Introduction
Cancer is caused by the genetic mutations induced by
various carcinogenic factors (1).
Lung cancer is the leading cause of death worldwide (2). The incidence of lung cancer is related
to smoking, and it is reported that 85% of male patients and 47% of
female lung cancer patients are smokers (3,4). Non-small
cell lung cancer (NSCLC) is the most common type of lung cancer
which accounts for >75% of all lung cancer cases (5). Lung cancer is a highly complicated and
heterogeneous disease with intricate genetic mutations (6). Therefore, further investigation of the
underlying mechanism of NSCLC is urgently needed.
MicroRNAs (miRNAs) are 21–28 nucleotides in length
and are highly conserved small non-coding RNAs that bind to mRNAs
of their target gene at their 3′-UTR to regulate target gene
expression at post-transcriptional (7,8). miRNAs
play an important role in various biological processes, such as
proliferation, metastasis, and apoptosis (9). In NSCLC, multiple miRNAs have been
identified as tumor suppressors, including miR-16, miR-23b, and
miR-143 (10–12). miR-96 is a member of the miR-183
family, which constituted a polycistronic paralogous miRNA cluster
(13). In breast cancer, miR-96
promoted cell invasion, migration, and proliferation in
vitro by silencing PTPN9 (14).
Ress et al reported that miR-96 promoted cell proliferation
and predicted poor prognosis in colorectal cancer (15). Recently, miR-96 has been reported to
be upregulated and acts as an oncogene in many cancers, including
NSCLC (16). The
reversion-inducing-cysteine-rich protein with kazal motifs (RECK)
is the functional target of miR-96 (17). The majority of human miRNAs were
imprecisely bound to mRNA of target genes (8). Thus, we considered there could be other
unknown targets of miR-96 in NSCLC. Jalvy-Delvaille et al
have shown that glypican-3 (GPC3) is a potential target of
miR-96 in hepatocellular carcinoma (HCC) cells (18).
GPC3, heparan sulfate proteoglycans (HSPGs)
which are the major part of extracellular matrix, play a pivotal
role in the regulation of heparin-binding growth factor and various
intracellular signaling pathways (19,20). It
has been found that GPC3 functions as a tumor suppressor in various
tumors. GPC3 was significantly downregulated in human malignant
mesothelioma cell lines and the ectopic expression of GPC3
suppressed the colony formation of human MM cells in vitro
(21). White et al reported
that GPC3 may participate in the tumorigenesis of some WT cases
(22).
In the present study, we investigated the role of
miR-96 and GPC3 in NSCLC. We found that miR-96 was upregulated in
lung cancer cells and NSCLC tissues compared with normal epithelium
cells and paracancerous tissues. The miR-96 overexpression
significantly promoted the migratory and invasive ability of lung
cancer cell lines. Moreover, we identified that GPC3 was a
direct target of miR-96 and miR-96 regulated the expression of
GPC3. GPC3 was decreased in lung cancer cell lines and a low
expression of GPC3 suppressed the proliferation of lung cancer
cells.
Materials and methods
Tissue samples and cell lines
In total, 57 human NSCLC samples and corresponding
paracancerous tissues from the patients who underwent surgery at
the First Affiliated Hospital of Jiamusi University (Jiamusi,
China) between January, 2012 and December, 2016 were collected. The
above experimental samples were obtained with informed consent of
the patients and verified by the Ethics Committee of the First
Affiliated Hospital of Jiamusi University.
Human lung cancer cells A549 and H460 and normal
lung epithelium cells MRC-5 were purchased from the American Type
Culture Collection (ATCC, Rockville, MD, USA) and cultured at 37°C
in a humidified atmosphere with 5% CO2 using RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Carlsbad, CA, USA),
and supplemented with 10% fetal bovine serum.
Western blot analysis
Total proteins were extracted from the cells and
tissues using the cell lysis buffer (Takara Biotechnology Co.,
Ltd., Dalian, China) according to the manufacturer's instructions.
The proteins were quantified using the Bradford assay kit (Takara
Biotechnology Co., Ltd.). The proteins were separated by SDS-PAGE,
and then transferred onto a PVDF membrane (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The membrane was blocked in Tween-20
containing 5% skimmed milk for 1 h and then incubated with primary
antibody (GPC3 rabbit polyclonal antibody, P51654; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C overnight. After
the membranes were washed three times (10 min each) with PBST, the
anti-rabbit secondary antibody (1:3,000; sc-362280; Santa Cruz
Biotechnology, Inc.) was incubated with the membranes for 1 h at
room temperature. The protein bands were visualized using the
Bio-Rad Gel Doc XR instrument (Bio-Rad Laboratories, Inc.). Each
experiment was performed three times in duplicate.
