Introduction
Hepatocellular carcinoma (HCC) is the most common
histological type in the majority of patients with liver cancer,
with increasing morbidity and mortality rates. Over the last few
decades, management of HCC, including surveillance programs,
diagnostic capacity and effective curative treatment methods, have
substantially improved, thereby improving patient survival times
and quality of life (1). However, the
high rate of 5-year relapse remains an important problem in
postoperative patients with HCC. In certain Asian countries, the
5-year cumulative rate of recurrence following primary hepatic
resection may be as high as 70–100% (2,3). Although
previous studies have provided evidence that certain biomarkers may
be used to predict the recurrence of HCC (4–6), little
has been identified regarding the crucial biomarkers required to
guide the target of potential treatments and to prevent HCC
recurrence.
It is now known that the majority of cancerous cells
undergo bioenergetic reprogramming, switching the maximal pyruvate
metabolism from mitochondrial oxidative phosphorylation to
cytoplasm glycolysis, in order to support neoplastic proliferation,
a process known as the ‘Warburg effect’ (7). During the conversion of tumor energy
metabolism, functional mitochondria are essential for the viability
of cancer cells (8). A few decades
ago, studies reported that the amount of pyruvate transportation
into the mitochondria and its utilization were significantly
decreased, and that the malfunction of mitochondrial pyruvate
carrier (MPC) activity was associated with the proliferation of
tumor cells (9,10). In line with this, using a specific
inhibitor of MPC slightly enhanced tumor growth (11).
MPC, the molecular identification and purification
of which were achieved in 2012 (12,13), is a
protein complex comprised of MPC1 (also known as BRP44L) and MPC2
(also known as BRP44) in humans. More recently, depletion or
extremely low levels of MPC1 protein were revealed to be common
features of multiple malignant cancer types and indicators of a
poorer prognosis (14). Studies
published thus far demonstrate that as a linker of glycolysis and
intra-mitochondrial pyruvate metabolism, MPC is likely to have
marked effects on the phenotypes of tumor metabolism and
proliferation. As mitochondria are highly abundant within liver
cells, the present study proposes that MPC may be associated with
HCC and thus may serve a vital role in its initiation and
progression.
The present study aimed to evaluate the association
between MPC1 and MPC2 and the clinicopathological parameters and
prognosis of HCC, and therefore to provide a potential biomarker
for the recurrence and prognosis of HCC.
Patients and methods
Sample collection
The present study was approved by the Ethics
Committee of the Cancer Institute of Tianjin Medical University
Cancer Institute and Hospital (Tianjin, China). Written informed
consent was obtained prior to participation in the study. A total
of 85 patient samples were used for immunohistochemistry (primary
HCC tissues and their adjacent non-cancerous tissues). The samples
were obtained from patients who had undergone a curative liver
resection between January 2011 and December 2012, following a
primary histopathologically confirmed HCC diagnosis. The relevant
clinicopathological characteristics of the patients with HCC are
presented in Table I. Follow-up
occurred between the date of the hepatectomy and November 2015.
Fresh hepatocarcinoma samples and para-carcinoma tissue were placed
in liquid nitrogen immediately when the specimens were isolated
between May 2015 and August 2015, to be used for mRNA and western
blot analysis. Recurrence-free survival (RFS) was determined
following a radiologically evident diagnosis of recurrence (using
computed tomography and/or magnetic resonance imaging). Overall
survival (OS) was the percentage of patients who survived since
curative liver resection.
 | Table I.Association between MPC protein
expression in tumor tissue and clinicopathological parameters in
hepatocellular carcinoma patients. |
Table I.
Association between MPC protein
expression in tumor tissue and clinicopathological parameters in
hepatocellular carcinoma patients.
