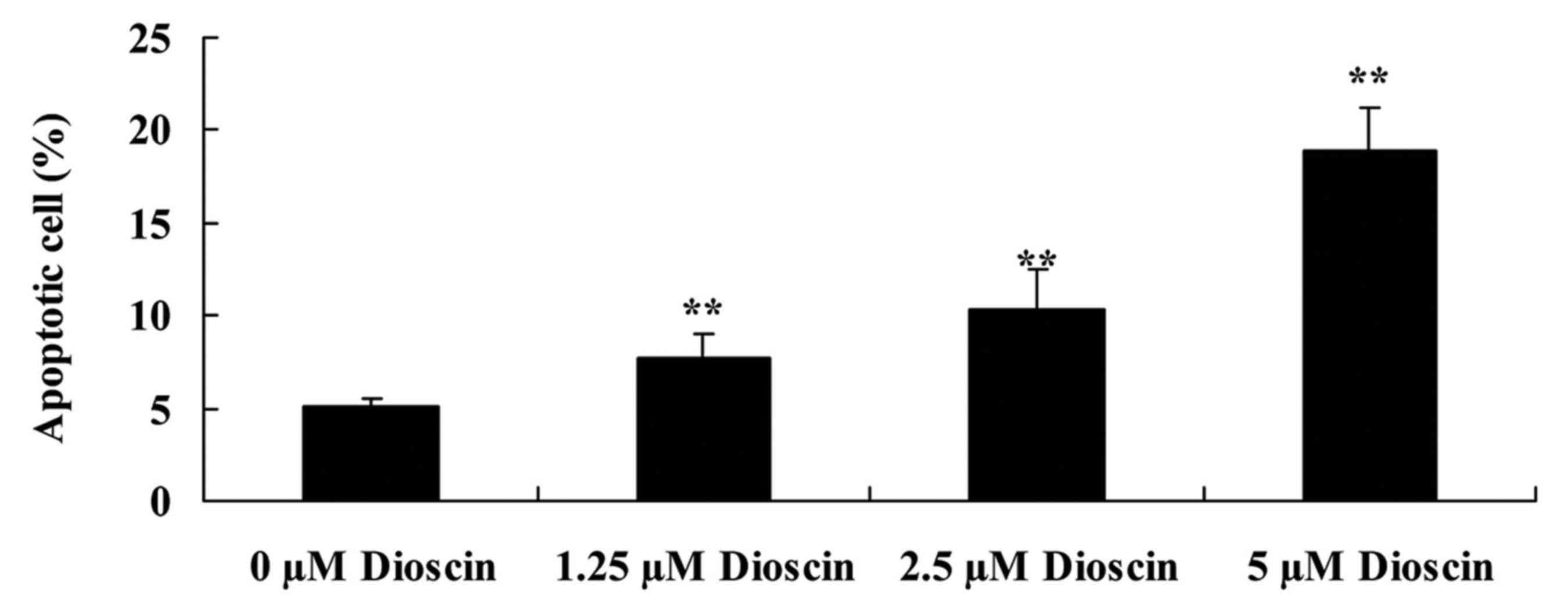
To study the anticancer effects of dioscin, the rate
of apoptosis in SKOV3 human ovarian cancer cells was measured
following treatment with 0, 1.25, 2.5 and 5 µM of dioscin for 48 h
with flow cytometry. Compared with the 0 µM control group, dioscin
significantly increased cellular apoptosis in a dose-dependent
manner (P<0.01; Fig. 3).