Plasmid construction and cell
transfection
miRNA vectors, including miR-96 mimic and LNA
anti-miR-96 and their control vectors were designed and purchased
from Shanghai GenePharma Co., Ltd. (Shanghai, China), which were
employed to overexpress or knock down miR-96. pcDNA3.1-GPC3 and
siRNA-GPC3 plasmids (purchased from Shanghai GenePharma Co., Ltd.)
were utilized to overexpress or knock down GPC3.
A549 cells were seeded onto 6-well plates and when
the confluence was ~70%, the plasmids were transfected using
Lipofectamine 2000 and RNAiMAX (Invitrogen; Thermo Fisher
Scientific, Inc., Carlsbad, CA, USA) according to the
manufacturer's instructions. Plasmids or Lipofectamine 2000 were
diluted with Opti-MEM Reduced-Serum Medium and set aside. After
mixing the two dilutions, and allowing to stand for 20 min, the
dilutions were added onto the 6-well plates containing cells. After
transfection for 4–6 h, fresh RPMI-1640 medium was added.
Total RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
The total RNA was extracted using the RNAprep Pure
Tissue kit (Tiangen Biotech Co., Ltd., Beijing, China) according to
the manufacturer's instructions. The cDNA was synthesized using the
FastKing RT kit (with gDNase) (Tiangen Biotech Co., Ltd.). GAPDH
mRNA levels were used for the normalization of GPC3. The primer
sequences were as follows: miR-96 forward,
5′-GCCCGCTTTGGCACTAGCACATT-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′;
GPC3 forward, 5′-CAGACTCGAGCTGCCTGGTGCCCAGC-3′ and reverse,
5′-GAGAGGTACCCAAAGAAATCCATGCAAAGAG-3′; GAPDH forward,
5′-CCACTCCTCCACCTTTGAC-3′ and reverse, 5′-ACCCTGTTGCTGTAGCCA-3′;
and U6 forward, 5′-CTTCGGCAGCACATATACT-3′ and reverse,
5′-AAAATATGGAACGCTTCACG-3′. RT-PCR was performed using the Roche
LightCycler 480 instrument (Roche Diagnostics, Basel, Switzerland).
The U6 snRNA levels were used for the normalization of miR-96.
Luciferase reporter assay
TargetScan website (www.targetscan.org) was used to predict the target
gene of miR-96, GPC3 was identified to be a potential target
of miR-96 and the binding site of GPC3 for miR-96 was at the
3′-UTR. The 3′-UTR sequence of GPC3 was amplified and cloned
into the pGL3 vector (pGL3-GPC3-WT; WT). The binding site of
GPC3 for miR-96 was mutated and cloned into the pcDNA3.1 vector
(pGL3-GPC3-MT; MT). Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to perform the luciferase
activity assay.
Migration and invasion assays
The migration of lung cancer A549 cell lines was
tested using Transwell chamber (8 µm pore; Corning, Inc., Corning,
NY, USA). Approximately 1×106 cells were added into the
upper chamber and cell medium was added into the lower chamber.
Cells were incubated for 24 h at 37°C. Crystal violet was used to
stain the migrated cells, which were visualized using an inverted
microscope. The upper chamber was filled with Matrigel (BD
Biosciences, San Jose, CA, USA) for the invasion test.
Cell proliferation assay
The 3-(4,5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT) was employed to investigate
cell proliferation. Before the experiment, the cells were seeded
onto 96-well plates, cultivated with RPMI-1640 medium for 24 h to
adherence and then MTT solution was added and incubated for 4 h at
37°C. After the supernatants were removed, the formazan crystals
were dissolved using DMSO (200 µl/well). The absorbance at 490 nm
was tested using the Thermo Scientific Evolution 300 instrument
(Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Statistical analysis
SPSS 18.0 software was used for statistical
analysis. Student's t-test or ANOVA and Scheffe's test were
employed to carry out the comparison between means of two groups or
multiple groups. Results were considered significant at
P<0.05.
