Introduction
Pancreatic cancer is one of the most frequently
malignant gastrointestinal cancer types and has a high mortality
rate for the majority of patients in advanced stages, with a median
survival of 3–6 months and a 5-year survival rate of less than 5%
(1). Pancreatic cancer ranks as the
tenth highest incidence, and the sixth most frequent cause of
mortality in China (2). In addition,
the incidence and mortality rate of pancreatic cancer has been
increasing yearly (3,4). The high mortality incidence is due to
the tumors being frequently metastatic at the time of initial
diagnosis, and despite an aggressive clinical approach systemic
therapies fail to treat pancreatic cancer (5). There are no specific clinical symptoms
in the early stages of the disease, and only mild symptoms will
appear in stage I pancreatic cancer, including epigastric
discomfort or indigestion. Surgery remains the primary treatment
for pancreatic cancer; however, only 5–25% of patients with
clinically diagnosed pancreatic cancer are eligible to receive
surgical treatment. Following complete resection of tumors and
adjuvant administration of gemcitabine, the median disease-free
survival for patients with pancreatic cancer is 13.4 months. The
5-year survival rate following pancreatic cancer resection is
<10%, and 50% of patients who have survived for 5 years succumb
to recurrence of disease within the subsequent 5 years (6,7). With
respect to the development of surgical treatments for pancreatic
cancer, novel therapy options remain challenging.
Patients who are not recommended for surgical
resection require adjuvant therapy, including chemotherapy and
radiotherapy, to extend their survival. Currently, the chemotherapy
drugs of choice for patients with pancreatic cancer are
gemcitabine, capecitabine, fluorouracil, mitomycin, adriamycin and
arsenic trioxide, and these are used in combination (8). However, as pancreatic cancer is
frequently insensitive to chemotherapy, the curative effect of
chemotherapy for this cancer type is poor (9). Therefore, it is important to identify
chemotherapy drugs to which patients with pancreatic cancer are
sensitive, or to identify alternative methods to improve the
sensitivity of patients to chemotherapeutic agents. It is also
important to reduce the side effects of radiation and chemotherapy
to improve the therapeutic effects and the prognosis of patients
with pancreatic cancer. There have been numerous previous studies
that have focused on natural anti-tumor drugs, and there is
increasing consideration of the anti-tumor effects of natural
products. A group of anti-cancer drugs from natural products,
including camptothecin and paclitaxel, have been established in
clinical practice and have demonstrated a curative effect for
certain types of cancer (10).
Silibinin is a flavonoid that is extracted from milk
thistle plants and has traditionally been used for detoxification
and in the treatment of liver diseases, as it is a natural drug
that protects the liver (11).
Silibinin has potential clinical value and has exhibited positive
effects in the treatment of liver diseases, diabetes, mushroom
poisoning, neurodegenerative diseases and numerous types of cancer
(12,13).
In vitro and in vivo studies,
silibinin has been established to have inhibitory effects in a
number of cancer types, including cutaneous carcinoma, breast,
cervical, prostate, colorectal, gastric carcinoma, bladder, lung,
ovarian, kidney, tongue, hepatocellular carcinoma and leukemia
(14). However, there have only been
a small number of studies on the inhibition effects of silibinin on
pancreatic cancer cells (15,16). Therefore, the present study used
pancreatic cancer SW1990 cells as a model to investigate the
inhibitory effects of silibinin on cell proliferation in pancreatic
cancer.
Materials and methods
Cell lines and reagents
The LO2 human hepatic cell line and the AsPC-1 and
SW1990 human pancreatic cancer cell lines were obtained from the
Department of Biochemistry, Medical College of Jinan University
(Guangzhou, China). All cells were maintained in RPMI-1640
supplemented with 5% fetal bovine serum at 37°C in 95% humidified
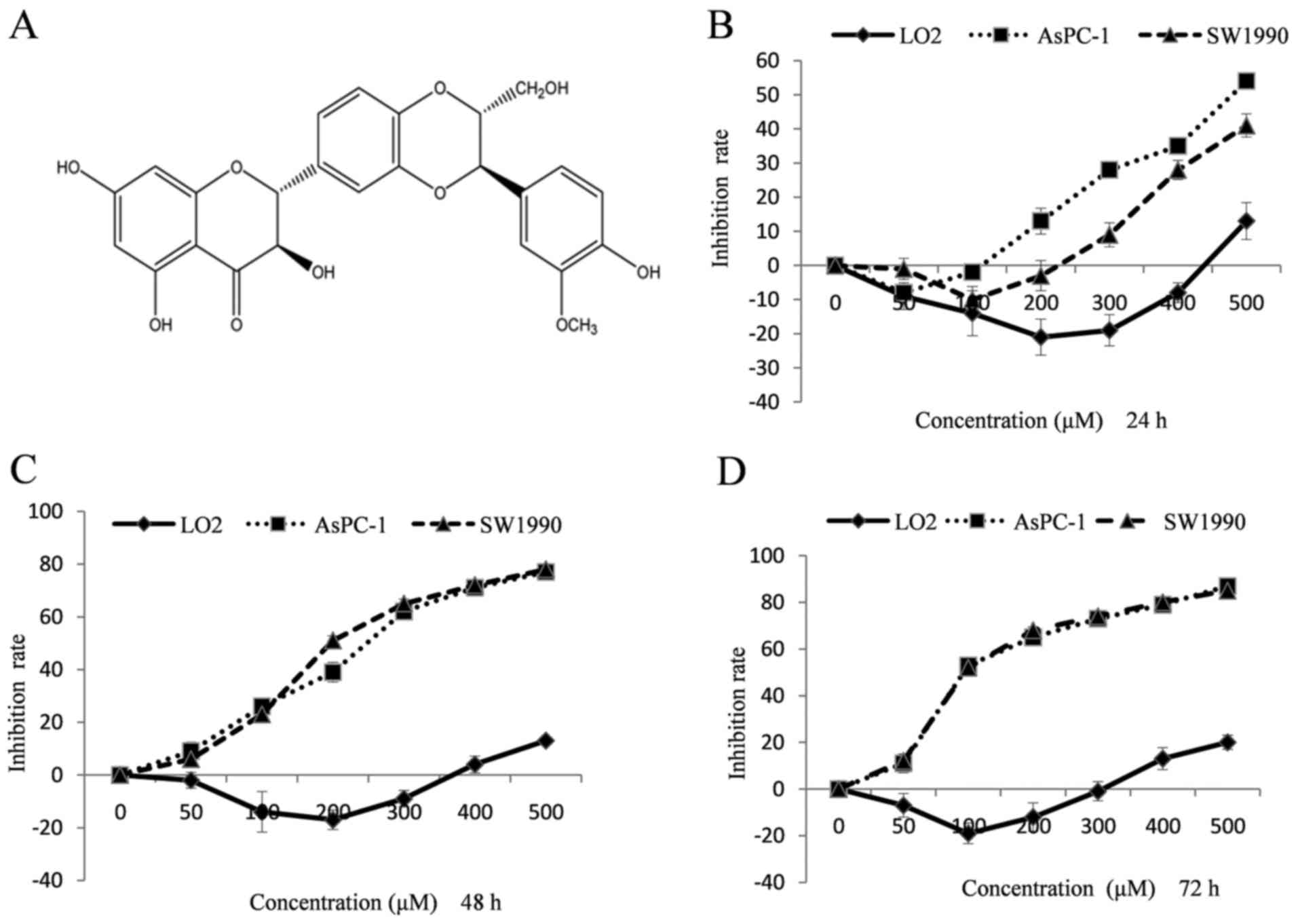
air containing 5% CO2. Silibinin (Fig. 1A) was purchased from Sichuan Weikeqi
Biological Technology Co., Ltd. (Chengdu, China).