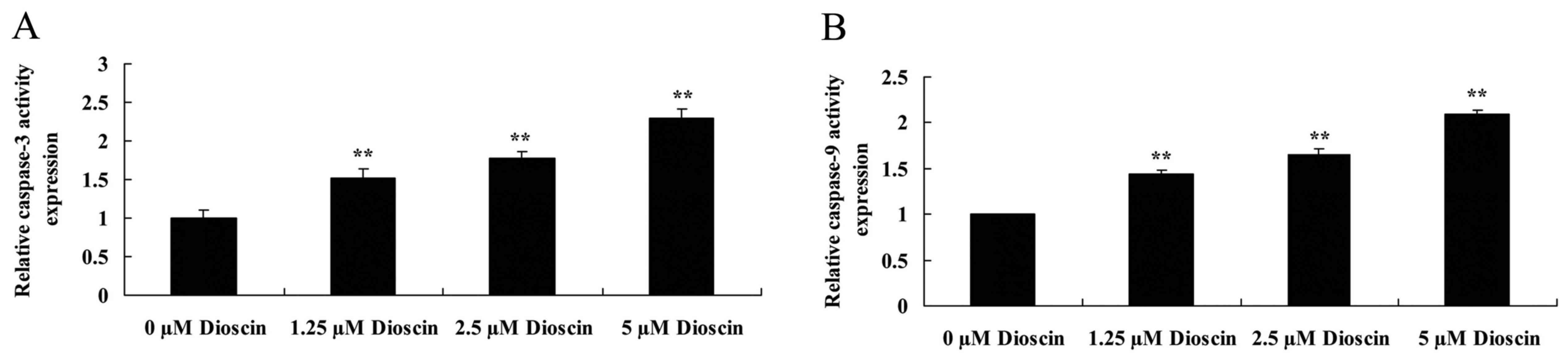
Dioscin treatment promoted caspase-3 and caspase-9
activity in SKOV3 cells. In order to investigate the endogenous
regulatory function of caspase-3 and caspase-9 in human ovarian
cancer, the present study measured caspase-3 and caspase-9 activity
in SKOV3 cells. As presented in Fig.
4, caspase-3 and caspase-9 activity in the SKOV3 cells was
significantly increased following treatment with 1.25, 2.5 or 5 µM
dioscin, compared with the untreated control (P<0.01).
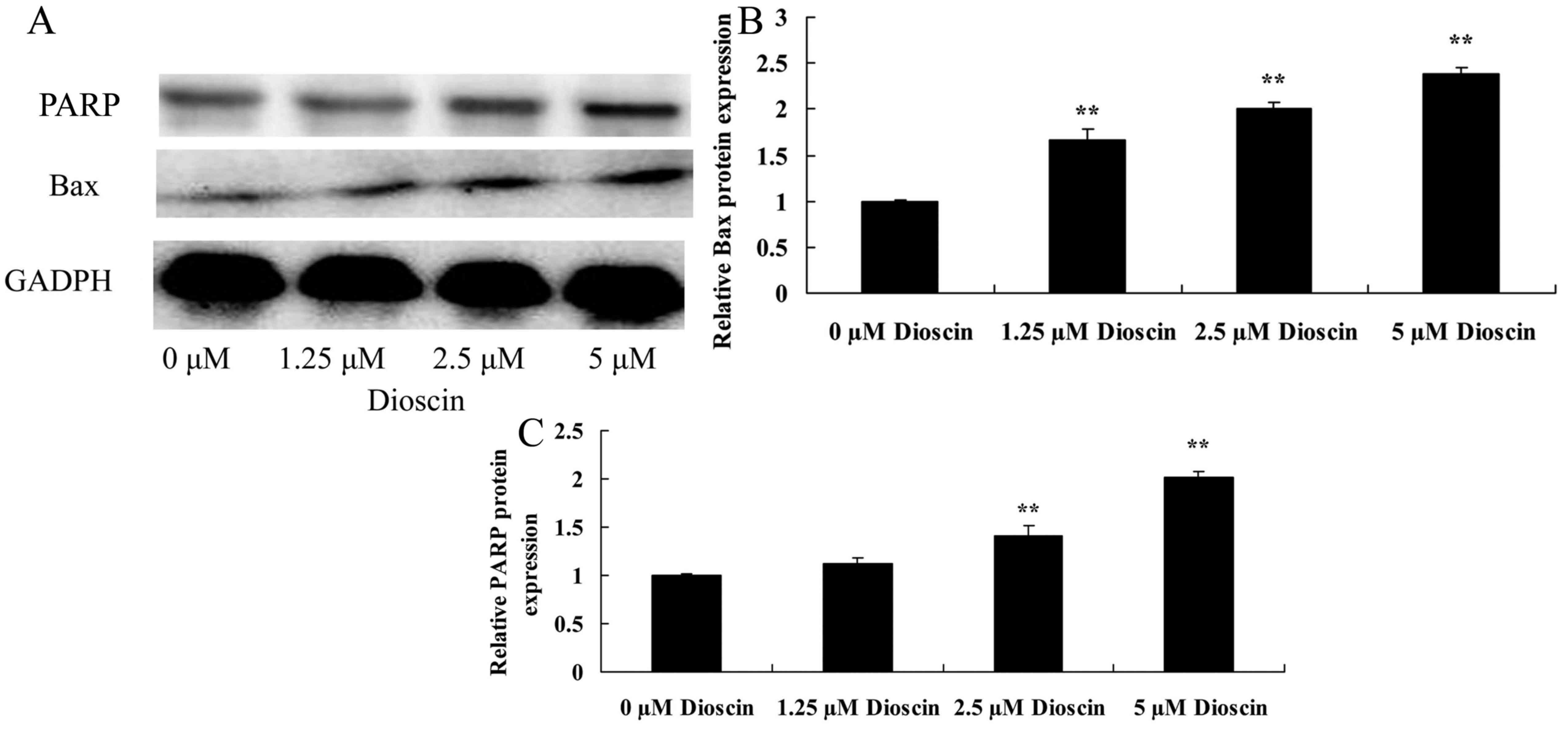
Dioscin treatment increased Bax and
cleaved PARP protein expression in SKOV3 cells
To explore the anticancer effects of dioscin in
human ovarian cancer, Bax protein expression level was determined
using a western blot analysis. Western blot analysis demonstrated
that the effects of dioscin (1.25, 2.5 or 5 µM) significantly
increased Bax and cleaved PARP protein expression levels in SKOV3
cells, compared with the untreated control group (P<0.01;
Fig. 5).
Dioscin treatment suppressed PI3K/AKT,
VEGFR2 protein expression and induced p-p38 protein expression in
SKOV3 cells
To investigate the role of PI3K/AKT, VEGFR2 and
p-p38 in the anticancer effects of dioscin treatment of SKOV3
cells, the present study performed western blot analysis. As
presented in Fig. 6, treatment with
2.5 µM or 5 µM dioscin significantly suppressed PI3K and
