Introduction
Smoking is the primary cause of lung cancer. The
proportion of cases attributable to smoking has reached 90% in
countries with ongoing high tobacco consumption (1). Cigarette smoke contains at least 69
carcinogens, including ammonia, cadmium, nickel, nicotine (2), and nitrosamines such as
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (3). Tobacco smoke components not only cause
cancer, but may also be involved in tumor invasiveness and
metastasis. Cigarette smoking is known to increase the risk of
prostate cancer metastasis (4), the
metastatic ability of breast cancer cells (5), and the risk of pulmonary metastasis of
breast cancer (6). Tobacco smoke may
also increase the spread of lung carcinoma cells (7); however, the mechanism by which this
happens is, to the best of our knowledge, unclear.
Metastasis is a well-regulated process (8) that depends on the invasion of cancer
cells into surrounding tissues; it is a leading cause of cancer
mortality (9), and is characteristic
of lung cancer. Transforming growth factor-β (TGF-β) may be a key
regulator of tumor cell invasion and metastasis. TGF-β1, TGF-β2 and
TGF-β3 are members of a superfamily of secreted cytokines that
regulate cellular processes, including proliferation,
differentiation, migration, survival, and immunity, by
ligand-receptor binding (10–12). TGF-β family members are ubiquitously
expressed. The TGF-β1-induced epithelial-mesenchymal transition in
lung cancer is a key first step in metastasis (13), and exposure of A549 cells to cigarette
smoke extract (CSE) induces the expression, release and activation
of TGF-β1 (14).
Matrix metalloproteinase (MMP) activity in tumor
cell metastasis includes degrading of basement membranes and the
extracellular matrix, which facilitates tumor invasion and
metastasis (15,16). TGF-β1 has been reported to stimulate
the expression of matrix metalloproteinase 3 (MMP3) in human
corneal epithelial cells (17), but
it is not known whether it has similar activity in lung cancer
cells. The present study investigated the effect of CSE on the
invasiveness of A549 cells and the possible involvement of TGF-β.
The proliferation and invasiveness of A549 cells increased
following CSE exposure. Expression of TGF-β1, mothers against
decapentaplegic homolog 2 (Smad2), and MMP3 was significantly
increased by CSE and partly abrogated by SB431542, a TGF-β1
receptor inhibitor. SB431542 inhibited the CSE-induced invasiveness
of A549 cells via the TGF-β1/MMP3 pathway.
Materials and methods
Cell culture and reagents
The A549 cell line was purchased from the American
Type Culture Collection (Manassas, VA, USA) and cultured in
Dulbecco's Modified Eagle's Medium (DMEM) (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (FBS) (Thermo Fisher Scientific, Inc.) at 37°C in a
humidified 5% CO2 atmosphere. The Cell Counting kit-8
(CCK-8) kit was obtained from Dojindo Molecular Technologies Inc.
(Kumamoto, Japan). GAPDH, TGF-β1, phosphorylated (p)-Smad2, and
MMP3 primary antibodies (dilution 1:1,000) were obtained from Abcam
(Cambridge, MA, USA). Solid SB431542 (cat. no. HY-10431) was
obtained from MedChemExpress (Monmouth Junction, NJ, USA). The
SB431542 was dissolved in 1 ml DMEM and the concentration was
adjusted to of 10 mmol/l, which was verified to have no effect on
the cell proliferation in preliminary experiments.
CSE preparation
Research cigarettes were purchased from Chengdu
Tobacco Industry Co., Ltd. (Chengdu, China); when burned, each
cigarette contained 11 mg tar, 17 mg carbon monoxide, and 1.1 mg
nicotine. CSE was prepared as described by Wirtz and Schmidt
(18). Briefly, the filters were
removed, cigarettes were installed on a pumping apparatus, and
completely combusted in 2 min. The smoke from ten cigarettes was
bubbled through a glass vessel containing 10 ml of serum-free DMEM,
which was then adjusted to pH 7.4 and filtered through a 0.22-µm
filter (EMD Millipore, Billerica, MA, USA) to remove particles and
bacteria. The CSE was standardized by measuring the absorbance at a
wavelength of 320 nm with a DU 640 spectrophotometer (Beckman
Coulter, Inc., Brea, CA, USA). DMEM was used as the blank control.