Fetal bovine serum, RPMI-1640, trypsin and EDTA were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). MTT, dimethyl sulfoxide (DMSO), Annexin V-fluorescein
isothiocyanate (FITC) and propidium iodide (PI) were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The antibody
against cleaved caspase-3 was purchased from Epitomics (Burlingame,
CA, USA). Antibodies against cyclin D1 (cat. no. 2978; dilution
1:1,000), cyclin E2 (cat. no. 4132; dilution 1:1,000), cyclin A
(cat. no. 4656; dilution 1:1,000), cyclin B2 (cat. no. 4135;
dilution 1:1,000), cyclin-dependent kinase (CDK)4 (cat. no. 12790;
dilution 1:1,000), CDK6 (cat. no. 13331; dilution 1:1,000), p15
(cat. no. 8762; dilution 1:1,000), p21 (cat. no. 2947; dilution
1:1,000), B-cell lymphoma 2 associated protein X (Bax; cat. no.
5023; dilution 1:1,000), B-cell lymphoma 2 (Bcl-2; cat. no. 15071;
dilution 1:1,000), Bcl-2-like 1 (Bcl-xl; cat. no. 2764; dilution
1:1,000), myeloid cell leukemia 1(Mcl-1; cat. no. 94296; dilution
1:1,000), Bcl-2-like 1 small (Bcl-xs; cat. no. 2764; dilution
1:1,000), Bcl-2-like 11 (Bim; cat. no. 2933; dilution 1:1,000), Jun
N-terminal kinase (JNK; cat. no. 9252; dilution 1:1,000),
phosphorylated JNK (p-JNK; cat. no. 4668; dilution 1:1,000),
caspase-9 (cat. no. 9508; dilution 1:1,000), caspase-3 (cat. no.
9665; dilution 1:1,000), poly (ADP-ribose) polymerase (PARP; cat.
no. 9532; dilution 1:1,000), GAPDH (cat. no. 5174; dilution
1:1,000) and horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G secondary antibody (cat. no. 7054; dilution,
1:5,000), and horseradish peroxidase-conjugated goat anti-mouse
immunoglobulin G secondary antibody (cat. no. 7056; dilution,
1:5,000) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA).
Cell growth and viability assay
Cell growth and viability were measured using an MTT
assay. The normal liver cell LO2 and human pancreatic cancer AsPC-1
and SW1990 cell lines in logarithmic growth phase were seeded into
96-well plates at a density of 5×103 cells/well and
incubated at 37°C overnight. Subsequently, the cells were treated
with varying concentrations of silibinin (50, 100, 200, 300, 400
and 500 µM) and DMSO (<0.1%) was used as the vehicle control (5
repeats were performed in each group). Cells were incubated for 24
to 72 h in standard culture conditions (37°C containing 5%
CO2). Subsequently, 20 µl MTT (5 mg/ml) reagent was
added to each well followed by an additional incubation of 4 h at
37°C. The supernatants were discarded and 150 µl of DMSO was added,
and the cells were agitated to dissolve the purple precipitate. A
spectrophotometer was used to measure the absorbance at a
wavelength of 570 nm. The cell inhibitory rate was calculated using
the following equation: Cell inhibitory
rate=(1-Atreatment/Ablank) ×100%, where A was
the absorbance.
Flow cytometry analysis for cell cycle
distribution
SW1990 cells in the logarithmic growth phase were
seeded at a density of 3×105 cells/well into 6-well
tissue culture plates. The cells were treated with various
concentrations of silibinin (50, 100 and 200 µM) and DMSO
(<0.1%) was used as the vehicle control. Following 48 h of
incubation at 37°C, cells were harvested using trypsin. The cells
were washed twice with ice-cold PBS and fixed in 70% ethanol
overnight at 4°C. Subsequently, the cell pellets were washed three
times with PBS and incubated with PI (500 g/l) and RNase (20 U/ml)
for 30 min at room temperature in the dark. The DNA content was
determined by flow cytometry by ModFit 2.0 software (FCM; Beckman
Coulter, Inc., Brea, CA, USA).
Annexin V-FITC/PI staining and flow
cytometric analysis to detect apoptosis
SW1990 cells in logarithmic growth phase were seeded
at a density of 3×105 per well into 6-well tissue
culture plates. Subsequently, cells were treated with various
concentrations of silibinin (50, 100 and 200 µM), and DMSO
(<0.1%) was used as the vehicle control. Following 48 h of
incubation at 37°C, the cells were harvested using trypsin. The
cells were washed twice with ice-cold PBS, re-suspended in 200 µl
of binding buffer (HEPES, NaCl and CaCl2) and incubated
with 10 µl Annexin V-FITC and 5 µl of PI for 15 min at room
temperature in the dark. A flow cytometer was used to analyze the
percentage of early and late apoptotic cells. A total of 10,000
cells were analyzed by WinMDI version 2.9 software (The Scripps
Research Institute, San Diego, CA, USA).
