Introduction
Schwannoma is a relatively rare tumor, comprising
~5% of benign soft tissue tumors (1).
Compared with other benign neurogenic tumors, schwannoma originates
from a nerve sheath, whereas neurofibroma arises from the nerve
itself. Because schwannomas are typically well encapsulated and
displace nerve fibers as they grow, it is thought that enucleation
can easily be performed without causing postoperative neurological
deficit. However, in certain cases it is difficult to enucleate the
tumor without causing nerve damage, even when using meticulous
operative techniques to preserve nerve fascicles, and such patients
may present with postoperative neurological symptoms. The reported
incidence of postoperative neurological deficit resulting from the
enucleation of schwannoma varies; notably, Park et al
(2) reported an incidence of ≤75% in
upper-extremity schwannoma. In addition, an association was
identified between the presence of Tinel's sign and increased tumor
volume with increased risk of postoperative nerve injury (2). One method for monitoring postoperative
neurologic symptoms is intraoperative motor-evoked potential (MEP).
MEP was originally developed as a method for monitoring cranial
nerve function during cerebral aneurysm surgery and brain tumor
resection; however, it is now widely applied in different types of
neurosurgery, and MEP monitoring is recommended as a precaution
against perioperative neuroparalysis in a spinal cord tumor
resection and scoliosis surgery (3–6). However,
to the best of our knowledge, no previous studies have attempted to
apply MEP for intraoperative neurological monitoring during the
enucleation of peripheral nerve schwannoma. The present study
examined the utility of MEP in predicting postoperative
neurological deficit following the surgical enucleation of
schwannoma.
Materials and methods
Patients
The current study included 23 patients [9 male, 14
female; age range, 29–78 years old (mean, 55 years old)] with
schwannoma of a peripheral nerve excluding a pure sensory nerve,
who underwent surgical enucleation at the department of orthopedic
surgery in Kagoshima University between 2011 and 2014 (Table I). Preoperative Magnetic Resonance
Imaging (MRI) was underwent for identifying the tumor's location in
all cases. The most frequently involved nerves were tibial (n=6)
and sciatic (n=5). A Tinel's-like sign or paresthesia that was
painful to percussion was identified preoperatively in 16/21 cases
(76%; excluding two cases of schwannoma originating from the lumbar
nerve root). All procedures were in accordance with the ethical
standards of the institutional Review Board of Kagoshima University
and with the 1964 Helsinki declaration and its later amendments or
comparable ethical standards. The patients were informed that data
from the case would be submitted for publication, and gave their
consent.
 | Table I.Clinicopathological characteristics of
the 23 patients with peripheral nerve schwannoma who underwent
surgical enucleation in the present study. |
Table I.
Clinicopathological characteristics of
the 23 patients with peripheral nerve schwannoma who underwent
surgical enucleation in the present study.
|
|
|
|
|
|
| Neurological
symptoms |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Case no. | Age (years) | Sex | Nerve involved with
the tumor | Pneumatic
tourniquet | Tinel's sign | Preoperative | Postoperative | ΔMEP <50% |
|---|
| 1 | 78 | M | Sciatic | − | − | No | No | − |
| 2 | 55 | F | Peroneal | − | + | Hypesthesia | Numbness, pain | − |
| 3 | 68 | M | Tibial | − | + | No | No | − |
| 4 | 49 | F | Tibial | + | + | No | No | + |
| 5 | 58 | M | Tibial | − | + | No | No | − |
| 6 | 63 | F | Brachial plexus | − | + | Ulnar nerve
palsy | No | − |
| 7 | 36 | F | Median | + | + | No | No | − |
| 8 | 50 | F | Femoral | − | + | No | No | − |
| 9 | 61 | M | Ulnar | − | + | No | Hypesthesia | − |
| 10 | 59 | F | Peroneal | − | + | No | Hypesthesia | − |
| 11 | 68 | F | Femoral | − | − | No | No | − |
| 12 | 50 | M | Tibial | + | − | No | No | + |
| 13 | 58 | F | Tibial | − | + | No | No | − |
| 14 | 55 | F | Brachial plexus | − | + | No | No | − |
| 15 | 51 | F | L4 nerve root | − | − | No | Muscle weakness | − |
| 16 | 70 | F | L3 nerve root | − | − | No | No | − |
| 17 | 39 | M | Sciatic | − | − | No | No | − |
| 18 | 63 | F | Sciatic | − | + | No | No | − |
| 19 | 62 | M | Median | − | + | No | No | − |
| 20 | 29 | F | Tibial | − | + | No | No | − |
| 21 | 51 | M | Sciatic | − | + | No | Peroneal nerve
palsy | + |
| 22 | 35 | F | Median | − | − | No | No | − |
| 23 | 60 | M | Sciatic | − | + | No | No | − |
Intraoperative MEP
MEP was performed with transcranial electrical
stimulation. The transcranial stimulation was typically delivered
in trains of five pulses with 2.0 msec interval at 500V (0.1 Hz
frequency) by stimulator (SEN-4100; Nihon Kohden, Tokyo, Japan).