The CSE spectrogram exhibited little variance (1.36±0.12 mmol/l)
across preparations. The concentration of the resulting solution
was designated as 100% and was diluted as required (0.1, 1.0 and
10.0%) for use in the experimental procedures. The CSE solutions
were freshly prepared, and used within 30 min of preparation.
Cell Counting kit-8 (CCK-8)
proliferation assay
In brief, A549 cells were treated with CSE, then
seeded into 96-well plates at a density of 5×104
cells/well in 100 µl DMEM and incubated at 37°C. When the cells
reached 70% confluence, the medium was replaced with an equal
volume containing CSE at concentrations of 0.1, 1.0 and 10.0% and
cultured for 12, 24, or 48 h before addition of 10 µl CCK-8
solution. After 1–2 h, absorbance was read at 490 nM using a
microplate reader (BioTek Instruments, Inc., Winooski, VT,
USA).
Cell motility assay
The invasiveness of A549 cells was assayed in
BioCoat Matrigel-coated invasion chambers (BD Biosciences, Franklin
Lakes, NJ, USA) with 8-µm-pore size polycarbonate membranes. Cells
were grown in serum-free DMEM at 37°C for 2 h, the medium was
removed and 750 µl DMEM with 10% FBS was added into the lower
chamber as a chemoattractant. A549 cells were treated with CSE or
SB431542, then added to each upper chamber at a density of
5×104 cells/well in 2 ml DMEM with 1% FBS. After 2 h,
1.0% CSE and 100 nmol/l SB431542 were added to the upper chambers.
The inserts and non-invasive cells were removed after 12 h. The
invasive cells on the lower surface of the membrane were then fixed
in 100% methanol for 15 min at room temperature, air dried, and
stained with crystal violet for 30 min at room temperature. The
numbers of cell in five random visual fields with a fluorescence
microscope (Olympus Corporation, Tokyo, Japan) at a magnification
of ×200 were recorded.
Western blot assays
Cells were treated with CSE or SB431542, then
separated by 1 ml 0.25% trypsin (Thermo Fisher Scientific, Inc.),
then disrupted in ice-cold lysis buffer containing protease and
phosphatase inhibitors (cat. no. FNN0011; Thermo Fisher Scientific,
Inc.) for 30 min, and then clarified by centrifugation at 2,000 × g
for 10 min at 4°C. Total protein concentration was determined using
a bicinchoninic acid assay, and the sample was boiled for 5 min
before loading. The cell lysate was resuspended in SDS buffer
(Beyotime Institute of Biotechnology, Haimen, China), and 40 µg
samples of protein were separated by 8% SDS-PAGE (Beyotime
Institute of Biotechnology). The proteins were transferred to
polyvinylidene difluoride membranes (EMD Millipore), blocked for 2
h with 5% bovine serum albumin (Beyotime Institute of
Biotechnology) incubated with primary antibodies: Antibodies of
GAPDH (cat. no. ab8245; dilution 1:1,000); TGF-β1 (cat. no.
ab92486; dilution 1:1,000); phosphorylated (p)-Smad2 (cat. no.
ab40855; dilution 1:1,000); and MMP3 (cat. no. ab53015; dilution
1:1,000) were obtained from Abcam. The incubation was overnight at
4°C, and then incubated with the corresponding horseradish
peroxidase-conjugated secondary antibody (cat. no. HP6023; dilution
1:1,000; Abcam) for 2 h at 20°C. Immunoreactivity was visualized by
SuperEnhanced chemiluminescence kit (Millipore, Bedford, MA, USA)
and the results were analyzed by Quantity One software v4.4.02
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Experimental procedures were performed in
triplicate, and the results were expressed as the mean ± standard
deviation. The significance of differences between the CSE groups
was assessed by one-way analysis of variance followed by Dunnett's
test. Student's t-test was used to compare the differences in
different treatment groups. Statistical analysis was performed with
GraphPad Prism version 5.01 (GraphPad Software, Inc., La Jolla, CA,
USA). P<0.05 was considered to indicated statistically
significant difference.
