Introduction
Epithelial ovarian cancer (EOC) is the most lethal
type of gynecological malignancy. In 2015, ~21,290 patients were
diagnosed with EOC, and 14,180 succumbed to the disease in the USA
(1). Despite the majority of patients
with EOC being asymptomatic in the early stages of the disease,
this malignancy is frequently associated with multiple
intraperitoneal disseminations and distant metastases (2–4). Due to
advances in therapeutic strategies, including the development of
maximal cytoreductive surgery and several types of effective
chemotherapy, it is possible to achieve clinical remission in
patients with advanced epithelial ovarian cancer (5). Furthermore, the short-term oncological
outcome of women with EOC appears to be more favorable when
compared with that of men; however, the majority of clinically
complete responders experience disease recurrence (2,3).
Hematopoietic lineage cell-specific protein 1 (HS1)
is a 75-kDa multi-domain protein that is primarily expressed within
the hematopoietic lineage (6–8). HS1 is frequently regarded as an F-actin
binding protein that activates the actin-related protein-2/3
(Arp2/3) complex involved in cytoskeleton rearrangement (6). HS1 is also a signal transducer that acts
via the non-receptor-type tyrosine kinases of the Src family
causing tyrosine residue phosphorylation (9). Previous studies have demonstrated that
HS1 is involved in cell growth, proliferation, adhesion and
migration, such as B cells (10),
dendritic cells (11), natural killer
cells (12), leukemic CLL cells
(13) and leukemic B cells (8) that are involved in intracellular
signaling. Furthermore, studies have identified that HS1 is
expressed on platelets and natural killer cells, as well as in
numerous types of hematological malignancies, including
acute/chronic leukemia and B-cell chronic lymphocytic leukemia
(CLL) (12,14–16).
As a hematopoietic homolog of cortactin, HS1 and
cortactin demonstrate a marked similarity in their amino acid
sequence and structure (17,18). HS1 and cortactin share an N-terminal
acidic domain containing the Arp2/3 complex and demonstrate F-actin
binding through a 37-amino-acid repeat domain containing three and
a half repeats of cortactin; the C-terminal of HS1 is highly
homologous (86%) at the Src homology 3 domain (6,17,19). Despite similarities in homology, HS1
and cortactin exert different biological functions. Cortactin is
widely expressed in all types of cell and/or tissue with the
exception of hematopoietic cells, and is associated with a poorer
clinical prognosis in patients with different types of cancer
including hepatocellular carcinoma, laryngeal cancer, ovarian
cancer, colon cancer and non-small cell lung cancer (7,20–25). However, to the best of our knowledge,
there have been no studies investigating an association between HS1
expression and oncological outcome in solid tumors.
In the present study, the expression levels of HS1,
and the potential association between HS1 expression and
clinicopathological features in four common types (serous, clear
cell, mucinous and endometrioid carcinoma) of EOC were investigated
in patients with EOC.
Materials and methods
Patients and tissue samples
Tissues were obtained from 195 patients who
underwent initial surgery at the Nagoya University Hospital
(Nagoya, Aichi, Japan) between January 1999 and December 2011.
Patients who underwent pre-surgical treatment including
radiotherapy and chemotherapy, were excluded from the present
study. All patients provided informed consent prior to recruitment.
Surgical treatment consisted of total hysterectomy, bilateral
salpingo-oophorectomy, omentectomy, and pelvic and para-aortic
lymphadenectomy. In the situation where residual tumor remained,
systemic lymphadenectomy was omitted. The histological type was
assigned according to criteria outlined by the World Health
Organization classification (2003) (26). Clinical staging was reviewed based on
staging criteria of the International Federation of Gynecology and
Obstetrics 1988 (FIGO) (27). All
tissue samples were fixed in 10% neutral buffered formalin for 24
to 48 h at room temperature, embedded in paraffin and routinely
stained with hematoxylin and eosin for histological
examination.
Immunohistochemical HS1 staining and
evaluation
Formalin-fixed, paraffin-embedded tissue blocks
(4-µm thick) were mounted on charged glass slides, de-paraffinized,
and rehydrated in a graded series of ethanol. Antigen retrieval was
conducted in 10 mM citrate solution (pH 6.0) (Wako Pure Chemical
Industries, Ltd., Osaka, Japan) at 98°C for 15 min. Endogenous
peroxidase activity was blocked with 3% hydrogen peroxide for 5 min
at room temperature. Blocking was performed in 10% normal goat
serum (Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) with
0.025% Triton X-100 in Tris-buffered saline (TBS-T) for 60 min.
