Introduction
Gastric cancer (GC), is among the most common types
of cancer in Asia (1). The 5-year
survival rate of patients with GC treated at an early stage is
90–95%. However, if GC develops to a late stage, the survival rate
is significantly decreased (2).
Despite advances in the treatment of GC, a considerable number of
patients remain with local recurrence or distant metastasis, and
the underlying molecular mechanism of GC metastasis remains unclear
(3). Therefore, investigation of the
regulatory mechanisms underlying the activation of GC cell
metastasis is required for the effective diagnosis and treatment of
GC.
Deregulation of epigenetic mechanisms contributes to
GC development and progression, including acetylation, methylation,
phosphorylation and ubiquitination (4). Histone methylation has attracted
increasing attention due to its participation in the process of
heterochromatin formation, gene imprinting, X chromosome
inactivation and gene transcriptional regulation (5,6). Histone
methyltransferases (HMTs) are a class of catalytic 1–3 ethyl group
transfer to histone lysine or arginine, and are classified into
histone lysine methyltransferases (HKMTs) and histone arginine
methyltransferases (7). HKMTs can be
further classified into 2 subgroups: SET domain and non-SET domain
(8). Aberrant HMT expression has been
identified in GC, and has been demonstrated to contribute to GC
metastasis and development by promoting oncogene expression or
inhibiting the expression of tumor suppressor genes. For example,
depletion of EHMT2 inhibits cell growth and induces apoptosis in
GC, indicating therapeutic potential in GC (9). High expression of SET domain containing
(lysine methyltransferase) 8 (SET8) was associated with a shortened
survival time in patients with GC, and the level of SET8 expression
was identified as an independent predictor of GC outcome (10). Enhancer of zeste2 polycomb repressive
complex 2 subunit can mediate the inhibition of S100A4 on
E-cadherin, and regulate the proliferation and migration of GC
cells (11). Abnormal EHMT1
expression in cancer tissues, including esophageal squamous cell
cancer (12) and breast cancer
(13), suggests that EHMT1 functions
in tumor pathogenesis and progression. However, whether EHMT1
serves a role in GC development remains unknown. The present study
aimed to elucidate the role of EHMT1 deregulation in GC
carcinogenesis, to characterize its putative oncogenic role and its
potential clinical impact.
Materials and methods
Tissues
The clinical research protocol of the present study
was approved by the Ethical Committee of Shanghai East Hospital
(Shanghai, China). GC tissues and matched non-tumor tissues were
obtained from 97 patients who underwent curative surgery between
March 2011 and September 2016 at the Department of Surgery,
Shanghai East Hospital. None of the patients had received
chemotherapy prior to surgery. Written informed consent was
obtained from all participants. All tissue samples were immediately
snap-frozen in liquid nitrogen or formalin-fixed (4% at 4°C for 24
h) and paraffin-embedded.
Cell culture
The immortalized normal gastric epithelial cell
line, GES-1, and the GC cell lines, BGC-803, AGS, KATO III and
NCI-N87 (all cells were purchased from the library of the Chinese
Academy of Sciences, Shanghai, China), were used in the present
study. All cells were cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
calf serum (Gibco; Thermo Fisher Scientific, Inc.), 100 µg/ml
streptomycin and 100 U/ml penicillin at 37°C in a humidified
incubator containing 5% CO2.
Xenograft model
All animal experiments were approved by the
Experimental Animal Ethics Committee of Shanghai East Hospital and
performed according to the Guide for the Institutional Animal Care
and Use Committee of Shanghai Tongji University (Shanghai China).
Specific pathogen-free grade, male, four-week-old, 10–12 g weight
male BALB/c nude mice were purchased from the Institute of Zoology,
Chinese Academy of Sciences (Beijing, China). Animals were housed
in cages with wood chip beddings in a temperature-controlled room
(20–22°C) with a 12-h light-dark cycle and 45–55% relative
humidity, and were permitted free access to food and drinking
water. In order to study the effect of EHMT1 on abdominal
metastasis of gastric cancer and the expression of E-cadherin in
the subcutaneous transplantation tumor, the mice were divided into
2 equal groups (five nude mice in each group), and subcutaneously
(6×105 cells) or abdominally (2×106 cells)
injected with either BGC-803/NC or BGC-803/sh1-EHMT1 cells. All
mice were sacrificed after 30 days. Subcutaneous tumor grafts were
removed, fixed in 4% formalin for 24 h in 4°C to obtain 5-µm thick
paraffin-embedded sections and analyzed by immunohistochemistry,
and peritoneal metastasis nodules were counted and further
analyzed. In the process, if the mice showed signs of cachexia or
excessive ascites affecting their diet and activity, the experiment
was promptly terminated.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from GC tissues and cell lines were
extracted using TRIzol (Thermo Fisher Scientific, Inc.), according
to the manufacturer's protocol. cDNA was synthesized from 1 µg RNA
using a reverse transcription kit (Promega Corporation, Madison,
WI, USA), according to the manufacturer's protocols. RT-qPCR was
performed using SYBR-Green PCR Master mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. GAPDH was used as an internal control. The PCR
primers were as follows: EHMT1 forward,
5′-CATGCAGCCAGTAAAGATCCC-3′, and reverse,
5′-CTGCTGTCGTCCAAAGTCAG-3′; and GAPDH forward,
5′-TTGGCATCGTTGAGGGTCT-3′ and reverse, 5′-CAGTGGGAACACGGAAAGC-3′.
