Introduction
Multiple myeloma (MM) is a hematologic malignancy
characterized by the development of a destructive and progressive
osteolytic bone disease, which is mainly associated with severe
bone pain, pathological fractures, osteoporosis, hypercalcemia and
spinal cord compression (1). Although
there have been numerous significant improvements in the
understanding of the pathophysiologic changes of MM, it remains an
incurable disease (2). Destructive
skeletal-related events (SREs) are the main clinical manifestations
in patients with MM (1,3). It was demonstrated that 70–80% of
patients presented with osteolytic bone lesions at diagnosis, and
during the course of MM, >90% of patients developed lytic
lesions (1–5). If no effective treatment was provided,
>50% of patients with Durie-Salmon (D-S) stage III MM would
suffer at least one SRE within 2 years (6). Frequently, one or more vertebral bodies
are detected to be affected by vertebral collapse and/or osteolytic
lesions, and long bone fractures more commonly occur in the
proximal locations of the upper arm and femora (7). In addition, occasionally soft tissue
mass appears in extramedullary tissue, resulting in severe pain and
reducing the quality of life. In recent years, surgical
consultation has been recommended for MM patients with intractable
pain, spinal instability and pathological fractures (8); however, the results of the surgery
performed on different sites are not definite. To date, no previous
studies have conducted a comparative analysis of different surgical
sites of MM patients.
To the best of our knowledge, the present study is
the first to compare the results of MM patients receiving surgery
for lesions located in the spine with those surgically treated for
long bone and soft tissue lesions.
Patients and methods
Patients and specimens
A total of 65 patients diagnosed with MM were
recruited in the present study, including 40 males and 25 females
with a mean age of 57.23 years (age range, 20–79 years). The
participants were consecutively surgically treated in our
institution (Beijing Chao-yang Hospital, Capital Medical
University, Beijing, China) over a 5-year period (January 2010 to
January 2015). Survival time was recorded from the date of surgery
to the last follow-up in June 2016. Informed consent was obtained
from the subjects for participation into the present study. Ethical
approval was obtained from The College Research Ethics Committee of
Beijing Chao-yang Hospital, Capital Medical University (Beijing,
China).
In this study, the cases were divided into two
groups. Group A comprised 33 patients (21 males and 12 females;
mean age, 58.32 years; age range, 20–79 years) with surgical sites
located in the spine, while Group B included 32 patients (19 males
and 13 females; mean age, 56.21 years; age range, 44–74 years)
whose surgical sites were in the long bone or soft tissue. The 8
soft tissue cases were initially diagnosed with MM at the
Department of Hematology, Beijing Chao-yang Hospital, Capital
Medical University (Beijing, China), and subsequently soft tissue
masses appeared with the progression of the disease. The D-S stage,
International Staging System (ISS) stage and type of MM were
recorded, and these data are listed in Table I (9,10). Type of
MM was determined using the classification system of the European
Society for Medical Oncology, according to the type of monoclonal
immunoglobulin secreted by multiple myeloma cells (11). Initially, 2 of the patients were
assessed at the Department of Orthopedics at Beijing Chao-yang
Hospital, Capital Medical University (Beijing, China) due to
experiencing severe pain, and were diagnosed with MM subsequent to
surgery and chemotherapy based on specimen examination. The
remaining 63 patients were diagnosed with MM upon admission, and
accepted treatment by surgery and chemotherapy at the Department of
Hematology at Beijing Chao-yang Hospital, Capital Medical
University (Beijing, China).
 | Table I.Common demographics of the enrolled
patients. |
Table I.
