Introduction
Prostate cancer (PCa) is the second highest cause of
cancer-associated mortality in males, particularly in Western
countries (1). In recent years, the
morbidity and mortality of PCa have been increasing in Chinese
males (2). The 5-year survival rate
of patients with localized PCa is ~100%, due to the availability of
effective treatments (3). However,
certain patients experience progression to metastatic
castrate-resistant prostate cancer, which is the final stage and
has a poor prognosis, eventually resulting in mortality (4). Therefore, identifying accurate
biomarkers for the early diagnosis of PCa, as well as efficient
targets for the therapy of advanced PCa, is urgently required.
MicroRNAs (miRNAs/miRs) are a group of short and
non-coding RNA sequences that serve an important role in the
post-transcriptional regulation of gene expression (5). Previous studies have demonstrated that
miRNAs may act as tumor suppressors or oncogenes during tumor
development, and that the aberrant expression of certain miRNAs is
closely associated with the progression of various types of cancer
(6,7).
Several studies have reported that the expression of miR-126 is
suppressed in multiple cancer types, and that it may act as a tumor
suppressor gene by inhibiting a number of potential oncogenes and
signaling pathways (8). In 2013, Feng
et al (9) reported that
miR-126 suppresses colon cancer progression via negatively
regulating the expression of C-X-C chemokine receptor type 4. CRK
was also confirmed as a direct target of miR-126 in gastric
carcinoma (9) and lung cancer
(10); this gene has a key role in
various signaling pathways regulating cell adhesion, proliferation
and migration (11,12). In non-small cell lung cancer, activity
of the phosphoinositide 3-kinase-AKT pathway was suppressed by
miR-126 (13). According to a
previous study, miR-126 expression may serve as an independent
prognostic predictor in patients with PCa subsequent to radical
prostatectomy (14). Another study
revealed that miR-126 could inhibit invasion and proliferation by
targeting phosphoinositide-3-kinase regulatory subunit 2 in PCa
cells (15). In the current study, it
was identified that miR-126 can reduce the expression of the ADAM
metalloproteinase domain 9 (ADAM9) protein, a member of the zinc
metalloprotease class, which is involved in the development of
various types of tumors (16). In
addition, ADAM9 has been demonstrated to modulate the epithelial
phenotype (17). In the current
study, the role of the miR-126/ADAM9 axis in the invasion,
proliferation, colony formation and epithelial-mesenchymal
transition (EMT) of PCa cells was demonstrated. These revelations
may improve the understanding of miR-126 in PCa progression, with
miR-126 potentially becoming a novel molecular target for advanced
PCa treatment.
Materials and methods
Patients and tissue microarrays
(TMAs)
A total of 93 PCa tissues were collected from
patients with a mean age of 69.9, ranging from 56 to 81 years old
who underwent radical prostatectomy between May 2008 and December
2011 at The First Affiliated Hospital of Nanjing Medical University
(Nanjing, China). All recruited patients provided informed consent
prior to recruitment. The Ethics Committee of The First Affiliated
Hospital of Nanjing Medical University approved the current study,
including the protocols. The clinical information of the 93
patients was summarized in Table I,
and the deadline for follow-up was April 2016. Triplicate tissue
cores with diameters of 0.6 mm were extracted from each PCa
tissue.
 | Table I.Association of ADAM9 expression and
clinicopathological characteristics of the patients. |
Table I.
Association of ADAM9 expression and
clinicopathological characteristics of the patients.
|
|
| ADAM9 expression, n
(%) |
|---|
|
|
|
|
|---|
| Variable | Total, n | Low (n=42) | High (n=51) | P-value |
|---|
| Age, years |
|
|
| 0.381 |
|
<60 | 6 | 3 (50.0) | 3 (50.0) |
|
|
60–70 | 42 | 22 (52.4) | 20 (47.6) |
|
|
>70 | 45 | 17 (37.8) | 28 (62.2) |
|
| Preoperative PSA
(ng/ml) |
|
|
| 0.083 |
|
<10 | 27 | 17 (63.0) | 10 (37.0) |
|
|
10–20 | 30 | 12 (40.0) | 18 (60.0) |
|
|
>20 | 36 | 13 (36.1) | 23 (63.9) |
|
| Gleason score |
|
|
| 0.853 |
|
<7 | 55 | 26 (47.3) | 29 (52.7) |
|
| =7 | 20 | 8 (40.0) | 12 (60.0) |
|
|
>7 | 18 | 8 (44.4) | 10 (55.6) |
|
| T stage |
|
|
| 0.564 |
|
pT2 | 79 | 37 (46.8) | 42 (53.2) |
|
|
pT3/T4 | 14 | 5 (35.7) | 9 (64.3) |
|
| Biochemical
recurrence |
|
|
| 0.030a |
|
Negative | 59 | 32 (54.2) | 27 (45.8) |
|
|
Positive | 34 | 10 (29.4) | 24 (70.6) |
|
Immunohistochemistry
Xylene was used to deparaffinize serial sections
from TMA blocks, which were then rehydrated through an ethanol
gradient; the serial sections were then blocked for 10 min in
hydrogen peroxide diluted (3%) in methanol. For antigen retrieval,
sections were incubated in a steam pressure cooker (210°C), for 2
min in citrate buffer (10 ml, pH 6.0). Subsequently, the samples
were blocked for 5 min, then incubated overnight at 4°C with
primary antibodies against ADAM9 (cat. no. ab186833; 1:100; Abcam,
Cambridge, UK). The samples were washed for 10 min in PBS and
incubated with the peroxidase-conjugated goat anti-rabbit IgG (H+L)
secondary antibody (dilution, 1:1,000; cat. no. ZB-2301; ZSGB-BIO,
Inc., Beijing, China) at 37°C for 30 min. Following another wash
with PBS for 10 min visualization (fluorescence microscope; ×100
and ×400 magnification) of the antibody reaction was conducted with
a fresh substrate solution containing 3,3′diaminobenzidine. The
sections were counterstained with hematoxylin at room temperature
for 2 min, dehydrated and coverslipped.
