Introduction
Salivary duct carcinoma (SDC) arises from the ductal
epithelium of the salivary gland and comprises rare tumors that
account for approximately 1–3% of all salivary gland malignancies
(1). SDC was first described by
Kleinsasser et al in 1968 owing to its histologic similarity
to invasive ductal carcinoma (IDC) (2). SDC constitutes one of the most
aggressive salivary gland malignancies and is resistant to
radiation therapy and chemotherapy (1,3). Although
extended resection and postoperative irradiation are performed as
standard treatments, the therapeutic outcome is not generally
improved (1,4). Considering the similarities with ductal
carcinoma of the breast and prostate cancer, overexpression of
androgen receptor (AR), epidermal growth factor receptor (EGFR),
and human epidermal growth factor receptor 2 (HER2) has also been
investigated in SDC (1,5–8). HER2
expression serves as a predictive factor in IDC as well (9); moreover, HER2 protein in IDC constitutes
the most important target for molecular targeted therapy.
Previously, rates of amplification of the HER2 gene and HER2
protein overexpression in SDC were reported to range widely from 15
to 100% (3,10,11).
Recently, androgen and/or estrogen deprivation therapy (12,13) and
molecular targeted therapy for HER2 have been attempted as adjuvant
therapies (14–17) with anti-HER2 therapy in particular
expected to become a useful tool for adjuvant therapy (15,17);
however, satisfactory results have not been obtained (16). Thus, additional novel therapeutic
strategies are required for SDC.
DNA methylation, i.e., the modification of cytosine
to form 5-methylcytosine, is essential for normal development but
is also associated with carcinogenesis. In many cases, suppression
of tumor suppressor genes by DNA hypermethylation of the promoter
region can induce carcinogenesis. Thus, elucidation of the DNA
methylation profile in SDC might facilitate the development of
novel therapeutic strategies for SDC.
Our previous studies demonstrated that DNA
methylation of several G-protein coupled receptors (GPCRs) was
associated with the survival rate of patients with head and neck
squamous cell (HNSCC) (18). The
galanin receptors, GALR1 and GALR2, are members of the GPCR
superfamily, and serve as important tumor suppressor genes for
HNSCC (19–21). Specifically, GALR1 mediates cell cycle
arrest (19) whereas GALR2 mediates
both cell cycle arrest and apoptosis (20) via common pathways including
p27kip1, p57kip2, and cyclin D1 (22). DNA methylation of GALR1 and
GALR2 promoters was significantly associated with the
survival and recurrence rates of patients with HNSCC and is
considered as a potential therapeutic target and prognostic factor
for HNSCC (23–25). GALR promoter methylation is observed
in other squamous cell carcinomas as well as adenocarcinomas such
as breast, colon, and hepatocellular carcinoma (26,27), and
thus appears to constitute a carcinoma type-independent prognostic
factor. The aim of the present study was therefore to first define
the GALR1, GALR2, and galanin methylation status in SDCs at
the time of diagnosis and then to evaluate its significance as a
biomarker for prognosis.
Patients and methods
Patient characteristics
Tumor specimens were obtained from 34 patients
diagnosed with SDC based on histological findings at the Department
of Otolaryngology-Head and Neck Surgery, Jichi Medical University,
School of Medicine, the Department of Otolaryngology-Head and Neck
Surgery, Hamamatsu University, School of Medicine, and the Division
of Head and Neck, Cancer Institute Hospital, Japanese Foundation of
Cancer Research, from March 1995 to March 2012. The present study
was approved by the Institutional Ethics Review Board of the ethics
committee of each of the three institutions that participated in
this study. The need to obtain informed consent was waived owing to
the retrospective nature of the analysis. In this study, we
analyzed only cases of de novo SDC; SDC ex pleomorphic adenomas
were excluded. Patient characteristics were also reviewed with
regard to sex, age, TNM classification, clinical stage, surgical
procedures, and additional adjuvant therapy.
Immunochemical analysis
The tissues were fixed in 10% formalin and embedded
in paraffin in a routine manner, and stained with hematoxylin and
eosin. All cases were histologically reviewed according to the
definition of SDC. Briefly, SDC showed a cribriform growth pattern,
Roman bridge formation, and comedonecrosis of tumor cells having
abundantly eosinophilic cytoplasm and a large pleomorphic nucleus
with prominent nucleoli and coarse chromatin. Immunohistochemistry
was performed on 4-µm sections from paraffin blocks using
antibodies directed against androgen receptor (AR) (mouse
monoclonal antibody clone AR441, Dako Corporation, Glostrup,
Denmark), estrogen receptor (ER) (clone 6F11, Leica Biosystems,
Nussloch, Germany), HER2 (rabbit polyclonal, HercepTest, Dako),
EGFR (clone 31G7, Nichirei Biosciences Inc., Tokyo, Japan),
p27Kip1 (clone Y236, GeneTex, Irvine, CA, USA),
p57Kip2 (clone: DO-7, Dako), and cyclin D1 (clone: SP4,
Thermo Scientific, Waltham, MA, USA). The results of
immunohistochemical staining were independently scored by two of
the authors (TK and YS). AR positively was evaluated in a manner
similar to ER according to the American Society of Clinical
Oncology/College of American Pathologist guideline (28) for evaluation of breast cancer
predictive factors: if ≥1% of tumor cell nuclei are immunoreactive,
the tumor was considered to be positive for AR. The evaluation of
HER2 expression was in accordance to the criteria for evaluating
responsiveness of breast carcinoma to anti-HER2 treatment, with a
score of 0–2 being considered as HER2 negative and a score of 3 was
considered as HER2 positive. For EGFR, according to the criteria
for evaluating responsiveness of colorectal carcinoma to anti-EGFR
treatment, a score of 0–2 was considered as EGFR negative and a
score of 3 was considered as EGFR positive. p27 scoring was
determined by the criteria of ovarian carcinoma: 1+ <5%, 2+
5–50%, 3+ >50%; p57 was in accordance to vulva carcinoma
criteria: 1+ <10%, 2+ 10–50%, 3+ >50%; and cyclin D1 was
scored according to breast carcinoma criteria (−<10%, +
≥10%).