Results
miR-96 is significantly upregulated in
NSCLC, the opposite of GPC3
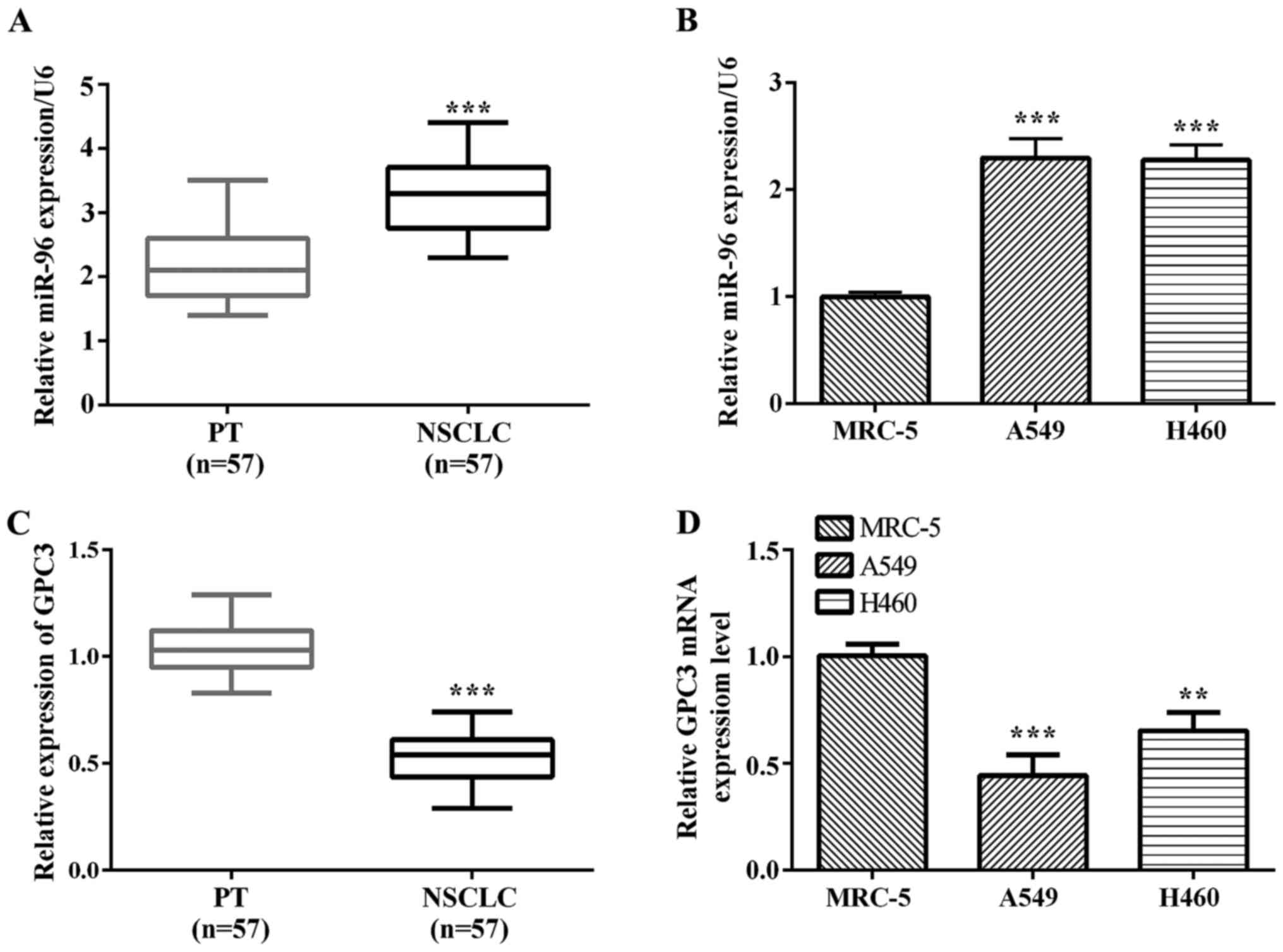
In this study, we investigated the miR-96 expression
level in tissues and cell lines by RT-qPCR. The results showed that
miR-96 was significantly upregulated in NSCLC tissues compared with
the corresponding paracancerous tissues (P<0.0001; Fig. 1A). We also found that miR-96 was
upregulated in A549 (P=0.0003) and H460 (P=0.0001) lung cancer cell
lines compared with normal lung epithelium MRC-5 cells (Fig. 1B). GPC3 mRNA expression was observed
in 53 cases of NSCLC samples and their matched normal paracancerous
tissues. We noted a trend of lower expression in NSCLC samples
compared to matched paracancerous tissues (Fig. 1C). Fig.
1D shows normal lung epithelium MRC-5 cells with the A549 and
H460 lung cancer cells, depicting decreased GPC3 mRNA in A549
(P=0.0009) and H460 (P=0.0039) lung cancer cells compared to
matched normal lung epithelium MRC-5 cells.