Western blotting analysis
SW1990 cells were seeded at a density of
3×105 per well into 6-well tissue culture plates. The
cells were incubated at 37°C with various concentrations of
silibinin (50, 100, 150 and 200 µM) for 48 h, and DMSO (<0.1%)
was used as the vehicle control. Subsequently, the cells were
harvested and washed twice with ice-cold PBS. The cells were lysed
in buffer (Biospec, Inc., Bartlesville, OK, USA) on ice for 30 min
and centrifuged at 12,000 × g for 15 min at 4°C. The cell proteins
were extracted and the concentrations were determined using a
bicinchoninic acid assay. A total of 30 µg of protein was denatured
by the addition of 5X reducing sample buffer (Biospec, Inc.),
incubated for 5 min at 100°C and separated by SDS-PAGE (10% or
15%). The proteins were transferred to polyvinylidene difluoride
membranes. Following blocking with 5% dried skimmed milk, the
membranes were incubated overnight at 4°C with the appropriate
primary antibodies. Subsequently, the membranes were washed 3 times
in TBST (10 mM Tris, 100 mM NaCl and 0.1% Tween 20) for 5 min each
and incubated for 1 h at room temperature with a secondary antibody
conjugated to horseradish peroxidase. Following washing in TBST,
the bound antibody complex was detected using an
electrochemiluminescence reagent (HRP Substrate Luminol Reagent and
HRP Substrate Peroxide Solution; Biospec, Inc.) and XAR film
(Kodak, Rochester, NY, USA) as described previously (ImageJ v1.46d;
National Institutes of Health, Bethesda, MD, USA) (17). The data were quantitated from at least
three separate experiments.
Statistical analysis
The results are expressed as the mean ± standard
error from 3 independent experiments using SPSS 16.0 software
(SPSS, Inc., Chicago, IL, USA). Student's t-tests were used to
determine the significance between the control and test groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Silibinin inhibits the proliferation
of LO2, AsPC-1 and SW1990 cells
To investigate the effects of silibinin on cell
proliferation, an MTT assay was used to analyze the proliferation
of LO2, AsPC-1 and SW1990 cells. The results demonstrated that
varying concentrations of silibinin had little effect on LO2 cells.
Notably, silibinin enhanced LO2 cell proliferation at
concentrations <300 µM (Fig.
1B-D). The proliferation of AsPC-1 and SW1990 cells was
significantly inhibited with silibinin treatment in a dose- and
time-dependent manner (Fig. 1B-D).
The half maximal inhibitory concentration (IC50) values
of silibinin on AsPC-1 at 48 and 72 h were 224.20 and 87.25 µM,
respectively. The IC50 values on SW1990 cells at 48 and
72 h were and 218.41 and 86.91 µM, respectively.
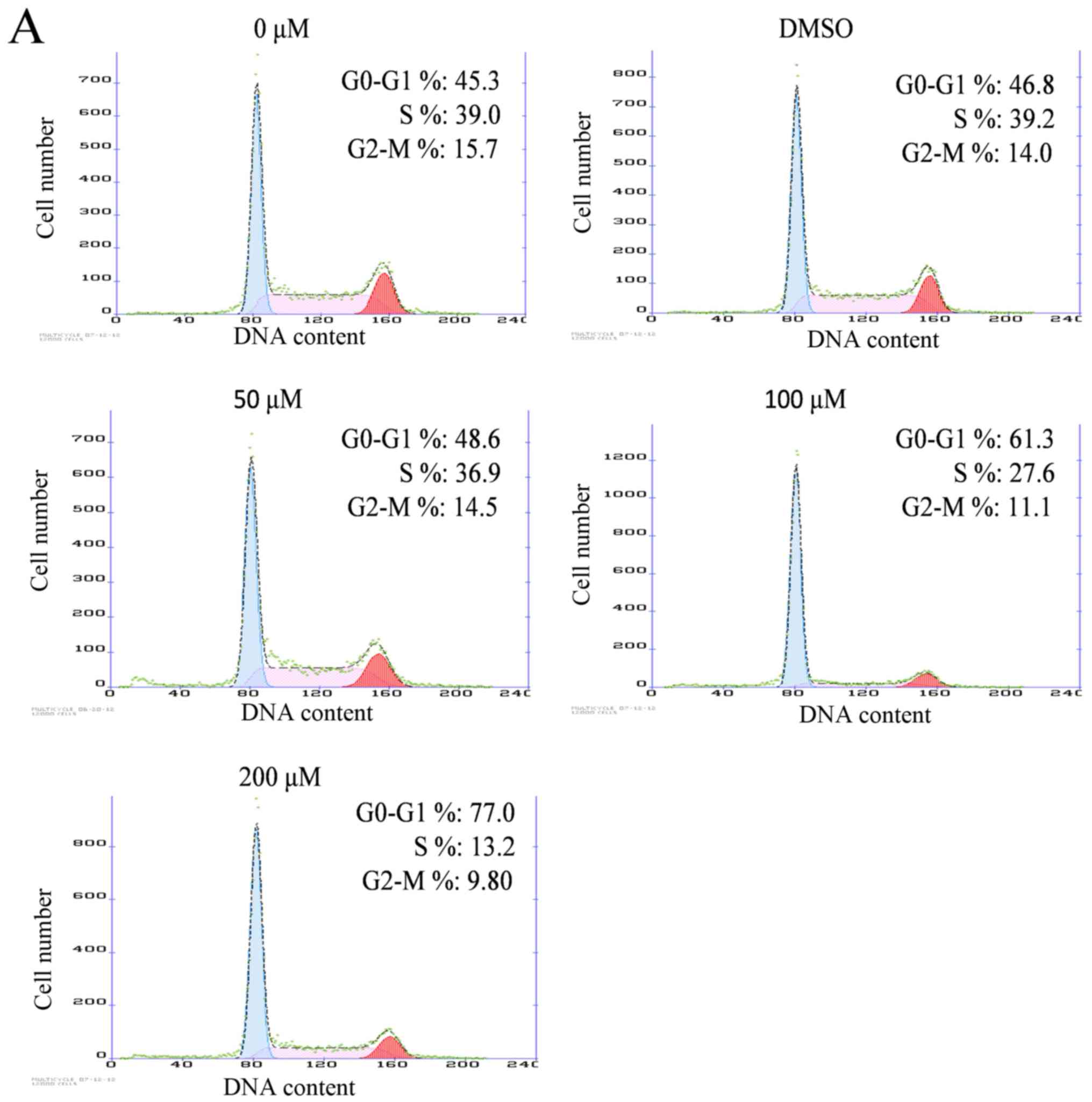
Effects of silibinin on cell cycle
distribution
As the decrease in cell growth was observed
following silibinin treatment, which may be due to the induction of
cell cycle arrest, the effect of silibinin on cell cycle
distribution was examined in SW1990 cells. Flow cytometry following
PI staining assay was used to analyze the effects of silibinin on
cell cycle distribution. As presented in Fig. 2A, the population of cells was arrested
in G1 phase (48.6–77.0% compared with 46.8% in DMSO-treated
controls). The populations of cells in S phase and G2-M phase were
reduced with increasing concentrations of silibinin. These results
suggest that cell cycle progression was arrested in G1 phase.