Evaluation of staining
Evaluation of immunohistochemical staining was
conducted by two experienced pathologists blinded to the clinical
data. The expression of ADAM9 was observed in the cytoplasm of
tumor cells at different intensities. The staining intensity was
evaluated with a semi-quantitative scoring system, as follows: 0,
negative staining; 1, weak staining; 2, moderate staining; and 3,
strong staining. The final immunohistochemical scores of each PCa
tissues were obtained by multiplying the percentage of tissue
stained (0–100%) and the corresponding intensity grade, resulting
in a score of 0–300. To distinguish between low and high levels of
ADAM9 expression, the median score was used as a cut-off value.
Cell culture and human clinical
samples
Human androgen-independent prostate cancer cell
lines (DU145 and PC3), normal prostate epithelial cells (RWPE-1)
and 293T cells were purchased from the Chinese Academy of Sciences
(Beijing, China). DU145 and PC3 cell lines were cultured in F-12K
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
RWPE-1 and 293T cells were cultured in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc.). The medium was
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), and cells well incubated in a humidified air
atmosphere at 37°C and 5% CO2.
A total of 18 pairs of PCa tissues and corresponding
adjacent normal tissues were obtained from patients with a mean age
of 68.1 years old, ranging from 57–77 years old, who underwent
radical prostatectomy between March 2014 and November 2014, with
informed consent, at The First Affiliated Hospital of Nanjing
Medical University. The pathologist confirmed their histological
characteristics, and the clinical characteristics were summarized
in Table II.
 | Table II.Characteristics of the 18 patients
treated by radical prostatectomy. |
Table II.
Characteristics of the 18 patients
treated by radical prostatectomy.
| Variable | Total, n | n, % |
|---|
| Age |
|
|
| Mean ±
SD (year) | 68.1±6.6 |
|
|
<60 | 1 |
5.6 |
|
60–70 | 8 | 44.4 |
|
>70 | 9 | 50.0 |
| Gleason score |
|
|
|
<7 | 13 | 72.2 |
| =7 | 2 | 11.1 |
|
>7 | 3 | 16.7 |
| T stage |
|
|
|
pT2 | 14 | 77.8 |
|
pT3/T4 | 4 | 22.2 |
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The RNA was extracted from cell lines and tumor
samples using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). miRNA was reversed transcribed into cDNA using
MiR-X™ miRNA First-Strand Synthesis (Takara Biotechnology Co.,
Ltd., Kusatsu, Japan). Total RNA was reversed transcribed into cDNA
using PrimeScript RT Master Mix (Takara Biotechnology Co., Ltd.).
RT-qPCR was performed using FastStart Universal SYBR Green Master
(Roche Diagnostics, Indianapolis, IN, USA) with the following
thermal cycling protocol: 95°C for 5 min, 95°C for 10 sec and 60°C
for 20 sec, for 40 cycles. U6 was used as the internal control for
miR-126, and β-actin was used as the internal control for ADAM9.
The primers for miR-126 were designed by and purchased from Tiangen
Biotech Co., Ltd. (Beijing, China). The following primer sequences
for miR-126 were used: Forward, 5′-CAGTGCGTGTCGTGGAGT-3′ and
reverse, 5′-GGGGCGTACCGTGAGTAAT-3′ (Realgene Biotech, Inc.,
Nanjing, China). The following primer sequences for U6 were used:
Forward, 5′-CGCTTCACGAATTTGCGTGTCAT-3′ and reverse,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ (Realgene Biotech, Inc.). The
following primer sequences for ADAM9 were used: Forward
5′-CCCCCAAATTGTGAGACTAAAG-3′ and reverse,
5′-TCCCGTCCCTCAATGCAGTAT-3′ (Realgene Biotech, Inc.). The following
primer sequences for β-actin were used: Forward
5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ and reverse,
5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′ (Realgene Biotech, Inc.). The
2−ΔΔCq method was used to analyze the relative gene
expression (18).
Transient transfection
The hsa-miR-126-3p mimics, non-specific miRNA
control (negative control), ADAM9 siRNA and scrambled siRNA
(negative control) were designed and chemically synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China). The following
sequences of siRNA for ADAM9 were used: Forward,
5′-CCAGAGAAGUUCCUAUAUA-3′, and reverse, 5′-UAUAUAGGAACUUCUCUGG-3′.
The following sequences of scrambled siRNA were used: Forward,
5′-UUCUCCGAACGUGUCACGUTT-3′, and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′. PCa cells were maintained until
mid-log phase, then digested with trypsin and seeded into 6-well
plates at a density of 1×105 cells/well. Following
culture for 48 h, when cells reached 60% confluence, cells were
processed for transfection. PCa cells were transfected with 2 µg
miR-126 mimics or negative control using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) in Opti-MEM
medium, according to the manufacturer's protocol. In the same way,
2.5 µg ADAM9 siRNA or scrambled siRNA were transfected into PCa
cells with Lipofectamine® 2000. Following transfection
for 4–6 h, the medium surrounding the cells was replaced with
F12-K.
ADAM9 overexpression plasmids were constructed by
Obio Technology Co., Ltd. (Shanghai, China). For co-transfection,
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used, and the PCa cells were divided into two
groups: One group was co-transfected with 2 µg miR-126 mimics and 5
µg pLenti-ADAM9, while the other group was co-transfected with 2 µg
non-specific miRNA control and 5 µg empty plasmids (Obio Technology
Co., Ltd.).
Luciferase reporter assay
The binding site of miR-126 in ADAM9 was predicted
by TargetScan (www.targetscan.org) and miRanda (www.microrna.org). To construct a luciferase reporter
plasmid with wild type ADAM9, the 3′ untranslated region (UTR) of
ADAM9, predicted to bind with miR-126, was chemosynthesized,
amplified and cloned into a pMIR-REPORT luciferase vector (Obio
Technology Co., Ltd.). The binding sequence was point-mutated to
form a control luciferase reporter plasmid by Obio Technology Co.,
Ltd.. For the luciferase reporter assay, the 293T cells were
co-transfected with miR-126 mimics or the miRNA control, and the
wild type or mutant-type ADAM9 plasmid. After incubation for 48 h,
a Dual-Luciferase® Reporter assay system (Promega
Corporation, Madison, WI, USA) was used to determine luciferase
activity on the LD400 Luminometer (Beckman Coulter, Inc., Brea, CA,
USA). Renilla luciferase activity was normalized to firefly
luciferase activity.