Overexpression of miR-96 promotes cell
migration and invasion in vitro
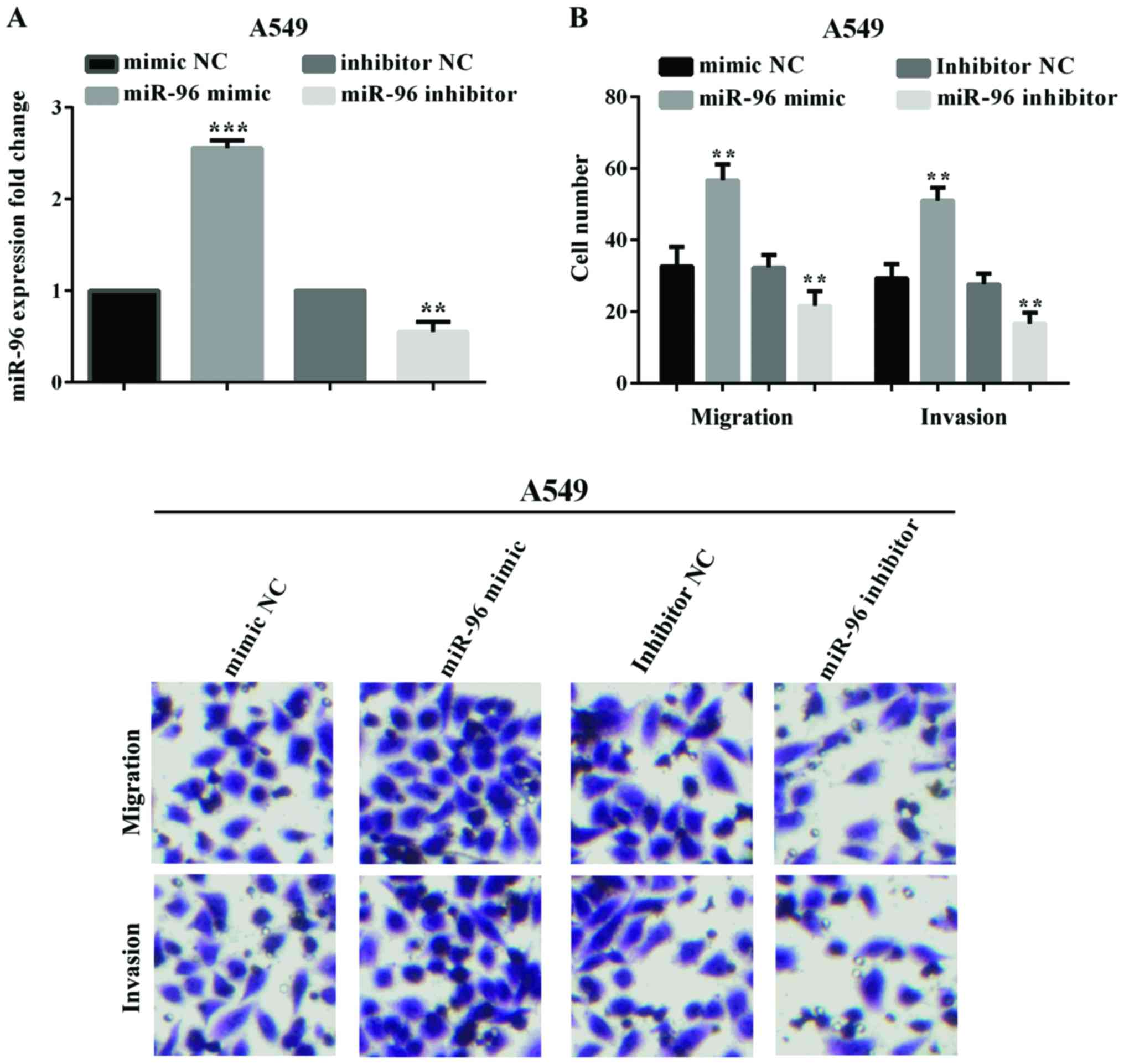
In order to investigate the influence of miR-96 on
cell migration and invasion, we transfected the miR-96 mimic, LNA
anti-miR-96 or negative control plasmid into A549 cell lines. The
effect of transfection of miR-96 mimic (P<0.0001) and
anti-miR-96 (P=0.0022) into the A549 cell line was confirmed by
RT-qPCR (Fig. 2A). The effect of
miR-96 on lung cancer cell migration and invasion was analyzed by
Transwell assay. The migratory and invasive ability of the
transfected cells was measured and compared with that of NC at 24,
48, 72 and 96 h post-transfection. As shown in Fig. 2B, upregulation of miR-96 significantly
promoted the migration (P=0.0043) and invasion (P=0.0023) of A549
cells, conversely, downregulation of miR-96 inhibited the cell
migration (P=0.0018) and invasion (P=0.0010).