DNA promoter methylation analysis
Genomic DNA was extracted from 8-µm sections of
paraffin blocks using the QIAamp DNA FFPE Tissue Kit (Qiagen,
Venlo, The Netherlands). Extracted DNA was bisulfite-modified using
the MethylEasy™ Xceed Rapid DNA Bisulphite Modification Kit
(TaKaRa Bio., Tokyo, Japan). Methylation in the region near the
transcription start site was assessed using bisulfite-treated DNA
polymerase chain reaction (PCR) amplified with methylation-specific
PCR primers (MSP) and unmethylation-specific PCR primers (UMSP)
using FastStart Taq DNA polymerase (Roche Lifescience Inc., Basel,
Switzerland). The primers are shown in Table I. The PCR conditions were 94°C for 5
min; optimal cycle numbers between 35 and 45 at 94°C for 30 sec,
60°C for 30 sec, and 72°C for 40 sec; and a final extension at 94°C
for 5 min. The PCR products were separated by 3% agarose gel
electrophoresis and stained with ethidium bromide. The PCR products
amplified by MSP or UMSP were visualized and quantified using Image
J software (http://imagej.nih.gov/ij/), and the
ratio of MSP/UMSP was defined as the methylation rate. Receiver
operating characteristic (ROC) curve analysis was performed using
the methylation rate for 34 SDC and 19 adjacent normal parotid
gland tissues. The cutoff value determined from this ROC curve was
applied to determine the frequently of GALR1, GALR2, and
galanin methylation in this study.
 | Table I.Sequences of primers used in this
study. |
Table I.
Sequences of primers used in this
study.
| Gene |
Methylation-specific primer sequence
(5′-3′) |
Unmethylation-specific primer sequence
(5′-3′) |
|---|
| Galanin | Forward:
TGACGCGATTTCGGGCGGTT | Forward:
TGATGTGATTTTGGGTGGTT |
|
| Reverse:
TATCCGCCGCCCGATATAAC | Reverse:
TATCCACCACCCAATATAAC |
| GALR1 | Forward:
GGTTCGCGGTATTCGGTAGT | Forward:
GGTTTGTGGTATTTGGTAGT |
|
| Reverse:
TCGCCGCCCACCTCCCGACTA A | Reverse:
TCACCACCCACCTCCCAACTAA |
| GALR2 | Forward:
CGATTGCGGGGGTTGGAGTTCGGA | Forward:
CCAACAACGACCGACGACGCTA |
|
| Reverse:
TGATTGTGGGGGTTGGAGTTTGGA | Reverse:
TTATCCCCAACAACAACCAACAACACTA |
Statistical analysis
For frequency analysis in contingency tables,
statistical analyses of association between variables were
performed using Fisher's exact test. To evaluate the galanin and
GALR pathway in SDC, the Pearson's correlation coefficients between
the methylation rate and expression score of p27, p57, and cyclin
D1 were calculated. Furthermore, the survival interval was
estimated as the length of time from the start of treatment to the
final date of confirmed survival. Overall survival (OS)
probabilities were estimated using the Kaplan-Meier method and the
log-rank test was applied to assess the significance of differences
among actuarial survival curves.
Results
Patient characteristics
Table II summarizes
the characteristics of the 34 patients with SDC evaluated in this
study. Men were predominant (20 cases, 58.8%) compared to women (14
cases, 41.2%). Median age was 63.4 years old (range, 45–79 years),
and median follow-up time was 32.3 months (range, 5–59 months).
Regarding tumor and nodal stage, T2, T4a, N0, and N2 were
predominant. Over half of cases (55.9%) were classified as Stage
IV. Surgery was performed for all cases with partial parotidectomy
in 7 cases (20.6%), total parotidectomy in 16 cases (47.1%), and
extended parotidectomy in 11 cases (32.4%). Postoperative
irradiation was applied for 28 cases (82.4%), whereas no cases
received preoperative irradiation.
 | Table II.Characteristics of patients with
salivary duct carcinoma of the parotid gland. |
Table II.
Characteristics of patients with
salivary duct carcinoma of the parotid gland.
|
Characteristics | No. (%) |
|---|
| Sex |
|
|
Male | 20 (58.8) |
|
Female | 14 (41.2) |
| Age |
|
|
Mean | 63.4 |
|
Range | 45–79 |
| Pathological T
classification |
|
| T1 | 3
(0.09) |
| T2 | 10 (24.9) |
| T3 | 7
(20.6) |
|
T4a | 14 (41.2) |
| Pathological N
classification |
|
| N0 | 11 (32.3) |
| N1 | 7
(20.6) |
| N2 | 16 (47.1) |
| Tumor stage |
|
| Stage
I | 3 (8.8) |
| Stage
II | 6
(17.6) |
| Stage
III | 6
(17.6) |
| Stage
IV | 19 (55.9) |
| Surgical
procedure |
|
| Partial
parotidectomy | 7
(20.6) |
| Total
parotidectomy | 16 (47.1) |
|
Extended parotidectomy | 11 (32.4) |
| Postoperative
irradiation |
|
|
Negative | 6
(17.6) |
|
Positive | 28 (82.5) |
Clinicopathological factors associated
with OS
The median OS was 37.2 months. The results of
univariate Kaplan-Meier survival analyses are summarized in
Table III. Increasing T stage, N
stage, tumor stage, tumor size, preoperative facial paralysis, and
resection margin status were negative prognostic factors for OS.