Silibinin modulates the expression of
cell cycle regulatory proteins
As significant cell cycle arrest was observed
following silibinin treatment, the expressions of cell cycle
regulatory proteins as Cyclins, CDKs and CDK inhibitors were
analyzed using western blotting analysis. The results demonstrated
that the protein expression levels of cyclin D1, cyclin E, cyclin
A, cyclin B1 and G1 phase-associated CDK4 and CDK6 were
significantly reduced following treatment with silibinin for 48 h.
However, the expression level of p15 increased, whereas the level
of p21 was not altered (Fig. 2B).
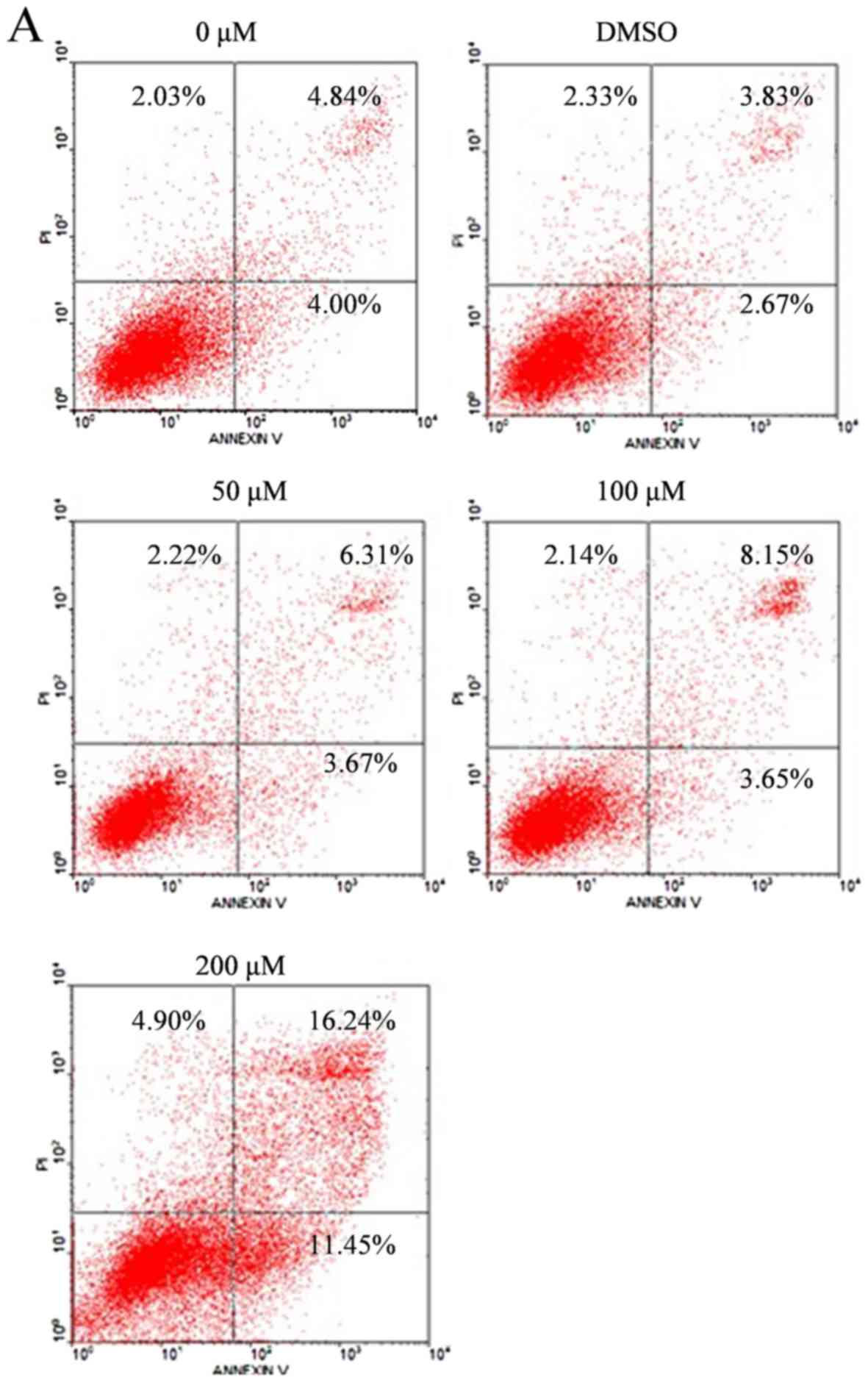
Silibinin induces apoptosis in human
pancreatic cancer SW1990 cells
To determine whether silibinin induces apoptosis,
the SW1990 cells were treated with silibinin at 0, 50, 100 or 200
µM or 0.1% DMSO for 48 h. Cells were double stained with Annexin
V-FITC and PI. Annexin V assay identified that the populations of
apoptotic cells increased with higher silibinin concentrations. The
apoptosis rate was 27.69% when the SW1990 cells were treated with
silibinin at a concentration of 200 µM for 48 h, while in the
control group, the apoptosis rate was 8.84% (Fig. 3A).
To investigate the underlying mechanism by which
silibinin induced apoptosis, western blotting analysis was
conducted with antibodies against caspase-3, caspase-9 or PARP.
Treatment with silibinin for 48 h promoted the activation of a
number of apoptotic proteins, including caspase-9, caspase-3 and
its substrate, PARP, via protein cleavage (Fig. 3B). Increased silibinin concentrations
resulted in a reduction of the intact proteins and an increase in
proteolytic cleavage bands in a concentration-dependent manner.