PCR reactions were performed with an initial denaturation at 95°C
for 5 min, followed by 30 cycles of 95°C for 30 sec, 57°C for 30
sec and 72°C for 30 sec, with a final extension at 72°C for 10 min.
Gene expression was quantified using the 2−ΔΔCq method
(14).
Immunohistochemistry (IHC)
Sections of a 5-µm thickness were sliced from the
paraffin-embedded tissues of the mice or patients, and
deparaffinized (100% xylene) and rehydrated in an ethanol series
(100–50%). The sections were then treated in 0.01 mol/l citrate
buffer (pH 6.0) for antigen retrieval. Endogenous peroxidase
activity was inhibited following incubation with methanol
containing 0.3% H2O2 for 30 min.
Subsequently, sections were incubated with antibodies detecting
EHMT1 (cat. no. ab41969; 1:200; Abcam, Cambridge, UK) and
E-cadherin (cat. no. ab76055; 1:200; Abcam) primary antibodies at
37°C for 2 h. Normal IgG (cat. no. ab6728; 1:200; Abcam) was used
as a negative control. The slides were washed with PBS and
incubated for 2 h at room temperature with an EnVision kit (cat.
no. GK500705; Dako; Agilent Technologies, Inc., Santa Clara, CA,
USA), according to the manufacturer's protocol. The percentage of
positive tumor cells was scored as follows: <10%, score 0;
10–25%, score 1; 26–50%, score 2; 51–75%, score 3; and >75%,
score 4. Intensity of staining was qualitatively evaluated as
follows: Negative, score 0; weak, score 1; moderate, score 2; or
strong, score 3. The percentage score was multiplied by the
intensity score to give a final staining score. Final scores of 0–4
were considered to indicate weak expression, whereas final scores
of 4–12 were considered to indicate strong expression.
Western blot analysis
Whole cell proteins were extracted from cells using
radioimmunoprecipitation buffer containing a Protease Inhibitor
Cocktail (Pierce; Thermo Fisher Scientific, Inc.). Protein was
quantified using a bicinchoninic Protein Assay kit (Pierce; Thermo
Fisher Scientific, Inc.). Cell extracts (50 µg) were separated by
10% SDS-PAGE, and transferred onto polyvinylidene fluoride
membranes. Membranes were blocked with 5% nonfat milk in
Tris-buffered saline (TBS) for 2 h at 25°C and incubated with
primary antibodies at 4°C overnight. The primary antibodies used
were mouse anti-EHMT1 (cat. no. ab41969; 1:1,000), mouse
anti-Ecadherin (cat. no. ab76055; 1:1,000) and mouse anti-GAPDH
(cat. no. ab8245; 1:1,000; all Abcam). Membranes were then washed
three times in 1xTBS-Tween solution for 15 min, and incubated with
anti-mouse IgG (horseradish peroxidase conjugated) secondary
antibodies for 2 h in 25°C (cat. no. ab193651; 1:5,000; Abcam).
Signals were visualized using an enhanced chemiluminescence kit
(cat. no. WBKLS0050; EMD Millipore, Billerica, MA, USA).
Plasmid construction and
transfection
EHMT1 short hairpin RNAs (shRNAs) and negative
control (NC) were obtained from OBIO (Shanghai, China). EHMT1
sh1RNA (targeting sequence, 5′-CGAGTCAATAACGCCAGCTAT-3′), sh2RNA
(targeting sequence, 5′-CCTCGGTTCTGAGTCGTATAA-3′) or NC (targeting
sequence, 5′-TTCTCCGAACGTGTCACGT-3′) were cloned into G418 plasmids
(Gibco; Thermo Fisher Scientific, Inc.). The plasmid products were
then transfected separately into BGC-803 cells (6×104
cells/well) at 1,500 µg/ml using Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. A
total of one month later following transfection, stably transfected
cells were detected using RT-qPCR and western blotting.
Wound healing assay
BGC-803/sh1, BGC-803/sh2 cells and NC cells were
cultured as a monolayer to 100% confluence in 6-well plates. The
cells were then scratched with a sterile pipette tip. The plates
were washed with PBS to remove the cellular debris, and then
cultured in RPMI-1640 serum-free medium (Gibco; Thermo Fisher
Scientific, Inc.). The extent of wound healing was observed at 0
and 48 h under an inverted phase contrast microscope.
Migration and invasion assays
The cell migration and invasion abilities were
measured using a 24-well Transwell chamber with 8-µm pore inserts
(Corning Inc., Corning, NY, USA). For the migration assay,
1.0×105 cells in 200 µl RPMI-1640 serumfree medium
(Gibco; Thermo Fisher Scientific, Inc.) were placed in the upper
chamber, whereas 600 µl media with 10% FBS was placed in the lower
chamber. After 24 h, cells remaining on the upper side of inserts
were gently scraped off and cells on the lower surface were fixed
in 100% methanol and stained with 0.5% crystal violet (37°C for 30
min). The invasion assay was performed according to the same
protocol as the migration assay; however, the upper chamber was
pre-coated with Matrigel. For the two assays, the stained cells
were counted in 5 randomly selected fields under an inverted light
microscope.