The thenar, flexor carpi ulnaris and brachioradialis muscles were
monitored in cases involving upper extremities. In cases associated
with lower extremities the anterior tibialis or gastrocnemius
muscle were monitored for the peroneal or tibial nerves,
respectively. MEP was measured prior to and following surgical
enucleation using four-channel electromyography (MEB-9140; Nihon
Kohden). A decrease in MEP of <50% of the preoperative value was
designated as alarm point indicating loss of motor function.
Surgical enucleation
General anesthesia was induced in all cases. Care
was taken not to influence the MEP by maintaining narcotic and
intravenous anesthesia rather than employing an inhaled anesthetic
and muscle relaxant. At the induction of anesthesia, propofol (0.5
mg/kg/10 sec), fentanyl (1.5~8 µg/kg) and vecuronium (0.1 mg/kg)
were used. Anesthesia was maintained using propofol (4~10 mg/kg/h)
and fentanyl (0.5~5 µg/kg/h). The surgery began with a longitudinal
incision over the tumor, followed by incision of the fascia to
expose the tumor. Prior to enucleation of the tumor body, the
connecting nerve at the proximal and distal parts of the tumor was
identified and dissected. This procedure loosens the involved nerve
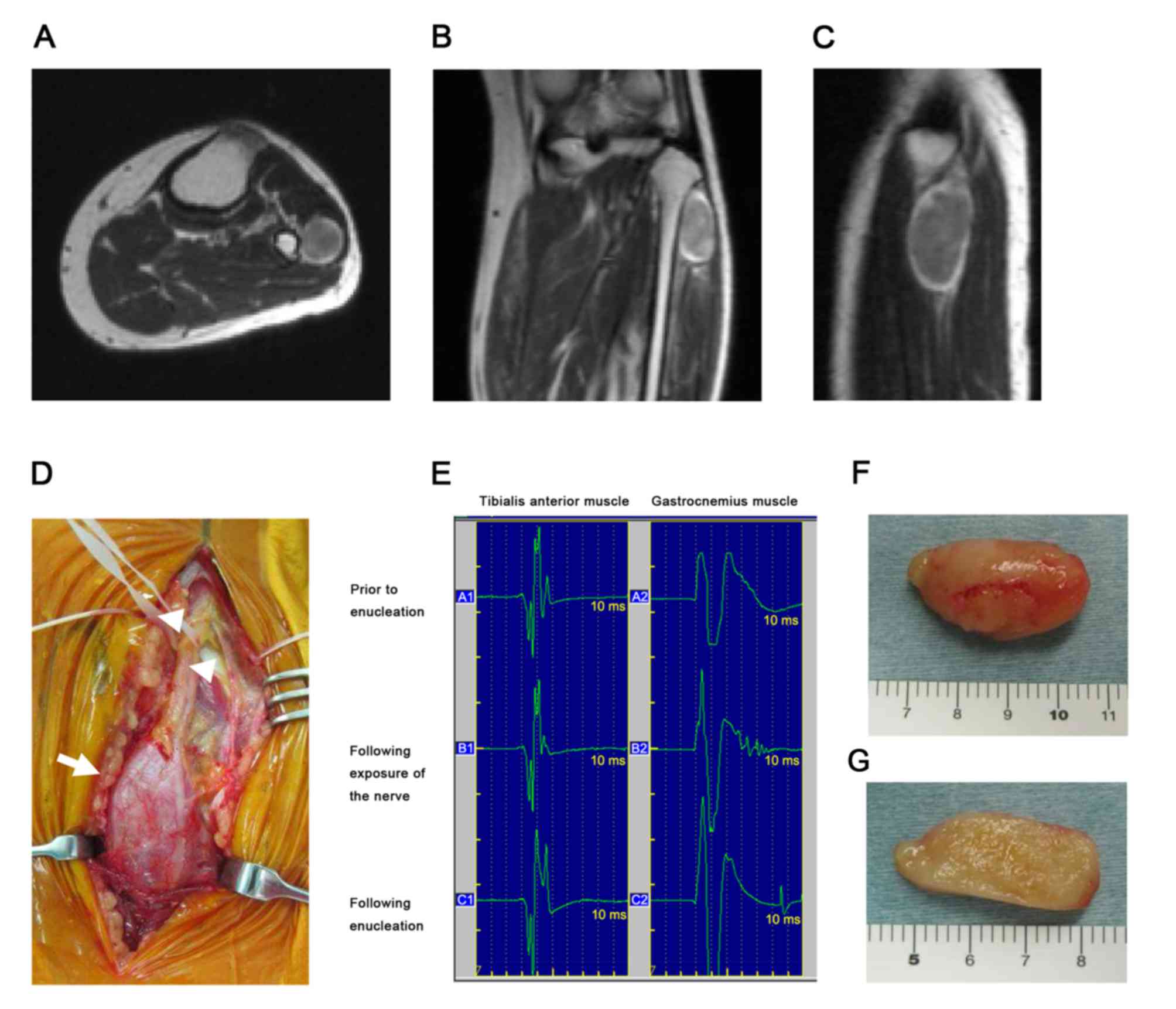
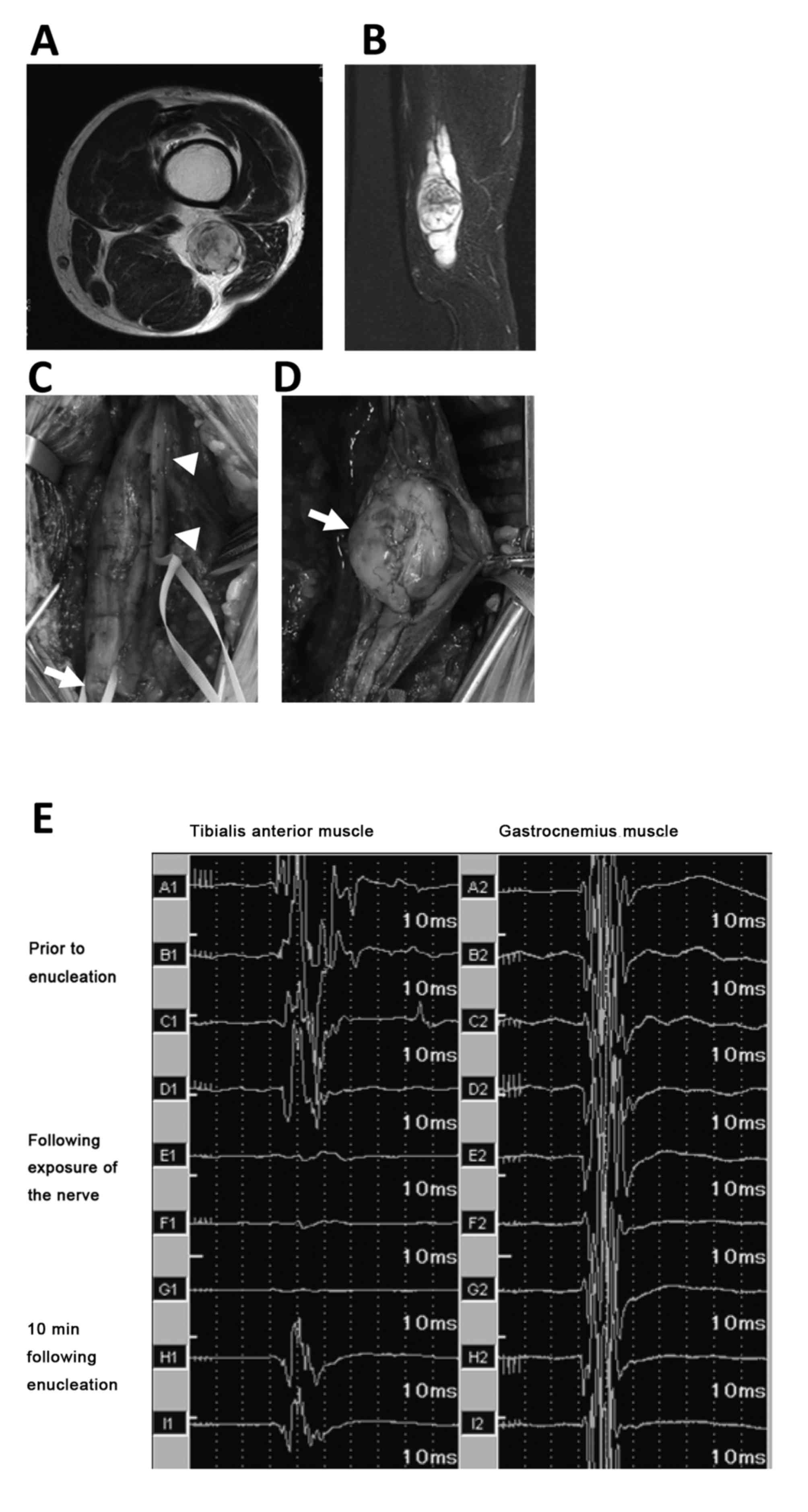
trunk and decreases the likelihood of nerve injury (Figs. 1D and 2C). Following dissection of the surrounding
connective tissue, a longitudinal incision was made on the tumor
capsule in a position that would affect the fascicle (Fig. 2D), exposing the yellowish tumor body.
Subsequently, a blunt dissection between the capsule and the tumor
resulted in en bloc enucleation of the schwannoma with preservation
of the nerve fascicles outside of the capsule. For cases in which a
tumor located in a limb was identified on preoperative MRI to be
close to a major blood vessel, the limb was exsanguinated and a
pneumatic tourniquet applied prior to incision.
Results
In 3/23 cases, MEP decreased to <50% of the
preoperative value (Table I). In
cases no. 4 and 12, the schwannoma occurred in the calf and
involved the common peroneal nerve and tibial nerve, respectively.
There was no postoperative neurological deficit in either case;
however, because a tourniquet was used during surgery, these
results were thought to be false positives. In another case where
MEP decreased by <50% (case no. 21), the tumor originated from
the sciatic nerve (Fig. 2A and B).
During surgical exposure of the tumor, and the tibial and peroneal
nerves, the MEP remained intact (Fig.
2E). Following enucleation of the tumor from the capsule, with
preservation of the affected nerve, the potential was completely
lost (Fig. 2E). After 10 min, the MEP
recovered to 61% of the preoperative MEP. The patient in this case
presented with common peroneal palsy postoperatively (Table I). In another case in which there was
postoperative motor loss (case no. 15), the schwannoma involved the
lumbar nerve root; however, the MEP did not change intraoperatively
(data not shown). The patient had loss of muscle strength around
the hip and knee that recovered 3 months following surgery. A total
of 3 other patients (case no. 2, 9 and 10) had sensory disturbance
in the area of the involved nerve (ulnar or peroneal)
postoperatively (Table I). The
sensory disturbance was transient in all 3 cases and gradually
resolved with 1–4 months. Preoperative neurological symptoms were
present in 2 cases (case no. 2 and 6). There were no postoperative
complications in either case. One patient (no. 2) had sensory
disturbance postoperatively described above, and the other (no. 6)
had no postoperative complications.