|
| MPC1 | MPC2 |
|---|
|
|
|
|
|---|
| Clinical
characteristics | High (n=43) | Low (n=42) | P-value | High (n=42) | Low (n=43) | P-value |
|---|
| Age, year | 54.8±9.5 | 54.7±11.2 | 0.426 | 55.7±10.9 | 53.9±9.8 | 0.386 |
| Gender
(male/female) | 33/10 | 37/5 | 0.255 | 33/9 | 37/6 | 0.407 |
| ALT (U/l) | 37.4±25.5 | 37.0±19.0 | 0.131 | 34.1±21.7 | 37.2±23.1 | 0.626 |
| AST (U/l) | 40.6±27.1 | 37.6±27.0 | 0.864 | 39.5±32.1 | 37.8±21.4 | 0.507 |
| TBIL (µmol/l) | 17.0±10.0 | 19.6±12.9 | 0.999 | 19.6±14.2 | 17.2±8.2 | 0.120 |
| ALB (g/l) | 46.3±4.1 | 43.5±6.6 | 0.187 | 44.0±6.6 | 45.8±4.4 | 0.431 |
| PT, sec | 11.2±1.1 | 11.8±3.1 | 0.296 | 11.7±3.2 | 11.4±1.2 | 0.441 |
| PLT,
×109/l | 158.0±69.8 | 167.9±48.9 | 0.473 | 162.6±80.4 | 163.6±69.1 | 0.411 |
| AFP (>200/≤200
ng/ml) | 16/27 | 15/27 | 1.000 | 17/25 | 14/29 | 0.504 |
| BCLC Stage
(0-A/B-C) | 23/20 | 15/27 | 0.128 | 18/24 | 20/23 | 0.828 |
| Tumor number
(1/>1) | 35/8 | 39/3 | 0.195 | 36/6 | 38/5 | 0.757 |
| Tumor size |
|
|
|
|
|
|
| Maximum
diameter (>5/≤5 cm) | 16/27 | 21/21 | 0.278 | 20/22 | 17/26 | 0.515 |
| Smallest
diameter (>3/≤3 cm) | 19/24 | 24/18 | 0.281 | 22/20 | 21/22 | 0.829 |
| Differentiation
(poor/moderate or well) | 16/27 | 13/29 | 0.649 | 14/28 | 15/28 | 0.880 |
| Microvascular
invasion (yes/no) | 23/20 | 25/17 | 0.663 | 23/19 | 25/18 | 0.828 |
| Micrometastases
(yes/no) | 14/29 | 18/24 | 0.375 | 17/26 | 16/27 | 0.824 |
Immunohistochemistry
All tissue samples were fixed with 10% neutral
formalin, embedding into paraffin sections (4 µm) after 24 h and
were subsequently deparaffinized with dimethylbenzene and
rehydrated through a concentration gradient of ethanol, prior to
antigen retrieval in a pressure cooker (pH=6.0 sodium citrate acid
repair solution, 121°C for 5 min. Samples were then washed in PBS 3
times, and endogenous peroxidase activity was inactivated in 3%
hydrogen peroxide solution for 10 min at room temperature. Sections
were incubated with an anti-BRP44L antibody (cat no., ab74871;
dilution, 1:50; Abcam, Cambridge, UK) and an anti-BRP44 antibody
(cat no., ab111380; dilution, 1:50; Abcam) overnight at 4°C.
Horseradish peroxidase-tagged antibody (cat no., PV-9000;
ready-to-use; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.,
Beijing, China) was added and incubated for 30 min at room
temperature, followed by positive staining with diaminobenzidine
(0.05%) for 1 min at room temperature, and counterstaining with
hematoxylin (0.1%) for 1 min at room temperature. The slides were
observed and imaged under a positive optical microscope. The
relative protein expression was quantified by Image-Pro Plus
version 5.0 software (Media Cybernetics Inc., Rockville, MD, USA)
and defined as follows: Density mean=density sum/area sum (15).
Western blot analysis
The proteins in the cells and tissues were lysed on
ice in a radioimmunoprecipitation assay lysis buffer (cat no.,
P0013-B; Beyotime Institute of Biotechnology, Haimen, China),
protein concentration was determined using a BCA Protein Assay kit
(Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
equal amounts of total protein (30 µg) were separated by SDS-PAGE
(12% gels) and transferred onto polyvinylidene difluoride membrane.
The membranes were blocked with 5% skimmed milk followed by
incubation with the specific primary antibodies and β-actin control
antibody (cat no., sc-47778; dilution, 1:1,000; Santa Cruz
Biotechnology, Inc., USA), anti-BRP44 L antibody (cat no., ab74871;
dilution, 1:500; Abcam) and anti-MPC2 polyclonal antibody (cat no.,
20049-1-AP; dilution, 1:500; ProteinTech Group, Inc., Chicago, IL,
USA), overnight at 4°C. The immunoreactivity signals were
visualized by enhanced chemiluminescence reagents according to the
manufacturer's protocols (EMD Millipore, Billerica, MA, USA)
following incubation with a horseradish peroxidase (HRP)-conjugated
goat anti-mouse immunoglobulin G (cat no. 10004302-1) and
HRP-conjugated goat anti-rabbit IgG (cat no A21020) secondary
antibodies (both, 1:5,000; Beijing Zhongshan Jinqiao Biotechnology
Co., Ltd., Beijing, China), for 60 min at room temperature.