Cell proliferation assays
Cell proliferation was assessed using a Cell
Counting Kit-8 (CCK-8) and colony formation assays. For the CCK-8
assay, 48 h subsequent to transfection, PC3 and DU145 cells were
seeded in 96-well plates at a density of 2,000 cells/well. CCK-8
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was used to
determine cell proliferation at 24, 48, 72 and 96 h after seeding.
Absorbance was determined at 450 nm by an Infinite M200 Pro
absorbance reader (Thermo Fisher Scientific, Inc.).
For the colony formation assay, at 48 h after
transfection, PC3 and DU145 cells were harvested and plated in cell
culture dishes at a density of 1,000 cells/dish. Following
incubation at 37°C for 14 days, the cells were stained with 0.005%
crystal violet for 2 h; the colonies were then counted under a
fluorescence-inverted microscope (×100 magnification).
Transwell migration and invasion
assays
The in vitro migration and invasion assays
were performed using 24-well chambers with 8-µm pore size PET
track-etched membranes (Corning, Inc., Corning, NY, USA). After
transfection for 48 h, a total of 5×104 transfected
cells were suspended in the upper chamber with serum-free medium,
while the lower chamber was filled with 20% FBS-containing medium.
The membranes were covered with Matrigel® (BD
Biosciences, San Jose, CA, USA) to form matrix barriers in the
invasion assays. After incubation at 37°C for 24 (migration assay)
or 48 h (invasion assay), a cotton swab was used to remove the
cells on the upper surface, while the cells on the bottom of the
membranes were fixed in 4% methanol solution for 1 h and stained
with 0.005% crystal violet for 2 h. These were then counted in
three random fields under a fluorescence-inverted microscope (×200
magnification).
Western blot assay
Total proteins were extracted from tumor cells using
protein lysis buffer (Beyotime Institute of Biotechnology, Nantong,
Jiangsu, China). The protein concentration was measured using a BCA
kit (Beyotime Institute of Biotechnology), according to the
manufacturer's protocols. For western blot analysis, 25 µg of each
protein were separated via 10% SDS-PAGE. Subsequently, the proteins
were transferred to PVDF membranes in transfer buffer, which
contains Tris-HCL SDS, Glycine and methanol. The membranes were
then incubated with monoclonal rabbit anti-ADAM9 (dilution,
1:1,000; cat. no. ab186833; Abcam), monoclonal rabbit
anti-E-cadherin (dilution, 1:1,000; cat. no. 3195), monoclonal
rabbit anti-N-cadherin (dilution, 1:1,000; cat. no. 13116) or