Statistical analysis
The association between EHMT1 expression and
clinicopathological characteristics was analyzed using Pearson's
χ2 test. The differences between 2 groups were analyzed
using Student's t-test, and the differences between ≥3 groups were
analyzed using one-way analysis of variance with Bonferroni's
post-hoc test. All statistical analyses were performed using SPSS
18.0 software (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
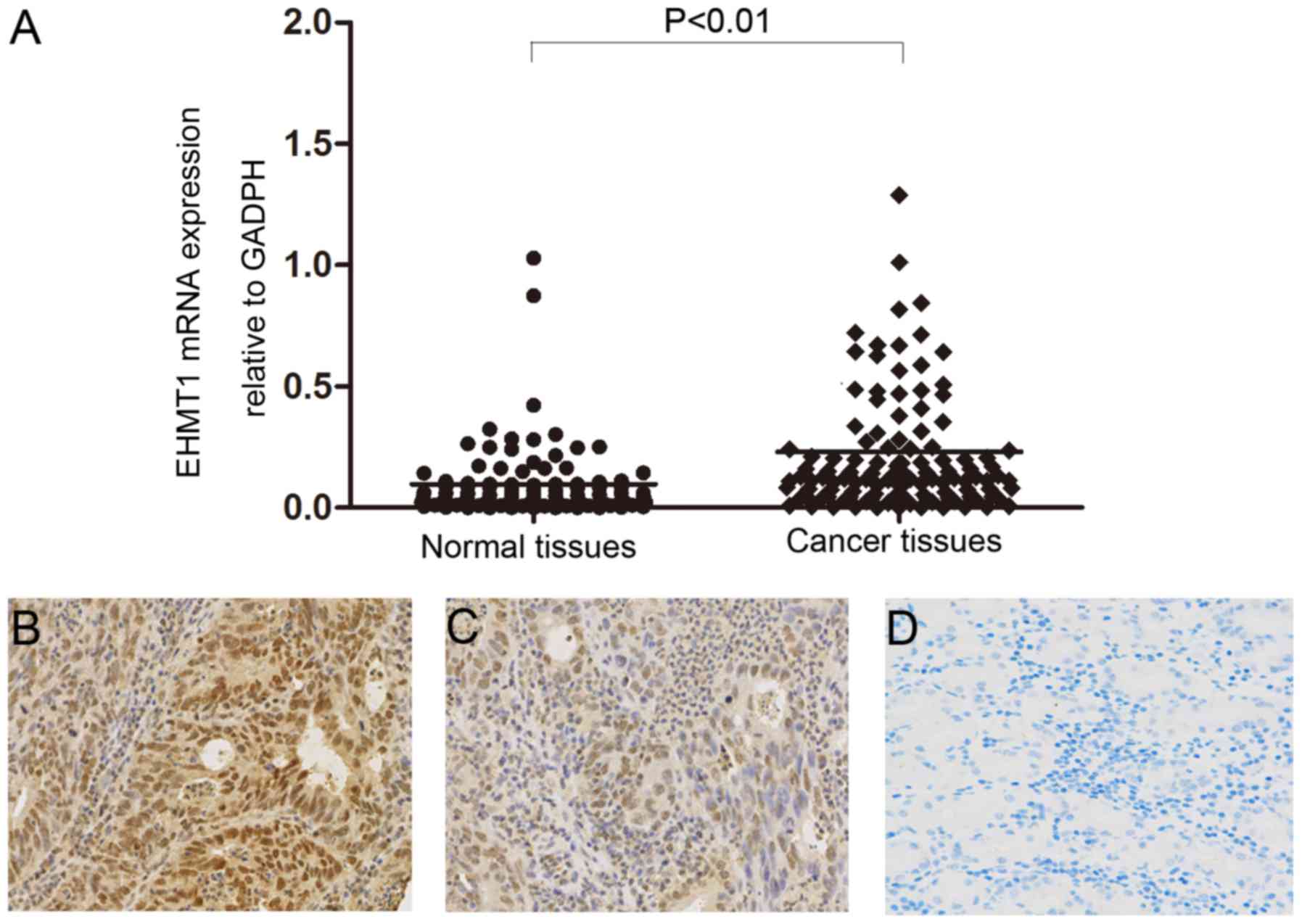
EHMT1 is overexpressed in GC tissues
and is associated with tumor stage and lymph node metastasis
RT-qPCR was performed to analyze mRNA expression of
EHMT1 in GC tumor tissues and matched non-tumor tissues. The mRNA
level of EHMT1 in GC tissues was significantly increased compared
with that of adjacent non-tumor tissues (P<0.01; Fig. 1A). The protein expression level of
EHMT1 in gastric tissues was analyzed by IHC, revealing increased
EHMT1 staining in GC tissues compared with that in non-tumor
tissues (Fig. 1B-D). The association
between EHMT1 protein expression and the clinicopathological
characteristics of 97 patients with GC are presented in Table I. χ2 analysis suggested
that high expression of EHMT1 in GC was significantly associated
with tumor stage (P=0.033) and lymph node metastasis (P=0.003).
However, there was no statistically significant association between
EHMT1 expression and other clinicopathological features, including
sex, age and tumor size.
 | Table I.Association between EHMT1 expression
and clinicopathological factors of gastric cancer patients. |
Table I.