RNA isolation, reverse
transcription-polymerase chain reaction (RT-PCR), and quantitative
PCR (qPCR)
Total RNA was extracted from tissues using TRIzol
reagent (Ambion, Thermo Fisher Scientific, Inc.) prior to being
reverse transcribed into cDNA using PrimeScript RT Master mix
(Takara Biotechnology Co., Ltd., Dalian, China), according to the
manufacturer's protocols. qPCR was performed using SYBR Premix Ex
Taq™ II (Takara Biotechnology Co., Ltd.) on a CFX96 Real-Time PCR
Detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Thermocycling conditions were as follows: 95°C for 30 sec, 45
cycles of melting at 95°C for 5 sec, followed by 60°C for 30 sec.
The mRNA expression level was normalized to GAPDH. The forward and
reward primer sequences were as follows: GAPDH forward,
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse, 5′-GAAGATGGTGATGGGATTTC-3′;
MPC1 forward, 5′-CGCGTTGGTGCGGAAAGCG-3′ and reverse,
5′-GGCAAATGTCATCCGCCCACTGA-3′; and MPC2 forward,
5′-TACCACCGGCTCCTCGATAAA-3′ and reverse,
5′-TATCAGCCAATCCAGCACACA-3′. Quantification of the MPC1, MPC2 and
GAPDH bands were calculated using the 2−∆∆Cq method
(16). The MPC1 and MPC2 mRNA
expression ratio of non-cancerous tissue to tumor tissues was
analyzed using log2.
Statistical analysis
Statistical analyses were performed by IBM SPSS
Statistics 22 (IBM Corp., Armonk, NY, USA). Statistical analyses of
continuous variables are expressed as the mean ± standard
deviation. Pearson's correlation analysis was used to estimate the
association between MPC1 and MPC2, at mRNA and protein level.
Student's t-test was used to analyze the association between
expression level and continuous variables, while the χ2
test was used for categorical variables of clinicopathological
characteristics. The Kaplan-Meier method was used to estimate the
RFS rates, and significant differences was assessed using the
log-rank test. Univariate and multivariate survival analyses were
performed using the Cox's proportional hazards model. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of MPC1 and MPC2 in HCC and
paired adjacent hepatic tissues
To examine whether MPC1 or MPC2 were dysregulated in
HCC, cancerous and adjacent non-cancerous tissues from patients
with HCC were surgically obtained. MPC1 and MPC2 protein expression
levels were examined in 85 tissues sections of patients with HCC