Tumors in T3-T4 stage were associated significantly worse OS than
those in T1-T2 stage. N2-N3 stage tumors had significantly worse OS
than N0-N1 stage tumors. Stage IV tumors had significantly worse OS
compared to Stage I–III tumors. Tumors over 30-mm diameter had
significantly worse OS than those less than 30-mm. Tumors with
preoperative facial paralysis had significantly worse OS than those
without paralysis. Tumors with a positive surgical margin had
significantly worse OS than negative tumors. Other factors such as
lymphovascular invasion and extra-nodal spread did not affect the
length of OS. Contrary to prior findings (15,16), there
was no association between HER2 positively and survival. Other
immunochemical factors such as EFGR, AR, and ER were also not
associated with survival. p27kip1, p57kip2,
and cyclin D1 are encoded by cell cycle associated genes, the
expression of which is controlled by GALR signaling in HNSCC
(19,20). Although cyclin D1 overexpression was
associated with the length of OS, p27kip1 and
p57kip2 expression did not affect OS.
 | Table III.Univariate analysis of
clinicopathological factors associated with overall survival. |
Table III.
Univariate analysis of
clinicopathological factors associated with overall survival.
| Variable | 4-year OS (%) | P-value |
|---|
| T stage |
|
0.00803a |
| T1-2
(n=20) | 65.7 |
|
| T3-4
(n=14) | 20.6 |
|
| N stage |
|
0.00098a |
| N0-1
(n=18) | 74.2 |
|
| T3-4
(n=16) | 11.8 |
|
| Disease stage |
| 6.1E-0.5 |
| Stage
I–III (n=14) | 90.9 |
|
| Stage
IV (n=19) |
9.4 |
|
| Tumor size |
|
0.00089a |
| <30
mm (n=20) | 68.4 |
|
| >30
mm (n=14) | 19.8 |
|
| Preoperative facial
paralysis |
|
0.00635a |
|
Negative (n=21) | 57.9 |
|
|
Positive (n=7) | 14.3 |
|
| Resection
margin |
|
0.00550a |
|
Negative (n=22) | 67.5 |
|
|
Positive (n=9) | 0 |
|
| Lymphovascular
invasion |
| 0.06100 |
|
Negative (n=8) | 77.3 |
|
|
Positive (n=20) | 54.6 |
|
| Extra-nodal
spread |
| 0.23000 |
|
Negative (n=18) | 54.3 |
|
|
Positive (n=13) |
41.04 |
|
| EGFR |
| 0.40320 |
| 0-2
(n=17) | 52.2 |
|
| 3
(n=17) | 44.9 |
|
| HER2 |
| 0.05100 |
| 0-2
(n=16) | 58.3 |
|
| 3
(n=18) | 29.6 |
|
| Androgen
receptor |
| 0.15900 |
|
Negative (n=12) | 62.3 |
|
|
Positive (n=22) | 39.0 |
|
| Estrogen
receptor |
| 0.05640 |
|
Negative (n=28) | 56.5 |
|
|
Positive (n=6) | 33.3 |
|
| p27 |
| 0.18465 |
| 1–2
(n=16) | 24.4 |
|
| 3
(n=18) | 60.0 |
|
| p57 |
| 0.28940 |
| 1–2
(n=25) |
40.99 |
|
| 3
(n=9) | 63.5 |
|
| Cyclin D1 |
|
0.03410b |
| 0
(n=25) | 57.4 |
|
| 1
(n=9) | 17.7 |
|
Promoter methylation of GALR1, GALR2,
and galanin
To investigate whether GALR1, GALR2, and
galanin were methylated in SDC, the methylation level of these
genes in tumor and normal tissue were compared. GALR1,
GALR2, and galanin promoter hypermethylation exhibited highly
discriminative ROC curve profiles, which clearly distinguished
HNSCC from normal mucosal tissues (23,24,29). The
ROC curve with corresponding area under the curve for GALR1,
GALR2, and galanin of SDC vs. normal mucosal tissues is
presented in Fig. 1. The methylation
rates of GALR1 in tumor tissues were significantly higher
(9.85-fold) than those in normal tissues (Fig. 1A). The cutoff methylation rate (0.2)
for GALR1 was chosen from the ROC curve to maximize
sensitivity (70.6%) and specificity (78.9%) (Fig. 1D). The cutoff methylation rate (0.34)
for GALR2 in tumor tissues was also significantly higher
(4.49-fold) than that in normal tissues (Fig. 1B). GALR2 methylation rates
yielded sensitivity (67.6%) and specificity (78.9%) (Fig. 1E). However, the cutoff methylation
rate of galanin was not determined because no significant
difference of methylation rate was observed between SDC and normal
tissue (Fig. 1C and F). According to
the cutoff values for GALR1 and GALR2, the tumors
were divided into methylated and unmethylated tumors.