Sections were treated with anti-HS1 rabbit monoclonal antibody in
blocking buffer at 4°C overnight (1:100; cat. no. 3890; Cell
Signaling Technology, Inc., Danvers, MA, USA). Following three
washes with TBS-T, sections were incubated with biotinylated goat
anti-rabbit (1:200; cat. no. PK-4001; Vector Laboratories, Inc.,
Burlingame, CA, USA) horseradish peroxidase for 1 h and incubated
with avidin-biotin complex reagent (cat. no. PK-4001; Vector
Laboratories, Inc.) for 30 min at room temperature. Peroxidase
activities were visualized using a 3,3′-diaminobenzidine peroxidase
substrate kit (cat. no SK-4100; Vector Laboratories, Inc.). Slides
were counterstained with Mayer's hematoxylin for 5 min at room
temperature (Wako Pure Chemical Industries, Ltd.). Sections were
dehydrated, cleared and mounted. Negative controls were run on all
sections in blocking buffer without the primary antibody. The
intensity of HS1 immunostaining was scored as follows: 0
(negative), 1 (weak), 2 (intermediate) and 3 (strong). The extent
of staining was scored as 0 (0–25%), 1 (26–50%), 2 (51–75%) or 3
(>76%) according to the percentage of medium and strong staining
in relation to the total cancer area. Specimens with a final
staining score of 0 or 1 indicated low HS1 expression, whereas a
final staining score of 2 or 3 indicated high HS1 expression. The
scoring procedure was performed twice by two independent observers
(each blinded to the other's scores) who had no knowledge of the
patients' clinical parameters and other prognostic factors. The
concordance rate was >95% between the observers. For the other
5% disagreement, it was adjusted by observer's consensus.
Statistical analysis
The χ2 test was performed to analyze the
association between low and high HS1 expression and patient
clinicopathological parameters. Survival curves were generated
using the Kaplan-Meier estimator method, which were then compared
using the log-rank test. Overall survival (OS) was defined as the
time between the date of initial therapy and the final follow-up or
the time of patient mortality due to any cause. The prognostic
significance of HS1 expression regarding other pathological
variables was analyzed using univariate and multivariate Cox
proportional hazards regression analysis. P<0.05 was considered
to indicate a statistically significant difference.
Results
Patient characteristics
In total, 195 patients with EOC were enrolled into
the present study. The age range of diagnosed patients was between
20 and 82 years (median, 56 years). According to the results
presented in Table I, the FIGO stage
distribution was as follows: 81 stage I patients (41.5%), 17 stage
II patients (8.7%), 60 stage III patients (30.8%) and 13 stage IV
patients (6.7%). Staging could not be determined in 24 patients
(12.3%), due to incomplete records. With regard to the pathological
type, the serous histological type was the most frequently
identified (81/195; 41.5%), followed by clear cell carcinoma
(61/195; 31.3%). A total of 162 (83.1%) patients were administered