using immunohistochemistry staining, and in fresh samples using
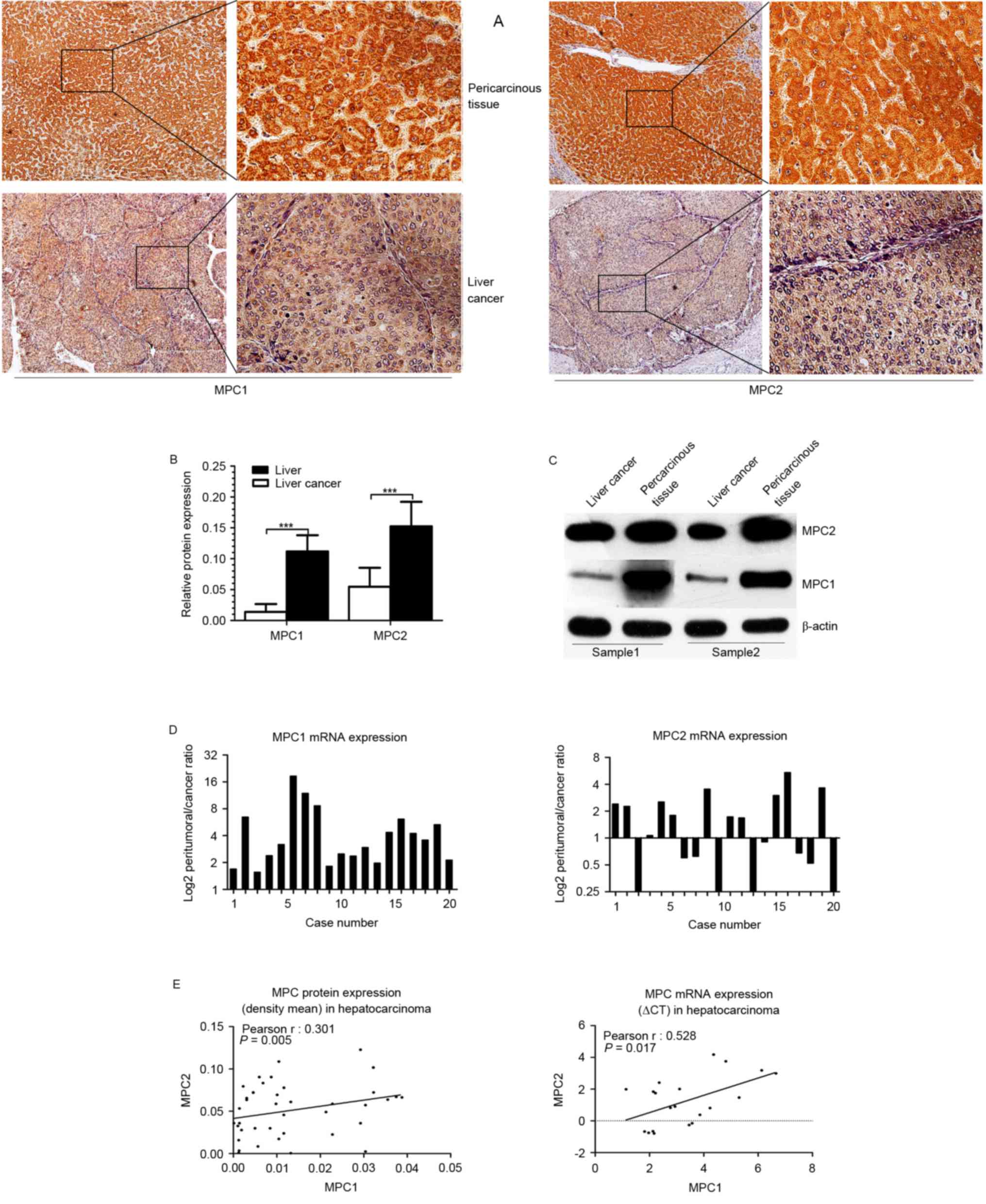
western blotting. Immunohistochemistry staining indicated that MPC1
and MPC2 protein expression was markedly lower in the tumor tissues
than that in the peritumoral liver tissues (Fig. 1A). The relative protein expression was
quantified by Image-Pro Plus software, and the results demonstrated
that MPC1 and MPC2 protein expression was markedly decreased in HCC
tissues (P<0.001) compared with that in the adjacent
non-cancerous counterparts. Furthermore, this difference was more
evident in MPC1 protein expression than in MPC2 protein expression
(Fig. 1B), and the same result was
obtained using western blotting (Fig.
1C). Additionally, the MPC1 and MPC2 mRNA expression level in
20 fresh harvested tumor specimens and their peripheral normal
liver tissues was analyzed using by reverse transcription PCR. In
line with previous results (14), the
present study observed that the mRNA level of MPC1 was decreased in
all the tumor tissues (20/20), but that the mRNA level of MPC2 was
inconsistently dysregulated; upregulated in 9/20 and downregulated
in 11/20 HCC tissues (Fig. 1D).
Although the expression of MPC2 mRNA was not consistent with its
protein expression, there was a positive statistical association
between MPC1 and MPC2 mRNA expression and between MPC1 and MPC2
protein expression (Fig. 1E). These
data suggest that there may be a difference in the regulation of
MPC1 and MPC2 at the mRNA level, and the two may serve a different
function in the occurrence and clinical significance of HCC.
MPC expression and association with
clinicopathological parameters
Based upon the consistently reduced expression of
MPC protein in HCC tissues, the clinical significance of MPC1 and
MPC2 expression levels were assessed. The density means of MPC1 and
MPC2 were calculated, and according to the median protein
expression in the group of tumor tissues, patients were split into
two groups (low expression and high expression). However, no
significant association was identified between patients'
clinicopathological characteristics and their MPC1 or MPC2 protein
expression levels (P>0.10; Table
I). Therefore, this led us to hypothesize that these clinical
and pathological parameters may not be associated with cancer
energy metabolism.