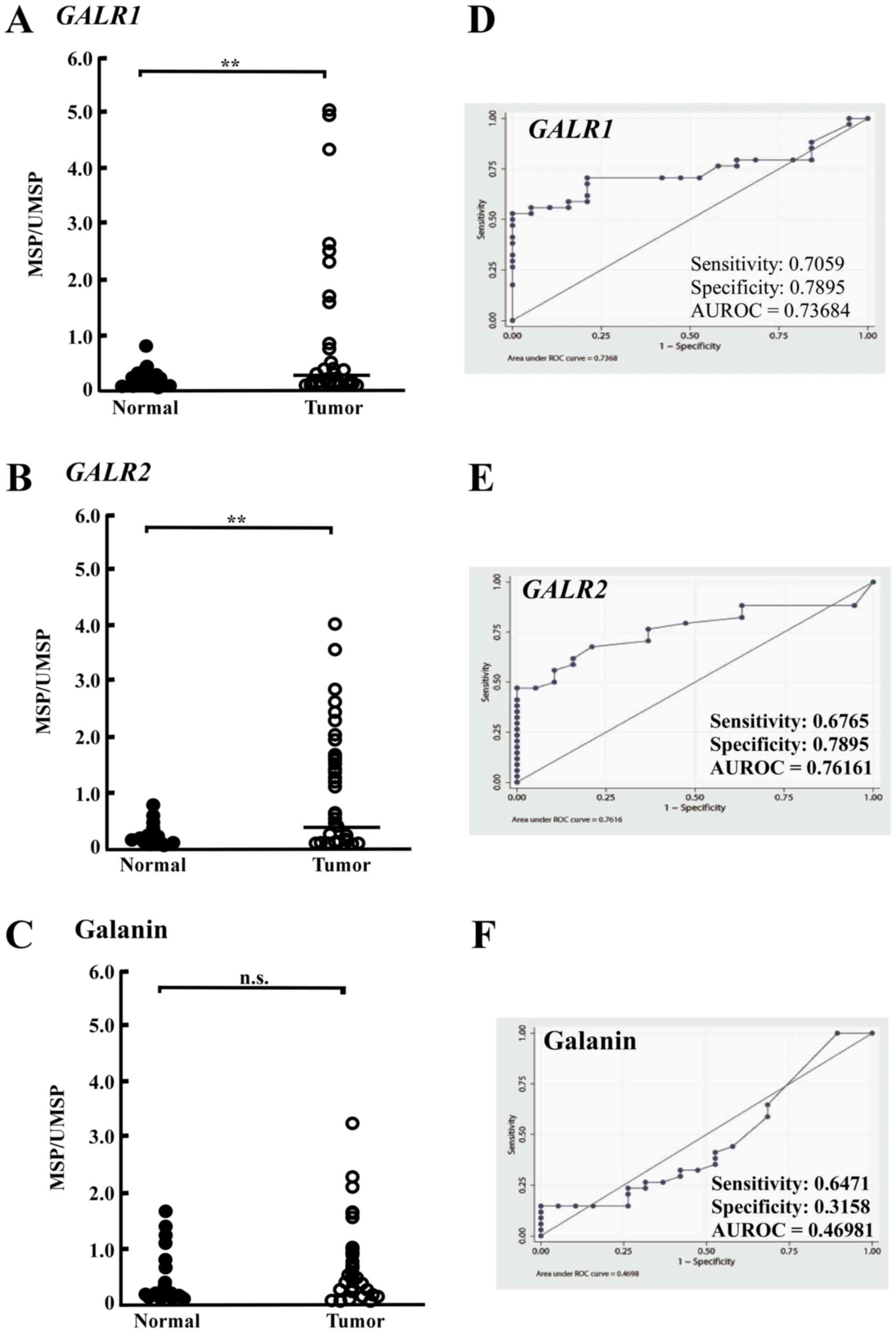 | Figure 1.GALR1, GALR2 and galanin
methylation analysis using quantitative methylation-specific PCR
(MSP) assay in SDC samples. Pattern of (A) GALR1, (B)
GALR2 and (C) galanin hypermethylation, respectively,
observed in matched pairs of salivary gland carcinoma and adjacent
normal mucosal tissues. ROC curve analysis in (D) GALR1, (E)
GALR2 and (F) galanin, respectively. AUROC indicates area
under the ROC curve. Asterisks mean significant differences
(**P<0.01). n.s. means no significant difference. SDC, salivary
duct carcinoma; PCR, polymerase chain reaction; MSP,
methylation-specific PCR primers; ROC, receiver operator
characteristics; GALR, galanin and galanin receptor. |
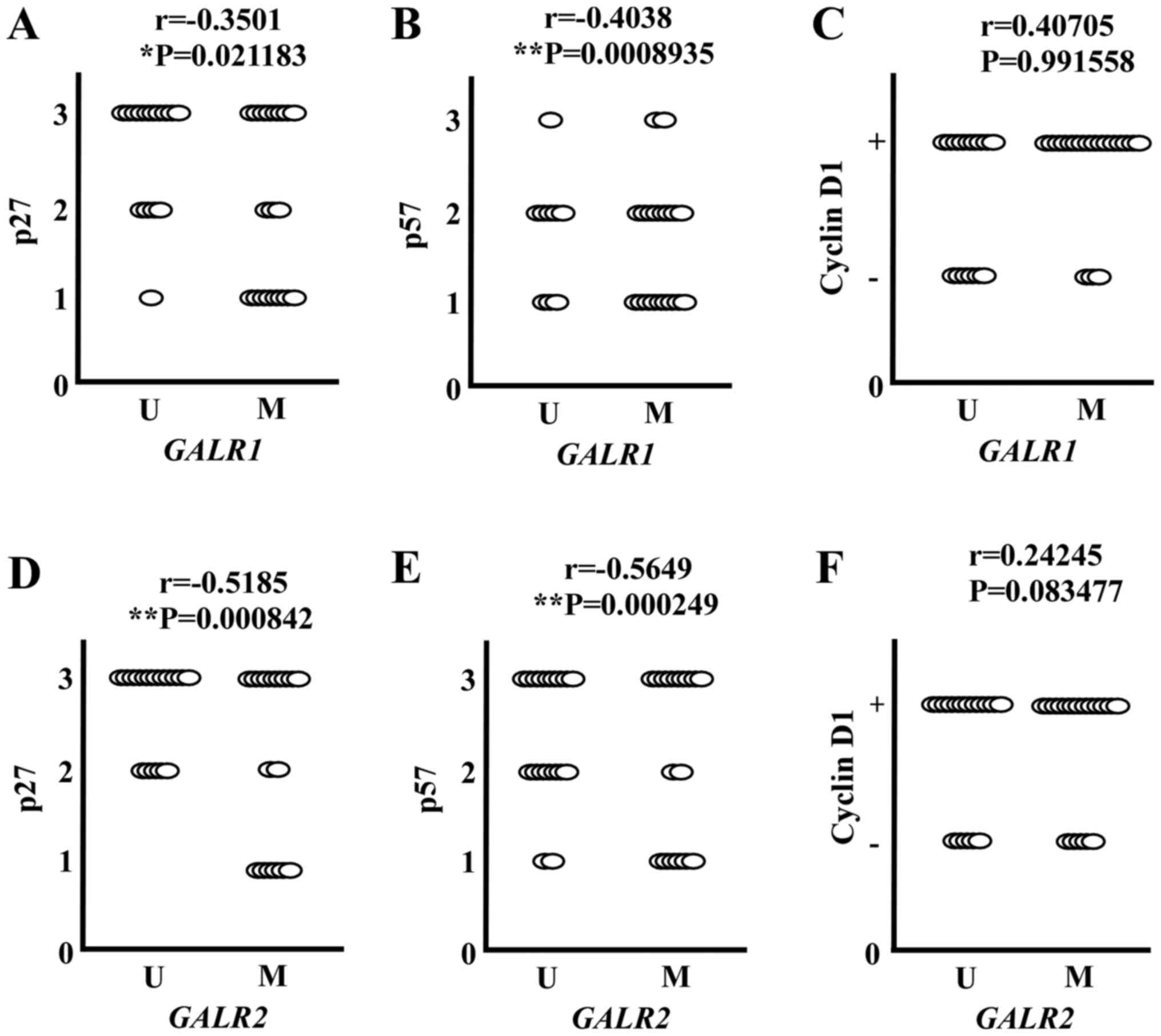
Correlation between GALR methylation
and expression of downstream proteins
Both GALR1 and GALR2 induced cell cycle arrest
though up-regulation of p27kip1 and p57kip2,
and down-regulation of cyclin D1 in HNSCC (19,20). To
confirm whether this pathway exists in SDC, the correlation between
GALR methylation and expression of these proteins was
evaluated. As shown in Fig. 2,