phosphorylated (p)-AKT, VEGFR2 protein expression, and induced
p-p38 protein expression in SKOV3 cell, compared with the control
group (P<0.01).
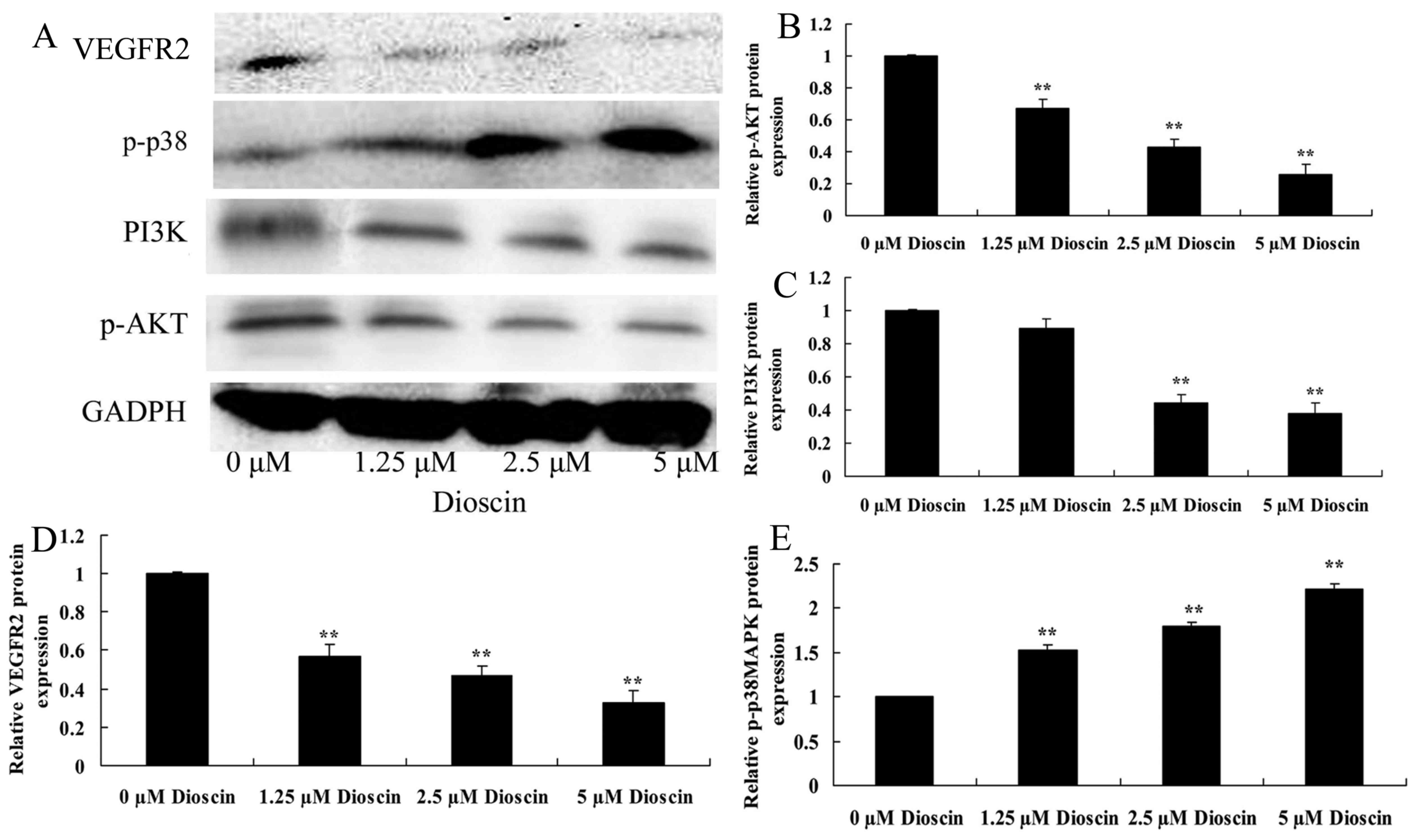 | Figure 6.Dioscin treatment suppressed PI3K/AKT,
VEGFR2 protein expression, and induced p-p38 protein expression in
SKOV3 human ovarian cancer cells. (A) The effect of dioscin
treatment on PI3K, p-AKT, VEGFR2 and p-p38 protein expression were
investigated using western blotting, which demonstrated that (B)
p-AKT, (C) PI3K, (D) VEGFR2 and (E) p-p38 protein expression were
decreased in SKOV3 human ovarian cancer cells treated with dioscin.
**P<0.01 vs. 0 µM dioscin group. PI3K, phosphoinositide
3-kinase; p-AKT, phosphorylated AKT; VEGFR2, vascular endothelial
growth factor receptor; p-, phosphorylated. |
Discussion
Primary epithelial ovarian cancer is the most fatal
type of malignant gynecological tumor, with a global fatality rate
of ~6.2% (4). In 2012, there were
~22,000 new cases of ovarian cancer and ~15,500 associated
mortalities in the United States (4).
This high mortality rate is due to chemotherapy resistance and the
lack of effective screening methods (18). Although >70% of patients with
ovarian cancer can be treated effectively with surgery,
chemotherapy and second-line chemotherapy, novel and more effective
pharmacological intervention is required due to the low overall
cure rates and serious adverse effects associated with chemotherapy
(19). The results of the present
study indicated that dioscin significantly promoted caspase-3 and
caspase-9 activity, increased Bax protein expression and enhanced
cleaved PARP protein expression in SKOV3 cells.
VEGF mediates cell synthesis, stimulates cellular
proliferation and migration, and enhances the permeability of blood
vessels through the effects of VEGFR on vascular endothelial cells
(20). Local increases in VEGF
concentration may promote the upregulation of the expression of
vascular endothelial cell VEGFR, which is an important mechanism to
allow VEGF in tumor tissue to promote angiogenesis (20). VEGFR2 is highly expressed on the
endothelial cell surface of new blood vessels in a tumor, and
promotes the growth of the new endothelial cells required by tumor
angiogenesis (21). The expression
percentage and expression intensity of VEGFR2 is considered to be
an important indicator for the evaluation of tumor prognosis
(22). Consequently, VEGFR2 is an
essential factor for tumor anti-angiogenesis therapy (22). The results of the present study
demonstrated that treatment with dioscin significantly suppressed
VEGFR2 protein expression in SKOV3 human ovarian cancer cells
compared with the untreated control group. A previous report by
Tong et al (15) suggested
that dioscin inhibits colon tumor growth through regulating VEGFR2
signaling pathways.