monoclonal rabbit anti-Vimentin (dilution, 1:1,000; cat. no. 5741)
(Cell Signaling Technology, Inc., Danvers, MA, USA), all at 4°C
overnight; rabbit monoclonal β-actin (Abcam) served as the internal
control. Following this, the membranes were washed three times in
TBST and incubated with an anti-rabbit secondary antibody solution
(dilution, 1:4,000) at room temperature for 2 h. The indicated
proteins were detected using an enhanced chemiluminescence western
blotting detection system (Bio-Rad ChemiDoc XRS+). The relative
protein expression was analyzed by Image-Lab software (version 6.0;
Bio-Rad Laboratories, Inc., Hercules, CA, USA), represented as the
density ratio vs. actin.
Statistical analyses
The associations between protein expression values
and clinicopathological factors were assessed using the
χ2-test. Kaplan-Meier curves and log-rank tests were
used to assess the association of ADAM9 expression with
recurrence-free survival. Results are expressed as the mean ±
standard deviation. Differences between two or three groups were
compared with the Student's t-test or one-way analysis of variance
followed by Dunnett's t-test. A value of P≤0.05 was considered to
indicate a statistically significant difference. All statistical
calculations were performed using SPSS v.13.0 software (SPSS, Inc.,
Chicago, IL, USA).
Results
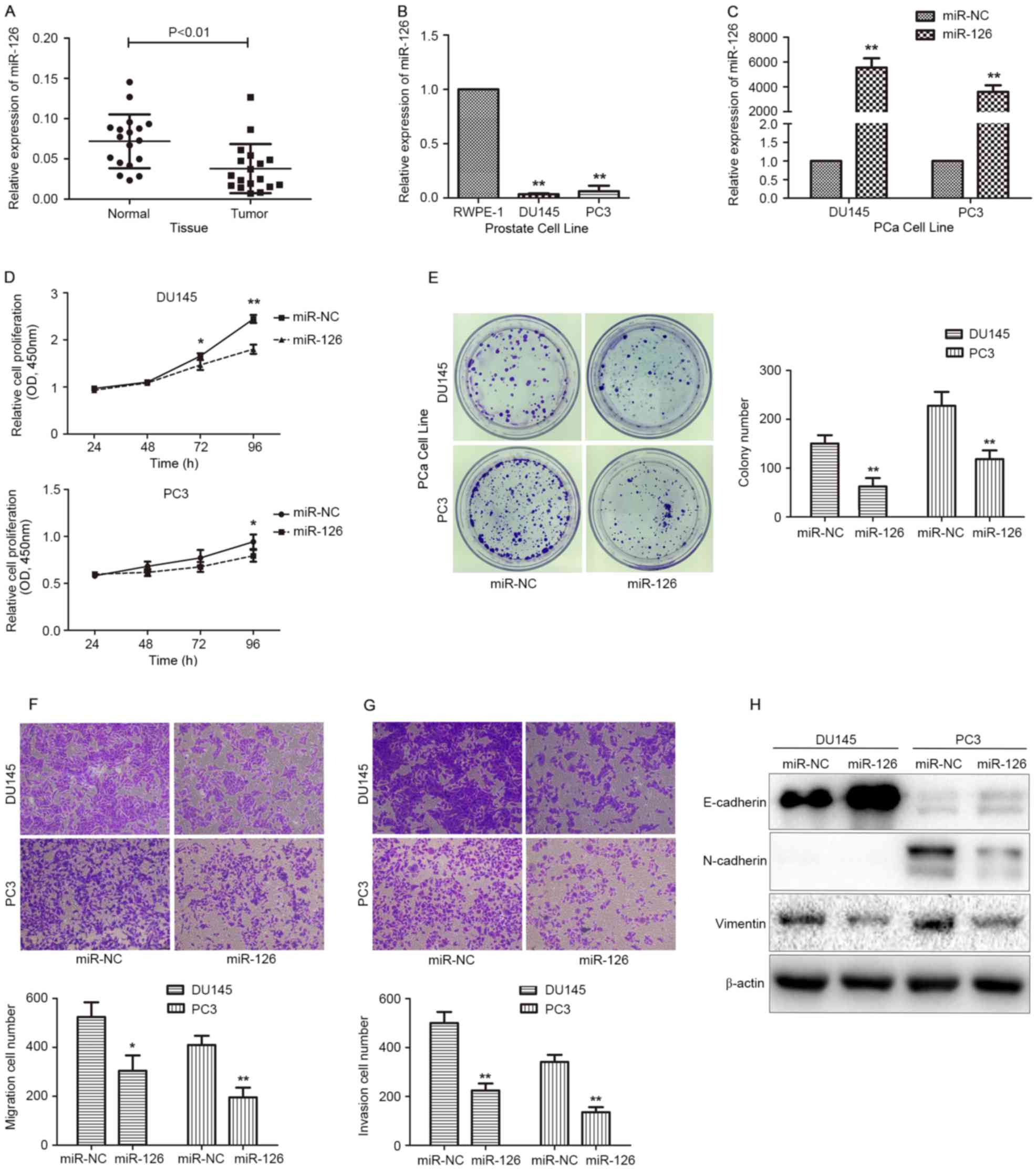
miR-126 expression is downregulated in
PCa tissues and cell lines
The expression levels of miR-126 in 36 frozen
samples from patients with PCa (18 cancerous and 18 corresponding
adjacent tissues) were examined by RT-qPCR. The results indicated
that, compared with adjacent normal tissues, miR-126 expression is
significantly reduced (P<0.01) in PCa tissues (Fig. 1A). Similarly, RT-qPCR was performed to
determine miR-126 expression levels in two PCa cell lines (PC3,
DU145) and normal prostate epithelial cells (RWPE-1). As depicted
in Fig. 1B, the expression level of
miR-126 was significantly lower in PC3 and DU145, compared with in
RWPE-1 cells (P<0.01). These results indicated that miR-126 may
function as a suppressive gene in the progression of PCa.
miR-126 overexpression suppresses PCa
cell proliferation, metastasis and the EMT process
To investigate the effect of miR-126 in PCa cell
function, miR-126 mimics were used to overexpress miR-126 in PC3
and DU145 cells (Fig. 1C).
Subsequently, a CCK-8 assay was performed to evaluate the cell
proliferation. It was observed that miR-126 overexpression
significantly suppressed the proliferation rate of PC3 and DU145
cells at 72 or 96 h (Fig. 1D).