using Taxane plus platinum chemotherapy (paclitaxel plus cisplatin,
paclitaxel plus carboplatin, docetaxel plus cisplatin, docetaxel
plus carboplatin), the remaining 33 (16.9%) cases were not treated.
In total, 79 (40.5%) patients underwent full staging and/or
complete surgery, and 116 (59.5%) underwent unstaged and/or
debulking surgery. Table I summarizes
all patient characteristics.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Clinical
parameter | n (%) |
|---|
| FIGO Stage |
|
| I | 81 (41.5) |
| II | 17 (8.7) |
| III | 60 (30.8) |
| IV | 13 (6.7) |
|
Unspecified | 24 (12.3) |
| Histological
type |
|
|
Serous | 81 (41.5) |
|
Clear-cell | 61 (31.3) |
|
Endometrioid | 39 (20.0) |
|
Mucinous | 14 (7.2) |
| Chemotherapy |
|
| Taxane
plus platinum | 162 (83.1) |
|
Others | 33 (16.9) |
| Surgery |
|
| Full
staging/complete | 79 (40.5) |
|
Non-staging/debulking | 116 (59.5) |
| HS1 expression |
|
|
Low | 89 (45.6) |
|
High | 106 (54.4) |
HS1 expression in EOC samples
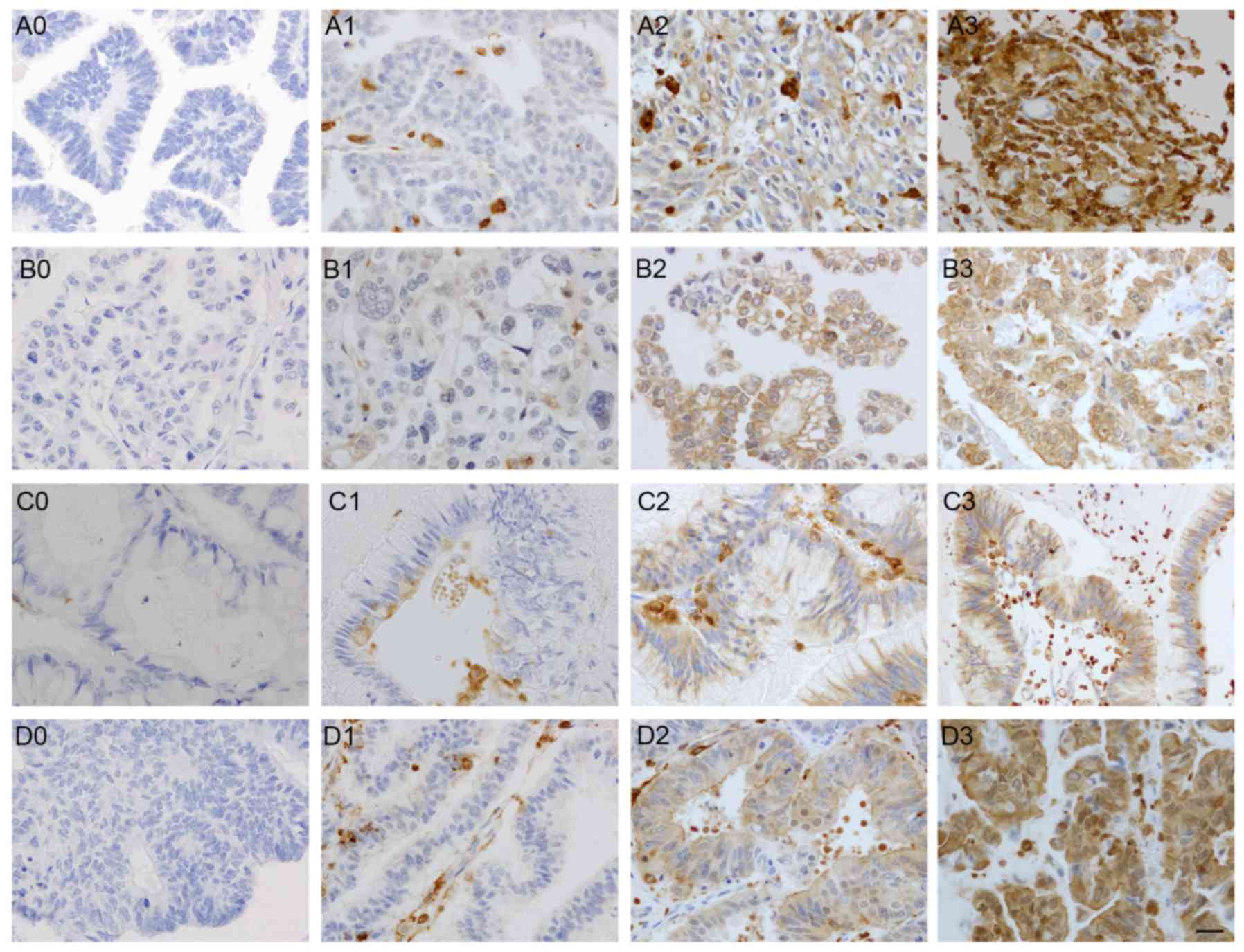
As presented in Fig.
1, high and low levels of HS1 expression were identified in EOC
samples. Results demonstrated that HS1 protein was localized in the
cytoplasm and membrane of the all stages tumor cells. According to
results present in Table II, the
distribution of the HS1 immunostaining intensity was as follows: 34
patients with negative staining (score 0; 17.4%), 55 patients with
weak staining (score 1; 28.2%), 86 patients with intermediate
staining (score 2; 44.1%) and 20 patients with strong staining
(score 3; 10.3%). Tumors were categorized based upon the staining
score. Low HS1 expression indicated a staining score of 0–1
(89/195; 45.6%) and high HS1 expression indicated a staining score
of 2–3 (106/195; 54.4%). As presented in Table II, high HS1 immunoreactivity was
detected in the following histological types: 43 serous (43/81;
53.1%), 41 clear cell (41/61; 67.2%), 18 endometrioid (18/39;
46.2%) and 4 mucinous (4/14; 28.6%) carcinoma cases. The HS1
immunoreactivity categorized into low vs. high expression was not
associated with any of the clinicopathological parameters,
including age, FIGO stage and type of chemotherapy or surgery
received; however, histological type was significantly associated
with HS1 expression (P=0.0303) (Table
III).
 | Table II.Distribution of hematopoietic lineage
cell-specific protein 1 immunoreactivity scores for each EOC
histological type. |
Table II.
Distribution of hematopoietic lineage
cell-specific protein 1 immunoreactivity scores for each EOC
histological type.
|
| Immunoreactivity
score, n (%) |
|---|
|
|
|
|---|
| Type of EOC | 0 | 1 | 2 | 3 |
|---|
| Serous | 12 (35.3) | 26 (47.3) | 30 (34.9) | 13 (65.0) |
| Clear cell | 4 (11.8) | 16 (29.1) | 36 (41.9) | 5 (25.0) |
| Endometrioid | 12 (35.3) | 9 (16.4) | 16 (18.6) | 2 (10.0) |
| Mucinous | 6 (17.6) | 4 (7.3) | 4 (4.7) | 0 (0.0) |
 | Table III.Distribution of several
clinicopathological factors according to HS1 expression. |
Table III.