GPC3 is a target of miR-96 and is
downregulated by miR-96
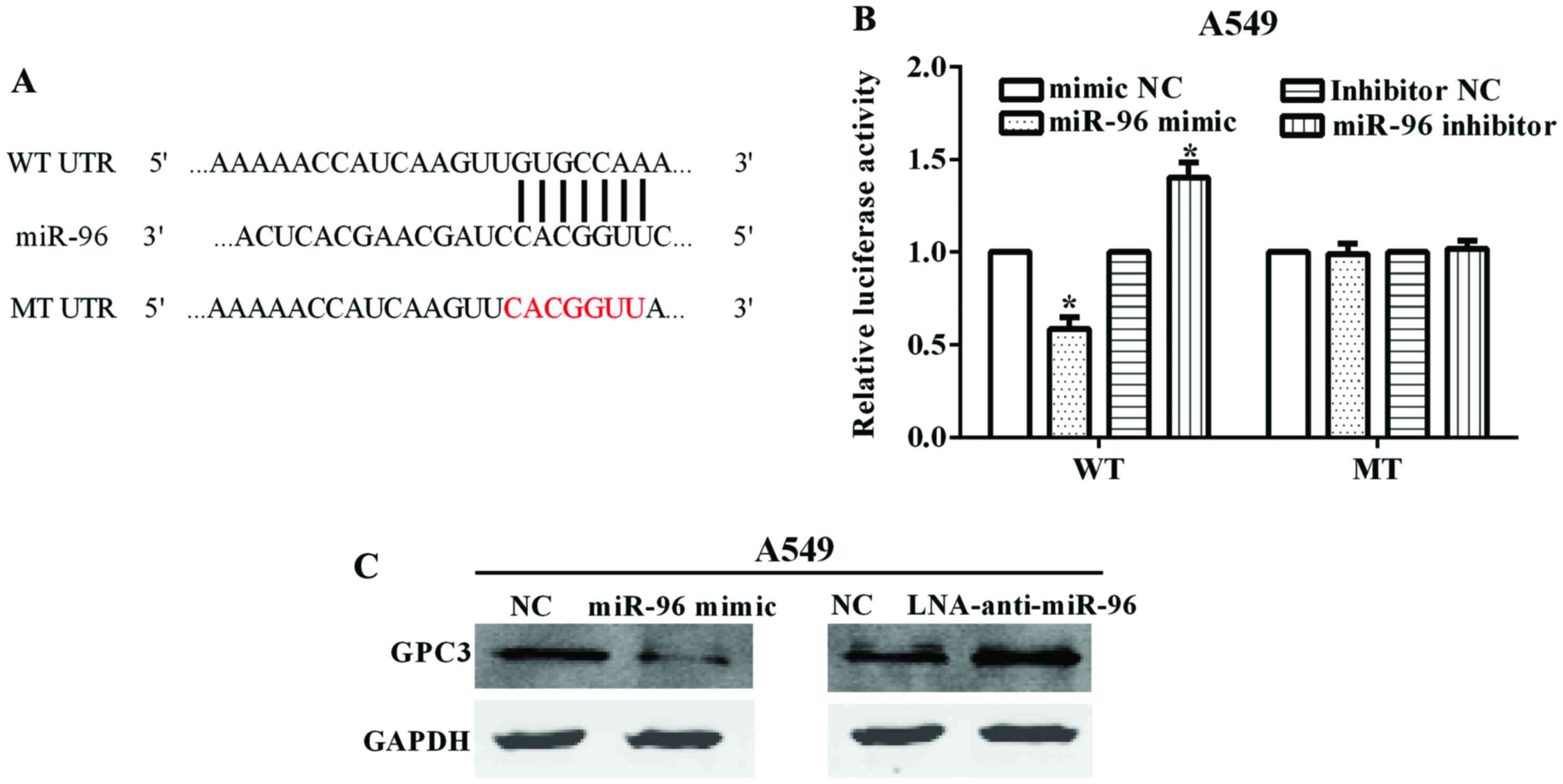
By the TargetScan online tool (www.targetscan.org/vert_71/), we identified
GPC3 as a potential target of miR-96. The binding site on
GPC3 of miR-96 locates at its 3′-UTR with binding site
5′-GUGCCAA-3′ (Fig. 3A). After
mutated the 3′-UTR from 5′-GUGCCAAA-3′ (WT) to 5′-CACGGAA-3′ (MT),
we constructed the Psicheck™-2-GPC3-WT and
Psicheck™-2-GPC3-MT plasmids. The luciferase reporter assay
demonstrated that the luciferase activity was significantly
inhibited when the A549 cell line was co-transfected with miR-96
mimic and Psicheck™-2-GPC3-WT (P=0.0116), while there was no
obvious change in transfection of miR-96 mimic and
Psicheck™-2-GPC3-MT (P=0.826) (Fig. 3B). By contrast, the luciferase
activity was increased when the cell lines were co-transfected with
miR-96 inhibitor and Psicheck™-2-GPC3-WT (P=0.0218), whereas
there was no obvious change in transfection of miR-96 inhibitor and
Psicheck™-2-GPC3-MT (P=0.574) (Fig. 3B). These results indicated that the
GPC3 was a target of miR-96.