Common demographics of the enrolled
patients.
| Characteristic | Group A (n=33) | Group B (n=32) | P-values |
|---|
| Male: female | 21:12 | 19:13 |
|
| Agea (years) | 58.3±12.7 | 56.2±8.2 | 0.429 |
| D-S stage of
MM |
|
|
|
| I
A/B | 0 | 0 |
|
| II
A/B | 3 | 4 |
|
| III
A/B | 27 | 26 |
|
| Missing
information | 3 | 2 |
|
| ISS stage of
MM |
|
|
|
| I | 2 | 2 |
|
| II | 13 | 12 |
|
|
III | 15 | 16 |
|
| Missing
information | 3 | 2 |
|
| Type of
MMc |
|
|
|
|
IgA-κ | 5 | 5 |
|
|
IgA-λ | 2 | 8 |
|
|
IgG-κ | 13 | 5 |
|
|
IgG-λ | 8 | 7 |
|
|
IgD-λ | 2 | 3 |
|
|
Nonsecretory | 0 | 2 |
|
|
Missing | 3 | 2 |
|
| Preoperative
chemotherapy |
|
|
|
|
Yes | 23 | 27 |
|
| No | 10 | 5 |
|
| Postoperative
chemotherapy |
|
|
|
|
Yes | 20 | 29 |
|
| No | 13 | 3 |
|
| Hospitalization
timea, days | 19.6±8.2 | 18.6±13.4 | 0.721 |
| Preoperative
duration of symptomsa
(months) | 18.4±16.3 | 20.5±17.1 | 0.623 |
| Surgery
durationa (min) | 180.0±74.6 | 119.7±45.0 |
<0.001b |
| Peri-operative
bleedinga (ml) | 343.7±74.1 | 253.2±73.0 | 0.108 |
| Survival
timea (months) | 24.3±20.2 | 20.6±14.4 | 0.397 |
| Preoperative
VASa (points) | 8.3±1.2 | 7.7±1.9 | 0.102 |
| VAS at 1 month
after surgerya
(points) | 5.5±1.9 | 3.3±1.3 |
<0.001b |
| VAS at 6 months
after surgerya
(points) | 2.8±2.5 | 1.4±0.6 |
<0.001b |
|
Plateletsa (×109/l) | 197.1±64.7 | 182.8±98.3 | 0.498 |
|
Hemoglobina (×1012/l) | 112.0±21.1 | 109.8±30.1 | 0.736 |
|
Albumina
(g/l) | 31.8±5.0 | 33.1±5.5 | 0.344 |
| Lactate
dehydrogenasea
(U/l) | 352.7±40.4 | 239.1±59.5 | 0.143 |
| Urine
proteina (mg/dl) | 24.4±7.6 | 14.3±6.6 | 0.332 |
Treatments
In group A, 23 patients (69.7%, 23/33) received
chemotherapy prior to surgery, while 27 patients (84.4%, 27/32)
received chemotherapy prior to surgery in group B. The remaining 10
patients in group A and 5 patients in group B accepted surgical
treatment without preoperative medical therapy. A total of 20
patients (60.6%, 20/33) in group A and 29 patients (90.6%, 29/32)
in group B continued to receive chemotherapy during the
postoperative course. The remaining 13 patients in group A and 3
patients in group B did not accepted further medical treatment due
to limited economic capacity or other reasons. The main
chemotherapy schedule was PCD (bortezomib + cyclophosphamide +
dexamethasone) or PAD (bortezomib + adriamycin + dexamethasone) in
the present study, as previously described (12,13). All
the cases receiving preoperative or postoperative chemotherapy
completed their chemotherapy courses. In addition, all patients
were informed of the benefits of pre- or postoperative radiation
therapy, however, the patients participating in the current study
selected only pre- or postoperative chemotherapy due to limited
understanding of the MM disease and their economic capability.
Lesion locations
In group A, the most common location of bone lesions
was in the spine (thoracic, 20 cases; lumbar spine, 5 cases;
sacrum, 3 cases; lumbar spine and sacrum, 3 cases; thoracic and
lumbar spine, 2 cases). In group B, the lesions were located in the
long bones and soft tissue (femur, 12 cases; humerus, 7 cases;
clavicle, 2 cases; tibia, 2 cases; radial bone, 1 case; soft
tissue, 8 cases).
Surgical procedures
The surgical approach and detailed procedure
performed were recorded in the surgeon's operative documents.