Consistently, the same trend in colony formation assays (Fig. 1E) was also noticed. These results
suggested that miR-126 overexpression decreased PCa cell
growth.
Transwell assays were performed to assess the
significance of miR-126 in PCa cell metastasis. As demonstrated in
Fig. 1F and G, the migration and
invasion rates were significantly inhibited after transfecting
miR-126 mimics into PC3 and DU145 cells (P<0.01). This suggested
that miR-126 may function as a suppressor in PCa metastasis.
EMT is a crucial process during tumor metastasis.
Based on the association between miR-126 expression and the
metastasis of PCa cells, a western blot assay was performed to
assess EMT-associated protein expression. Following transfection
with miR-126 mimics, increased expression of the epithelial marker
E-cadherin, and decreased expression of the mesenchymal markers
N-cadherin and Vimentin (Fig. 1H)
were observed; however, N-cadherin was only expressed in PC3, and
not in DU145 cells, which is consistent with the results previously
reported by Nalla et al (19).
The results of western blot analysis for EMT indicated that miR-126
overexpression reversed the EMT process in PCa cells.
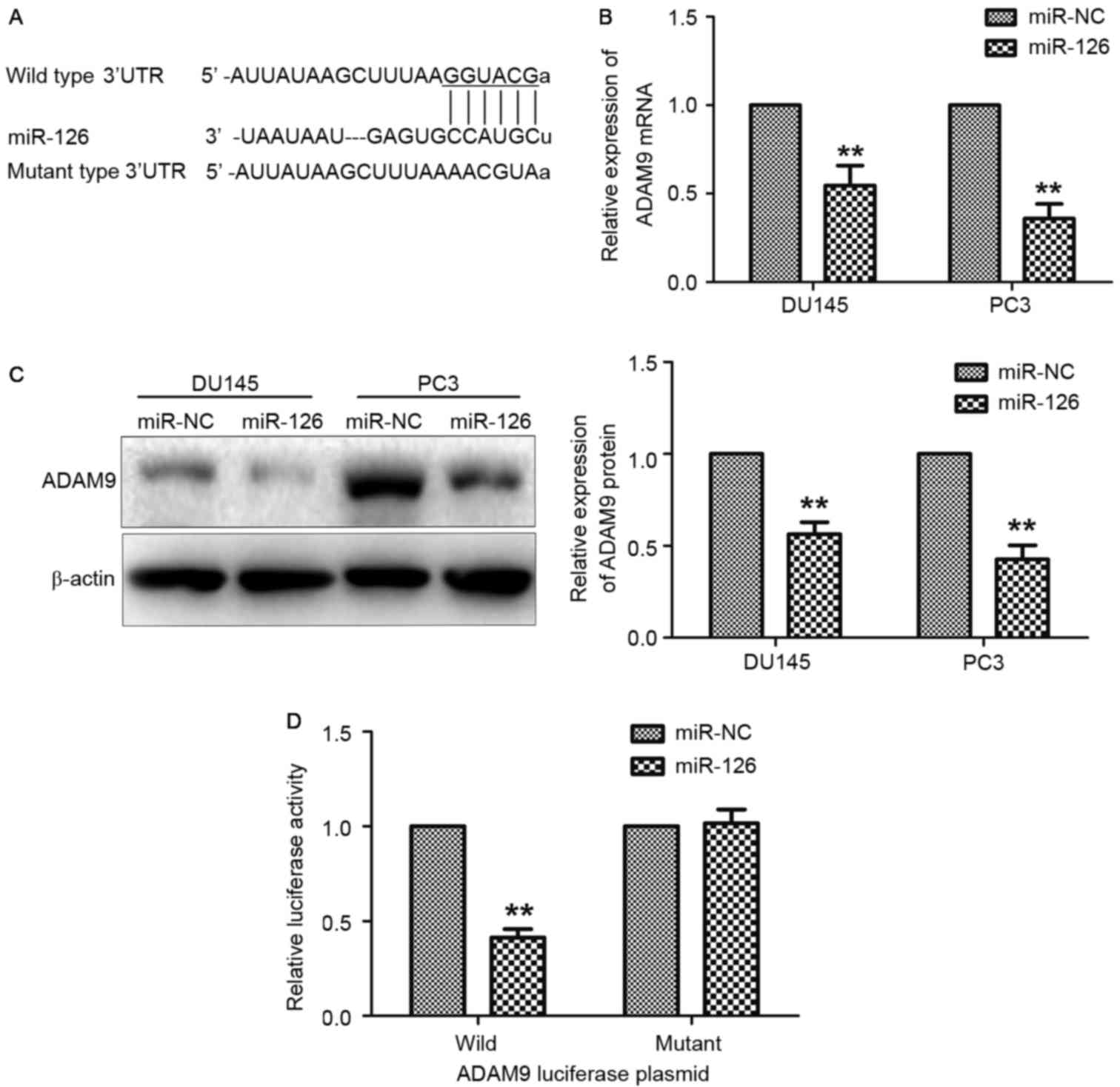
ADAM9 is a direct target gene of
miR-126
In previous literature, it was revealed that miRNA
regulates gene expression by binding to the 3′UTR of target mRNAs
(20). The 3′UTR of ADAM9 was
predicted to be a potential target of miR-126 via comprehensive
analysis using TargetScan and miRanda (Fig. 2A). To identify the influence of
miR-126 on ADAM9 expression, RT-qPCR and western blot analyses were
performed on PC3 and DU145 cells transfected with miR-126. As
depicted in Fig. 2B and C, the mRNA
and protein expression levels of ADAM9 in PC3 and DU145 cells were
significantly reduced following transfection with miR-126 mimics
(P<0.01). To further verify the direct interaction of miR-126
and ADAM9, a luciferase reporter assay in 293T cells was conducted.
As demonstrated in Fig. 2D, miR-126
overexpression decreased the luciferase activity of the wild-type
ADAM9 plasmid, whereas no effects were observed with the ADAM9
mutant plasmid.
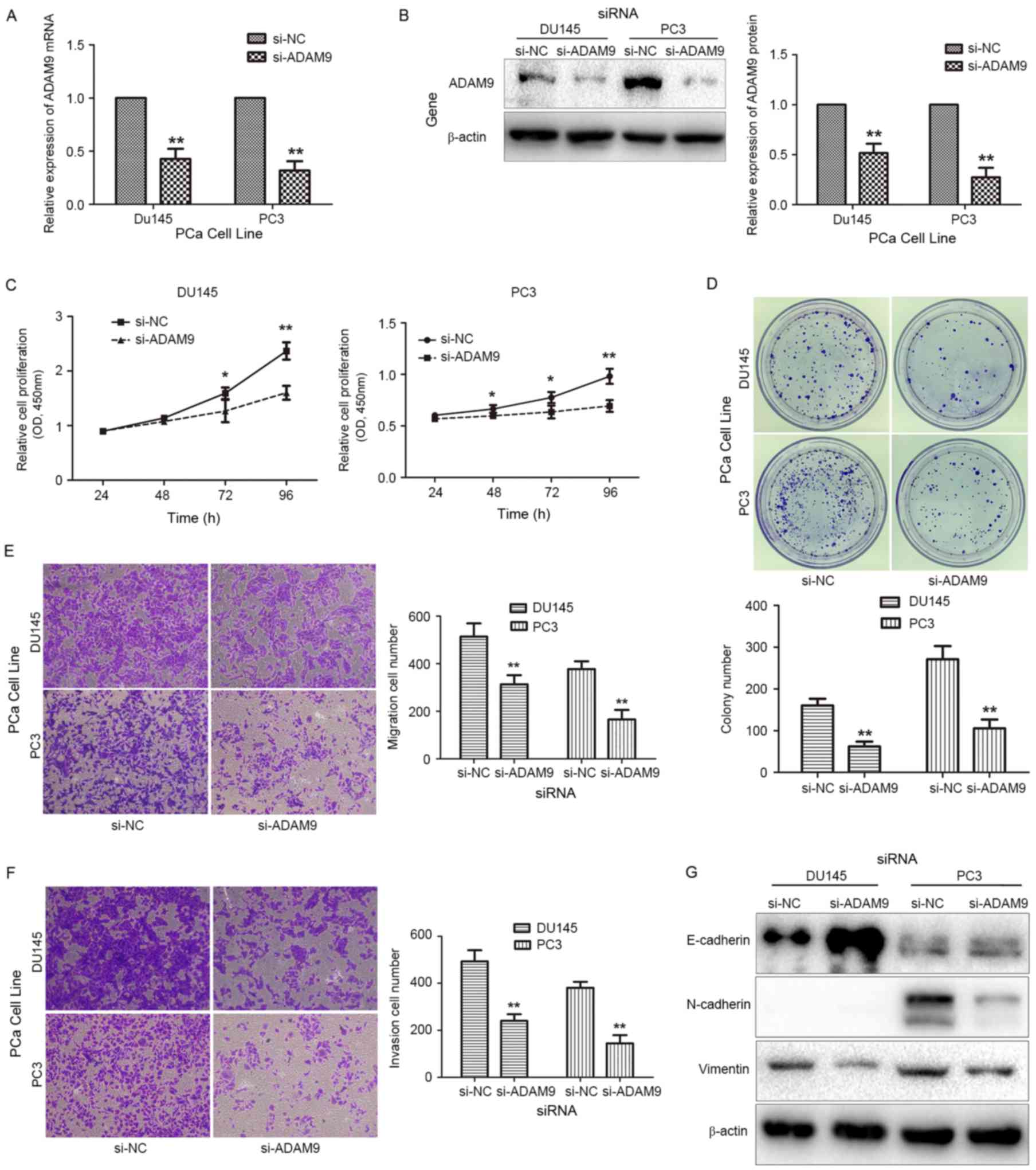
miR-126 functions as a
tumor-suppressor gene by reducing ADAM9 expression
To determine whether miR-126 suppresses PCa
progression through the downregulation of ADAM9 expression, the
role of ADAM9 in PCa cell function was investigated. ADAM9 siRNA
was transfected into DU145 and PC3 cells to decrease endogenous
ADAM9 expression (Fig. 3A and B).