Results
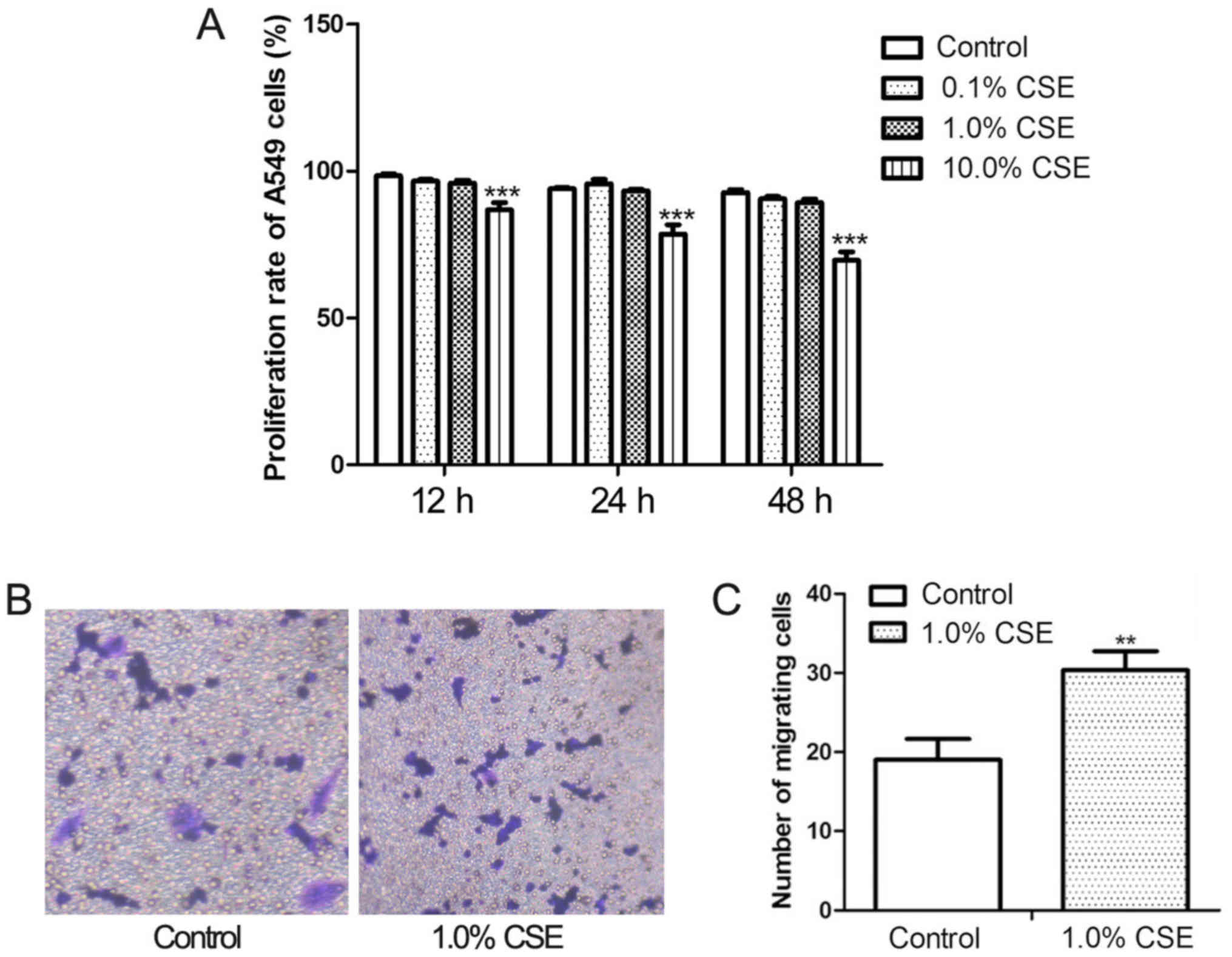
CSE treatment increases the
invasiveness of A549 cells
The proliferation and invasiveness of A549 cells
were assayed following CSE treatment at concentrations of 0.1, 1.0,
and 10.0% for 12, 24, and 48 h (Fig.
1A). At a concentration of 10.0%, CSE significantly decreased
the proliferation of A549 cell; however, proliferation was not
significantly affected by 1.0% CSE. Therefore, the effect of CSE on
invasiveness was evaluated at a concentration of 1.0%. The results
of the Transwell invasion assay (Fig.