Discussion
Enucleation of schwannoma is possible because the
tumor occurs in a nerve sheath and does not involve the nerve
fibers. However, various rates of postoperative neurological
deficit have been demonstrated. Artico et al (7), reported that among 73 cases of
resectable schwannoma, preoperative symptoms improved in 41%,
worsened 6.8% and remained unchanged in 52% of patients
postoperatively. Oberle et al (8) identified that sensory disturbance
occurred immediately following surgery in 6/12 patients. Notably,
Donner et al (9) reported
that, out of 31 patients with schwannoma who had preoperative
muscle weakness, 13% experienced postoperative loss of muscle
strength. In the present study, postoperative neurological deficit
occurred in 22% of patients, which is similar to that of previous
reports. The cause of postoperative neurological deficit in
schwannoma remains unclear, although several mechanisms have been
proposed, including preoperative nerve compression by the tumor,
mechanical nerve injury during surgery, or ischemia of the nerve
associated with the surgical procedure. Although postoperative
neurological symptoms in patients with peripheral schwannoma are
transient in the majority cases, it can be a problem in terms of
patient satisfaction with the surgery.
Sawada et al (10), reported that 4/17 cases of schwannoma
occurring in the limbs were located in the subclavicular area or
brachial plexus and could not be enucleated. Due to the anatomical
complexity of subclavicular and brachial plexus schwannomas, an
adequate operative field may be difficult to secure, which can
result in incomplete enucleation and a higher risk of recurrence.
In such cases, sufficient exposure of the nerve and tumor should be
conducted with a form of nerve monitoring to predict intraoperative
nerve injury. In the present study, two cases involved the brachial
plexus. In one of these cases, the middle part of the clavicle was
transected to obtain adequate exposure of the major blood vessels
and nerves. Although there is the risk of delayed union of a
repositioned clavicle, this was a good option for reducing the risk
of vessel or nerve injury in the current study.
Various methods of perioperative neurological
monitoring have been developed, including spontaneous
electromyography (spEMG) and somatosensory evoked potential (SSEP),
in addition to MEP. In a large study of 1,055 patients who
underwent cervical spinal surgery, the sensitivities and
specificities of spEMG, SSEP, and MEP were 46 and 73%, 52 and 100%,
and 100 and 96%, respectively (11).
MEP is recognized as the most useful type of neurological
monitoring and is recommended for use alone or in combination with
other monitoring modalities, depending on the risks of the surgery
being undertaken (11). Therefore,
neurological monitoring using MEP has been widely used for brain
and spinal surgeries. For example, in corrective surgery for highly
deformed spinal columns, the risk of neurological complications can
be as high as 27% (12). To reduce
the risk of such complications, the utility of perioperative nerve
monitoring with MEP as a predictor of postoperative neurologic
deficit has been studied (13,14). The
Japanese Society for Spine Surgery and Related Research conducted a
multicenter study of intraoperative never monitoring with MEP in
959 spinal surgeries to determine a warning threshold a cut-off
percent for the change between pre- and postoperative MEP (15).