Patients involved by MM were all medically stable for surgery and
complied with the selection criteria for surgical intervention,
with the exception of 3 patients in group A (lesions located in
T7-9, T4-5 and T5, respectively) who were in a serious condition
with irreversible neurological impairment when admitted to the
hospital. The preoperative condition of these patients was
evaluated via X-ray examination, computed tomography (CT), magnetic
resonance imaging (MRI) and blood tests, while ultrasound
examination was also required in certain cases with soft tissue
lesions.
Different surgical techniques were performed
according to the sites of lesions and the surgeon's preference. In
group A, 24 patients were treated by lesion resection, posterior
decompression and dorsal stabilization with pedicle screw systems.
In addition, lesions located in the vertebral body were resected as
much as possible, and the defect was filled with bone cement
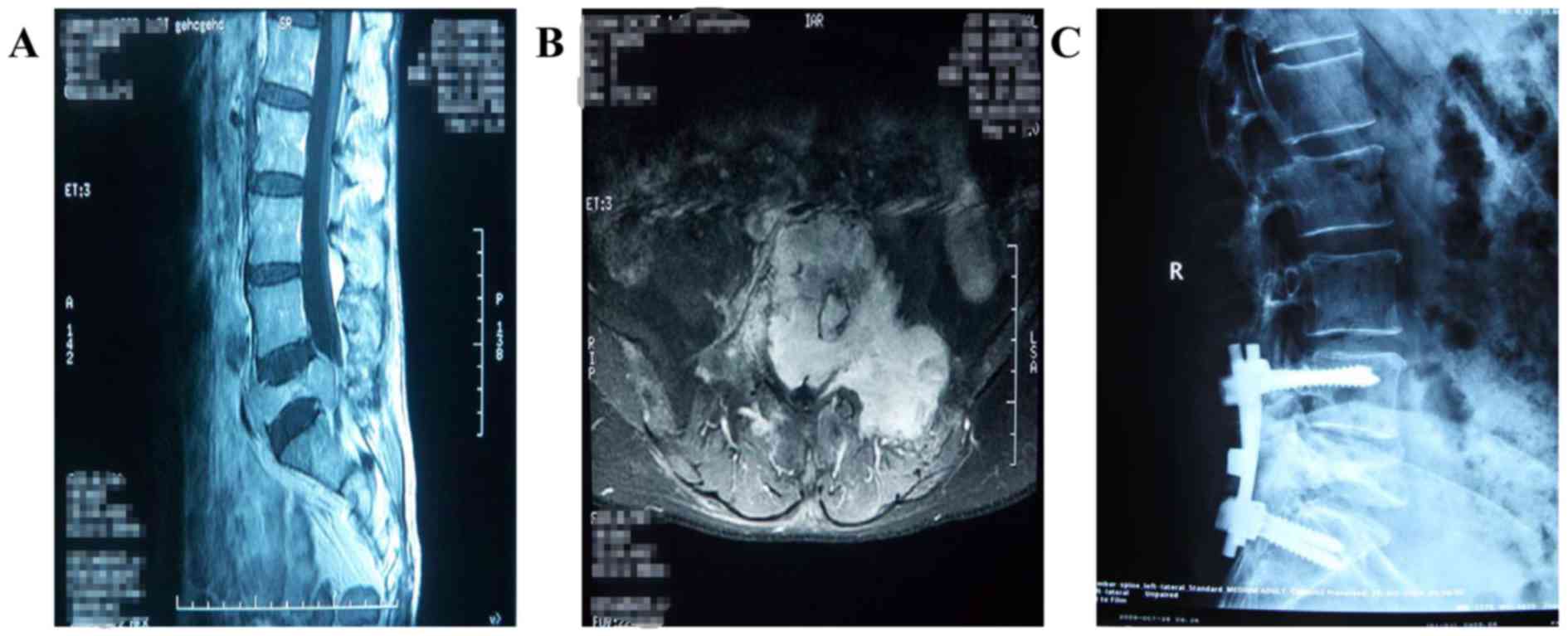
(Fig. 1); a total of 3 patients
received this treatment. A total of 5 patients were diagnosed with
a vertebral body compression fracture, and percutaneous kyphoplasty
(PKP) was performed on the lesion levels. There were 3 patients
whose lesions were located in the sacrum causing cauda equina
compression; of these, 2 cases were treated by lesion resection and
reconstruction with bone cement and a pedicle screw system, while
radiofrequency ablation, tumor resection and reconstruction with
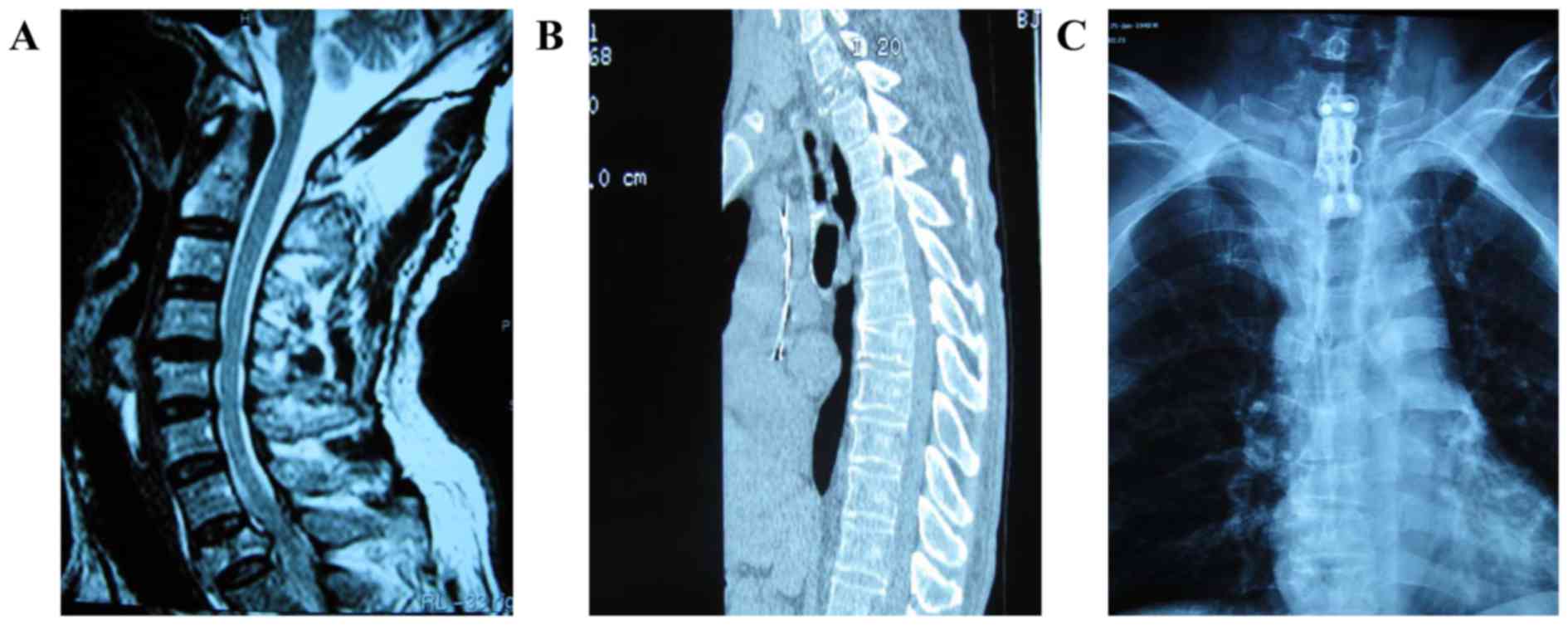
bone cement was performed in the other case. Furthermore, 1 case
with a lesion located in the ventral vertebral body of the first
thoracic was treated by vertebral body resection and reconstruction
with a titanium cage and bone cement, as well as instrumentation
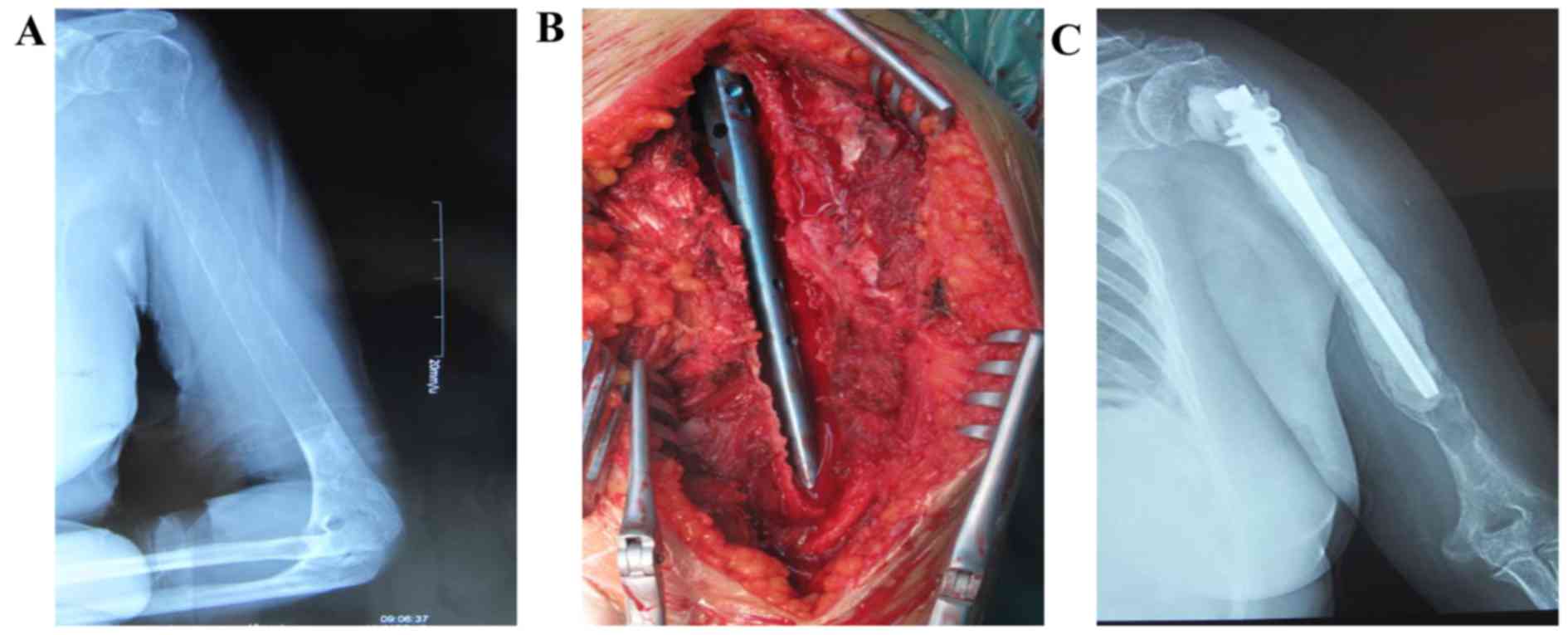
with a vertebral body screw through the anterior approach (Fig. 2). In group B, surgical procedures
including tumor resection and reconstruction with bone cement,
titanium plates and screws were performed in 20 patients. In
addition, 1 patient with a lesion located near the proximal humerus
was treated by tumor resection and reconstruction with bone cement
and intramedullary nailing (Fig. 3).
In 2 cases, a lesion in the femoral head was resected, and
replacement of endoprosthesis was performed. Furthermore, 1 case
with an intertrochanteric fracture was treated by implantation of
intramedullary nailing. There were 8 patients whose surgical sites
were in the soft tissue (lower limb, 2 cases; upper limb, 2 cases;
buttock, 2 cases; groin, 1 case; back, 1 case). Among these 8
cases, tumor resection alone was performed in 6 patients, and the
remaining 2 patients were treated with both tumor resection and
nerve decompression.