Observations of cell proliferation (Fig.
3C), colony formation (Fig. 3D),
migration (Fig. 3E) and invasion
(Fig. 3F) capabilities revealed that
each function was suppressed, and that the process of EMT was
reversed (Fig. 3G) following the
transient knockdown of ADAM9 in PCa cells. These results are
consistent with those of previous experiments, which indicated the
overexpression of miR-126. To further identify whether miR-126
suppresses PCa cell functions by reducing ADAM9 expression,
co-transfection of ADAM9 overexpression plasmids and miR-126 mimics
into PCa cells was conducted, and whether cell functions could be
reversed by overexpression of ADAM9 was assessed. As depicted in
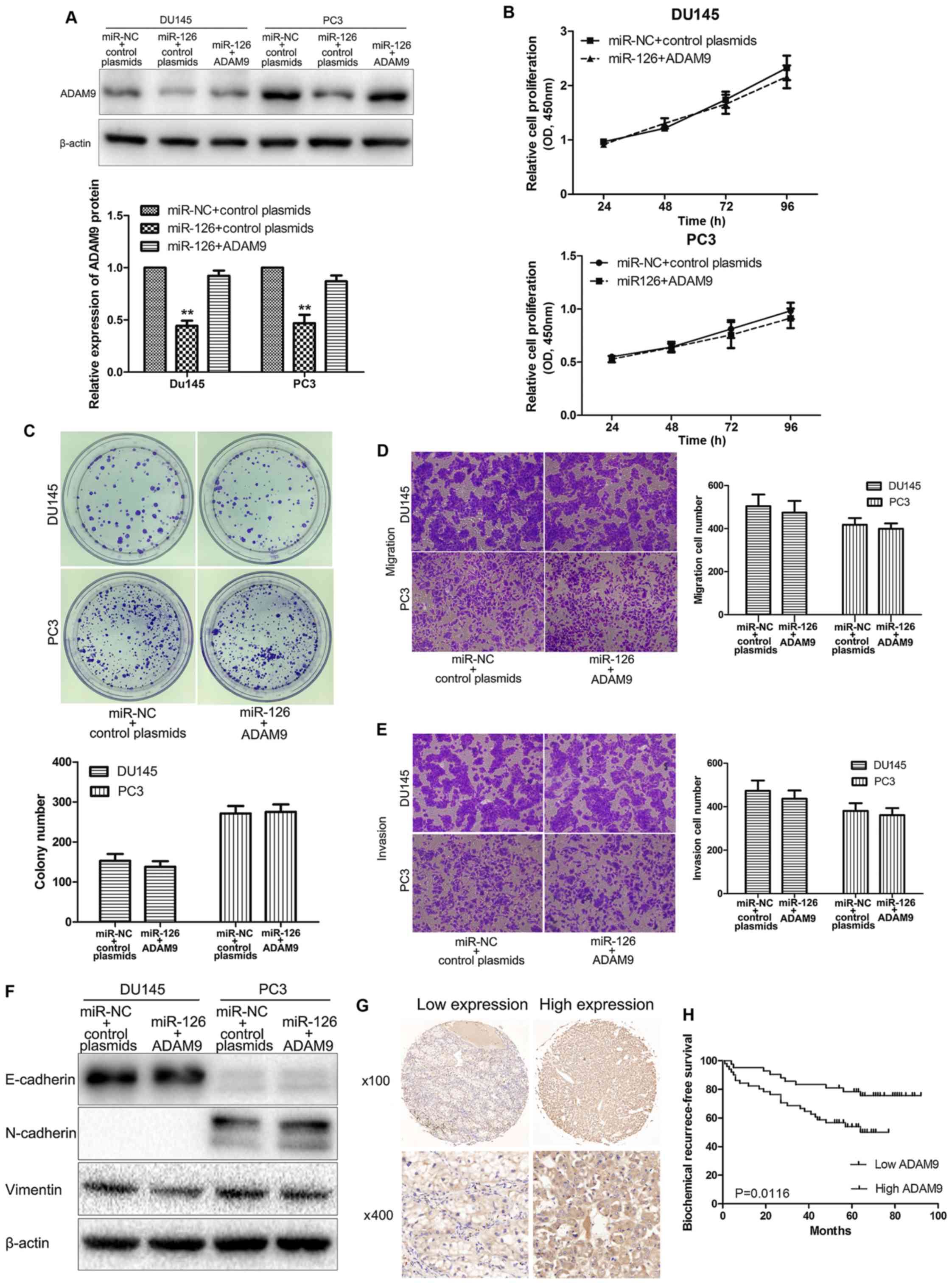
Fig. 4A, ADAM9 protein was markedly
increased following transfection with ADAM9 overexpression
plasmids. Furthermore, restoration of ADAM9 in
miR-126-overexpressing PCa cells showed no significant differences
in proliferation (Fig. 4B), colony
formation (Fig. 4C), migration
(Fig. 4D), invasion (Fig. 4E), and the EMT process (Fig. 4F), compared with the control
group.
ADAM9 may act as a prognostic
indicator for PCa
The expression of ADAM9 protein in 93 PCa tissue
samples was evaluated to analyze the role of ADAM9 in PCa
progression (Fig. 4G). As
demonstrated by the χ2 test results (Table I), higher ADAM9 protein expression was
significantly associated with biochemical recurrence (P=0.030). In
addition, the Kaplan-Meier curves indicated a significantly shorter
biochemical recurrence-free survival time for those patients with
high ADAM9 expression (P=0.0116; Fig.
4H). These results indicated that high expression of ADAM9 was
associated with poor prognosis in patients with PCa.