Few studies have applied nerve monitoring to
surgeries involving peripheral nerves. Several studies used
perioperative neurological monitoring of the peripheral nerves to
predict cervical spinal nerve 5 (C5) paralysis during cervical
spinal surgery (16,17). Jimenez et al (16), reported that perioperative monitoring
with spEMG was an effective predictor of C5 paralysis following
cervical spinal surgery, and other studies demonstrated similar
results (17,18). Bose et al (18), evaluated whether MEP monitoring could
predict C5 neuroparalysis by defining a cut-off value as a decrease
in MEP of <50% of the baseline value. The results revealed that
MEP was able to detect C5 paralysis with a sensitivity and
specificity of 91 and 89%, respectively. In contrast, the
sensitivity and specificity of spEMG (42 and 85%) and SSEP (0 and
98%) led to the conclusion that MEP was the most useful form of
nerve monitoring. Bhalodia et al (19), examined the ability of SSEP and MEP to
predict postoperative C5 paralysis, and reported that it was
difficult to predict with either modality, whether used alone or in
combination. The low sensitivity of SSEP was attributed to the
differing structures of cranial and peripheral nerves. Another
disadvantage of MEP is that it frequently produces false positive
results (17).
No other studies have investigated MEP as a method
for monitoring peripheral nerves during the surgical enucleation of
schwannoma, to the best of our knowledge. In the present study,
there were two cases with false positive results; however, in these
cases a pneumatic tourniquet was used intraoperatively. This
suggests that the MEP level may depend on blood flow to peripheral
nerves. It has previously been reported that MEP is a sensitive
indicator of spinal cord ischemia (20). Therefore, even if a nerve is not
transected or injured, traction or compression of a peripheral
nerve may induce ischemia, which can affect the MEP. In one case in
the present study, the MEP suddenly decreased following enucleation
of the tumor and the patient developed transient but complete
peroneal nerve palsy postoperatively. Although the nerve trunk was
preserved, intraoperative ischemia caused by traction or
compression of the nerve may have been responsible. This suggests
that great care should be taken when preserving the vessels around
the nerve and that the MEP should be checked frequently when
handling vessels near the schwannoma. Nevertheless, MEP alone was
not able to predict postoperative motor loss, suggesting that
further combined monitoring with free-run electromyography or
direct electrical stimulation (21)
may aid in the accurate prediction of nerve injury.
In conclusion, the present study examined the
utility of MEP as a perioperative nerve monitoring technique during
the enucleation of peripheral nerve schwannomas. Decreased blood
flow caused by the pneumatic tourniquet was observed to result in a
decrease in MEP. Although MEP alone was not able to predict
postoperative transient sensory or motor deficits following the
enucleation of schwannoma, the combination of MEP with other
methods of neurological monitoring may improve the accuracy of
nerve monitoring and should be investigated in future studies.
Acknowledgments
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kransdorf MJ: Benign soft-tissue tumors in
a large referral population: Distribution of specific diagnoses by
age, sex, and location. AJR Am J Roentgenol. 164:395–402. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park MJ, Seo KN and Kang HJ: Neurological
deficit after surgical enucleation of schwannomas of the upper
limb. J Bone Joint Surg Br. 91:1482–1486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hyun SJ and Rhim SC: Combined motor and
somatosensory evoked potential monitoring for intramedullary spinal
cord tumor surgery: Correlation of clinical and neurophysiological
data in 17 consecutive procedures. Br J Neurosurg. 23:393–400.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Epstein NE: The need to add motor evoked
potential monitoring to somatosensory and electromyographic
monitoring in cervical spine surgery. Surg Neurol Int. 4 Suppl
5:S383–S391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feng B, Qiu G, Shen J, Zhang J, Tian Y, Li
S, Zhao H and Zhao Y: Impact of multimodal intraoperative
monitoring during surgery for spine deformity and potential risk
factors for neurological monitoring changes. J Spinal Disord Tech.