Follow-up and assessments
The follow-up investigation was conducted by phone
or out-patient review. The mean follow-up time was 24.7 months
(ranging from 3 to 60 months). Neurological impairment was assessed
according to the Frankel classification which provided an
assessment of spinal cord function and was used as a tool for
spinal cord injury (14). It was
defined as five grades (Frankel A, B, C, D and E) according to
different motor and sensory function following spinal cord injury.
Postoperative radiographs were judged based on local tumor
recurrence and the stability of instrumentations. The preoperative
visual analogue scale (VAS) score (15), as well as the postoperative VAS scores
at 1 and 6 months after surgery were retrospectively compared
between the two groups.
Statistical analysis
Groups A and B were compared in terms of the age,
hospitalization time, preoperative duration of symptoms, surgery
duration, peri-operative bleeding, survival time and laboratory
examinations, with differences between the two groups assessed by
independent sample t-test and correlation analysis. The
postoperative complications and mortality rate between groups A and
B were analyzed using an χ2-test. The survival time was
estimated using the Kaplan-Meier method. Cox regression analysis
was used to estimate the effect of factors on the prediction of
survival. The threshold for a statistically significant difference
was set at P<0.05. Statistical analysis was performed with SPSS
version 17.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
The clinicopathological data of patients in groups A
and B are presented in Table I. No
statistical significance was observed in the age, hospitalization
time, preoperative duration of symptoms, peri-operative bleeding,
survival time, preoperative VAS score, and in the levels of
platelets, hemoglobin, albumin, lactate dehydrogenase (LDH) and
urine protein between the two groups. However, there was a
statistically significant difference in the surgery duration
(P<0.001), as well as in the postoperative VAS scores at 1 and 6
months after surgery (both P<0.001) between groups A and B.
Treatment outcome and survival
In group A, 18 patients succumbed to the disease and
15 patients were alive at the last follow-up, while 14 patients
succumbed and 18 were alive in group B. The mortality rate of
groups A and B was analyzed by χ2-test, and no
significant difference was detected (χ2=0.552, P=0.458).
Among the 8 soft tissue cases, 4 patients succumbed and 4 patients
were alive at the last follow-up. Pain relief and improvement in
the quality of life were obtained in all the patients. The mean VAS
scores for the 65 enrolled patients decreased from 7.97 prior to
surgery to a value of 4.34 at 1 month after surgery and 2.08 at 6
months after surgery. However, the decrease in the VAS score was
significantly greater in group A when compared with that in group B
(P<0.001; Table I).
Furthermore, the neurological function improved by
different degrees subsequent to the surgical intervention in the
majority of patients in group A. Among the 33 MM patients with
preoperative neurological dysfunction, 27 patients improved from
grade D to E after surgery according to the Frankel classification,
while 3 patients improved from Frankel grade C to D. In addition, 3
patients remained at the same state as that upon admission (Frankel
grade C), as their neurological function was already severely and
irreversibly impaired, and these patients finally succumbed to the
disease at 10, 10 and 23 days after surgery, respectively. In group
A, 30 out of the 33 patients (90.9%, 30/33) demonstrated
improvement in neurological impairment following surgery, and no
patient developed progressive neurological impairment.
Following surgical intervention, local recurrence
was not detected in these patients via associated postoperative
imaging examinations, including X-ray plain film, CT and MRI
examinations. In group A, 2 patients (6.1%, 2/33) were complicated
with pulmonary infection and 1 case (3.0%, 1/33) was complicated
with septic shock, resulting in a complication rate of 9.1% (3/33)
in group A. In group B, only 1 patient (3.1%, 1/32) was complicated
with cerebral infarction, pulmonary infection and urinary infection
continuously. The total complication rate in the present study was
6.2% (4/65). In addition, there was no significant difference in
the postoperative complications between groups A and B
(χ2=0.338, P=0.561; Table
II). The median postoperative survival time in groups A and B
was 36 and 60 months, respectively, as determined by the
Kaplan-Meier method. When the 8 soft tissue cases were analyzed
separately, the median postoperative survival time appeared to be
28 months. The overall survival time of the 8 soft tissue cases was
51.4 months, whereas that of the total 65 cases was 60.2 months.