Prognostic potential of MPC
To further clarify the exact function of the MPC in
HCC following hepatectomy, the associations between the protein
level of MPC1 and MPC2 and their respective prognostic functions
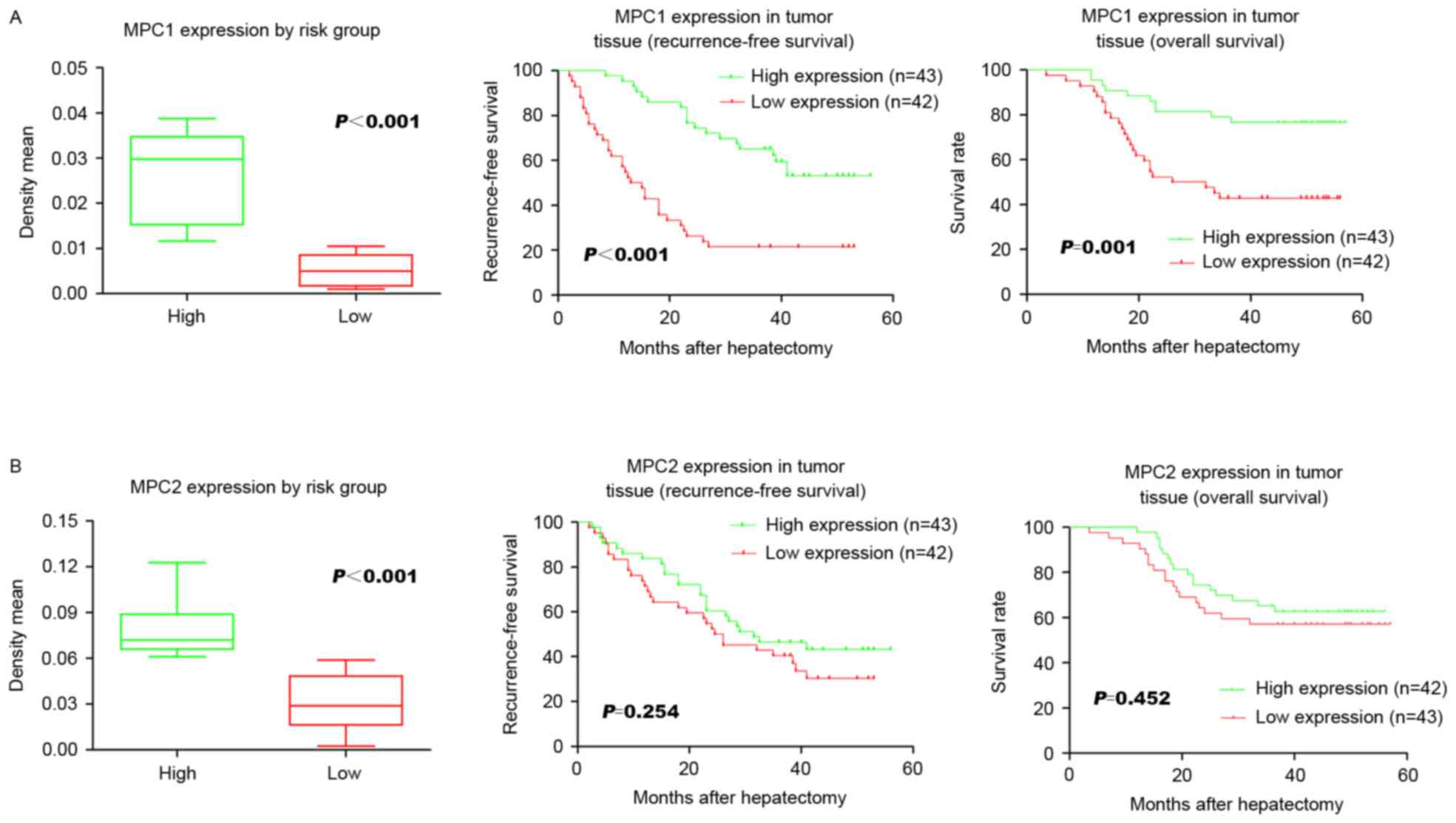
were analyzed. The low MPC1 expression group had a significantly
lower 3-year RFS rate following surgery compared with the high
expression group (P<0.001; 21.4 and 55.8%, respectively;
Fig. 2A). The low MPC1 expression
group was also associated with a significantly (P=0.001) shorter
3-year OS compared with the high expression group (41.9 and 78.6%,
respectively; Fig. 2A). However, no
significant association was identified between either RFS or OS and
MPC2 expression levels (P=0.254 and P=0.452, respectively; Fig. 2B). Univariate analysis revealed that
Barcelona clinic liver cancer stage (17), and MPC1 expression were significant
prognostic factors for RFS (Table
II). Multivariate analysis using the Cox's proportional hazards
model revealed that microvascular invasion [hazard ratio (HR),
2.115; 95% confidence interval (CI), 1.143–3.913; P=0.017; Table II] and MPC1 expression (HR, 3.773;
95% CI, 2.113–6.737; P=0.000; Table
II) were independent recurrence risk factors in HCC patients
who had undergone hepatectomy. These data demonstrate that a loss
of MPC1 protein is strongly associated with cancer relapse and a
poor prognosis.
 | Table II.Univariate and multivariate analysis
of the risk factors of hepatocellular carcinoma recurrence. |
Table II.