25:E108–E114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pastorelli F, Di Silvestre M, Plasmati R,
Michelucci R, Greggi T, Morigi A, Bacchin MR, Bonarelli S, Cioni A,
Vommaro F, et al: The prevention of neural complications in the
surgical treatment of scoliosis: The role of the neurophysiological
intraoperative monitoring. Eur Spine J. 20 Suppl 1:S105–S114. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Artico M, Cervoni L, Wierzbicki V,
D'Andrea V and Nucci F: Benign neural sheath tumours of major
nerves: Characteristics in 119 surgical cases. Acta Neurochir
(Wien). 139:1108–1116. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oberle J, Kahamba J and Richter HP:
Peripheral nerve schwannomas-an analysis of 16 patients. Acta
Neurochir (Wien). 139:949–953. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Donner TR, Voorhies RM and Kline DG:
Neural sheath tumors of major nerves. J Neurosurg. 81:362–373.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sawada T, Sano M, Ogihara H, Omura T,
Miura K and Nagano A: The relationship between pre-operative
symptoms, operative findings and postoperative complications in
schwannomas. J Hand Surg Br. 31:629–634. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kelleher MO, Tan G, Sarjeant R and
Fehlings MG: Predictive value of intraoperative neurophysiological
monitoring during cervical spine surgery: A prospective analysis of
1055 consecutive patients. J Neurosurg Spine. 8:215–221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lenke LG, Newton PO, Sucato DJ,
Shufflebarger HL, Emans JB, Sponseller PD, Shah SA, Sides BA and
Blanke KM: Complications after 147 consecutive vertebral column
resections for severe pediatric spinal deformity: A multicenter
analysis. Spine (Phila Pa 1976). 38:119–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hilibrand AS, Schwartz DM, Sethuraman V,
Vaccaro AR and Albert TJ: Comparison of transcranial electric motor
and somatosensory evoked potential monitoring during cervical spine
surgery. J Bone Joint Surg Am. 86-A:1248–1253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schwartz DM, Sestokas AK, Dormans JP,
Vaccaro AR, Hilibrand AS, Flynn JM, Li PM, Shah SA, Welch W,
Drummond DS and Albert TJ: Transcranial electric motor evoked
potential monitoring during spine surgery: Is it safe? Spine (Phila
Pa 1976). 36:1046–1049. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kobayashi S, Matsuyama Y, Shinomiya K,
Kawabata S, Ando M, Kanchiku T, Saito T, Takahashi M, Ito Z,
Muramoto A, et al: A new alarm point of transcranial electrical
stimulation motor evoked potentials for intraoperative spinal cord
monitoring: A prospective multicenter study from the spinal cord
monitoring working group of the Japanese society for spine surgery
and related research. J Neurosurg Spine. 20:102–107. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jimenez JC, Sani S, Braverman B, Deutsch H
and Ratliff JK: Palsies of the fifth cervical nerve root after
cervical decompression: Prevention using continuous intraoperative
electromyography monitoring. J Neurosurg Spine. 3:92–97. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan D, Schwartz DM, Vaccaro AR, Hilibrand
AS and Albert TJ: Intraoperative neurophysiologic detection of
iatrogenic C5 nerve root injury during laminectomy for cervical
compression myelopathy. Spine (Phila Pa 1976). 27:2499–2502. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bose B, Sestokas AK and Schwartz DM:
Neurophysiological detection of iatrogenic C-5 nerve deficit during
anterior cervical spinal surgery. J Neurosurg Spine. 6:381–385.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhalodia VM, Schwartz DM, Sestokas AK,
Bloomgarden G, Arkins T, Tomak P, Gorelick J, Wijesekera S, Beiner
J and Goodrich I: Efficacy of intraoperative monitoring of
transcranial electrical stimulation-induced motor evoked potentials
and spontaneous electromyography activity to identify acute-versus
delayed-onset C-5 nerve root palsy during cervical spine surgery:
Clinical article. J Neurosurg Spine. 19:395–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kai Y, Owen JH, Allen BT, Dobras M and
Davis C: Relationship between evoked potentials and clinical status
in spinal cord ischemia. Spine (Phila Pa 1976). 20:291–296. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leppanen RE: Intraoperative monitoring of
segmental spinal nerve root function with free-run and
electrically-triggered electromyography and spinal cord function
with reflexes and F-responses. A position statement by the American
Society of Neurophysiological Monitoring. J Clin Monit Comput.
19:437–461. 2005. View Article : Google Scholar : PubMed/NCBI
|