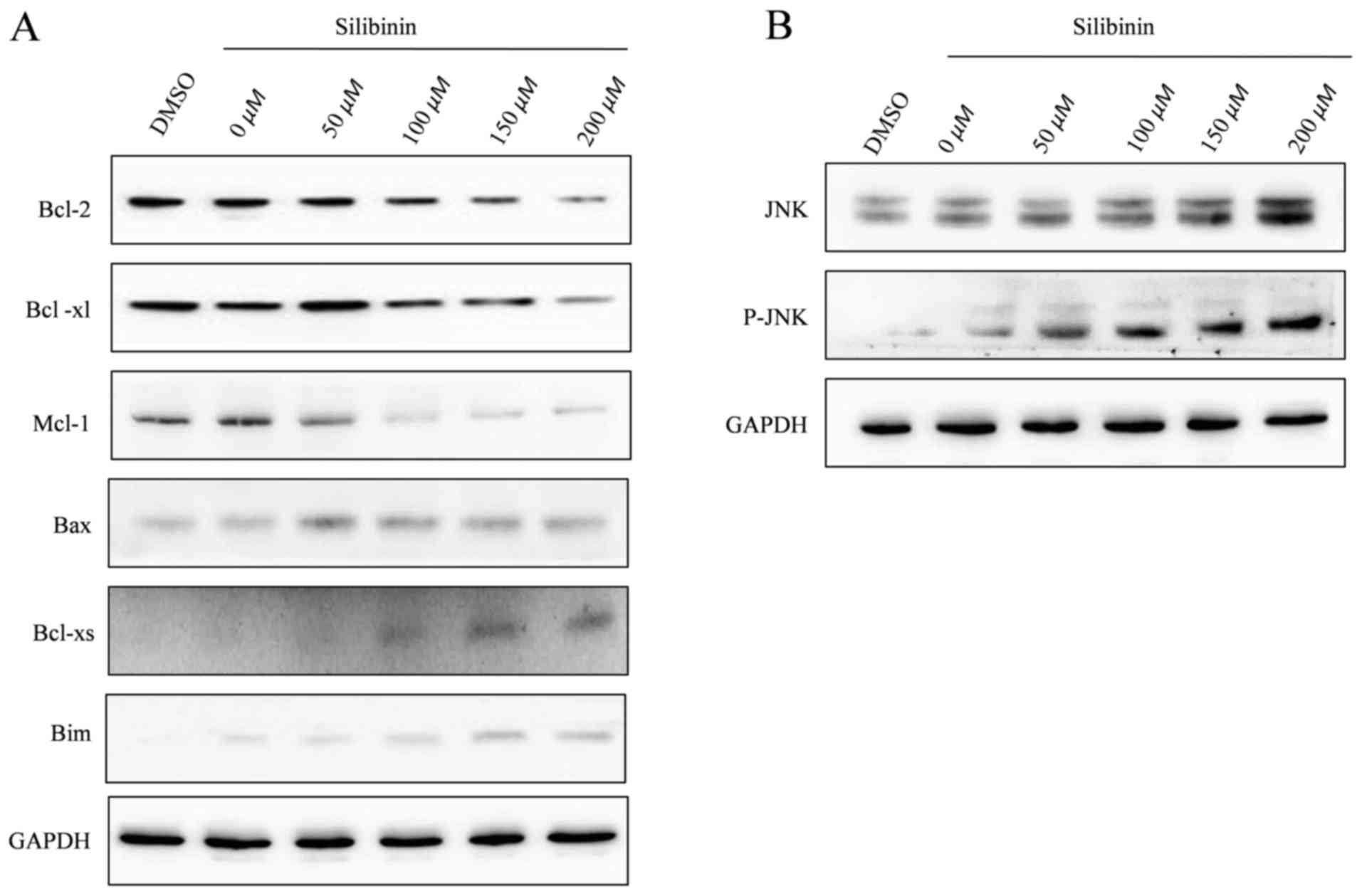
Detection of the protein expression
levels of Bcl-2 family proteins and JNK using western blotting
analysis
The mitochondrial death pathway is controlled by
members of the Bcl-2 family. The Bcl-2 family serves a central
regulatory role in deciding the fate of cells via the interaction
between pro- and anti-apoptotic members (18). Therefore, whether the
mitochondrial-mediated apoptosis in SW1990 cells induced by
silibinin treatment was modulated by Bcl-2 family members was
investigated.
Silibinin suppressed the expression levels of Bcl-2,
Mcl-1 and Bcl-xl, but did not alter the expression of Bax. The
expression of pro-apoptotic protein (Bcl-xs) and the BH3-only
protein, Bim, increased (Fig. 4A). As
a result of these effects, the ratios of Bcl-2/Bax, Mcl-1/Bax and
Bcl-xl/Bax were reduced during apoptosis. The protein expression
levels of JNK and p-JNK increased with higher concentrations of
silibinin (Fig. 4B). These results
suggest that silibinin induces apoptosis in SW1990 cells through
the activation of JNK and altering the expression levels of the
Bcl-2 anti-apoptotic proteins and BH3-only proteins triggering the
mitochondrial apoptosis signaling pathway.
Discussion
Certain anti-cancer agents have been extracted and
purified from plants, including taxol and camptothecin. These drugs
have been established in the clinic as effective chemotherapy
agents and have demonstrated beneficial effects in certain types of
carcinoma. In addition, numerous plant extracts including, tea
polyphenol, resveratrol, ginger extract and soy isoflavones, have
demonstrated potential anti-tumor effects, which may provide a
novel direction for the study of potential anti-cancer drugs.
Pancreatic cancer has poor outcomes with regards to surgical
resection and chemotherapy (19,20). In
China, the incidence of pancreatic cancer has continued to increase
yearly (21). The search for an
effective chemotherapeutic drug with low toxicity is an important
focus of studying pancreatic cancer treatment. Silibinin serves a
role in anti-oxidation, anti-fibrosis, anti-inflammation,
immunoregulation and in the promotion of hepatocyte regeneration
(22). It has been used in the clinic
for >20 years for the treatment of hepatitis, fatty liver,
cirrhosis and other diseases (23).
Apart from its hepatoprotective effects, silibinin has received
increased interest due to its anti-tumor efficacy. It was
identified that silymarin and silibinin exhibit anti-tumor effects
and may inhibit the growth of prostate, skin, bladder, lung, colon,
breast, ovarian, renal, liver and tongue cancer (24–29).
Notably, silibinin has been used in phase I clinical trial for the
treatment of prostate cancer. Silymarin and silibinin exhibit low
toxicity, and consequently their median lethal dosage values are
unable to be measured in animal experiments. Additionally,
silibinin is used as an antioxidant food additive in the United
States and Europe (30,31).