We also investigated the GPC3 protein levels when
changed by the expression of miR-96. The results showed that GPC3
was significantly decreased when A549 cell line was transfected
with miR-96 mimic. By contrast, the GPC3 protein level was
increased when the A549 cell line was transfected with LNA
anti-miR-96 (Fig. 3C).
GPC3 reverses partial fuction of
miR-96 on proliferation
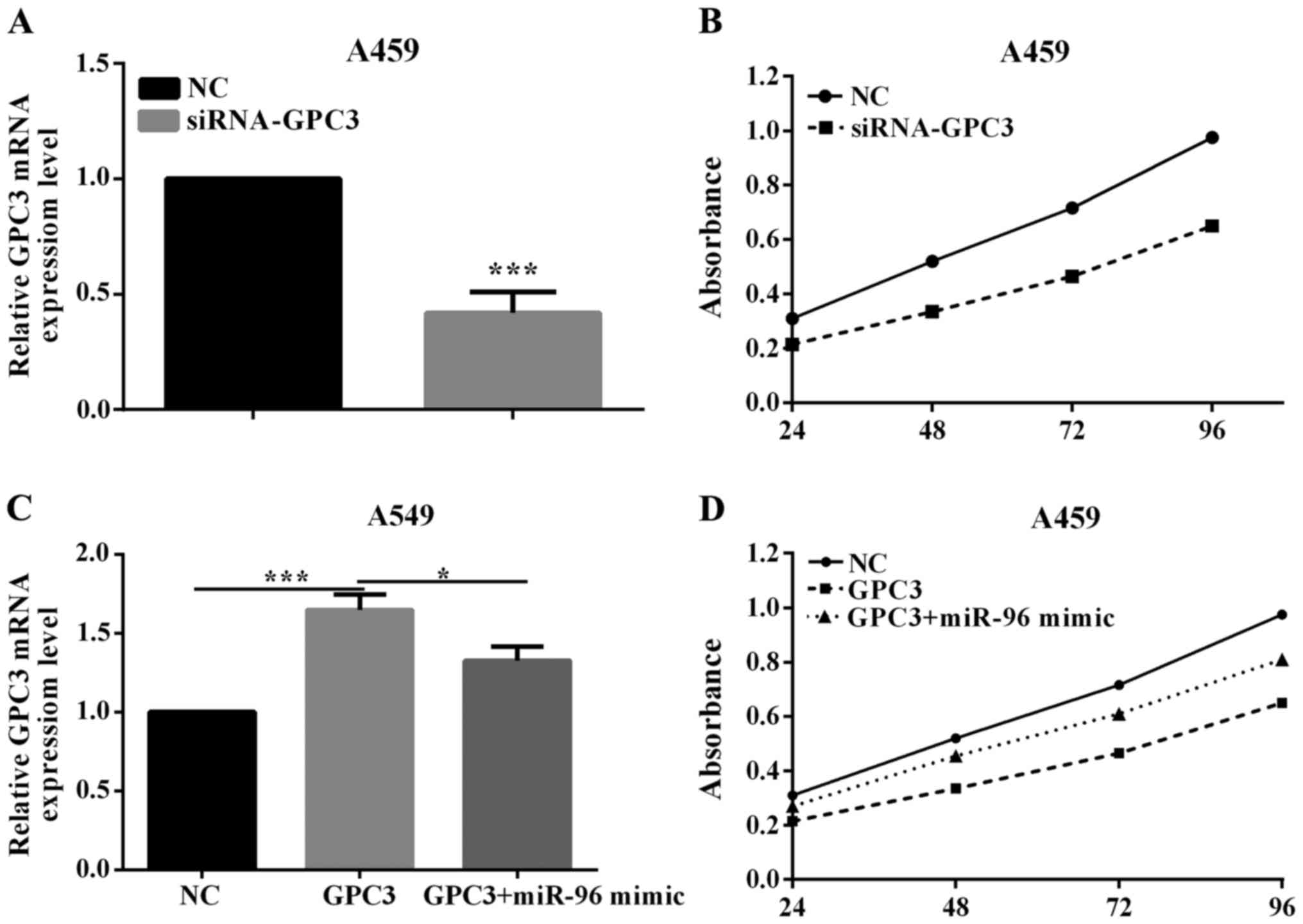
To investigate whether miR-96 may promote NSCLC
tumorigenesis by silencing GPC3, we assessed the role of PTPN9 on
cell proliferation. In order to knock down GPC3 gene, the
siRNA-GPC3 or negative control (siRNA-NC) was transfected
into A549 cell lines. The efficient knockdown of GPC3 mRNA in A549
cells (P=0.0048) is shown in Fig. 4A.