1B) revealed that there were significantly more invasive cells
on the lower membrane surface following CSE treatment compared with
control cultures (P<0.05).
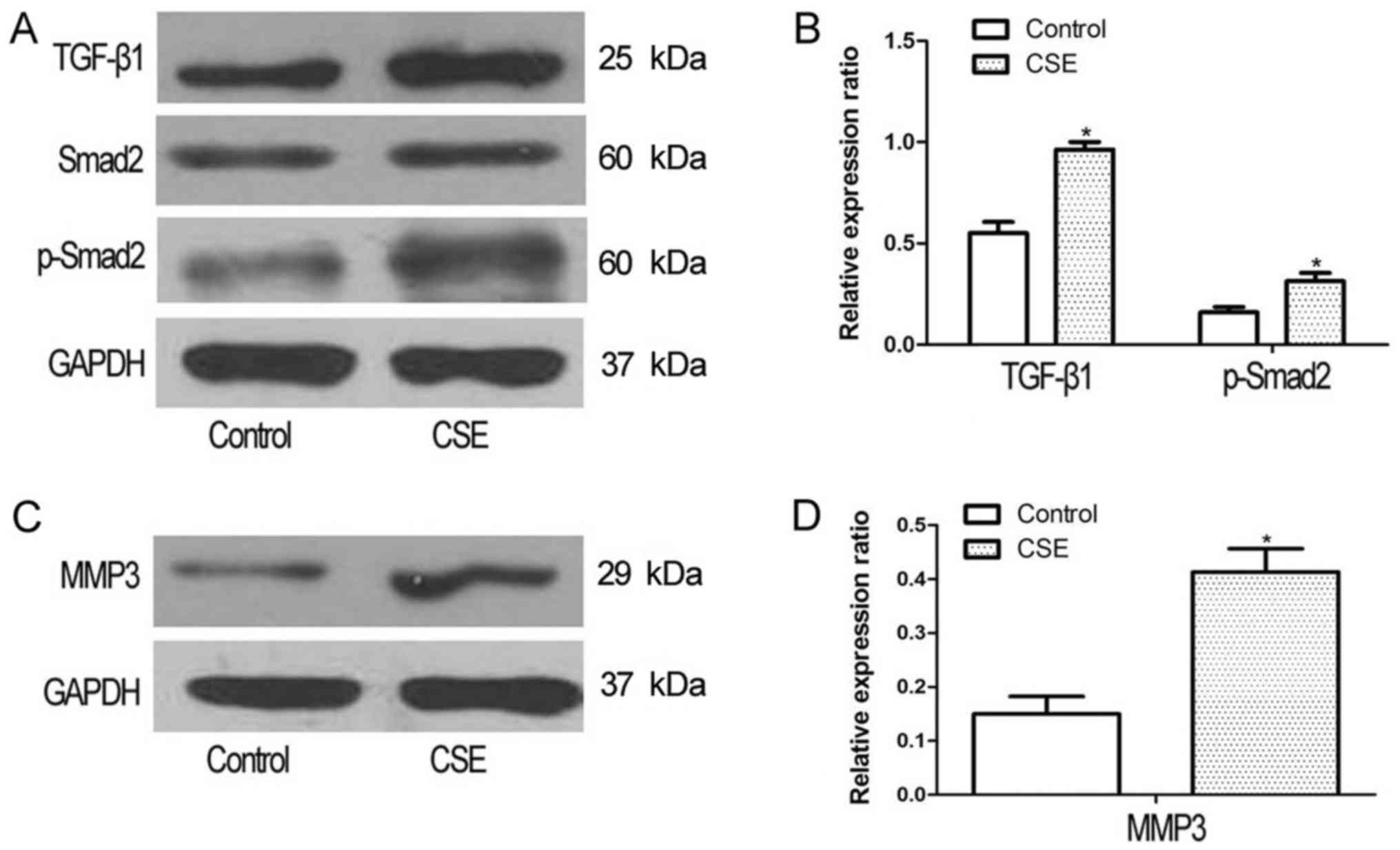
CSE exposure significantly increases
TGF-β1, Smad2, and MMP3 expression in A549 cells
To determine whether CSE promoted the activation of
the TGF-β1 pathway, A549 cells were treated with 1.0% CSE for 48 h,
and the expression of TGF-β1 and Smad2, which mediates TGF-β1
signaling, was assessed. The expression levels of TGF-β1 and Smad2
were significantly increased following CSE exposure (Fig. 2A and B). The expression of MMP3, an
indicator of metastasis, was also significantly increased in A549
cells following exposure to CSE (Fig. 2C
and D).