The present study investigated the inhibitory
effects of silibinin on the proliferation of SW1990 pancreatic
cancer cells and the potential underlying mechanisms of action. An
MTT assay was used to detect the inhibitory effect of varying
concentrations of silibinin on the proliferation of LO2 normal
human liver cell line and on AsPC-1 and SW1990 pancreatic cancer
cell lines. The results demonstrated that there was no marked
inhibitory effect of silibinin on the proliferation of LO2 cells,
whereas the inhibition of proliferation of AsPC-1 and SW1990 cells
was significantly altered and occurred in a time- and
dose-dependent manner.
The effects of silibinin treatment on cell cycle
distribution differ between cell types. For example, silibinin
promotes a G2 phase cell cycle arrest in human gastric carcinoma
SGC7901 cells (32); however, it also
triggers G2/M and G1 arrest in the TCC-SUP and T-24 human bladder
transitional cell carcinoma cell lines, respectively (33). Silibinin promotes AsPC-1 human
pancreatic cancer cells to arrest in G1 phase but does not markedly
affect BxPC-3 and Panc-1 cells (17).
Kaur et al (34) indicated
that silibinin treatment notably inhibits the growth of Lovo cells
and induces increased levels of the apoptosis-associated cleaved
caspases (caspases 3 and 9) and cleaved PARP, and therefore
promotes cell cycle arrest at G1 phase.
Using flow cytometry assay, a significant G1 phase
arrest in the silibinin group was identified and western blotting
analysis demonstrated that silibinin increased the expression of
p15INK4B and did not alter the expression of
p21Cip1 in SW1990 cells. p15, which is upstream of
CDK4/CDK6, inhibits CDK4/CDK6 activity, as well as inhibits the
activity of the CDK4/cyclin D and CDK6/cyclin D complexes. In the
current study, a decrease in the expression of the G1
phase-associated cell cycle proteins cyclin D1 and the
cyclin-dependent kinases CDK4 and CDK6 was revealed using western
blotting analysis. As the majority of cells cannot proceed to the
next phase, the expression of late G1 phase-, S phase- and G2
phase-associated cell cycle proteins, including cyclin E2, cyclin A
and cyclin B1, was also reduced in the cells that were treated with
silibinin. These results are consistent with G1 phase arrest, as
well as with the results of the flow cytometry experiments.
Previous studies have demonstrated that apoptosis
may occur via three signaling pathways, including the death
receptor-mediated pathway, the mitochondrial-mediated pathway and
the endoplasmic reticulum-associated pathway (35,36).
Caspase-9 is linked to the mitochondrial death pathway and once the
mitochondrial pathway is initiated, pro-caspase-9 is cleaved into
its active form. Activated caspase-9 cleaves its downstream
signaling protein pro-caspase-3, which promotes caspase-3
activation. PARP, an enzyme that is involved in the recognition of
genomic integrity and DNA repair, is inactivated by caspase
cleavage and is therefore regarded as a molecular marker of
apoptosis (29,37–39).
In the present study, SW1990 cells were stained with
Annexin V-FITC and PI and the cells in the silibinin-treated groups
were apoptotic; the percentage of apoptotic cells increased with
higher concentrations of silibinin. These results also demonstrated
that SW1990 cells treated with silibinin have activated
pro-caspase-9 and pro-caspase-3, and PARP was also cleaved into its
active form. These data suggest that silibinin induces apoptosis
via the mitochondrial-mediated signaling pathway in SW1990 cells
and this was consistent with the results of flow cytometry
analysis.
The mitochondrial-mediated apoptotic signaling
pathway is associated with the expression and activation of the
Bcl-2 protein family. Bcl-2 proteins are grouped into three
classes: Those that inhibit apoptosis (Bcl-2, Bcl-xl, Bcl-2-like 2,
Mcl-1, Bcl-10, Boo/Diva, NR-13 and Bcl-2-related protein A1); those
that promote apoptosis (Bak, Bax, Bcl-xs, Bcl2-related ovarian
killer); and the pro-apoptotic BH3-only proteins (Bad, Bid,
Bcl2-interacting killer, Bim, B lymphocyte kinase, Bcl2-modifiying
factor (Bmf), harakiki Bcl2-interacting factor, Bcl2-interacting
protein 23, NIP3-like protein X and Noxa) that bind and regulate
the anti-apoptotic Bcl-2 proteins (40,41). In
the present study, western blotting analysis demonstrated that with
increasing concentrations of silibinin, the expression of the
anti-apoptotic Bcl-2 family of proteins Bcl-2, Bcl-xl and Mcl-1 was
decreased, whereas the expression of the BH3-only protein Bim was
increased in a concentration-dependent manner. The expression of
the pro-apoptotic protein Bcl-xs was increased, whereas the
expression of another pro-apoptotic protein Bax was unaltered.
These results suggest that Bim may form a heterodimer with Bcl-2 or
Mcl-1 and promote dissociation of Bax/Bcl-2 or Bax/Mcl-1
heterodimers. This event promotes Bax oligomerization and
cytochrome-c release from the mitochondria, which initiates
the mitochondrial-mediated apoptotic signaling pathway. The
expression of the pro-apoptotic protein Bcl-xs was increased, and
therefore Bcl-xs may be able to activate Bak or Bax through
inhibiting the function of voltage-dependent anion channel 2 and
Bcl-xl (42). Although the protein
levels of anti-apoptotic proteins, including Bcl-2, Mcl-1 and
Bcl-xl, were decreased, the ratios of Bax/Bcl-2, Bax/Mcl-1 and
Bax/Bcl-xl were increased, resulting in an increased quantity of
unbound Bax and initiation of the mitochondrial-mediated apoptotic
signaling pathway.
JNK serves an important role in the death
receptor-initiated extrinsic apoptotic signaling pathway and the
mitochondrial intrinsic apoptotic signaling pathway. JNK promotes
apoptosis through two actions. Firstly, activated JNK locates to
the nucleus and activates transcription factors, including c-Jun.
This activation of c-Jun/AP1 or p53/p73, leads to the increased
expression of pro-apoptotic genes. The second action requires JNK
to locate into the mitochondria and phosphorylate BH3-only Bcl-2
proteins, including Bad Ser128, which inhibits 14-3-3 protein
interactions and therefore reducing the anti-apoptotic activity of
Bcl-2 and Bcl-xl. Phosphorylation of 14-3-3ξ at Ser184 by JNK does
not allow 14-3-3ξ to relocate. JNK is able to induce the cleavage
of Bid, independently of caspase-8, in HeLa cells during
JNK-induced apoptosis. The 21 kDa Bid fragment promotes the release
of the second mitochondria-derived activator of caspase, which is a
pro-apoptotic protein (43).