Univariate and multivariate analysis
of the risk factors of hepatocellular carcinoma recurrence.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (>60/≤60
years) | 1.096
(0.594–2.023) | 0.769 |
|
|
| Gender
(male/female) | 0.677
(0.318–1.439) | 0.311 |
|
|
| AFP (>200/≤200
ng/ml) | 0.690
(0.396–1.201) | 0.190 |
|
|
| BCLC stage
(0-A/B-C) | 0.497
(0.282–0.878) | 0.016 | 1.288
(0.657–3.622) | 0.461 |
| Tumor number
(1/>1) | 1.080
(0.487–2.397) | 0.850 |
|
|
| Tumor size |
|
|
|
|
| Maximum
diameter (>5/≤5 cm) | 1.615
(0.936–2.785) | 0.085 | 0.697
(0.369–1.315) | 0.264 |
|
Smallest diameter (>3/≤3
cm) | 1.228
(0.713–2.117) | 0.459 |
|
|
| Differentiation
(poor/moderate or well) | 0.714
(0.414–1.233) | 0.229 |
|
|
| Microvascular
invasion (presence/absence) | 1.732
(0.983–3.053) | 0.057 | 2.115
(1.143–3.913) | 0.017a |
| Micrometastases
(presence/absence) | 1.083
(0.606–1.933) | 0.788 |
|
|
| MPC1 expression
(low/high) | 3.926
(2.205–6.989) | 0.000 | 3.773
(2.113–6.737) | 0.001a |
| MPC2 expression
(low/high) | 1.397
(0.809–2.411) | 0.230 |
|
|
Discussion
In humans, the two MPC complex proteins, MPC1 and
MPC2, are required in order to facilitate pyruvate transport across
the mitochondrial membrane. Since its discovery, dysfunction of MPC
has been observed in several cancer types, and in light of this,
loss of the MPC has also been demonstrated to serve important
functions in the development of tumors in liver cell lines,
including normal rat liver, Ehrlich ascites tumor cells, Morris
hepatoma 44 and Morris hepatoma 3924A cells (10,14).
However, the clinical significance of MPC expression in patients
with HCC remains unknown. The present study identified that the
protein expression of MPC1 and MPC2 was downregulated in HCC tumor
tissues. Notably, the results indicated that low expression of
these two proteins had no significant association with the clinical
and pathological characteristics of the disease. Furthermore, the
present study also demonstrated that low MPC1 may be an unfavorable
prognostic biomarker, due to the fact that it is an independent
risk factor for RFS and OS in patients with HCC who have undergone
a hepatectomy.
It is likely that mRNA expression is not a direct
indication of protein expression, as the latter can be regulated
not only at the transcription level, but also at the translational
and turnover levels. The staining abundance of MPC1 and MPC2
protein expression in the present study differed significantly
between tumor and non-cancerous tissues. The mRNA expression levels
of MPC2 were associated with MPC1 in HCC tumor tissues, but unlike
the consistent low expression of MPC1, the expression of MPC2 in
hepatocellular carcinoma tissue was uneven. There are two
potiential explanations for this discrepancy. One hypothesis is
that certain MPC2 mRNAs are translated only once the blocking
protein is removed by a certain signal. Another possibility is that
MPC2 protein expression is regulated by certain modifications,
including phosphorylation, acetylation, hydroxylation or changes in
the space position (18). The
mechanism(s) underlying the results of the present study remain
unknown. Due to the fact that MPC is most abundant in the liver, it
is indispensable for mitochondrial pyruvate import (19), and thus likely to constitute a
critical feature of cancer cells. Further investigations are
required to fill this gap, to survey and to evaluate if and how MPC
modification affects its activity.
It should be noted that there are numerous factors,
including tumor-, patient-, liver- and treatment-associated
factors, in addition to the risk stratification schemes, which may
be used to assess and improve the ability of clinicians to select
patients and treatment methods, and a number of these have
significant prognostic value and potential in improving patient
outcomes (20,21). The present study is the first to
evaluate the association between MPC expression levels and clinical
and tumor pathological characteristics, despite the fact that no
association was observed. This lack of statistical significance may
be due in part to the small sample size used (n=85), the fact that
other potentially associated factors may not have been included in
the study or the fact that the tumor characteristics and clinical
traits evaluated here have no association with the energy
metabolism of tumor cells. Further investigation on the function of
MPC in HCC is required in order for these mechanisms to be fully
elucidated.