Increased TGF-β1, Smad2, MMP3
expression and cell invasiveness in response to CSE is partly
inhibited by SB431542
A549 cells were exposed to SB431542, a TGF-β1
receptor antagonist, to assess the involvement of TGF-β1/Smad2, and
MMP3 following CSE treatment. At a density of 10 mmol/l, which was
verified to have no effect on the cell proliferation in preliminary
experiments (data not shown), to block the TGF-β1 receptor,
SB431542 significantly decreased TGF-β1, Smad2 and MMP3 expression
(Fig. 3A and B). In the Transwell
invasion assay, SB431542 inhibited the effect of CSE on the
invasiveness of A549 cells. The number of invasive CSE-exposed A549
cells was significantly decreased by treatment with SB431542
(Fig. 3C and D).
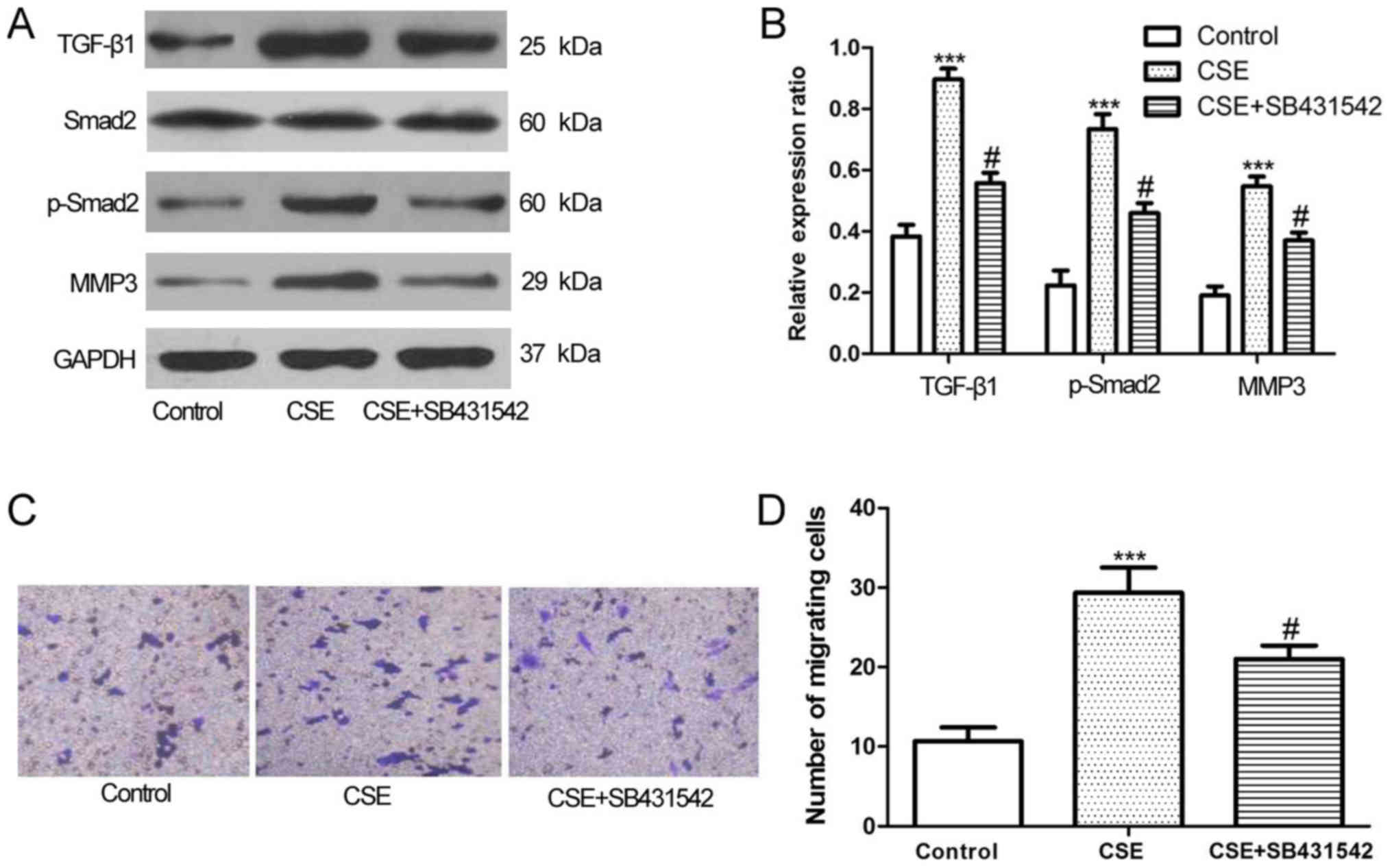 | Figure 3.Levels of TGF-β1, p-Smad2, and MMP3
increased by CSE treatment in A549 cells was significantly
inhibited by SB431542. (A) A549 cells were treated with 1.0% CSE
with or without 10.0 mmol/l SB431542 for 48 h, the levels of
TGF-β1, p-Smad2, and MMP3 was assayed by western blot analysis,
with (B) densitometry analysis confirming this. (C) The
invasiveness of A549 cells was determined by Transwell assays and
(D) quantified. ***P<0.001 vs. control; #P<0.05,
CSE+SB431542 vs. CSE only. CSE, cigarette smoke extract; TGF-β1,
transforming growth factor-β1; p-Smad2, phosphorylated mothers
against decapentaplegic homolog 2; MMP3, matrix metalloproteinase
3. |
Discussion
CSE exposure increased the proliferation and
invasiveness of A549 cells; it also increased MMP3 production and
TGF-β1/Smad2 pathway activity, which were inhibited by SB431542, a
known TGF-β1 receptor antagonist.
The smoke generated from the tobacco in cigarettes
exposes the smoker to upwards of 4,000 different xenobiotic
chemicals (19,20), and exposure to cigarette smoke
increases the risk of lung cancer (21). Cigarette smoke has also been
associated with pancreatic cancer metastasis (22) and with the increased metastatic
ability of breast cancer cells via promotion of the
epithelial-mesenchymal transition (5). CSE has also been reported to enhance the
metastatic ability and invasiveness of lung cancer cells (7). In the present study CSE increased the