EGFR and its ligands extensively exist on
epithelium, stroma, original nerve cells and various
microstructures (23). EGFR is
activated by dimerization and phosphorylation following the binding
of exogenous substrates; in turn, it activates the downstream
Ras/Raf/MAPK and Ras/PI3K/AKT/mTOR signaling pathways, thus
controlling a variety of the biological responses of mature tissues
(24). In addition, EGFR and the
associated pathways are essential in embryonic development and
tissue differentiation (24). EGFR
serves an important function in regulating the growth and
differentiation of normal cells as well as promoting the growth of
tumor and mesenchymal cells, tumor cell adhesion and apoptosis
resistance, and tumor metastasis and angiogenesis (25). In addition, previous studies have
demonstrated that the overexpression of EGFR is associated with
drug resistance in ovarian cancer (25). The results of the present study
revealed that dioscin significantly suppressed PI3K and p-AKT
protein expression in SKOV3 cells, compared with the untreated
control group. Hsieh et al (26) demonstrated that dioscin induced human
lung cancer cell apoptosis through modulation of the PI3K/Akt
signaling pathway.
The effect of number of growth factors and receptors
associated with tumor growth and metastasis processes is mediated
via the p38MAPK signaling pathway (27). Tumors are generated as a response to
the dysregulation of intracellular signaling (27); the p38MAPK pathway is an essential
signaling pathway for the transduction of signals to the nucleus
(28), with functions in cell growth,
proliferation, cell death, cell cycle, inflammation and stress
reactions (29). In addition, the
p38MAPK signal transduction pathway may inhibit tumor formation
(30) and regulate the synthesis of
interleukin (IL)-1β precursor, thus promoting IL-1β synthesis
(31). IL-1β coordinates
immunological function, activating lymphocytes to promote
proliferation, promoting the generation of antibodies and activated
cytokines, enhancing the cytotoxicity of natural killer cells and
creating a positive feedback effect that effectively kills tumor
cells (31). The results of the
present study indicated that treatment with dioscin significantly
induced the p-p38 MAPK protein expression of SKOV3 human ovarian
cancer cells compared with the untreated control group. In accord
with this result, Wang et al (17) demonstrated that dioscin induced the
apoptosis of HL-60 acute myeloid leukemia cells through the
activation of p38MAPK and Jun N-terminal kinase.
In conclusion, the results of the present study
demonstrated for the first time, to the best of our knowledge, that
dioscin suppresses cellular viability and induces apoptosis in
ovarian cancer cells through the suppression of the VEGFR2 and
PI3K/AKT/p38 MAPK signaling pathways. Thus, dioscin may have
potential as an anticancer drug, and the VEGFR2 and PI3K/AKT/p38
MAPK signaling pathway may represent a potential target for
anticancer agents.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
XD designed the study. XG performed the experiments.
XG and XD analyzed the data. XD wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ren Y, Shi T, Jiang R, Yin S, Wang P and
Zang R: Multiple cycles of neoadjuvant chemotherapy associated with
poor survival in bulky stage IIIC and IV ovarian cancer. Int J
Gynecol Cancer. 25:1398–1404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Falandry C, Weber B, Savoye AM, Tinquaut
F, Tredan O, Sevin E, Stefani L, Savinelli F, Atlassi M, Salvat J,
et al: Development of a geriatric vulnerability score in elderly
patients with advanced ovarian cancer treated with first-line
carboplatin: A GINECO prospective trial. Ann Oncol. 24:2808–2813.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fisher M and Gore M: Cost-effectiveness of
trabectedin plus pegylated liposomal doxorubicin for the treatment
of women with relapsed platinum-sensitive ovarian cancer in the UK:
Analysis based on the final survival data of the OVA-301 trial.