Apoptosis may also be initiated by JNK activation of Bax or Bak via
the phosphorylation of Bim and Bmf, which has been demonstrated in
HEK293T cells during UV-induced apoptosis (44). Finally, JNK phosphorylates Bcl-2 at
Ser70 and causes the dissociation of the Bcl-2/Bax heterodimer and
promotes cell apoptosis (45). The
present study identified that following treatment with silibinin,
the expression levels of JNK and p-JNK were increased in SW1990
cells, which was consistent with the results of Duan et al
(46). The results of the present
study indicated that the pro-apoptotic effects on SW1990 cells were
mediated via the JNK signaling pathway, and the activation of this
signaling pathway may activate the downstream
mitochondrial-associated apoptotic signaling pathway to induce cell
apoptosis.
In conclusion, silibinin suppresses the
proliferation of pancreatic cancer cells by the induction of G1
phase cell cycle arrest and through an increase in the expression
of p15INK4B. In addition, silibinin induced JNK
activation and initiated the mitochondrial-associated apoptotic
signaling pathway, which promoted the pancreatic cancer SW1990
cells to undergo apoptosis. The signaling pathway by which
silibinin upregulates p15INK4B in pancreatic cancer
SW1990 cells remains to be elucidated. Additionally, whether the
activation of JNK by silibinin promotes the dissociation of
Bcl-2/Bax or Bcl-xl/Bax and the oligomerization of Bax, and whether
silibinin suppresses the growth of pancreatic cancer in SW1990
xenograft mice are yet to be determined.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Sci-Tech Project Foundation of Guangdong Province in China (grant
no. 2011B031800012), the Medical Science Research Foundation of
Guangdong Province in China (grant no. A2013338) and the Natural
Science Foundation of Guangdong Province in China (grant no.
2014A030313356).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
JJ and MC contributed to the conception of the
study, and conceived and designed the experiments. XZ performed the
experiments and data analyses and wrote the manuscript. JL, PZ and
LD helped perform the analysis with constructive discussions, and
contributed significantly to analysis and manuscript preparation.
ZW and LW contributed reagents, materials and analysis tools and
helped perform the analysis with constructive discussions. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Quan JL, Feng Y, Chen J and Fu DL: Current
status and progress of pancreatic cancer in China. World J
Gastroenterol. 21:7988–8003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen WQ, Li H, Sun KX, Zheng RS, Zhang SW,
Zeng HM, Zou XN, Gu XY and He J: Report of cancer incidence and
mortality in different areas of China, 2014. Zhonghua Zhong Liu Za
Zhi. 40:5–13. 2018.(In Chinese). PubMed/NCBI
|
|
3
|
Kongkam P, Benjasupattananun P, Taytawat
P, Navicharoen P, Sriuranpong V, Vajragupta L, Klaikaew N, Ridtitid
W, Treeprasertsuk S, Rerknimitr R and Kullavanijaya P: Pancreatic
cancer in an Asian population. Endosc Ultrasound. 4:56–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gall TMH, Tsakok M, Wasan H and Jiao LR:
Pancreatic cancer: Current management and treatment strategies.
Postgrad Med J. 91:601–607. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pliarchopoulou K and Pectasides D:
Pancreatic cancer: Current and future treatment strategies. Cancer
Treat Rev. 35:431–436. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo G, Guo M, Liu Z, Xiao Z, Jin K, Long
J, Liu L, Liu C, Xu J, Ni Q and Yu X: Blood neutrophil-lymphocyte
ratio predicts survival in patients with advanced pancreatic cancer
treated with chemotherapy. Ann Surg Oncol. 22:670–676. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Foley K, Kim V, Jaffee E and Zheng L:
Current progress in immunotherapy for pancreatic cancer. Cancer
Lett. 381:244–251. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi S, Yao W, Xu J, Long J, Liu C and Yu
X: Combinational therapy: New hope for pancreatic cancer? Cancer
Lett. 317:127–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seicean A, Petrusel L and Seicean R: New
targeted therapies in pancreatic cancer. World J Gastroenterol.
21:6127–6145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodeiro I, Magarino Y, Ocejo O, Garrido G
and Delgado R: Use of natural products in anti-cancer alternative
therapy: Risk of interactions with conventional anti-cancer drugs.
Bol. Latinoam Caribe Plant Med Aromaticas. 7:332–344. 2008.
|
|
11
|
Sozen H, Celik OI, Cetin ES, Yilmaz N,
Aksozek A, Topal Y, Cigerci IH and Beydilli H: Evaluation of the
protective effect of silibinin in rats with liver damage caused by
itraconazole. Cell Biochem Biophys. 71:1215–1223. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheung CW, Gibbons N, Johnson DW and Nicol
DL: Silibinin-a promising new treatment for cancer. Anti Cancer
Agents Med Chem. 10:186–195. 2010. View Article : Google Scholar
|
|
13
|
Gazák R, Walterová D and Kren V: New and
emerging applications in medicine. Curr Med Chem. 14:315–338. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tiwari P and Kaushala PM: Silibinin in
cancer therapy: A promising prospect. Cancer Res Front. 1:303–318.
2015. View Article : Google Scholar
|
|
15
|
Ge Y, Zhang Y, Chen Y, Li Q, Chen J, Dong
Y and Shi W: Silibinin causes apoptosis and cell cycle arrest in
some human pancreatic cancer cells. Int J Mol Sci. 12:4861–4871.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nambiar D, Prajapati V, Agarwal R and
Singh RP: In vitro and in vivo anticancer efficacy of silibinin
against human pancreatic cancer BxPC-3 and PANC-1 cells. Cancer
Lett. 334:109–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng R, Jun T, Xia LP, Li DD, Zhou WJ,
Wang LL, Feng GK, Zeng YX, Gao YH and Zhu XF: ExcisaninA, a
diterpenoid compound purified from Isodon MacrocalyxinD, induces
tumor cells apoptosis and suppresses tumor growth through
inhibition of PKB/AKT kinase activity and blockade of its signal
pathway. Mol Cancer Ther. 8:873–882. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Conroy T, Bachet JB, Ayav A, Huguet F,
Lambert A, Caramella C, Maréchal R, Van Laethem JL and Ducreux M:
Current standards and new innovative approaches for treatment of
pancreatic cancer. Eur J Cancer. 57:10–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang X, Hao J, Zhu CH, Niu YY, Ding XL,
Liu C and Wu XZ: Survival benefits of western and traditional
Chinese medicine treatment for patients with pancreatic cancer.