Association between EHMT1 expression
and clinicopathological factors of gastric cancer patients.
|
|
| EHMT1 protein
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
factor | n | Positive (n=65) | Negative (n=32) | P-value |
|---|
| Sex |
|
|
| 0.420 |
| Male | 74 | 48 | 26 |
|
|
Female | 23 | 17 | 6 |
|
| Age, years |
|
|
| 0.497 |
| ≥60 | 59 | 38 | 21 |
|
|
<60 | 38 | 27 | 11 |
|
| Tumor
differentiation |
|
|
| 0.517 |
| Well to
moderate | 38 | 24 | 14 |
|
| Poor | 59 | 41 | 18 |
|
| Tumor location |
|
|
| 0.295 |
| Gastric
fundus | 3 | 1 | 2 |
|
| Gastric
corpus | 41 | 30 | 11 |
|
|
Pylorus | 53 | 34 | 19 |
|
| Tumor size, cm |
|
|
| 0.430 |
| ≤3 | 46 | 29 | 17 |
|
|
>3 | 51 | 36 | 15 |
|
| Tumor stage |
|
|
| 0.033 |
|
T1-T2 | 37 | 20 | 17 |
|
|
T3-T4 | 60 | 45 | 15 |
|
| Lymph node
metastasis |
|
|
| 0.003 |
|
Negative | 43 | 22 | 21 |
|
|
Positive | 54 | 43 | 11 |
|
| Distant
metastasis |
|
|
| 0.316 |
|
Negative | 95 | 63 | 32 |
|
|
Positive | 2 | 2 | 0 |
|
| TNM stage |
|
|
| 0.837 |
|
I+II | 38 | 25 | 13 |
|
|
III+IV | 59 | 40 | 19 |
|
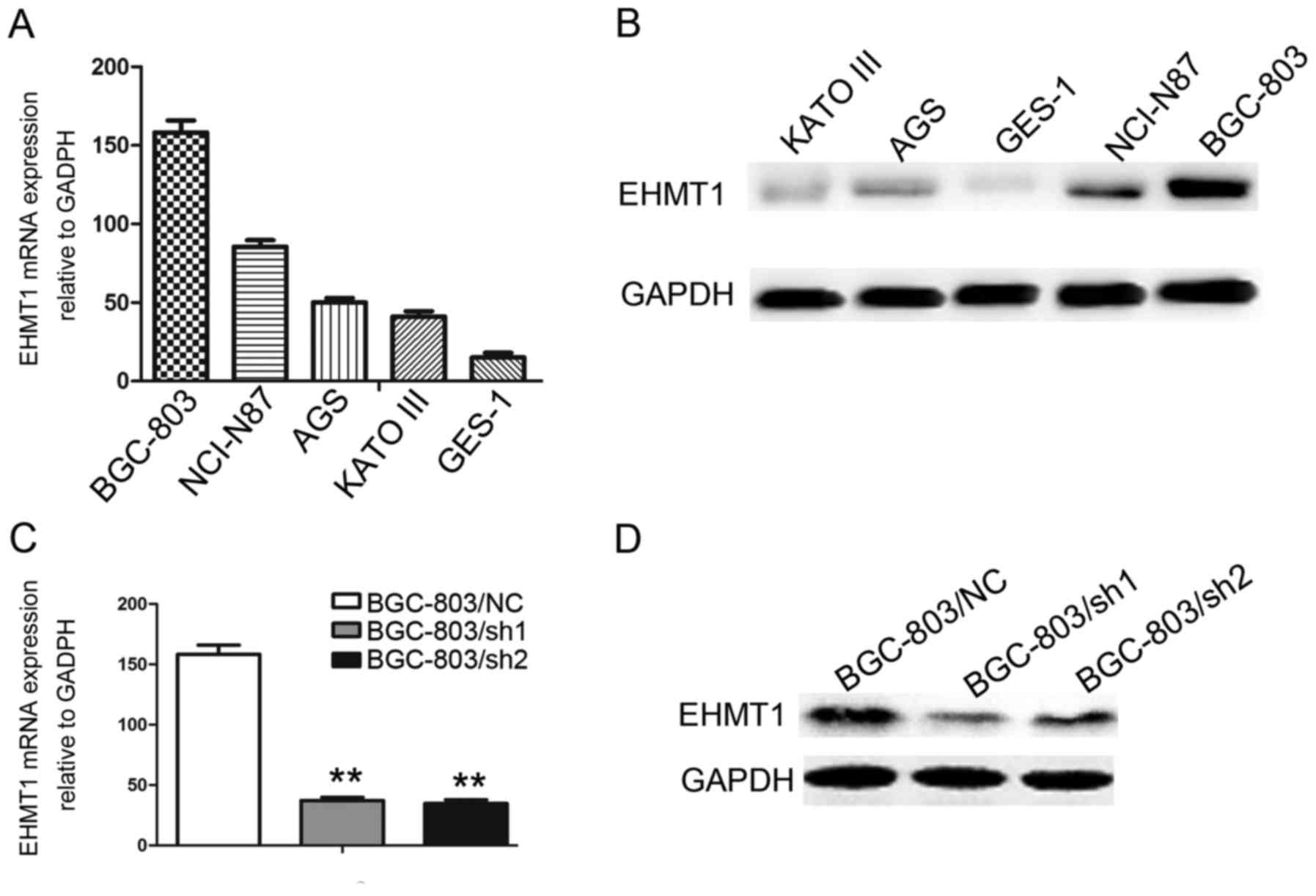
Construction of stable EHMT1-knockdown
GC cell lines
EHMT1 expression was investigated in the
immortalized gastric epithelial cell line, GES-1, and in a series
of GC cell lines, including BGC-803, NCI-N87, AGS and KATO-III.