GALR1 methylation showed a significant inverse association
with p27kip1 and p57kip2. The
p27kip1 or p57kip2 lower expressing tumors
were more often observed among GALR1 methylated tumors than
unmethylated tumors (Fig. 2A and B).
Similarly, GALR2 methylation was also significantly
inversely associated with p27kip1 and
p57kip2. p27kip1 or p57kip2 higher
expressing tumors were more often observed among GALR2
unmethylated tumors than methylated tumors (Fig. 2D and E). However, a significant
correlation between cyclin D1 expression and GALR
methylation was not observed (Fig. 2C and
F). These results indicate that GALR1 and GALR2 signaling
pathways likely act as tumor suppressors in SDC.
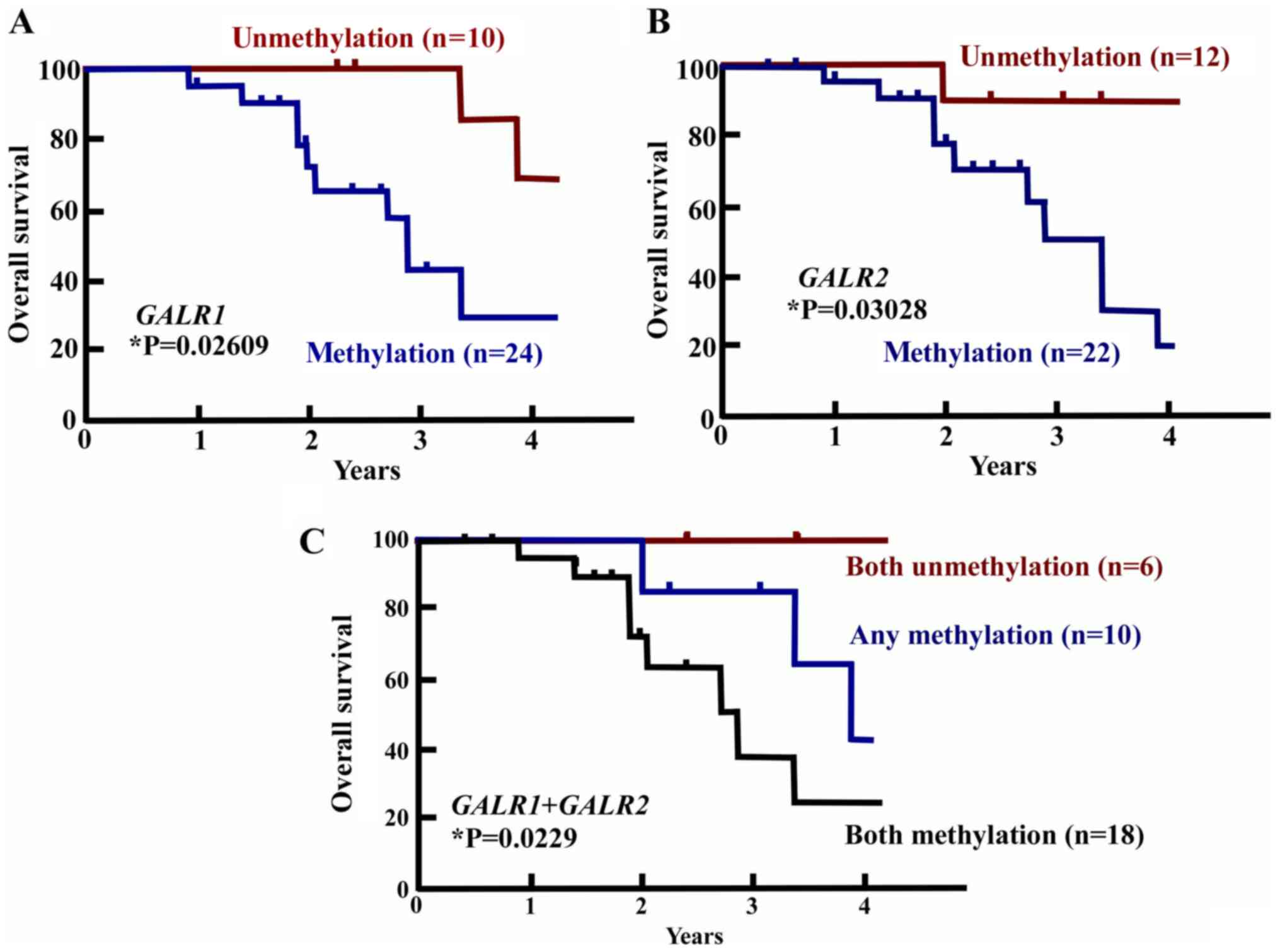
Prognostic value of GALR1 and/or GALR2
promoter methylation status
To examine the prognostic value of GALR1
and/or GALR2 promoter methylation status, the OS of
methylated and unmethylated tumors were compared. GALR1
methylation was associated with a statistically significant
decrease in OS (log-rank test, P=0.02609) (Fig. 3A). The OS of GALR1 methylated
tumors was 27.5% and of unmethylated tumors was 67.5% at 4 years.