Value Health. 16:507–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kehoe S, Hook J, Nankivell M, Jayson GC,
Kitchener H, Lopes T, Luesley D, Perren T, Bannoo S, Mascarenhas M,
et al: Primary chemotherapy versus primary surgery for newly
diagnosed advanced ovarian cancer (CHORUS): An open-label,
randomised, controlled, non-inferiority trial. Lancet. 386:249–257.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyake T, Kumasawa K, Sato N, Takiuchi T,
Nakamura H and Kimura T: Soluble VEGF receptor 1 (sFLT1) induces
non-apoptotic death in ovarian and colorectal cancer cells. Sci
Rep. 6:248532016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Camerin GR, Brito AB, Vassallo J, Derchain
SF and Lima CS: VEGF gene polymorphisms and outcome of epithelial
ovarian cancer patients. Future Oncol. 13:409–414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim BR, Yoon K, Byun HJ, Seo SH, Lee SH
and Rho SB: The anti-tumor activator sMEK1 and paclitaxel
additively decrease expression of HIF-1α and VEGF via
mTORC1-S6K/4E-BP-dependent signaling pathways. Oncotarget.
5:6540–6551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ganta S, Singh A, Patel NR, Cacaccio J,
Rawal YH, Davis BJ, Amiji MM and Coleman TP: Development of
EGFR-targeted nanoemulsion for imaging and novel platinum therapy
of ovarian cancer. Pharm Res. 31:2490–2502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ranjbar R, Nejatollahi F, Ahmadi Nedaei
AS, Hafezi H and Safaie A: Expression of vascular endothelial
growth factor (VEGF) and epidermal growth factor receptor (EGFR) in
patients with serous ovarian carcinoma and their clinical
significance. Iran J Cancer Prev. 8:e34282015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kodigepalli KM, Dutta PS, Bauckman KA and
Nanjundan M: SnoN/SkiL expression is modulated via arsenic
trioxide-induced activation of the PI3K/AKT pathway in ovarian
cancer cells. FEBS Lett. 587:5–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Glaysher S, Bolton LM, Johnson P, Atkey N,
Dyson M, Torrance C and Cree IA: Targeting EGFR and PI3K pathways
in ovarian cancer. Br J Cancer. 109:1786–1794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu M, Chen X, Lou J, Zhang S, Zhang X,
Huang L, Sun R, Huang P, Wang F and Pan S: TGF-β1 contributes to
CD8+ Treg induction through p38 MAPK signaling in ovarian cancer
microenvironment. Oncotarget,. 7:44534–44544. 2016.
|
|
13
|
Wang F, Chang Z, Fan Q and Wang L:
Epigallocatechin-3-gallate inhibits the proliferation and migration
of human ovarian carcinoma cells by modulating p38 kinase and
matrix metalloproteinase-2. Mol Med Rep. 9:1085–1089. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu M, Xiao L, Hu J, Deng S and Xu Y:
Targeting of p38 mitogen-activated protein kinases to early growth
response gene 1 (EGR-1) in the human paclitaxel-resistance ovarian
carcinoma cells. J Huazhong Univ Sci Technolog Med Sci. 28:451–455.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tong Q, Qing Y, Wu Y, Hu X, Jiang L and Wu
X: Dioscin inhibits colon tumor growth and tumor angiogenesis
through regulating VEGFR2 and AKT/MAPK signaling pathways. Toxicol
Appl Pharmacol. 281:166–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qi M, Yin L, Xu L, Tao X, Qi Y, Han X,
Wang C, Xu Y, Sun H, Liu K and Peng J: Dioscin alleviates
lipopolysaccharide-induced inflammatory kidney injury via the
microRNA let-7i/TLR4/MyD88 signaling pathway. Pharmacol Res.
111:509–522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, He QY and Chiu JF: Dioscin induced
activation of p38 MAPK and JNK via mitochondrial pathway in HL-60
cell line. Eur J Pharmacol. 735:52–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith HO, Moon J, Wilczynski SP, Tiersten
AD, Hannigan EV, Robinson WR, Rivkin SE, Anderson GL, Liu PY and
Markman M: Southwest oncology group trial S9912: Intraperitoneal
cisplatin and paclitaxel plus intravenous paclitaxel and pegylated
liposomal doxorubicin as primary chemotherapy of small-volume
residual stage III ovarian cancer. Gynecol Oncol. 114:206–209.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Noonan AM, Bunch KP, Chen JQ, Herrmann MA,
Lee JM, Kohn EC, O'Sullivan CC, Jordan E, Houston N, Takebe N, et
al: Pharmacodynamic markers and clinical results from the phase 2
study of the SMAC mimetic birinapant in women with relapsed
platinum-resistant or -refractory epithelial ovarian cancer.