Among these cell lines, BGC-803, NCI-N87, AGS and KATO-III
exhibited increased EHMT1 expression compared with GES-1
(P<0.01; Fig. 2A and B). To
further explore the functions of EHMT1 in GC cells, knockdown of
EHMT1 expression was performed in BGC-803 cell lines, and
experimentally validated (P<0.01; Fig.
2C and D).
EHMT1 promotes wound healing,
migration and invasion of GC cells
Wound healing assays were performed to investigate
the effect of EHMT1 on GC cell motility. It was observed that the
distance between wound edges in the BGC-803/sh1-EHMT1 and
BGC-803/sh2-EHMT1 cells was large compared with that in the control
cells (P<0.01; Fig. 3A and B).
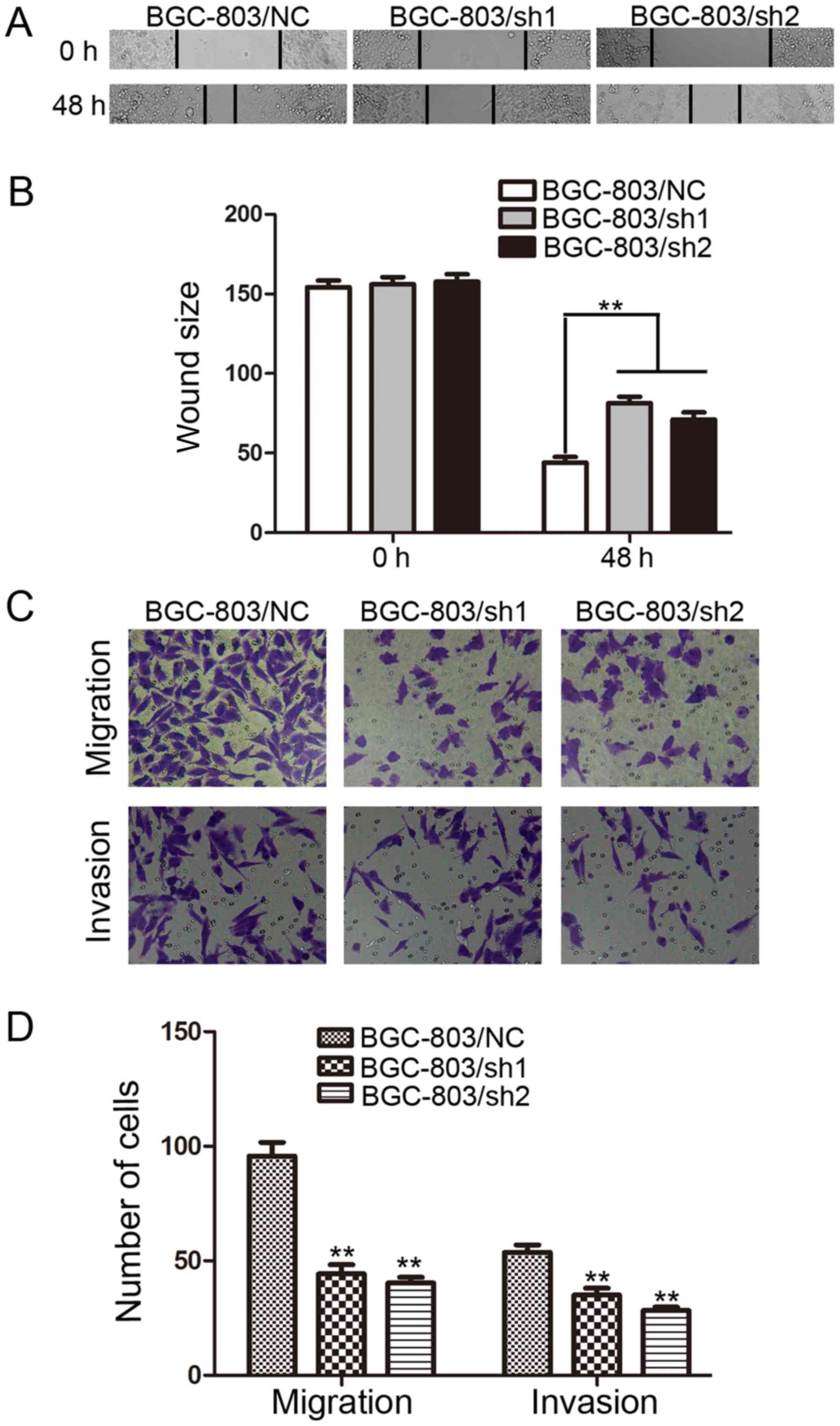 | Figure 3.EHMT1 promotes the migration and
invasion of GC cells. (A) BGC-803 cells were transfected with EHMT1
shRNA1/2 or NC shRNA, and representative images of wound healing
assays were captured at 0 and 48 h post-wounding (magnification,
×200). (B) Quantification of the distance between wound edges of
BGC-803/NC, BGC-803/sh1 and BGC-803/sh2 cells. (C) Representative
images of migratory and invasive cells that traversed the micropore
membrane following transfection with crystal violet staining
(magnification, ×200). (D) Average number of migratory cells and
invasive cells from 5 fields of view. Data are represented as the
mean ± standard deviation of 3 independent experiments. **P<0.01
vs. control. EHMT1, euchromatic histone lysine methyltransferase 1;
GC, gastric cancer; shRNA, short hairpin RNA; NC, negative control;
sh1, EHMT1 shRNA 1; sh2, EHMT1 shRNA 2. |
The effect of EHMT1 on GC cell migration and
invasion, which are key determinants of malignant progression and
metastasis, was also assessed using Transwell assays. After 24 h of
incubation, cells were counted under an inverted microscope. As
illustrated in Fig. 3C and D, the
number of cells that migrated into the lower chamber was
significantly lower in BGC-803/sh1-EHMT1 and BGC-803/sh2-EHMT1
cells compared with that in BGC-803/NC cells in the migration and
invasion assays (all P<0.01).