invasiveness of A549 cells (Fig. 1B and
C).
Metastasis is a complex multistep process and a
leading cause of cancer-associated mortality (9). MMP3 is a proteolytic enzyme that is
active in metastasis, capable of degrading structural components of
the extracellular matrix (23) and
disrupting intercellular and cell-extracellular matrix adhesions
(24). MMP3 activity contributes to
tumor invasion and metastasis, and is indicative of a poor survival
prognosis (25). CSE exposure
increased the expression of MMP3 in A549 cells (Fig. 2C and D) and may have increased the
invasiveness of lung cancer cells by upregulating the MMP3
expression, which is in line with previous reports of MMP3 activity
in lung cancer metastasis (26). As
there are several other MMPs involved in the cancer metastasis,
their roles should be studied in future experiments.
TGF-β is a mediator of cancer invasion and
metastasis (27). TGF-β signals are
transferred to the nucleus via TGF-β type I or type II receptors
that phosphorylate canonical Smad2/3 downstream effectors (28). In the present study, CSE increased
TGF-β1 and Smad2 activity (Fig. 2A and
B). In vitro, treatment with an anti-MMP3 antibody was
found to result in a dose-dependent decrease in active TGF-β1
(27). Activated TGF-β can regulate
the secretion, expression, and activation of MMP3, resulting in a
bidirectional regulatory loop (29).
SB431542 is a TGF-β1 receptor kinase inhibitor that
interrupts the activation of downstream signaling pathways
(30). SB431542 has previously been
reported by Tanaka et al (31)
to induce an in vivo antitumor immune response associated
with TGF-β activity. Matsuyama et al (32) reported that SB431542 exerted antitumor
activity by inhibiting the proliferation of osteosarcoma cells. Xi
et al (33) revealed that
SB431542 inhibited the invasiveness of RPMI 8226 cells by
decreasing the expression of MMP3. In the present study, SB431542
significantly inhibited the activity of the TGFβ1/Smad2 pathway and
decreased MMP3 expression in A549 cells exposed to CSE (Fig. 3A and B), and reduced the invasiveness
of CSE-treated A549 cells (Fig. 3C and
D).