Discussion
PCa has increasing morbidity and mortality rates,
and the prognosis becomes worse as the disease develops to an
advanced stage or metastasizes. Various studies have demonstrated
that miRNAs may function as tumor suppressors or oncogenes during
tumor development, according to the roles of their target genes
(6,21). In PCa, several miRNAs have been
reported to serve critical roles in the regulation of basic
biological processes, including cell growth, apoptosis, metastasis
and drug resistance (21,22). These findings suggest that miRNAs have
promising diagnostic and therapeutic values for patients with
PCa.
miR-126 expression is suppressed in the majority of
human cancer types, and always functions as a tumor suppressor
gene, to the best of our knowledge (8); however, in PCa, the functional role and
the potential prognostic value of miR-126 remain to be elucidated.
In the present study, RT-qPCR was used to identify that the
expression of miR-126 was significantly suppressed in PCa tissues.
Studying the androgen-independent PCa cell lines PC3 and DU145
revealed that miR-126 overexpression could inhibit their
proliferative and metastatic capabilities. In addition, it was
verified that miR-126 functioned as the tumor suppressor by
downregulating the expression of ADAM9, a member of the ADAM
family.
ADAMs are involved in cell adhesion, cell migration
and tissue remodeling (23); these
processes are important for tumor progression. Previous studies
have demonstrated that various ADAMs represent promising diagnostic
and prognostic markers in tumors (24). ADAM9 has been reported to be
associated with cancer metastasis (25,26); in
addition, evidence indicates that ADAM9 serves a role in cancer
proliferation by activating the epidermal growth factor receptor
signaling pathway (27–29). The data from the present study
indicate that miR-126 acts as a tumor suppressor by targeting ADAM9
in PCa. Previous studies have revealed that ADAM9 promotes the
metastasis of cancer by regulating E-cadherin and integrins
(30,31). In the current study, it was revealed
that miR-126 reversed the epithelial phenotype and repressed the
mesenchymal phenotype via decreasing the expression of ADAM9 in
PCa. EMT is a complex multistep process and serves a critical role
in cancer metastasis and tumor invasion (32). Recent studies have identified that EMT
serves a vital role in chemoresistance, tumor metastasis, disease
aggressiveness and poor prognosis of prostate cancer (33,34).
Therefore, the present study suggested that miR-126 may be a novel
therapeutic target to combat aggressive PCa.
To the best of our knowledge, the present study is
the first to identify ADAM9 as a potential target of miR-126 in PCa
cells. Furthermore, it was speculated that the reduced level of
miR-126 could lead to the accumulation of ADAM9 in PCa tissues,
which may result in a poor prognosis for patients with PCa. To test
this hypothesis, the expression levels of ADAM9 were evaluated in
TMAs constructed from 93 PCa samples. The present data suggest that
ADAM9 may be a prognostic marker for the biochemical recurrence of
PCa.
In summary, the results of the current study
provided evidence that miR-126 may function as a tumor-suppressive
gene by targeting ADAM9 to inhibit PCa cell proliferation,
migration, invasion and colony formation, as well as the EMT
process. It was also identified that a high level of ADAM9
expression is an important marker for the poor prognosis of
patients with PCa. These results suggested that miR-126 may be a
potential therapeutic target for the treatment of advanced PCa.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81550010 and
81672532) and the Graduate International Exchange and Cooperation
Projects of Nanjing Medical University (grant no. 201601C006).
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conception and design was conducted by ZW and JL.
Acquisition of data was completed by YH, CL, CM, ZW and BL.
Analysis and interpretation of data was conducted by SW, SS, PS,
MB, AX and JZ. Drafting the manuscript was completed by YH, JZ and
AX. All authors approved the final version.
Ethics approval and consent to
participate
All patients gave informed consent to the study
(pathological characteristics and immunohistochemical staining of
PCa tissues obtained after curative resection and study of
statistical associations with clinicopathological data). This study
was approved by the medical ethics committee of the hospital and
conducted in accordance with the Chinese laws and regulations.
Consent for publication
Written informed consent for the publication of any
associated data and accompanying images has been obtained from all
participants.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PCa
|
prostate cancer
|
|
EMT
|
epithelial-mesenchymal transition
|
|
TMA
|
tissue microarray
|
|
FBS
|
fetal bovine serum
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
UTR
|
untranslated region
|
|
CCK-8
|
Cell Counting Kit-8
|
References
|
1
|
Yamamoto S, Kawakami S, Yonese J, Fujii Y,
Urakami S, Masuda H, Numao N, Ishikawa Y, Kohno A and Fukui I:
Long-term oncological outcome and risk stratification in men with
high-risk prostate cancer treated with radical prostatectomy. Jpn J
Clin Oncol. 42:541–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peyromaure EM, Mao K, Sun Y, Xia S, Jiang
N, Zhang S, Wang G, Liu Z and Debré B: A comparative study of
prostate cancer detection and management in China and in France.
Can J Urol. 16:4472–4477. 2009.PubMed/NCBI
|
|
3
|
Walz J, Gallina A, Saad F, Montorsi F,
Perrotte P, Shariat SF, Jeldres C, Graefen M, Bénard F, McCormack
M, et al: A nomogram predicting 10-year life expectancy in
candidates for radical prostatectomy or radiotherapy for prostate
cancer. J Clin Oncol. 25:3576–3581. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoang DT, Iczkowski KA, Kilari D, See W
and Nevalainen MT: Androgen receptor-dependent and -independent
mechanisms driving prostate cancer progression: Opportunities for
therapeutic targeting from multiple angles. Oncotarget.
8:3724–3745. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nana-Sinkam SP and Croce CM: Clinical
applications for microRNAs in cancer. Clin Pharmacol Ther.