Cancer. 122:588–597. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adham SA, Sher I and Coomber BL: Molecular
blockade of VEGFR2 in human epithelial ovarian carcinoma cells. Lab
Invest. 90:709–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu W, Chen H, Yel F, Wang F and Xie X:
VEGF induces phosphorylation of STAT3 through binding VEGFR2 in
ovarian carcinoma cells in vitro. Eur J Gynaecol Oncol. 27:363–369.
2006.PubMed/NCBI
|
|
22
|
Barua A, Yellapa A, Bahr JM, Machado SA,
Bitterman P, Basu S, Sharma S and Abramowicz JS: VEGFR2-targeted
ultrasound imaging agent enhances the detection of ovarian tumors
at early stage in laying hens, a preclinical model of spontaneous
ovarian cancer. Ultrason Imaging. 37:224–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ye J, Chen W, Wu ZY, Zhang JH, Fei H,
Zhang LW, Wang YH, Chen YP and Yang XM: Upregulated CTHRC1 promotes
human epithelial ovarian cancer invasion through activating EGFR
signaling. Oncol Rep. 36:3588–3596. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baron AT, Wilken JA, Haggstrom DE,
Goodrich ST and Maihle NJ: Clinical implementation of soluble EGFR
(sEGFR) as a theragnostic serum biomarker of breast, lung and
ovarian cancer. IDrugs. 12:302–308. 2009.PubMed/NCBI
|
|
25
|
Zhao J, Klausen C, Qiu X, Cheng JC, Chang
HM and Leung PC: Betacellulin induces slug-mediated down-regulation
of E-cadherin and cell migration in ovarian cancer cells.
Oncotarget. 7:28881–28890. 2016.PubMed/NCBI
|
|
26
|
Hsieh MJ, Tsai TL, Hsieh YS, Wang CJ and
Chiou HL: Dioscin-induced autophagy mitigates cell apoptosis
through modulation of PI3K/Akt and ERK and JNK signaling pathways
in human lung cancer cell lines. Arch Toxicol. 87:1927–1937. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Malm SW, Hanke NT, Gill A, Carbajal L and
Baker AF: The anti-tumor efficacy of 2-deoxyglucose and D-allose
are enhanced with p38 inhibition in pancreatic and ovarian cell
lines. J Exp Clin Cancer Res. 34:312015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu W, Gu J, Ren Q, Shi Y, Xia Q and Wang
J, Wang S, Wang Y and Wang J: NFATC1 promotes cell growth and
tumorigenesis in ovarian cancer up-regulating c-Myc through
ERK1/2/p38 MAPK signal pathway. Tumour Biol. 37:4493–4500. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watson JL, Greenshields A, Hill R, Hilchie
A, Lee PW, Giacomantonio CA and Hoskin DW: Curcumin-induced
apoptosis in ovarian carcinoma cells is p53-independent and
involves p38 mitogen-activated protein kinase activation and
downregulation of Bcl-2 and survivin expression and Akt signaling.
Mol Carcinog. 49:13–24. 2010.PubMed/NCBI
|
|
30
|
Zhang B, Wang X, Cai F, Chen W, Loesch U
and Zhong XY: Antitumor properties of salinomycin on
cisplatin-resistant human ovarian cancer cells in vitro and in
vivo: Involvement of p38 MAPK activation. Oncol Rep. 29:1371–1378.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Buldak RJ, Polaniak R, Buldak L,
Mielanczyk L, Kukla M, Skonieczna M, Dulawa-Buldak A, Matysiak N
and Zwirska-Korczala K: Exogenous administration of visfatin
affects cytokine secretion and increases oxidative stress in human
malignant melanoma Me45 cells. J Physiol Pharmacol. 64:377–385.
2013.PubMed/NCBI
|