In the current study, the promotion of the
invasiveness of lung cancer cells by CSE was associated with the
activation of the TGFβ1/Smad2 pathway and regulation of MMP3
expression. The effects of CSE were partially reversed by SB431542,
a TGFβ1 receptor antagonist that may have therapeutic potential in
cancer, which could be proven in other in vitro models, such
as HAC-84 and GLC-15 cells, or in vivo experiments in the
future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All the data and materials are available upon
reasonable request.
Authors' contributions
KL contributed to the study design and contributed
to data analysis, CY contributed to performing experiments and KH
contributed to data analysis.
Ethics approval and consent to publish
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pesch B, Kendzia B, Gustavsson P, Jöckel
KH, Johnen G, Pohlabeln H, Olsson A, Ahrens W, Gross IM, Brüske I,
et al: Cigarette smoking and lung cancer-relative risk estimates
for the major histological types from a pooled analysis of
case-control studies. Int J Cancer. 131:1210–1219. 1012. View Article : Google Scholar
|
|
2
|
Song MA, Marian C, Brasky TM, Reisinger S,
Djordjevic M and Shields PG: Chemical and toxicological
characteristics of conventional and low-TSNA moist snuff tobacco
products. Toxicol Lett. 245:68–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hecht SS: It is time to regulate
carcinogenic tobacco-specific nitrosamines in cigarette tobacco.
Cancer Prev Res (Phila). 7:639–647. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moreira DM, Aronson WJ, Terris MK, Kane
CJ, Amling CL, Cooperberg MR, Boffetta P and Freedland SJ:
Cigarette smoking is associated with an increased risk of
biochemical disease recurrence, metastasis, castration-resistant
prostate cancer, and mortality after radical prostatectomy: Results
from the SEARCH database. Cancer. 120:197–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di Cello F, Flowers VL, Li H,
Vecchio-Pagán B, Gordon B, Harbom K, Shin J, Beaty R, Wang W,
Brayton C, et al: Cigarette smoke induces epithelial to mesenchymal
transition and increases the metastatic ability of breast cancer
cells. Mol Cancer. 12:902013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murin S and Inciardi J: Cigarette smoking
and the risk of pulmonary metastasis from breast cancer. Chest.
119:1635–1640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gopalakrishna R, Chen ZH and Gundimeda U:
Tobacco smoke tumor promoters, catechol and hydroquinone, induce
oxidative regulation of protein kinase C and influence invasion and
metastasis of lung carcinoma cells. Proc Natl Acad Sci USA.
91:12233–12237. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y, Li Y, Liu J, Fan Y, Li X, Dong M,
Liu H and Chen J: Expression levels of microRNA-145 and
microRNA-10b are associated with metastasis in non-small cell lung
cancer. Cancer Biol The. 17:272–279. 2016. View Article : Google Scholar
|
|
9
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Imamura T, Hikita A and Inoue Y: The roles
of TGF-β signaling in carcinogenesis and breast cancer metastasis.
Breast cancer. 19:118–124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bierie B and Moses HL: Tumour
microenvironment: TGFbeta: The molecular Jekyll and Hyde of cancer.
Nat Rev Cancer. 6:506–620. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akhurst RJ and Derynck R: TGF-beta
signaling in cancer-a double-edged sword. Trends Cell Biol.
11:S44–S51. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu RY, Zeng Y, Lei Z, Wang L, Yang H, Liu
Z, Zhao J and Zhang HT: JAK/STAT3 signaling is required for
TGF-β-induced epithelial-mesenchymal transition in lung cancer
cells. Int J Oncol. 44:1643–1651. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Checa M, Hagood JS, Velazquez-Cruz R, Ruiz
V, Garcia-De-Alba C, Rangel-Escareño C, Urrea F, Becerril C,
Montaño M, García-Trejo S, et al: Cigarette smoke enhances the
expression of profibrotic molecules in alveolar epithelial cells.