The proliferation assay demonstrated that knockdown of GPC3
could significantly promote the proliferation of A549 cell lines
(P=0.0352; Fig. 4B). This indicated
that GPC3 was a tumor suppressor in NSCLC.
To explore whether miR-96 regulation of cell
proliferation is executed in a GPC3-dependent manner, we
co-transfected A549 cells with miR-96 mimic and
pcDNA3.1-GPC3 plasmid. Compared with cells transfected with
pcDNA3.1-GPC3, the cells transfected with both miR-96 mimic
and pcDNA3.1-GPC3 exhibited a lower GPC3 mRNA level
(P=0.0003; Fig. 4C). Consequently,
the cells transfected with both miR-96 mimic and
pcDNA3.1-GPC3 exhibited an obviously lower proliferation
(P=0.0131; Fig. 4D), suggesting that
miR-96-resistant GPC3 can attenuate the proliferative effect of
GPC3 on lung cells. The results indicate that miR-96 may regulate
the proliferation of lung cancer cells in a GPC3-dependent
manner.
Discussion
Lung cancer is the leading cause of mortality in
men, and the second leading cause of mortality in women worldwide.
NSCLC is one of the most common histological subtypes of lung
cancer, which can be divided into three subtypes, which are large
cell carcinoma (LCC), lung adenocarcinoma (LAD), and lung squamous
cell carcinoma (LSCC) (23). Although
cancer treatments have improved in recent years, the outcomes of
patients with GC remain unsatisfactory. Thus, to find new and
effective therapeutic methods for treatment of NSCLC is
imperative.
miRNAs have been found to play an important role in
the development of diverse diseases, including cancer. As reported
in previous studies, several miRNAs play an important role in
tumorigenesis of human NSCLC due to their overexpression in tumor
tissues. miR-96 is a member of the miR-183 family, which
constituted a polycistronic paralogous miRNA cluster (13). miR-96 was found to be highly
upregulated in different kinds of tumors, including breast cancer
(14), and colorectal cancer
(15). Similarly, in our study,
miR-96 was found to be upregulated in 53 pairs of NSCLC tissues and
lung cancer cells. Upregulation of miR-96 could promote lung cancer
cell A549 migration and invasion, while downregulation suppressed
A549 cell migratory and invasive ability. In addition, GPC3 was
found to be downregulated in NSCLC tissues and lung cancer cells.
Hong et al reported that miR-96 promoted cell invasion,
migration, and proliferation in breast cancer (14). Considering these results, we strongly
believe that the impact of miR-96 on migration and invasion may be
through direct inhibition of GPC3.
GPC3 is an HSPG playing a pivotal role in the
regulation of heparin-binding growth factor and various
intracellular signaling pathways (24). The glypican proteins use a
glycosylphosphatidylinositol anchor to link to the cytoplasmic
membrane (25). GPC3 was
significantly downregulated in human malignant mesothelioma cell
lines and ectopic expression of GPC3 suppressed colony formation of
human MM cells (21). In accordance
with these reports, we identified that knockdown of GPC3 by siRNA
promoted proliferative ability in lung cancer A549 cell line. In
HCC, GPC3 is a transcriptor target of c-Myc and GPC3 can also
regulate the expression of c-Myc (26). In the present study, we found that
GPC3 was a direct target of miR-96 and regulated by miR-96.
Moreover, when GPC3 was overexpressed, the cell proliferative
ability increased. In co-transfection of GPC3 and miR-96 mimic,
proliferative ability was reduced compared with only transfection
with GPC3. Thus, we propose that miR-96 and GPC3 had a relationship
with NSCLN proliferation.
There still exist some disadvantages in our
research. For example, the number of patient samples was small. Our
research did not refer to the clinical experiments. In our further
study, we will perform the above experiments.
Taken together, we identified that miR-96 promoted
migration and invasion of lung cancer cells by targeting
GPC3. miR-96 functioned as an oncogene in NSCLC and GPC3
could reverse partial fuction of miR-96 on proliferation. The
identified miR-96/GPC3 axis may provide a meaningful therapeutic
method for the treatment of NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request
Authors' contributions
SL contributed to the conception of the study. XF
contributed significantly in performing the experiment and helped
to write the manuscript. JZ wrote the manuscript and helped to
perform the experiment. YZ performed the data analyses. MS and HZ
helped perform the analysis with constructive discussions. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All the samples were obtained with informed consent
of the patients and verified by the Ethics Committee of the First
Affiliated Hospital of Jiamusi University (Jiamusi, China).