Medicine (Baltimore). 94:e10082015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sultana A, Smith Tudur C, Cunningham D,
Starling N, Tait D, Neoptolemos JP and Ghaneh P: Systematic review,
including meta-analyses, on the management of locally advanced
pancreatic cancer using radiation/combined modality therapy. Brit J
Cancer. 96:1183–1190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pradhan S and Girish C: Hepatoprotective
herbal drug, silymarin from experimental pharmacology to clinical
medicine. Indian J Med Res. 124:491–504. 2006.PubMed/NCBI
|
|
23
|
Magliulo E, Gagliardi B and Fiori GP:
Results of a double blind study on the effect of silymarin in the
treatment of acute viral hepatitis, carried out at two medical
centres (author's transl). Med Klin. 73:1060–1065. 1978.(In
German). PubMed/NCBI
|
|
24
|
Deep G and Agarwal R: Antimetastatic
efficacy of silibinin: Molecular mechanisms and therapeutic
potential against cancer. Cancer Metastasis Rev. 29:447–463. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng D, Wang Y, Zhang D, Liu Z, Duan C,
Jia L, Wang F, Liu Y, Liu G, Hao L and Zhang Q: In vitro antitumor
activity of silybin nanosuspension in PC-3 cells. Cancer Lett.
307:158–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu KJ, Zeng J, Zhu GD, Zhang LL, Zhang D,
Li L, Fan JH, Wang XY and He DL: Silibinin inhibits prostate cancer
invasion, motility and migration by suppressing vimentin and MMP-2
expression. Acta Pharmacol Sin. 30:1162–1168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hogan FS, Krishnegowda NK, Mikhailova M
and Kahlenberg MS: Flavonoid, silibinin inhibits proliferation and
promotes cell-cycle arrest of human colon cancer. J Surg Res.
143:58–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Li Q, Ge Y, Chen Y, Chen J, Dong
Y and Shi W: Silibinin triggers apoptosis and cell-cycle arrest of
SGC7901 cells. Phytother Res. 27:397–403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lah JJ, Cui W and Hu KQ: Effects and
mechanisms of silibinin on human hepatoma cell lines. World J
Gastroenterol. 13:5299–5305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu K, Zeng J, Li L, Fan J, Zhang D, Xue Y,
Zhu G, Yang L, Wang X and He D: Silibinin reverses
epithelial-to-mesenchymal transition in metastatic prostate cancer
cells by targeting transcription factors. Oncol Rep. 23:1545–1552.
2010.PubMed/NCBI
|
|
31
|
Tyagi A, Bhatia N, Condon MS, Bosland MC,
Agarwal C and Agarwal R: Antiproliferative and apoptotic effects of
silibinin in rat prostate cancer cells. Prostate. 53:211–217. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Ge Y, Chen Y, Li Q, Chen J, Dong
Y and Shi W: Cellular and molecular mechanisms of silibinin induces
cell-cycle arrest and apoptosis on HeLa cells. Cell Biochem Funct.
30:243–248. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tyagi A, Agarwal C, Harrison G, Glode LM
and Agarwal R: Silibinin causes cell cycle arrest and apoptosis in
human bladder transitional cell carcinoma cells by regulating
CDKI-CDK-cyclin cascade, and caspase 3 and PARP cleavages.
Carcinogenesis. 25:1711–1720. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaur M, Velmurugan B, Tyagi A, Deep G,
Katiyar S, Agarwal C and Agarwal R: Silibinin suppresses growth and
induces apoptotic death of human colorectal carcinoma LoVo cells in
culture and tumor xenograft. Mol Cancer Ther. 8:2366–2374. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Indran IR, Tufo G, Pervaiz S and Brenner
C: Recent advances in apoptosis, mitochondria and drug resistance
in cancer cells. Biochem Biophys Acta. 1807:735–745.
2011.PubMed/NCBI
|
|
36
|
Gogvadze V, Orrenius S and Zhivotovsky B:
Mitochondria as targets for cancer chemotherapy. Semin Cancer Biol.
19:57–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Costantini P, Bruey JM, Castedo M,
Métivier D, Loeffler M, Susin SA, Ravagnan L, Zamzami N, Garrido C
and Kroemer G: Pre-processed caspase-9 contained in mitochondria
participates in apoptosis. Cell Death Differ. 9:82–88. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bialik S, Zalckvar E, Ber Y, Rubinstein AD
and Kimchi A: Systems biology analysis of programmed cell death.
Trends Biochem Sci. 35:556–564. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lüthi AU and Martin SJ: The CASBAH: A
searchable database of caspase substrates. Cell Death Differ.
14:641–650. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kang MH and Reynolds CP: Bcl-2 inhibitors:
Targeting mitochondrial apoptotic pathways in cancer therapy. Clin
Cancer Res. 15:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Plötz M, Gillissen B, Hossini AM, Daniel
PT and Eberle J: Disruption of the VDAC2-Bak interaction by
Bcl-x(S) mediates efficient induction of apoptosis in melanoma
cells. Cell Death Differ. 19:1928–1938. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Deng Y, Ren X, Yang L, Lin Y and Wu X: A
JNK-dependent pathway is required for TNFalpha-induced apoptosis.
Cell. 115:61–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Letai A, Bassik MC, Walensky LD,
Sorcinelli MD, Weiler S and Korsmeyer SJ: Distinct BH3 domains
either sensitize or activate mitochondrial apoptosis, serving as
prototype cancer therapeutics. Cancer Cell. 2:183–192. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wei Y, Sinha S and Lebive B: Dual role of
JNK1-mediated phosphorylation of Bcl-2 in autophagy and apoptosis
regulation. Autophagy. 4:949–951. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Duan WJ, Li QS, Xia MY, Tashiro S, Onodera
S and Ikejima T: Silibinin activated p53 and induced autophagic
death in human fibrosarcoma HT1080 cells via reactive oxygen
species-p38 and c-Jun N-terminal kinase pathways. Biol Pharm Bull.
34:47–53. 2011. View Article : Google Scholar : PubMed/NCBI
|