PLoS One. 11:e01503832016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim HS, Shang T, Chen Z, Pflugfelder SC
and Li DQ: TGF-beta1 stimulates production of gelatinase (MMP-9),
collagenases (MMP-1, −13) and stromelysins (MMP-3, −10, −11) by
human corneal epithelial cells. Exp Eye Res. 79:263–274. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wirtz HR and Schmidt M: Acute influence of
cigarette smoke on secretion of pulmonary surfactant in rat
alveolar type II cells in culture. Eur Respir J. 9:24–32. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fowles J and Dybing E: Application of
toxicological risk assessment principles to the chemical
constituents of cigarette smoke. Tob Control. 12:424–430, 203.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Johnson MD, Schilz J, Djordjevic MV, Rice
JR and Shields PG: Evaluation of in vitro assays for assessing the
toxicity of cigarette smoke and smokeless tobacco. Cancer Epidemiol
Biomarkers Prev. 18:3263–3304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sundar IK, Nevid MZ, Friedman AE and
Rahman I: Cigarette smoke induces distinct histone modifications in
lung cells: Implications for the pathogenesis of COPD and lung
cancer. J Proteome Res. 13:982–996. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Momi N, Ponnusamy MP, Kaur S, Rachagani S,
Kunigal SS, Chellappan S, Ouellette MM and Batra SK:
Nicotine/cigarette smoke promotes metastasis of pancreatic cancer
through α7nAChR-mediated MUC4 upregulation. Oncogene. 32:1384–1395.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Radisky DC, Levy DD, Littlepage LE, Liu H,
Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, et
al: Rac1b and reactive oxygen species mediate MMP-3-induced EMT and
genomic instability. Nature. 436:123–127. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mehner C, Miller E, Nassar A, Bamlet WR,
Radisky ES and Radisky DC: Tumor cell expression of MMP3 as a
prognostic factor for poor survival in pancreatic, pulmonary, and
mammary carcinoma. Genes Cancer. 6:480–489. 2015.PubMed/NCBI
|
|
26
|
Jiang YN, Yan HQ, Huang XB, Wang YN, Li Q
and Gao FG: Interleukin 6 trigged ataxia-telangiectasia mutated
activation facilitates lung cancer metastasis via MMP-3/MMP-13
up-regulation. Oncotarget. 6:40719–40733. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Costanza B, Umelo IA, Bellier J,
Castronovo V and Turtoi A: Stromal modulators of TGF-β in cancer. J
Clin Med. 6:pii: E72017. View Article : Google Scholar
|
|
28
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krstic J and Santibanez JF: Transforming
growth factor-beta and matrix metalloproteinases: Functional
interactions in tumor stroma-infiltrating myeloid cells.
ScientificWorldJournal. 2014:5217542014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Badalucco S, Di Buduo CA, Campanelli R,
Pallotta I, Catarsi P, Rosti V, Kaplan DL, Barosi G, Massa M and
Balduini A: Involvement of TGFβ1 in autocrine regulation of
proplatelet formation in healthy subjects and patients with primary
myelofibrosis. Haematologica. 98:514–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanaka H, Shinto O, Yashiro M, Yamazoe S,
Iwauchi T, Muguruma K, Kubo N, Ohira M and Hirakawa K: Transforming
growth factor β signaling inhibitor, SB-431542, induces maturation
of dendritic cells and enhances anti-tumor activity. Oncol Rep.
24:1637–1643. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matsuyama S, Iwadate M, Kondo M, Saitoh M,
Hanyu A, Shimizu K, Aburatani H, Mishima HK, Imamura T, Miyazono K
and Miyazawa K: SB-431542 and Gleevec inhibit transforming growth
factor-beta-induced proliferation of human osteosarcoma cells.
Cancer Res. 63:7791–7798. 2003.PubMed/NCBI
|
|
33
|
Xi H, Shuai QG and Shao LL: Involvement of
the TGFβ1/Smad2/MMP3 signaling pathway in SB431542-induced
inhibition of cell invasion in multiple myeloma RPMI 8226 cells.
Oncol Lett. 14:541–546. 2017. View Article : Google Scholar : PubMed/NCBI
|