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Pao W and Girard N: New driver mutations
in non-small-cell lung cancer. Lancet Oncol. 12:175–180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Islami F, Torre LA and Jemal A: Global
trends of lung cancer mortality and smoking prevalence. Transl Lung
Cancer Res. 4:327–338. 2015.PubMed/NCBI
|
|
4
|
Subramanian J and Govindan R: Lung cancer
in never smokers: A review. J Clin Oncol. 25:561–570. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Wang DC, Shi L, Zhu B, Min Z and
Jin J: Genome analyses identify the genetic modification of lung
cancer subtypes. Semin Cancer Biol. 42:20–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sittka A and Schmeck B: MicroRNAs in the
lung. Adv Exp Med Biol. 774:121–134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ke Y, Zhao W, Xiong J and Cao R:
Downregulation of miR-16 promotes growth and motility by targeting
HDGF in non-small cell lung cancer cells. FEBS Lett. 587:3153–3157.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han H, Yang J, Wang Y, Chen W, Chen J,
Yang Y and Li Q: Nucleobase-modified polyamidoamine-mediated
miR-23b delivery to inhibit the proliferation and migration of lung
cancer. Biomater Sci. 5:2268–2275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma Q, Jiang Q, Pu Q, Zhang X, Yang W, Wang
Y, Ye S, Wu S, Zhong G, Ren J, et al: MicroRNA-143 inhibits
migration and invasion of human non-small-cell lung cancer and its
relative mechanism. Int J Biol Sci. 9:680–692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu S, Witmer PD, Lumayag S, Kovacs B and
Valle D: MicroRNA (miRNA) transcriptome of mouse retina and
identification of a sensory organ-specific miRNA cluster. J Biol
Chem. 282:25053–25066. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hong Y, Liang H, Uzair-Ur-Rehman, Wang Y,
Zhang W, Zhou Y, Chen S, Yu M, Cui S, Liu M, et al: miR-96 promotes
cell proliferation, migration and invasion by targeting PTPN9 in
breast cancer. Sci Rep. 6:374212016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ress AL, Stiegelbauer V, Winter E,
Schwarzenbacher D, Kiesslich T, Lax S, Jahn S, Deutsch A,
Bauernhofer T, Ling H, et al: MiR-96-5p influences cellular growth
and is associated with poor survival in colorectal cancer patients.
Mol Carcinog. 54:1442–1450. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu L, Pu X, Wang Q, Cao J, Xu F, Xu LI and
Li K: miR-96 induces cisplatin chemoresistance in non-small cell
lung cancer cells by downregulating SAMD9. Oncol Lett. 11:945–952.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo H, Li Q, Li W, Zheng T, Zhao S and Liu
Z: MiR-96 downregulates RECK to promote growth and motility of
non-small cell lung cancer cells. Mol Cell Biochem. 390:155–160.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jalvy-Delvaille S, Maurel M, Majo V,
Pierre N, Chabas S, Combe C, Rosenbaum J, Sagliocco F and Grosset
CF: Molecular basis of differential target regulation by miR-96 and
miR-182: The Glypican-3 as a model. Nucleic Acids Res.
40:1356–1365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai Z, Grobe K and Zhang X: Role of
heparan sulfate proteoglycans in optic disc and stalk
morphogenesis. Dev Dyn. 243:1310–1316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bernfield M, Götte M, Park PW, Reizes O,
Fitzgerald ML, Lincecum J and Zako M: Functions of cell surface
heparan sulfate proteoglycans. Annu Rev Biochem. 68:729–777. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murthy SS, Shen T, De Rienzo A, Lee WC,
Ferriola PC, Jhanwar SC, Mossman BT, Filmus J and Testa JR:
Expression of GPC3, an X-linked recessive overgrowth gene, is
silenced in malignant mesothelioma. Oncogene. 19:410–416. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
White GR, Kelsey AM, Varley JM and Birch
JM: Somatic glypican 3 (GPC3) mutations in Wilms' tumour. Br J
Cancer. 86:1920–1922. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Filmus J: Glypicans in growth control and
cancer. Glycobiology. 11:19R–23R. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ho M and Kim H: Glypican-3: A new target
for cancer immunotherapy. Eur J Cancer. 47:333–338. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li L, Jin R, Zhang X, Lv F, Liu L, Liu D,
Liu K, Li N and Chen D: Oncogenic activation of glypican-3 by c-Myc
in human hepatocellular carcinoma. Hepatology. 56:1380–1390. 2012.
View Article : Google Scholar : PubMed/NCBI
|