Distribution of several
clinicopathological factors according to HS1 expression.
|
| HS1 expression, n
(%) |
|---|
|
|
|
|---|
| Factor | Low | High | P-value |
|---|
| Age, years |
|
| 0.334 |
|
≤55 | 27 (30.3) | 24 (22.6) |
|
|
>55 | 54 (60.7) | 75 (70.8) |
|
|
Unspecifieda | 8 (9.0) | 7 (6.6) |
|
| FIGO stage |
|
| 0.705 |
| I | 38 (42.7) | 43 (40.6) |
|
| II | 8 (9.0) | 9 (8.5) |
|
|
III | 24 (27.0) | 36 (34.0) |
|
| IV | 8 (9.0) | 5 (4.7) |
|
|
Unspecifieda | 11 (12.4) | 13 (12.3) |
|
| Histological
type |
|
| 0.0303 |
|
Serous | 38 (42.7) | 43 (40.6) |
|
| Clear
cell | 20 (22.5) | 41 (38.7) |
|
|
Endometrioid | 21 (23.6) | 18 (17.0) |
|
|
Mucinous | 10 (11.2) | 4 (3.8) |
|
| Chemotherapy |
|
| 0.719 |
| Taxane
plus platinum | 73 (82.0) | 89 (84.0) |
|
|
Others | 16 (18.0) | 17 (16.0) |
|
| Surgery |
|
| 0.547 |
| Full
staging/complete | 34 (38.2) | 45 (42.5) |
|
|
Non-staging/debulking | 55 (61.8) | 61 (57.5) |
|
Association between HS1
immunoreactivity and oncological outcome of patients with EOC
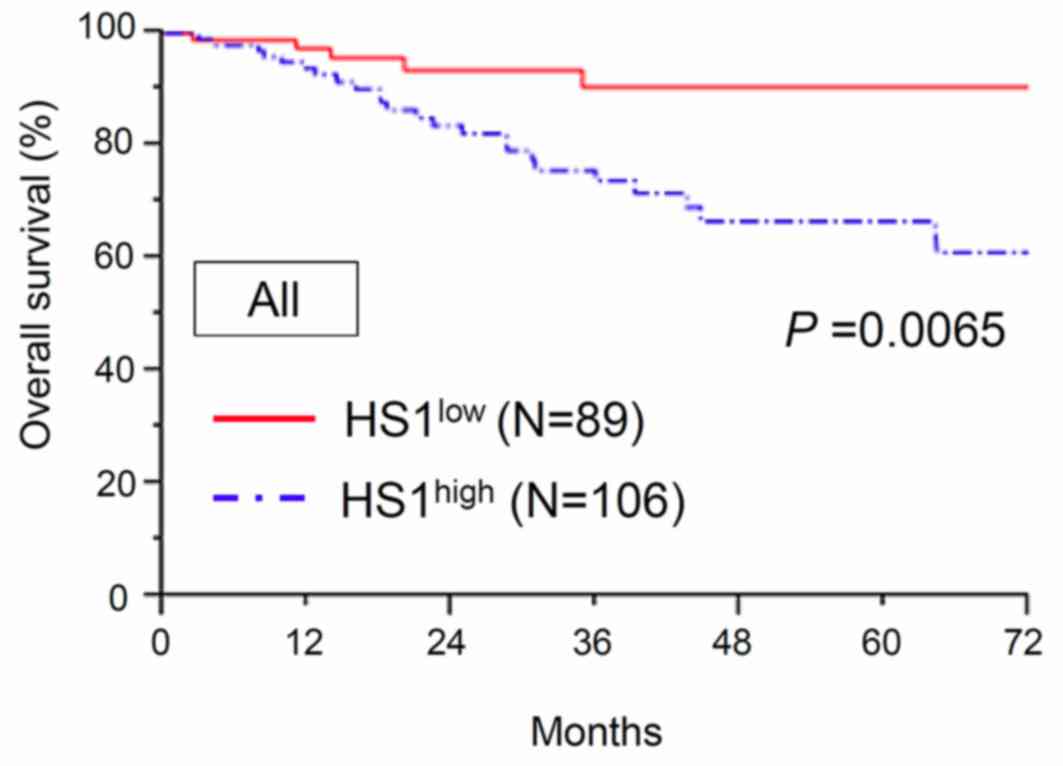
The association between HS1 expression and OS was
investigated. The median follow-up time of the patients was 67.2
months. At the end of the follow-up period, 166/195 (85.1%)
patients remained alive and 29/195 (14.9%) patients had succumbed
to the disease. The 3- and 5-year OS rates of all patients were
80.3 and 75.8%, respectively. The 5-year OS rates of patients with
low (n=89) and high (n=106) expression of HS1 were 90.4 and 66.7%,
respectively. The OS time in patients with high HS1 expression was
significantly shorter compared with that in patients with low HS1
expression (P=0.0065; Fig. 2).