Furthermore, the postoperative 1- and 3-year overall survival rates
of group A were 67.2 and 59.5%, respectively, while these were 68.9
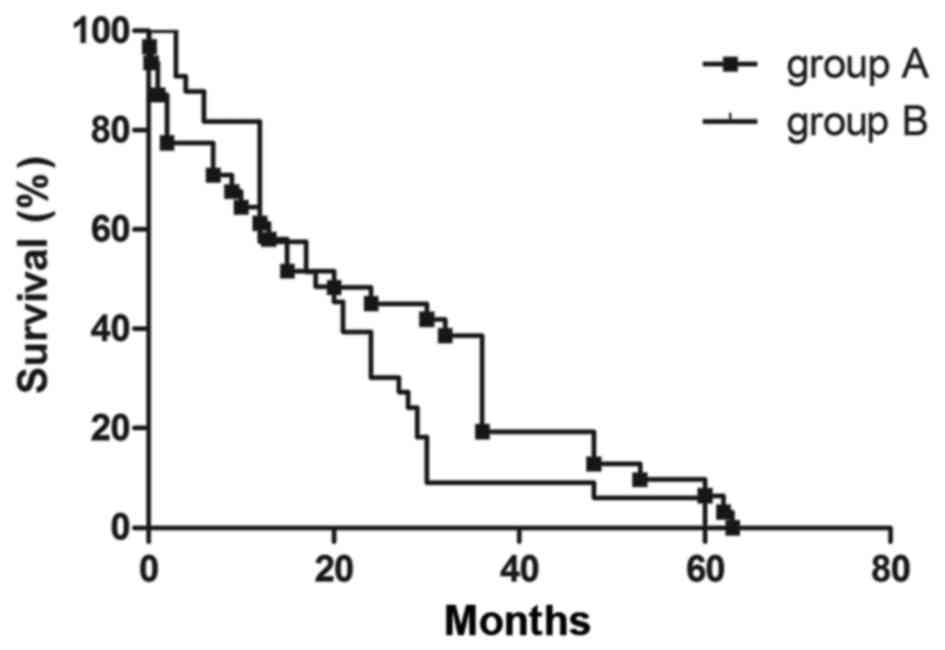
and 58.3%, respectively, in group B. The survival curves of the two
groups were compared, as shown in Fig.
4. There was no significant difference in mortality rate of
groups A and B (χ2=0.552, P=0.458).
 | Table II.Postoperative complications in the
two groups. |
Table II.
Postoperative complications in the
two groups.
| Postoperative
complication | Group A | Group B | Total |
|---|
| Pulmonary
infection | 2 | 0 | 2 |
| Septic shock | 1 | 0 | 1 |
| Cerebral
infarction, pulmonary infection and urinary infection | 0 | 1 | 1 |
| Total | 3 | 1 | 4 |
Risk factors
Multivariate Cox regression analysis revealed the
significant survival risk factors, and these included the
preoperative VAS score (RR=1.731, P=0.025), postoperative
chemotherapy (RR=5.241, P=0.048), prothrombin time activity (PTA;
RR=0.63, P=0.008), albumin (RR=0.586, P=0.006), LDH (RR=1.000,
P=0.037) and urine protein level (RR=1.037, P=0.026; Table III). Evidence of instrumentation
failure and local recurrence was not found in the patients enrolled
during the follow-up period.
 | Table III.Multivariate Cox regression
analysis. |
Table III.