The high recurrence rate following HCC hepatectomy,
which is a common occurrence posing a threat to patient outcomes,
remains an unsolved problem (22).
Regardless of any association between MPC and clinicopathological
characteristics, however, it was observed that low MPC protein
expression may serve as a definitive prognostic biomarker to
monitor tumor relapse (RFS) over the time period following
resection. In the present study, low MPC1 expression had a marked
association with the risk of future recurrence and a shorter OS
time. However, there was no statistically significant difference in
either the RFS or OS rates observed between the HCC samples and
their non-cancerous counterparts in the low MPC2 expression group.
The present study indicated the high prognostic value of diminished
MPC expression for determining the outcome of patients with HCC
following resection.
Previous studies have demonstrated that inhibition
of MPC activity has a profound impact on cell glucose and pyruvate
metabolism, and that it induces glutaminolysis in the Krebs cycle
(11,23). Furthermore, various cancer cells
appear to delete or suppress MPC expression and the study discussed
herein (14) provide information
regarding the function served by aberrant MPC activity in cancer
metabolism. Additionally, gluconeogenesis is considered to be the
reverse of glycolysis and is now recognized as a common hallmark of
cancer. Gluconeogenesis is an important feature of hepatocytes, and
pyruvate is the major substrate of gluconeogenesis. Silencing of
liver MPC1 resulted in abolished hepatic MPC activity and a
subsequent marked decrease in gluconeogenesis (24). Notably, in HCC tissues that have lost
the ability to perform gluconeogenesis, focusing on the switch from
glycolysis to gluconeogenesis may be an efficacious method for HCC
treatment (25). Taken together, the
results indicate that restoring the MPC function in patients with
HCC may be a vital effective treatment. MPC activity has emerged to
be inhibited by the insulin sensitizers, thiazolidinediones
(26), and phosphodiesterase
inhibitor Zaprinast (27). Drug
targets to increase the efficacy of MPC remain elusive, although a
previous study demonstrated that indirectly increasing the
concentration of intracellular pyruvate can reverse the low MPC
activity and cell respiration in various cell types (28).
In conclusion, MPC may serve a crucial role in
repressing HCC recurrence by inhibiting pyruvate oxidative
metabolism, and may be a promising attractive biomarker for
patients with HCC following hepatectomy. Furthermore, the functions
of MPC in liver glucose metabolism and in HCC onset or development
require further investigation.
Acknowledgements
The authors would like to thank Dr Guo Hua, Dr Luo
Yi, Dr Sun Bo, Dr Fu Hui, Dr Chen Lu, Miss Guo Piao and Miss Xi
Qing from the Laboratory of Cancer Cell Biology of Tianjin Medical
University Cancer Institute and Hospital for providing assistance
with the experimental technique and for information support.
References
|
1
|
Fitzmorris P, Shoreibah M, Anand BS and
Singal AK: Management of hepatocellular carcinoma. J Cancer Res
Clin Oncol. 141:861–876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takayama T: Surgical treatment for
hepatocellular carcinoma. Jpn J Clin Oncol. 41:447–454. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sengupta B and Siddiqi SA: Hepatocellular
carcinoma: Important biomarkers and their significance in molecular
diagnostics and therapy. Curr Med Chem. 19:3722–3729. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schütte K, Schulz C, Link A and
Malfertheiner P: Current biomarkers for hepatocellular carcinoma:
Surveillance, diagnosis and prediction of prognosis. World J
Hepatol. 7:139–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saito Y, Shimada M, Utsunomiya T, Morine
Y, Imura S, Ikemoto T, Mori H, Hanaoka J, Yamada S and Asanoma M:
Prediction of recurrence of hepatocellular carcinoma after curative
hepatectomy using preoperative Lens culinaris agglutinin-reactive
fraction of alpha-fetoprotein. Hepatol Res. 42:887–894. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wallace DC: Mitochondria and cancer. Nat
Rev Cancer. 12:685–698. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eboli ML, Paradies G, Galeotti T and Papa
S: Pyruvate transport in tumour-cell mitochondria. Biochim Biophys
Acta. 460:183–187. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paradies G, Capuano F, Palombini G,
Galeotti T and Papa S: Transport of pyruvate in mitochondria from
different tumor cells. Cancer Res. 43:5068–5071. 1983.PubMed/NCBI
|
|
11
|
Yang C, Ko B, Hensley CT, Jiang L, Wasti
AT, Kim J, Sudderth J, Calvaruso MA, Lumata L, Mitsche M, et al:
Glutamine oxidation maintains the TCA cycle and cell survival
during impaired mitochondrial pyruvate transport. Mol Cell.