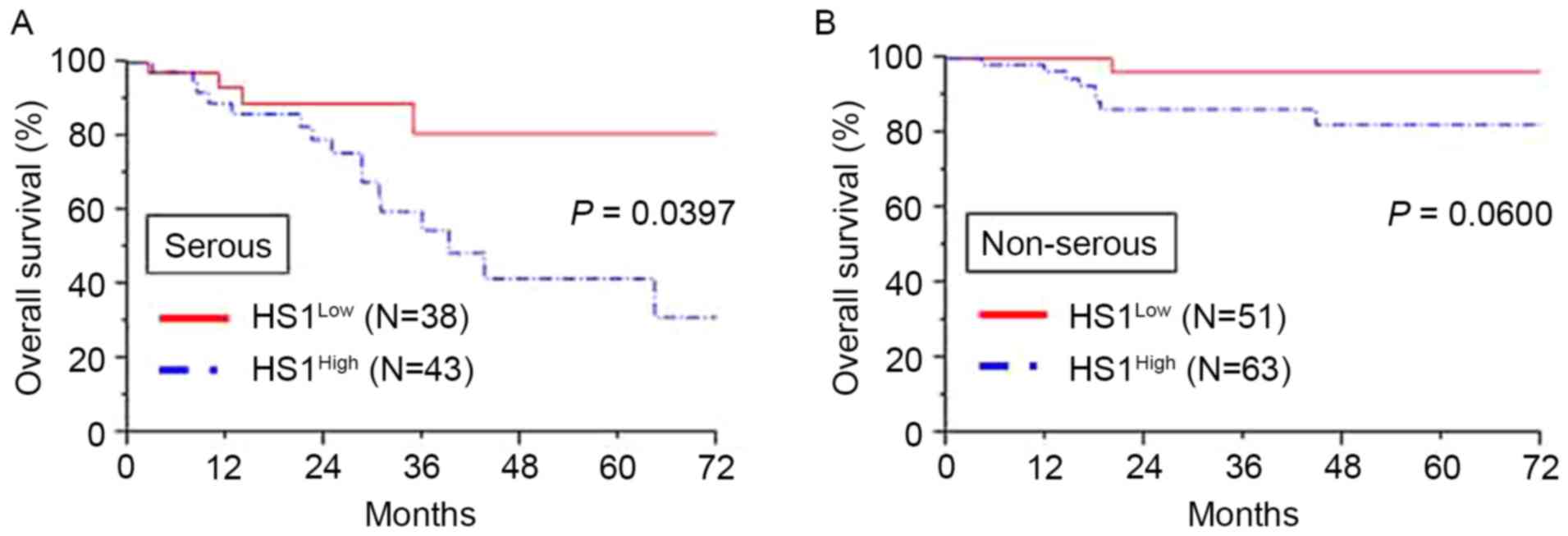
Additionally, analysis of patients with a serous histological type
revealed that significantly poorer OS was demonstrated in patients
with high HS1 expression compared with that in patients with low
HS1 expression (P=0.0397; Fig. 3A).
Furthermore, analysis of patients with a non-serous histological
type demonstrated similar tendencies in the long-term clinical
outcome, although the result was not significant (P=0.0600;
Fig. 3B).
Furthermore, univariate and multivariate analyses
were performed on 170 patients with complete clinical information
including age, FIGO stage, histological type, type of chemotherapy
and type of surgery; 25 patients with limited clinical information
are excluded from this analysis. Results from the univariate
analyses demonstrated that the FIGO stage III/IV, serous
histological type, non-staging surgical procedure and high HS1
immunoreactivity significantly predicted a poor OS outcome
(P<0.0001, P=0.0237, P=0.0143 and P=0.0320, respectively). For
multivariate analyses, age, FIGO stage, histological type, type of
chemotherapy, type of surgery and HS1 immunoreactivity were
recruited into the Cox proportional hazards model. Results
demonstrated that FIGO staging (hazard ratio, 30.114; 95%
confidence interval, 7.226–205.835; P<0.0001) and HS1 expression
(hazard ratio, 3.539; 95% confidence interval, 1.221–12.811;
P=0.0187) were significant prognostic factors for the prediction of
a poor OS outcome (Table IV).
 | Table IV.Univariate and multivariate
analysisa of
clinicopathological parameters in association with overall
survivalb. |
Table IV.
Univariate and multivariate
analysisa of
clinicopathological parameters in association with overall
survivalb.
|
| Overall
survival |
|---|
|
|
|
|---|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
| 0.734 |
| 0.887 |
|
≤55 | 1 |
| 1 |
|
|
>55 | 0.855
(0.340–2.123) |
| 0.932
(0.347–2.485) |
|
| FIGO stage |
| <0.0001 |
| <0.0001 |
|
I/II | 1 |
| 1 |
|
|
III/IV | 21.611
(6.155–136.684) |
| 30.114
(7.226–205.835) |
|
| Histological
type |
| 0.0237 |
| 0.232 |
|
Serous | 1 |
| 1 |
|
|
Non-serous | 0.348
(0.138–0.865) |
| 1.938
(0.647–5.734) |
|
| Chemotherapy |
| 0.489 |
| 0.182 |
| Taxane
plus platinum | 1 |
| 1 |
|
|
Others | 1.587
(0.367–4.822) |
| 2.821
(0.576–10.832) |
|
| Surgery |
| 0.0143 |
| 0.199 |
| Full
staging/complete | 1 |
| 1 |
|
|
Non-staging/debulking | 3.294
(1.258–10.207) |
| 2.038
(0.698–6.906) |
|
| HS1 |
| 0.0320 |
| 0.0187 |
|
Low | 1 |
| 1 |
|
|
High | 3.013
(1.093–10.573) |
| 3.539
(1.221–12.811) |
|
Discussion
To the best of our knowledge, the present study is
the first to investigate and identify that HS1 is highly expressed
in four representative types (serous, clear cell, mucinous and
endometrioid carcinoma) of EOC samples, and that high expression is
associated with a poor prognosis. van Rossum et al (19) reported that HS1 demonstrated a genomic
organization similar to cortactin; however, the comparison between
HS1 and cortactin levels in solid tumors is yet to be
investigated.