Methylation of GALR2 was also significantly associated with
OS: The OS of GALR2 methylated tumors at 4 years was 21.2%
and that of unmethylated tumors was 96.2%. GALR2 methylation
was thus significantly associated with OS decrease (log-rank test,
P=0.03028) (Fig. 3B). Methylation in
both GALR1 and GALR2 was associated with an OS rate
of 22.2%, as compared with an OS rate of 42.1% for any methylation
and 100% for unmethylation of both GALR1 and GALR2
(log-rank test, P=0.0229) (Fig. 3C).
These results indicate that GALR1 and GALR2
methylation status would be sufficient to determine the prognosis
for SDC.
Discussion
Limited knowledge is available regarding SDC, a rare
tumor arising mainly from the salivary gland. A large study by
Jayaprakash et al described that negative factors for SDC
comprised age 50 years or older, tumor size, and lymph node
involvement, with no apparent survival benefit of radiation therapy
(30). In the present study, age and
gender did not affect the survival rate and were not prognostic
factors. Conversely, clinocopathological factors were important
prognostic factors in SDC, similar to other carcinomas. T stage, N
stage, disease stage, tumor size, preoperative facial paralysis,
and positive resection margin significantly decreased the survival
rate. These results provide important information for therapeutic
selection, suggesting that extended surgery should be chosen for
locoregional advanced cases. As facial nerve paralysis was observed
in 7 of 34 cases, the local invasive potential of SDC appears very
aggressive. However, the surgical margin is limited by anatomical
necessity, as the site is close to the skull base, cervical
vertebra, and carotid artery. Thus, effective adjuvant therapies
are required.
Alternatively, genetic alterations in SDCs have been
reported, leading to the investigation of HER2, EGFR, ER, and AR as
therapeutic targets and prognostic factors (1,4–8). In the present study, 61.7% of cases
expressed a high level EGFR (3+), 52.9% expressed a high level HER2
(3+), 5.9% expressed ER, and 64.7% of cases expressed AR. However,
although the expression of these proteins was also observed in this
study, significant correlations to survival rates were not
observed. In comparison, HER2 positively is considered to be a
predictor of poor prognosis in breast cancer, wherein the
determination of HER2 status is reported to be crucial to select
patients who may benefit from HER2-targeted therapy. Based on
previous results, HER2-targeted therapy may therefore not have
received sufficient evaluation as a standard therapy in SDC
(16). In SDCs, however, although
Jaehne et al (3) reported that
HER2 overexpression was linked to poor survival in their analysis
of 50 cases, it remains unclear whether HER2 gene amplification
and/or protein overexpression are predictors of poor prognosis in
carcinomas other than breast cancer. In particular, a recent report
indicates that HER2 is not a prognostic factor in SDC (1). Thus, molecular targeted therapies based
on the reported genetic alterations require further
investigation.
To develop novel therapeutic strategies for HNSCC,
we have previously investigated the epigenetic silencing of tumor
suppressor genes, with the most promising tumor suppressor genes
being GALR1 and GALR2 (19,20). The
effects of GALR1 and GALR2 are clearly reflected in clinical
outcome (23,24,29). Our
previous experiments using HNSCC cell lines demonstrated that
GALR1 and GALR2 promoter methylation is significantly
correlated with a decrease of the respective mRNA expression
(23). GALR1 promoter
methylation was significantly correlated with reduced survival
rates, tumor stage, lymph-node status, increased tumor size, cyclin
D1 expression, and p16 methylation (23). However, in multivariate analysis, only
GALR1 methylation and tumor stage were significant
predictors of poor survival (23,31).
GALR2 promoter methylation was significantly related to
methylation of COL1A, H-cadherin, DAPK, GALR1, and
galanin (24). GALR2 promoter
methylation was also related to a significant decrease in disease
free survival. Specifically, in a multivariate logistic regression
analysis, GALR2 promoter methylation in the primary tumor
was related to an adjusted odds ratio for recurrence of 3.12
(24,31).
Based on these results, we investigated the promoter
methylation status of galanin, GALR1, and GALR2 in
SDC to confirm the value as prognostic biomarkers in this disorder.
The methylation rates of GALR1 in SDC tumor tissues were
significantly higher (10.31-fold) than those in normal tissues.
GALR2 promoter methylation in tumor tissues was also
significantly higher (4.51-fold) than that in normal tissues.
GALR1 methylation further showed a significant inverse
association with p27kip1 and p57kip2.
p27kip1 or p57kip2 lower expressing tumors
were more often observed among GALR1 methylated tumors than
unmethylated tumors. Similarly, GALR2 methylation was
significantly inversely associated with p27kip1 and
p57kip2. p27kip1 or p57kip2 higher
expression tumors were more often observed among GALR2 unmethylated
tumors than in methylated tumors. These results suggested that
GALR1 and GALR2 pathways likely exist in SDC and that their
methylation states may constitute potential prognostic biomarkers.