56:414–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herzig S, Raemy E, Montessuit S, Veuthey
JL, Zamboni N, Westermann B, Kunji ER and Martinou JC:
Identification and functional expression of the mitochondrial
pyruvate carrier. Science. 337:93–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bricker DK, Taylor EB, Schell JC, Orsak T,
Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N,
et al: A mitochondrial pyruvate carrier required for pyruvate
uptake in yeast, Drosophila, and humans. Science. 337:96–100. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schell JC, Olson KA, Jiang L, Hawkins AJ,
Van Vranken JG, Xie J, Egnatchik RA, Earl EG, DeBerardinis RJ and
Rutter J: A role for the mitochondrial pyruvate carrier as a
repressor of the Warburg effect and colon cancer cell growth. Mol
Cell. 56:400–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang CJ, Zhou ZG, Holmqvist A, Zhang H, Li
Y, Adell G and Sun XF: Survivin expression quantified by image
pro-plus compared with visual assessment. Appl Immunohistochem Mol
Morphol. 17:530–535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Llovet JM, Brú C and Bruix J: Prognosis of
hepatocellular carcinoma: The BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Sousa Abreu R, Penalva LO, Marcotte EM
and Vogel C: Global signatures of protein and mRNA expression
levels. Mol Biosyst. 5:1512–1526. 2009.PubMed/NCBI
|
|
19
|
Vigueira PA, McCommis KS, Schweitzer GG,
Remedi MS, Chambers KT, Fu X, McDonald WG, Cole SL, Colca JR,
Kletzien RF, et al: Mitochondrial pyruvate carrier 2 hypomorphism
in mice leads to defects in glucose-stimulated insulin secretion.
Cell Rep. 7:2042–2053. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maluccio M and Covey A: Recent progress in
understanding, diagnosing, and treating hepatocellular carcinoma.
CA Cancer J Clin. 62:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gluer AM, Cocco N, Laurence JM, Johnston
ES, Hollands MJ, Pleass HC, Richardson AJ and Lam VW: Systematic
review of actual 10-year survival following resection for
hepatocellular carcinoma. HPB (Oxford). 14:285–290. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morise Z, Kawabe N, Tomishige H, Nagata H,
Kawase J, Arakawa S, Yoshida R and Isetani M: Recent advances in
the surgical treatment of hepatocellular carcinoma. World J
Gastroenterol. 20:14381–14392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vacanti NM, Divakaruni AS, Green CR,
Parker SJ, Henry RR, Ciaraldi TP, Murphy AN and Metallo CM:
Regulation of substrate utilization by the mitochondrial pyruvate
carrier. Mol Cell. 56:425–435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gray LR, Sultana MR, Rauckhorst AJ,
Oonthonpan L, Tompkins SC, Sharma A, Fu X, Miao R, Pewa AD, Brown
KS, et al: Hepatic mitochondrial pyruvate carrier 1 is required for
efficient regulation of gluconeogenesis and whole-body glucose
homeostasis. Cell Metab. 22:669–681. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma R, Zhang W, Tang K, Zhang H, Zhang Y,
Li D, Li Y, Xu P, Luo S, Cai W, et al: Switch of glycolysis to
gluconeogenesis by dexamethasone for treatment of hepatocarcinoma.
Nat Commun. 4:25082013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Divakaruni AS, Wiley SE, Rogers GW,
Andreyev AY, Petrosyan S, Loviscach M, Wall EA, Yadava N, Heuck AP,
Ferrick DA, et al: Thiazolidinediones are acute, specific
inhibitors of the mitochondrial pyruvate carrier. Proc Natl Acad
Sci USA. 110:5422–5427. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Du J, Cleghorn WM, Contreras L, Lindsay K,
Rountree AM, Chertov AO, Turner SJ, Sahaboglu A, Linton J, Sadilek
M, et al: Inhibition of mitochondrial pyruvate transport by
zaprinast causes massive accumulation of aspartate at the expense
of glutamate in the retina. J Biol Chem. 288:36129–36140. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Compan V, Pierredon S, Vanderperre B,
Krznar P, Marchiq I, Zamboni N, Pouyssegur J and Martinou JC:
Monitoring mitochondrial pyruvate carrier activity in real time
using a BRET-based biosensor: Investigation of the warburg effect.
Mol Cell. 59:491–501. 2015. View Article : Google Scholar : PubMed/NCBI
|