It was previously hypothesized that HS1 and
cortactin genes exhibit differing expression patterns; however, in
platelets (28) and megakaryocytes
(29), cortactin is expressed
similarly to HS1. In macrophages (30) and carcinoma cells (31), HS1 and cortactin are accumulated in
podosomes. A recent study demonstrated that cortactin is highly
expressed and regulates splicing in patients with B-cell CLL, and
is associated with a poor prognosis (32). These contradictory findings suggest
that the HS1 gene may possess multiple functions due to being
present in different pathological lineages.
Results from the present study demonstrated that HS1
was not expressed in normal ovarian tissue, and the positive
expression rate in melanoma was high, with a staining score of 3
(data not shown). Xu et al (33) demonstrated that the aberrant
expression of cortactin is associated with melanocytic tumor
progression. In the present study HS1 expression was primarily
identified in the cytoplasm of serous, clear cell, endometrioid and
mucinous carcinoma cells. Additionally, results obtained from the
Kaplan-Meier survival analysis indicated that high HS1 expression
was significantly associated with a decreased survival rate
compared with low HS1 expression. In total, ~80% of ovarian
carcinoma samples were of the serous type, and results demonstrated
that high HS1 expression was associated with a significant decrease
in OS rate compared with low HS1 expression (34). Furthermore, univariate analysis was
performed in order to assess associations between prognosis and
several clinical characteristics, including the FIGO stage,
histological type, type of chemotherapy, type of surgery and HS1
expression level status. Results demonstrated that the advanced
stage (stages III and IV), serous histological type, non-staging
surgery and high expression of HS1 were significant prognostic
markers for poor OS for patients with EOC. Multivariate analysis
performed on these parameters using the Cox proportional hazards
model revealed that high HS1 expression, EOC tumor type and the
FIGO stage were independent prognostic factors for EOC. We
hypothesize that HS1 functions similarly in serous and non-serous
EOC; however, analysis of the association between OS and non-serous
EOC could not be performed due to unbalanced stage distribution (in
clear cell carcinoma, there were 47 stage I/II specimens (31 high
vs. 16 low HS1 expression) and 13 stage III/IV specimens (10 high
vs. 3 low HS1 expression). The unbalanced stage distribution may
result in type II error (also known as a ‘false negative’ finding).
We hypothesize that if a larger number of non-serous carcinoma
specimens were reanalyzed, HS1 expression may be significantly
associated with a poor prognosis.
A previous study demonstrated that HS1 was
associated with the remodeling of the actin cytoskeleton (17). Scielzo et al (16) demonstrated that HS1 served important
functions in cell migration, bone marrow infiltration and
chemotherapy resistance, and therefore was associated with a poor
prognosis. Additionally, it was identified that patients with
B-cell CLL who exhibited the worst prognosis exhibited highly
phosphorylated HS1 protein that was able to infiltrate the bone
marrow (8). In a previous animal
model, silencing of HS1 expression exerted abnormal cell adhesion
and reduced cell migration in immunodeficient mice (8). HS1-deficient mice also demonstrated
antigen-receptor-induced apoptosis and a proliferative response of
splenic B and T cells (35). Despite
no direct evidence of HS1 expression in other types of solid
carcinoma, several biomarkers associated with F-actin-bundling
protein and anti-apoptosis, including cortactin, fascin and
survivin, were expressed and associated with a poor prognosis in
patients with EOC (23,36). A previous study demonstrated that
fascin is upregulated in numerous types of human carcinoma, and is
associated with tumor aggressiveness and poor OS (37). Additionally, previous studies reported
that cortactin and HS1 are actin-binding molecules involved in
adhesion and cellular migration via actin skeleton remodeling in
the majority of cell types (7,17,19,20,38).
Cortactin overexpression is associated with invasiveness of various
solid tumor cells and a poor prognosis, such as human
hepatocellular carcinoma (21),
laryngeal carcinomas (22), ovarian
cancer (23), colon cancer (24), non-small cell lung cancer (25) and hematopoietic lineages such as in
platelets (28), megakaryocytes
(29) and B-cell CLL (32). As HS1 is a homolog of cortactin, we
hypothesized that HS1 may exhibit a similar role to cortactin with
regard to its biological properties and mechanisms in EOC (17,19).
Peritoneal metastasis is the most frequent clinical presentation
demonstrated in EOC and is composed of multiple stages including
release from the original ovarian neoplasm, attachment to the
mesothelium and subsequent migration/invasion into the
subperitoneal tissue. Future studies are required to investigate
the underlying molecular mechanisms of HS1 in ovarian cancer
metastasis.
In summary, the results of the present study
demonstrate that HS1 is expressed in four common types of EOC and
is associated with the OS of patients with EOC. Furthermore, to the
best of our knowledge, this is the first report investigating the
clinical significance of HS1 in EOC. The present study demonstrates
that the immunoreactivity of HS1 is an independent prognostic
marker for patients with EOC. In order to assess the role of the
HS1 gene further, early clinical detection and novel therapeutic
approaches are required in vivo and in vitro.