Furthermore, GALR1 methylation was associated with a
statistically significant decrease in OS: 38.8% for GALR1
methylated tumors vs. 68.2% for unmethylated tumors. Methylation of
GALR2 was also associated with OS, with the OS of
GALR2 methylated tumors being 21.2% and compared to 96.2%
for unmethylated tumors. GALR2 methylation thus was
associated with significantly decreased OS. Methylation in both
GALR1 and GALR2 was associated with an OS rate of
22.2%, as compared with that of 42.1% for any methylation and of
100% for both promoters being unmethylated. Thus, GALR1 and
GALR2 resemble other major tumor suppressor genes in terms
of frequency of aberrant promoter methylation in vivo. The
survival curves clearly show the correlation between methylation
status of GALRs and OS, however, the downstream proteins
expressions such as p27 and p57, and OS are not related. Cyclin D1
overexpression was related to the length of OS, but not associated
with GALR methylation status.
Although this discrepancy was not fully understood,
other signaling pathways and many kinds of molecules controlled by
GALR would be related to survival of SDC. Further investigation
about GALRs signaling pathway in SDC are required. In summary, in
this study, we showed for the first time, to our knowledge, that
silencing of the GALR1 and GALR2 genes by methylation
may constitute a critical event in SDC. The current data further
suggest that GALR1 and GALR2 are potentially
significant therapeutic targets and prognostic factors in SDC.
Acknowledgements
This paper was orally presented at the 41st annual
meeting of Japan Society for Head and Neck Cancer on June 8, 2017
in Kyoto, Japan.
Funding
A Grant-in-Aid for Scientific Research (grant nos.
26462620, 17K11403 and 17K11402) from the Ministry of Education,
Culture, Sports, Science, and Technology of Japan.
Availability of data and materials
The datasets during and/or analysed during the
current study available from the corresponding author on reasonable
request.
Authors' contributions
TK, SI and HN saw the patients and drafted the
clinical detail at Jichi Medical University. KM, YM and HM saw the
patients and drafted the clinical details at Hamamatsu University
School of Medicine. HF and KK saw the patients and collected the
clinical data at Cancer Institute, Japanese Foundation of Cancer
Research. YS made pathological diagnosis with GK and TK. MM, TK and
GK performed the DNA methylation experiments. TEC supervised this
study. TK primarily compiled the data into this report. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethics Review Board of the ethics committee of each of the three
institutions that participated in this study. Jichi Medical
University, Hamamatsu University, School of Medicine, and Cancer
Institute Hospital, Japanese Foundation of Cancer Research. The
need to obtain informed consent was waived owing to the
retrospective nature of the analysis, however, consent was obtained
from patients at the time of data collection.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gilbert MR, Sharma A, Schmitt NC, Johnson
JT, Ferris RL, Duvvuri U and Kim S: A 20-year review of 75 cases of
salivary duct carcinoma. JAMA Otolaryngol Head Neck Surgery.
142:489–495. 2016. View Article : Google Scholar
|
|
2
|
Kleinsasser O, Klein HJ and Hübner G:
Salivary duct carcinoma. A group of salivary gland tumors analogous
to mammary duct carcinoma. Arch Klin Exp Ohren Nasen
Kehlkopfheilkd. 192:100–105. 1968.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jaehne M, Roeser K, Jaekel T, Schepers JD,
Albert N and Löning T: Clinical and immunohistologic typing of
salivary duct carcinoma: A report of 50 cases. Cancer.
103:2526–2533. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takase S, Kano S, Tada Y, Kawakita D,
Shimura T, Hirai H, Tsukahara K, Shimizu A, Imanishi Y, Ozawa H, et
al: Biomarker immunoprofile in salivary duct carcinomas:
clinicopathological and prognostic implications with evaluation of
the revised classification. Oncotarget. 8:59023–59035. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Masubuchi T, Tada Y, Maruya S, Osamura Y,
Kamata SE, Miura K, Fushimi C, Takahashi H, Kawakita D, Dishimoto
S, et al: Clinicopathological significance of androgen receptor,
HER2, Ki-67 and EGFR expressions in salivary duct carcinoma. Int J
Clin Oncol. 20:35–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di Palma S, Simpson RH, Marchiò C, Skálová
A, Ungari M, Sandison A, Whitaker S, Parry S and Reis-Filho JS:
Salivary duct carcinomas can be classified into luminal androgen
receptor-positive, HER2 and basal-like phenotypes. Histopathology.
61:629–643. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kondo Y, Kikuchi T, Esteban JC, Kumaki N,
Ogura G, Inomoto C, Hirabayashi K, Kajiwara H, Sakai A, Sugimoto R,
et al: Intratumoral heterogeneity of HER2 protein and amplification
of HER2 gene in salivary duct carcinoma. Pathol Int. 64:453–459.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto N, Minami S and Fujii M:
Clinicopathologic study of salivary duct carcinoma and the efficacy
of androgen deprivation therapy. Am J Otolaryngol. 35:731–735.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Allgayer H, Babic R, Gruetzner KU,
Tarabichi A, Schildberg FW and Heiss MM: c-erbB-2 is of independent
prognostic relevance in gastric cancer and is associated with the
expression of tumor-associated protease systems. J Clin Oncol.