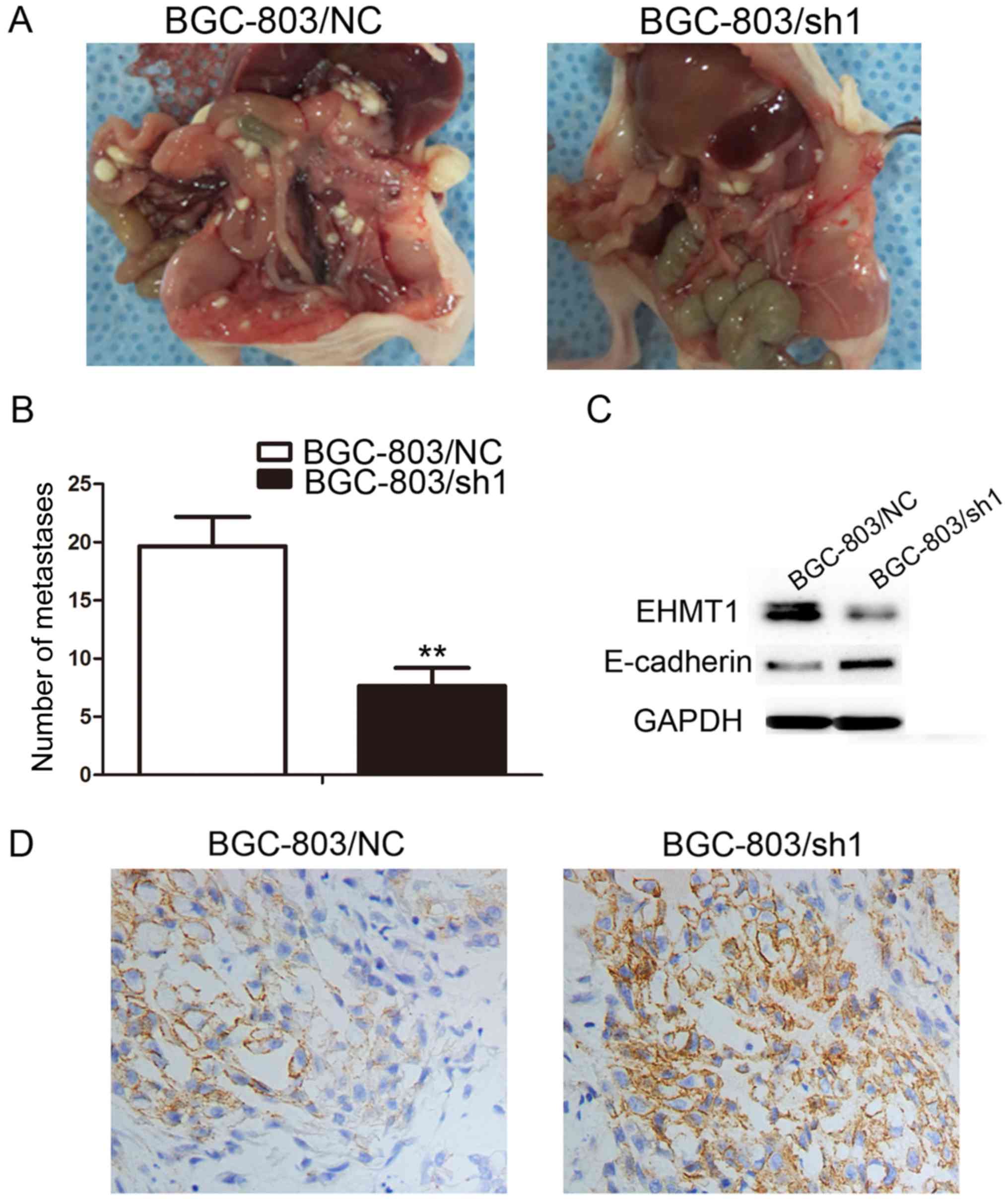
EHMT1 expression promotes peritoneal
metastasis of GC cells
Based on the aforementioned in vitro results,
the in vivo function of overexpressed EHMT1 was investigated
by abdominally injecting BGC-803/sh1-EHMT1 and negative control
cells into nude mice. A total of 30 days following injection, the
mice were sacrificed and the peritoneal nodules were evaluated. As
demonstrated in Fig. 4A, increased
peritoneal spread was observed in the negative control group
compared with that in the BGC-803/sh1-EHMT1 group. Following
counting of the nodules, it was evident that there were
significantly fewer peritoneal nodules in mice injected with the
BGC-803/sh1-EHMT1 cells compared with the number in mice injected
with negative control cells (P<0.01; Fig. 4B). The xenograft model experiment was,
therefore, consistent with the in vitro results, confirming
that EHMT1 expression in GC promotes GC metastasis.