93:98–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baer C, Claus R and Plass C: Genome-wide
epigenetic regulation of miRNAs in cancer. Cancer Res. 73:473–477.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ebrahimi F, Gopalan V, Smith RA and Lam
AK: miR-126 in human cancers: Clinical roles and current
perspectives. Exp Mol Pathol. 96:98–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng R, Chen X, Yu Y, Su L, Yu B, Li J,
Cai Q, Yan M, Liu B and Zhu Z: miR-126 functions as a tumour
suppressor in human gastric cancer. Cancer Lett. 298:50–63. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Crawford M, Brawner E, Batte K, Yu L,
Hunter MG, Otterson GA, Nuovo G, Marsh CB and Nana-Sinkam SP:
MicroRNA-126 inhibits invasion in non-small cell lung carcinoma
cell lines. Biochem Biophys Res Commun. 373:607–612. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar S, Davra VE, Obr A, Geng KL, Wood T
and Birge R: Crk adaptor protein promotes PD-L1 expression, EMT and
immune evasion in a murine model of triple-negative breast cancer.
Oncoimmunology. 7:e13761552017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsuda M and Tanaka S: Roles for crk in
cancer metastasis and invasion. Genes Cancer. 3:334–340. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang J, Lan H, Huang X, Liu B and Tong Y:
MicroRNA-126 inhibits tumor cell growth and its expression level
correlates with poor survival in non-small cell lung cancer
patients. PLoS One. 7:e429782012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun X, Liu Z, Yang Z, Xiao L, Wang F, He
Y, Su P, Wang J and Jing B: Association of microRNA-126 expression
with clinicopathological features and the risk of biochemical
recurrence in prostate cancer patients undergoing radical
prostatectomy. Diagn Pathol. 8:2082013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song L, Xie X, Yu S, Peng F and Peng L:
MicroRNA-126 inhibits proliferation and metastasis by targeting
pik3r2 in prostate cancer. Mol Med Rep. 13:1204–1210. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duffy MJ, McKiernan E, O'Donovan N and
McGowan PM: Role of ADAMs in cancer formation and progression. Clin
Cancer Res. 15:1140–1144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Josson S, Anderson CS, Sung SY, Johnstone
PA, Kubo H, Hsieh CL, Arnold R, Gururajan M, Yates C and Chung LW:
Inhibition of ADAM9 expression induces epithelial phenotypic
alterations and sensitizes human prostate cancer cells to radiation
and chemotherapy. Prostate. 71:232–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nalla AK, Estes N, Patel J and Rao JS:
N-cadherin mediates angiogenesis by regulating monocyte
chemoattractant protein-1 expression via PI3K/Akt signaling in
prostate cancer cells. Exp Cell Res. 317:2512–2521. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Agostini M, Pucciarelli S, Calore F, Bedin
C, Enzo M and Nitti D: miRNAs in colon and rectal cancer: A
consensus for their true clinical value. Clin Chim Acta.
411:1181–1186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian L, Fang YX, Xue JL and Chen JZ: Four
microRNAs promote prostate cell proliferation with regulation of
PTEN and its downstream signals in vitro. PLoS One. 8:e758852013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kopczyńska E: Role of microRNAs in the
resistance of prostate cancer to docetaxel and paclitaxel. Contemp
Oncol (Pozn). 19:423–427. 2015.PubMed/NCBI
|
|
23
|
Nath D, Slocombe PM, Webster A, Stephens
PE, Docherty AJ and Murphy G: Meltrin gamma(ADAM-9) mediates
cellular adhesion through alpha(6)beta(1)integrin, leading to a
marked induction of fibroblast cell motility. J Cell Sci.
113:2319–2328. 2000.PubMed/NCBI
|
|
24
|
Lendeckel U, Kohl J, Arndt M, Carl-McGrath
S, Donat H and Röcken C: Increased expression of ADAM family
members in human breast cancer and breast cancer cell lines. J
Cancer Res Clin Oncol. 131:41–48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shintani Y, Higashiyama S, Ohta M,
Hirabayashi H, Yamamoto S, Yoshimasu T, Matsuda H and Matsuura N:
Overexpression of ADAM9 in non-small cell lung cancer correlates
with brain metastasis. Cancer Res. 64:4190–4196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mazzocca A, Coppari R, De Franco R, Cho
JY, Libermann TA, Pinzani M and Toker A: A secreted form of ADAM9
promotes carcinoma invasion through tumor-stromal interactions.
Cancer Res. 65:4728–4738. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim JM, Jeung HC, Rha SY, Yu EJ, Kim TS,
Shin YK, Zhang X, Park KH, Park SW, Chung HC and Powis G: The
effect of disintegrin-metalloproteinase ADAM9 in gastric cancer
progression. Mol Cancer Ther. 13:3074–3085. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fry JL and Toker A: Secreted and
membrane-bound isoforms of protease ADAM9 have opposing effects on
breast cancer cell migration. Cancer Res. 70:8187–8198. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Itabashi H, Maesawa C, Oikawa H, Kotani K,
Sakurai E, Kato K, Komatsu H, Nitta H, Kawamura H, Wakabayashi G
and Masuda T: Angiotensin II and epidermal growth factor receptor
cross-talk mediated by a disintegrin and metalloprotease
accelerates tumor cell proliferation of hepatocellular carcinoma
cell lines. Hepatol Res. 38:601–613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hirao T, Nanba D, Tanaka M, Ishiguro H,
Kinugasa Y, Doki Y, Yano M, Matsuura N, Monden M and Higashiyama S:
Overexpression of ADAM9 enhances growth factor-mediated recycling
of E-cadherin in human colon cancer cell line HT29 cells. Exp Cell
Res. 312:331–339. 2006.PubMed/NCBI
|
|
31
|
Mahimkar RM, Visaya O, Pollock AS and
Lovett DH: The disintegrin domain of ADAM9: A ligand for multiple
beta1 renal integrins. Biochem J. 385:461–468. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Demirkan BM: The roles of
epithelial-to-mesenchymal transition (EMT) and
mesenchymal-to-epithelial transition (MET) in breast cancer bone
metastasis: Potential targets for prevention and treatment. J Clin
Med. 2:264–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie Y, Liu S, Lu W, Yang Q, Williams KD,
Binhazim AA, Carver BS, Matusik RJ and Chen Z: Slug regulates
E-cadherin repression via p19Arf in prostate tumorigenesis. Mol
Oncol. 8:1355–1364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cui Y and Yamada S: N-cadherin dependent
collective cell invasion of prostate cancer cells is regulated by
the N-terminus of α-catenin. PLoS One. 8:e550692013. View Article : Google Scholar : PubMed/NCBI
|