Acknowledgements
The authors would like to thank the laboratory team
from the Department of Obstetrics and Gynecology, Nagoya University
Graduate School of Medicine, for providing tissue samples and
technical support.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kajiyama H, Shibata K, Mizuno M, Umezu T,
Suzuki S, Yamamoto E, Fujiwara S, Kawai M, Nagasaka T and Kikkawa
F: Long-term clinical outcome of patients with recurrent epithelial
ovarian carcinoma: Is it the same for each histological type? Int J
Gynecol Cancer. 22:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kikkawa F, Nawa A, Ino K, Shibata K,
Kajiyama H and Nomura S: Advances in treatment of epithelial
ovarian cancer. Nagoya J Med Sci. 68:19–26. 2006.PubMed/NCBI
|
|
4
|
Yoshikawa N, Kajiyama H, Mizuno M, Shibata
K, Kawai M, Nagasaka T and Kikkawa F: Clinicopathologic features of
epithelial ovarian carcinoma in younger vs. older patients:
Analysis in Japanese women. J Gynecol Oncol. 25:118–123. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eisenkop SM, Friedman RL and Wang HJ:
Complete cytoreductive surgery is feasible and maximizes survival
in patients with advanced epithelial ovarian cancer: A prospective
study. Gynecol Oncol. 69:103–108. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kitamura D, Kaneko H, Miyagoe Y, Ariyasu T
and Watanabe T: Isolation and characterization of a novel human
gene expressed specifically in the cells of hematopoietic lineage.
Nucleic Acids Res. 17:9367–9379. 1989.PubMed/NCBI
|
|
7
|
Kitamura D, Kaneko H, Taniuchi I, Akagi K,
Yamamura K and Watanabe T: Molecular cloning and characterization
of mouse HS1. Biochem Biophys Res Commun. 208:1137–1146. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scielzo C, Bertilaccio MT, Simonetti G,
Dagklis A, Ten Hacken E, Fazi C, Muzio M, Caiolfa V, Kitamura D,
Restuccia U, et al: HS1 has a central role in the trafficking and
homing of leukemic B cells. Blood. 116:3537–3546. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamamoto T, Yamanashi Y and Toyoshima K:
Association of Src-family kinase Lyn with B-cell antigen receptor.
Immunol Rev. 132:187–206. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamanashi Y, Okada M, Semba T, Yamori T,
Umemori H, Tsunasawa S, Toyoshima K, Kitamura D, Watanabe T and
Yamamoto T: Identification of HS1 protein as a major substrate of
protein-tyrosine kinase(s) upon B-cell antigen receptor-mediated
signaling. Proc Natl Acad Sci USA. 90:3631–3635. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dehring DA, Clarke F, Ricart BG, Huang Y,
Gomez TS, Williamson EK, Hammer DA, Billadeau DD, Argon Y and
Burkhardt JK: Hematopoietic lineage cell-specific protein 1
functions in concert with the Wiskott-Aldrich syndrome protein to
promote podosome array organization and chemotaxis in dendritic
cells. J Immunol. 186:4805–4818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Butler B, Kastendieck DH and Cooper JA:
Differently phosphorylated forms of the cortactin homolog HS1
mediate distinct functions in natural killer cells. Nat Immunol.
9:887–897. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caligaris-Cappio F, Bergui L, Tesio L,
Corbascio G, Tousco F and Marchisio PC: Cytoskeleton organization
is aberrantly rearranged in the cells of B chronic lymphocytic
leukemia and hairy cell leukemia. Blood. 67:233–239.
1986.PubMed/NCBI
|
|
14
|
Thomas SG, Calaminus SD, Auger JM, Watson
SP and Machesky LM: Studies on the actin-binding protein HS1 in
platelets. BMC Cell Biol. 8:462007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hussein K, von Neuhoff N, Büsche G, Buhr
T, Kreipe H and Bock O: Opposite expression pattern of Src kinase
Lyn in acute and chronic haematological malignancies. Ann Hematol.
88:1059–1067. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scielzo C, Ghia P, Conti A, Bachi A, Guida
G, Geuna M, Alessio M and Caligaris-Cappio F: HS1 protein is
differentially expressed in chronic lymphocytic leukemia patient
subsets with good or poor prognoses. J Clin Invest. 115:1644–1650.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hao JJ, Zhu J, Zhou K, Smith N and Zhan X:
The coiled-coil domain is required for HS1 to bind to F-actin and
activate Arp2/3 complex. J Biol Chem. 280:37988–37994. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He H: HS1 and EMS1. Gan To Kagaku Ryoho.
24:1448–1453. 1997.(In Japanese). PubMed/NCBI
|
|
19
|
van Rossum AG, Schuuring-Scholtes E, van
Buuren-van Seggelen V, Kluin PM and Schuuring E: Comparative genome
analysis of cortactin and HS1: The significance of the F-actin
binding repeat domain. BMC Genomics. 6:152005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schuuring E, van Damme H,
Schuuring-Scholtes E, Verhoeven E, Michalides R, Geelen E, de Boer
C, Brok H, van Buuren V and Kluin P: Characterization of the EMS1
gene and its product, human Cortactin. Cell Adhes Commun.