EHMT1 promotes GC invasion and
metastasis by silencing E-cadherin
E-cadherin is a marker of tumor metastasis, and
E-cadherin protein expression was upregulated in BGC-803/sh1-EHMT1
cells compared with that in BGC-803/NC cells (Fig. 4C). To further verify the effect of
EHMT1 on E-cadherin, BGC-803/sh1-EHMT1 and BGC-803/NC cells were
subcutaneously injected into BALB/c nude mice. The maximum tumor
volume of xenografts derived from the BGC-803/sh1-EHMT1 and
BGC-803/NC groups was 0.991 and 0.861 cm3, respectively.
In addition, the maximum weight of the tumors derived from the
BGC-803/sh1-EHMT1 and BGC-803/NC groups was 0.661 and 0.574 g,
respectively. IHC suggested that EHMT1 silenced E-cadherin
expression in subcutaneous tumors, thus promoting tumor metastasis
(Fig. 4D).
Discussion
Histone modifications, including ubiquitination,
phosphorylation, acetylation and methylation serve critical roles
in transcriptional repression and activation through the regulation
of chromatin structure (15). EHMT1
has been reported to be upregulated in esophageal squamous cell
cancer (12) and breast cancer
(13). However, to the best of our
knowledge, the expression level of EHMT1 in GC remains unknown. In
the present study, the significant upregulation of EHMT1 in GC was
demonstrated using RT-qPCR and IHC, and its expression was
associated with lymph node metastasis and tumor stage. It was also
demonstrated that EHMT1 promotes the metastasis ability of GC cells
and suppresses E-cadherin expression, which may contribute to the
existing understanding of GC development.
The transcriptional activity of a gene is often
unaffected by DNA methylation, but is determined by chromatinstate
(16). Methylated histones allow
close DNA packing and a dense chromatin structure, resulting in a
decrease in the transcriptional activity of the gene in question
(13). Fritsch et al
established that EHMT1 can methylate the histone and promoter of
specific genes, leading to decreased transcriptional activity
(17). EHMT1 serves an important role
in the methylation and dimethylation of histone H3 lysine 9 (H3K9)
euchromatin, activating a series of downstream reactions and
producing corresponding physiological functions. In the majority of
cases, EHMT1 will first form a heterodimer with G9a (18). The G9a/EHMT1 complex can inhibit the
expression of p53 by demethylating K373 at the C-terminus of the
p53 gene, indicating a promotive effect of EHMT1 in cancer
(19). In the Mage-a tumor stem cell
line, H3K9 demethylation (H3K9me2) mediated by the EHMT1/G9a
complex has an inhibitory effect on the expression of its antigenic
gene (target of tumor vaccine currently undergoing clinical
evaluation world-wide) (20). The
surface antigens of tumor cells are targets of therapeutic drugs
(21), and the EHMT1/G9a complex may
function in immune avoidance and therapeutic resistance effects.
Epithelial-mesenchymal transition (EMT) refers to the biological
process by which epithelial cells are transformed into mesenchymal
cells, and is considered to be an important marker of tumor
progression. The loss of E-cadherin expression is indicative of the
migration and invasion of malignant tumor cells (22–24).
Previous studies have demonstrated that G9a forms a complex with
Snail and binds to the E-cadherin promoter, resulting in H3K9me2
activity and increased potential for EMT and metastasis (25,26).
Considering that EHMT1 and G9a exist as dimers, the present study
investigated whether EHMT1 can affect the expression of E-cadherin.
As expected, EHMT1 inhibited the expression of E-cadherin in
vitro and in vivo. Thus, EHMT1 may promote the process
of GC metastasis by influencing EMT in GC cells.
In conclusion, the present study demonstrated that
EHMT1 was highly expressed in GC, and was associated with lymph
node metastasis and tumor stage. Depletion of EHMT1 expression
inhibited GC cell wound healing, migration and invasion. These
results highlight the potential of EHMT1 as a potential therapeutic
target for GC.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Science and Technology Development Fund of Pudong New District
(grant no. PKJ2016-Y60) and the Young Talent program of Tongji
University (grant no. 1507219045).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article..
Authors' contributions
TD and YY designed the research. YY, JFS and DY were
responsible for the implementation of the experiment and
acquisition of data. BY, SZ and JW analysed and interpreted the
data. TD, YY and JFS wrote, reviewed and revised the manuscript.
The final version of the manuscript has been read and approved by
all authors.