18:2201–2209. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Williams MD, Roberts DB, Kies MS, Mao L,
Weber RS and El-Naggar AK: Genetic and expression analysis of HER-2
and EGFR genes in salivary duct carcinoma: empirical and
therapeutic significance. Clin Cancer Res. 16:2266–2274. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nabili V, Tan JW, Bhuta S, Sercarz JA and
Head CS: Salivary duct carcinoma: A clinical and histologic review
with implications for trastuzumab therapy. Head Neck. 29:907–912.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soper MS, Iganej S and Thompson LD:
Definitive treatment of androgen receptor-positive salivary duct
carcinoma with androgen deprivation therapy and external beam
radiotherapy. Head Neck. 36:E4–E7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Campos-Gómez S, Flores-Arredondo JH,
Dorantes-Heredia R, Chapa-Ibargüengoitia M and de la Peña-Lopez R:
Case report: Anti-hormonal therapy in the treatment of ductal
carcinoma of the parotid gland. BMC Cancer. 14:7012014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JS, Kwon OJ, Park JJ and Seo JH:
Salivary duct carcinoma of the parotid gland: Is adjuvant
HER-2-targeted therapy required? J Oral Maxillofac Surg.
72:1023–1031. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krishnamurthy J, Krishnamurty DM, Baker
JJ, Zhen W, Lydiatt D and Ganti AK: Salivary duct carcinoma
responding to trastuzumab-based therapy: Case report and review of
the literature. Head Neck. 35:E372–E375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Perissinotti AJ, Lee Pierce M, Pace MB,
El-Naggar A, Kies MS and Kupferman M: The role of trastuzumab in
the management of salivary ductal carcinomas. Anticancer Res.
33:2587–2591. 2013.PubMed/NCBI
|
|
17
|
Limaye SA, Posner MR, Krane JF, Fonfria M,
Lorch JH, Dillon DA, Shreenivas AV, Tishler RB and Haddad RI:
Trastuzumab for the treatment of salivary duct carcinoma.
Oncologist. 18:294–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanazawa T, Misawa K, Misawa Y, Uehara T,
Fukushima H, Kusaka G, Maruta M and Carey TE: G-Protein-coupled
receptors: Next generation therapeutic targets in head and neck
cancer? Toxins (Basel). 7:2959–2984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanazawa T, Iwashita T, Kommareddi P, Nair
T, Misawa K, Misawa Y, Ueda Y, Tono T and Carey TE: Galanin and
galanin receptor type 1 suppress proliferation in squamous
carcinoma cells: Activation of the extracellular signal regulated
kinase pathway and induction of cyclin-dependent kinase inhibitors.
Oncogene. 26:5762–5771. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanazawa T, Kommareddi PK, Iwashita T,
Kumar B, Misawa K, Misawa Y, Jang I, Nair TS, Iino Y and Carey TE:
Galanin receptor subtype 2 suppresses cell proliferation and
induces apoptosis in p53 mutant head and neck cancer cells. Clin
Cancer Res. 15:2222–2230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kanazawa T, Misawa K and Carey TE: Galanin
receptor subtypes 1 and 2 as therapeutic targets in head and neck
squamous cell carcinoma. Expert Opin Ther Targets. 14:289–302.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanazawa T, Misawa K, Misawa Y, Maruta M,
Uehara T, Kawada K, Nagatomo T and Ichimura K: Galanin receptor 2
utilizes distinct signaling pathways to suppress cell proliferation
and induce apoptosis in HNSCC. Mol Med Rep. 10:1289–1294. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Misawa K, Ueda Y, Kanazawa T, Misawa Y,
Jang I, Brenner JC, Ogawa T, Takebayashi S, Grenman RA, Herman JG,
et al: Epigenetic inactivation of galanin receptor 1 in head and
neck cancer. Clin Cancer Res. 14:7604–7613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Misawa Y, Misawa K, Kanazawa T, Uehara T,
Endo S, Mochizuki D, Yamatodani T, Carey TE and Mineta H: Tumor
suppressor activity and inactivation of galanin receptor type 2 by
aberrant promoter methylation in head and neck cancer. Cancer.
120:205–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Misawa K, Mochizuki D, Endo S, Mima M,
Misawa Y, Imai A, Shinmura K, Kanazawa T, Carey TE and Mineta H:
Site-specific methylation patterns of the GAL and GALR1/2 genes in
head and neck cancer: Potential utility as biomarkers for
prognosis. Mol Carcinog. 56:1107–1116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chung W, Kwabi-Addo B, Ittmann M, Jelinek
J, Shen L, Yu Y and Issa JP: Identification of novel tumor markers
in prostate, colon and breast cancer by unbiased methylation
profiling. PLoS One. 3:e20792008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu J, Zhang HY, Ma ZZ, Lu W, Wang YF and
Zhu JD: Methylation profiling of twenty four genes and the
concordant methylation behaviours of nineteen genes that may
contribute to hepatocellular carcinogenesis. Cell Res. 13:319–333.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hammond ME, Hayes DF, Wolff AC, Mangu PB
and Temin S: American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. J Oncol Pract. 6:195–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Misawa K, Kanazawa T, Misawa Y, Uehara T,
Imai A, Takahashi G, Takebayashi S, Cole A, Carey TE and Mineta H:
Galanin has tumor suppressor activity and is frequently inactivated
by aberrant promoter methylation in head and neck cancer. Transl
Oncol. 6:338–346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jayaprakash V, Merzianu M, Warren GW,
Arshad H, Hicks WL Jr, Rigual NR, Sullivan MA, Seshadri M, Marshall
JR, Cohan DM, et al: Survival rates and prognostic factors for
infiltrating salivary duct carcinoma: Analysis of 228 cases from
the Surveillance, Epidemiology and End Results database. Head Neck.
36:694–701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Misawa K, Misawa Y, Kanazawa T, Mochizuki
D, Imai A, Endo S, Carey TE and Mineta H: Epigenetic inactivation
of galanin and GALR1/2 is associated with early recurrence in head
and neck cancer. Clin Exp Metastasis. 33:187–195. 2016. View Article : Google Scholar : PubMed/NCBI
|