6:185–209. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao G, Huang ZM, Kong YL, Wen DQ, Li Y,
Ren L and Zhang HY: Cortactin is a sensitive biomarker relative to
the poor prognosis of human hepatocellular carcinoma. World J Surg
Oncol. 11:742013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ambrosio EP, Rosa FE, Domingues MA,
Villacis RA, Coudry Rde A, Tagliarini JV, Soares FA, Kowalski LP
and Rogatto SR: Cortactin is associated with perineural invasion in
the deep invasive front area of laryngeal carcinomas. Hum Pathol.
42:1221–1229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin CK, Chao TK, Yu CP, Yu MH and Jin JS:
The expression of six biomarkers in the four most common ovarian
cancers: Correlation with clinicopathological parameters. APMIS.
117:162–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ni QF, Yu JW, Qian F, Sun NZ, Xiao JJ and
Zhu JW: Cortactin promotes colon cancer progression by regulating
ERK pathway. Int J Oncol. 47:1034–1042. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Noh SJ, Baek HA, Park HS, Jang KY, Moon
WS, Kang MJ, Lee DG, Kim MH, Lee JH and Chung MJ: Expression of
SIRT1 and cortactin is associated with progression of non-small
cell lung cancer. Pathol Res Pract. 209:365–370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tavassoli FA and Devilee Peter: Pathology
and genetics of tumours of the breast and female genital organs.
Lyon: IAPS Press, 2003; 2003
|
|
27
|
Heintz AP, Odicino F, Maisonneuve P,
Beller U, Benedet JL, Creasman WT, Ngan HY, Sideri M and Pecorelli
S: Carcinoma of the ovary. J Epidemiol Biostat. 6:107–138.
2001.PubMed/NCBI
|
|
28
|
Miglarese MR, Mannion-Henderson J, Wu H,
Parsons JT and Bender TP: The protein tyrosine kinase substrate
cortactin is differentially expressed in murine B lymphoid tumors.
Oncogene. 9:1989–1997. 1994.PubMed/NCBI
|
|
29
|
Zhan X, Haudenschild CC, Ni Y, Smith E and
Huang C: Upregulation of cortactin expression during the maturation
of megakaryocytes. Blood. 89:457–464. 1997.PubMed/NCBI
|
|
30
|
Mizutani K, Miki H, He H, Maruta H and
Takenawa T: Essential role of neural Wiskott-Aldrich syndrome
protein in podosome formation and degradation of extracellular
matrix in src-transformed fibroblasts. Cancer Res. 62:669–674.
2002.PubMed/NCBI
|
|
31
|
Schuuring E, Verhoeven E, Litvinov S and
Michalides RJ: The product of the EMS1 gene, amplified and
overexpressed in human carcinomas, is homologous to a v-src
substrate and is located in cell-substratum contact sites. Mol Cell
Biol. 13:2891–2898. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gattazzo C, Martini V, Frezzato F,
Trimarco V, Tibaldi E, Castelli M, Facco M, Zonta F, Brunati AM,
Zambello R, et al: Cortactin, another player in the Lyn signaling
pathway, is over-expressed and alternatively spliced in leukemic
cells from patients with B-cell chronic lymphocytic leukemia.
Haematologica. 99:1069–1077. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu XZ, Garcia MV, Li TY, Khor LY,
Gajapathy RS, Spittle C, Weed S, Lessin SR and Wu H: Cytoskeleton
alterations in melanoma: Aberrant expression of cortactin, an
actin-binding adapter protein, correlates with melanocytic tumor
progression. Mod Pathol. 23:187–196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Soslow RA: Histologic subtypes of ovarian
carcinoma: An overview. Int J Gynecol Pathol. 27:161–174.
2008.PubMed/NCBI
|
|
35
|
Taniuchi I, Kitamura D, Maekawa Y, Fukuda
T, Kishi H and Watanabe T: Antigen-receptor induced clonal
expansion and deletion of lymphocytes are impaired in mice lacking
HS1 protein, a substrate of the antigen-receptor-coupled tyrosine
kinases. EMBO J. 14:3664–3678. 1995.PubMed/NCBI
|
|
36
|
Park SH, Song JY, Kim YK, Heo JH, Kang H,
Kim G, An HJ and Kim TH: Fascin1 expression in high-grade serous
ovarian carcinoma is a prognostic marker and knockdown of fascin1
suppresses the proliferation of ovarian cancer cells. Int J Oncol.
44:637–646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hashimoto Y, Skacel M and Adams JC: Roles
of fascin in human carcinoma motility and signaling: Prospects for
a novel biomarker? Int J Biochem Cell Biol. 37:1787–1804. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rottner K and Stradal TE: Actin dynamics
and turnover in cell motility. Curr Opin Cell Biol. 23:569–578.
2011. View Article : Google Scholar : PubMed/NCBI
|