Ethics approval and consent to
participate
Experiments using human tissues were approved by the
Ethical Committee of Shanghai East Hospital (Shanghai, China), and
written informed consent was obtained from all participants. All
animal experiments were approved by the Experimental Animal Ethics
Committee of Shanghai East Hospital and performed according to the
Guide for the Institutional Animal Care and Use Committee of
Shanghai Tongji University (Shanghai, China).
Consent for publication
All patients provided written informed consent for
the publication of data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GC
|
gastric cancer
|
|
HMTs
|
histone methyltransferases
|
|
EMT
|
epithelial-mesenchymal transition
|
References
|
1
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Correa P: Gastric cancer: Overview.
Gastroenterol Clin North Am. 42:211–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patel TN, Roy S and Ravi R: Gastric cancer
and related epigenetic alterations. Ecancermedicalscience.
11:7142017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang L and Xu AM: SET and MYND domain
containing protein 3 in cancer. Am J Transl Res. 9:1–14.
2017.PubMed/NCBI
|
|
6
|
Greer EL and Shi Y: Histone methylation: A
dynamic mark in health, disease and inheritance. Nat Rev Genet.
13:343–357. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Albert M and Helin K: Histone
methyltransferases in cancer. Semin Cell Dev Biol. 21:209–220.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wood A and Shilatifard A:
Posttranslational modifications of histones by methylation. Adv
Protein Chem. 67:201–222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin X, Huang Y, Zou Y, Chen X and Ma X:
Depletion of G9a gene induces cell apoptosis in human gastric
carcinoma. Oncol Rep. 35:3041–3049. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi XL, Guo ZJ, Wang XL, Liu XL and Shi
GF: SET8 expression is associated with overall survival in gastric
cancer. Genet Mol Res. 14:15609–15615. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu S, Chen D, Shen W, Chen L, Yu A, Fu H,
Sun K and Sun X: EZH2 mediates the regulation of S100A4 on
E-cadherin expression and the proliferation, migration of gastric
cancer cells. Hepatogastroenterology. 62:737–741. 2015.PubMed/NCBI
|
|
12
|
Guan X, Zhong X, Men W, Gong S, Zhang L
and Han Y: Analysis of EHMT1 expression and its correlations with
clinical significance in esophageal squamous cell cancer. Mol Clin
Oncol. 2:76–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cebrian A, Pharoah PD, Ahmed S, Ropero S,
Fraga MF, Smith PL, Conroy D, Luben R, Perkins B, Easton DF, et al:
Genetic variants in epigenetic genes and breast cancer risk.
Carcinogenesis. 27:1661–1669. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peterson CL and Laniel MA: Histones and
histone modifications. Curr Biol. 14:R546–R551. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ordway JM and Curran T: Methylation
matters: Modeling a manageable genome. Cell Growth Differ.
13:149–162. 2002.PubMed/NCBI
|
|
17
|
Fritsch L, Robin P, Mathieu JR, Souidi M,
Hinaux H, Rougeulle C, Harel-Bellan A, Ameyar-Zazoua M and
Ait-Si-Ali S: A subset of the histone H3 lysine 9
methyltransferases Suv39h1, G9a, GLP, and SETDB1 participate in a
multimeric complex. Mol Cell. 37:46–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiong Y, Li F, Babault N, Dong A, Zeng H,
Wu H, Chen X, Arrowsmith CH, Brown PJ, Liu J, et al: Discovery of
potent and selective inhibitors for G9a-Like protein (GLP) lysine
methyltransferase. J Med Chem. 60:1876–1891. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang J, Dorsey J, Chuikov S, Pérez-Burgos
L, Zhang X, Jenuwein T, Reinberg D and Berger SL: G9a and Glp
methylate lysine 373 in the tumor suppressor p53. J Biol Chem.
285:9636–9641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Link PA, Gangisetty O, James SR,
Woloszynska-Read A, Tachibana M, Shinkai Y and Karpf AR: Distinct
roles for histone methyltransferases G9a and GLP in cancer
germ-line antigen gene regulation in human cancer cells and murine
embryonic stem cells. Mol Cancer Res. 7:851–862. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simpson AJ, Caballero OL, Jungbluth A,
Chen YT and Old LJ: Cancer/testis antigens, gametogenesis and
cancer. Nat Rev Cancer. 5:615–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Biddle A and Mackenzie IC: Cancer stem
cells and EMT in carcinoma. Cancer metastasis Rev. 2012.(Epub Ahead
of Print). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang L, Wu RL and Xu AM:
Epithelial-mesenchymal transition in gastric cancer. Am J Transl
Res. 7:2141–2158. 2015.PubMed/NCBI
|
|
25
|
Liu S, Ye D, Guo W, Yu W, He Y, Hu J, Wang
Y, Zhang L, Liao Y, Song H, et al: G9a is essential for
EMT-mediated metastasis and maintenance of cancer stem cell-like
characters in head and neck squamous cell carcinoma. Oncotarget.
6:6887–6901. 2015.PubMed/NCBI
|
|
26
|
Dong C, Wu Y, Yao J, Wang Y, Yu Y,
Rychahou PG, Evers BM and Zhou BP: G9a interacts with Snail and is
critical for Snail-mediated E-cadherin repression in human breast
cancer. J Clin Invest. 122:1469–1486. 2012. View Article : Google Scholar : PubMed/NCBI
|