Multivariate Cox regression
analysis.
| Parameter | Risk ratio | 95% confidence
interval | P-value |
|---|
| Sex | 3.459 | 0.190–62.984 | 0.402 |
| Age | 0.914 | 0.798–1.048 | 0.197 |
| Preoperative VAS
score | 1.731 | 1.070–2.800 | 0.025a |
| Hospitalization
time | 0.964 | 0.884–1.052 | 0.409 |
| Preoperative
duration of symptoms | 1.086 | 0.976–1.209 | 0.132 |
| Preoperative
chemotherapy | 1.218 | 0.042–35.379 | 0.908 |
| Postoperative
chemotherapy | 5.241 | 1.017–27.014 | 0.048a |
| Stage (D-S) |
|
|
|
| I | 0.000 | 0.000–1.095 | 0.053 |
| II | 23367 | 0.000–1.142 | 0.377 |
|
III | 2.128 | 0.014–315.35 | 0.767 |
| Bleeding during
operation | 0.999 | 0.995–1.002 | 0.438 |
| PTA | 0.63 | 0.447–0.886 | 0.008a |
| PT | 0.205 | 0.014–2.990 | 0.247 |
| APTT | 0.858 | 0.715–1.029 | 0.098 |
| TT | 1.112 | 0.621–1.990 | 0.720 |
| Albumin | 0.586 | 0.401–0.857 | 0.006a |
| Hemoglobin | 0.966 | 0.904–1.033 | 0.312 |
| LDH | 1.000 | 0.997–1.003 | 0.037a |
| Urine protein | 1.037 | 1.004–1.071 | 0.026a |
Discussion
MM is the most common primary tumor of the spine,
and its typical localization in the vertebral body is in the lower
thoracic or lumbar spine (16). Among
the SREs secondary to MM, spinal pathologic fractures are
considered to be the most common complication (17). However, MM also occurs in the long
bone and the soft tissue. Tumor enlargement, pathologic fractures
and neurological symptoms are relatively common in MM patients.
Apart from treatment approaches including radiotherapy,
chemotherapy, bisphosphonates and supportive treatment that are
useful (18,19), Dimopoulos et al (11) reported a case with acute bony spinal
cord compression and neurological impairment in which the patient
was successfully treated with a non-operative approach. However,
surgical treatment is also proven to be effective in pain relief
and improvement of the life quality for the majority of MM patients
with SREs and soft tissue mass. The aim of the present study was to
compare MM patients with different presentation sites who were
surgically treated.
Based on recent progress (1,20,17), the understanding of the
osteoclastogenic and osteoblastic factors involved in the
development of myeloma bone disease has improved. The myeloma cells
are located adjacent to sites of active bone resorption, which
suggests that the mechanism for osteoclastic bone destruction in
myeloma bone disease is locally mediated (21). In cases of neurological impairment,
radiation therapy and chemotherapy are often effective to diminish
the local tumor lesion; however, these strategies do not
sufficiently treat spinal instability. It is evident that the
combination of surgical and adjuvant treatment is necessary to
promote promising outcomes, whether the location of the lesion is
in the spine, long bone or soft tissue. Therefore, a primary target
in the treatment of MM bone disease is the preservation or
restoration of spinal stability, which is similar to the goal in
the treatment of metastasis (22,23).
To date, only a few studies have been published
reporting a comparative analysis of MM patients with different
surgical sites. For instance, Zeifang et al (24) reported that a tumor in long
weight-bearing bones was associated with a reduced survival rate as
compared with a spinal tumor location (21 vs. 66 months,
respectively). However, in the present study, the median survival
time of patients with lesions located in the long bone and soft
tissue was longer in comparison with that of patients with lesions
located in the spine (60 vs. 36 months, respectively), which is not
consistent with previous findings reported in the literature. A
statistical difference was not evident in MM patients with
different anatomical sites of osteolytic bone lesions in the
present study. It can be assumed that plasma cells initially
infiltrate the axial skeleton, leading to the compression of
marrow. With increased cellular proliferation, extensive bone
destruction, pathological fractures, hypercalcemia and osteolyses
in long weight-bearing bones become evident, indicating an advanced
stage of the disease (25). However,
in our opinion, the surgery conducted on the spine is a larger
invasive procedure compared with procedures on the long bone and
soft tissue, which leads to a longer period of time before the
patient is able to walk. Thus, it may result in more postoperative
complications, including pulmonary infection, deep venous
thrombosis and bedsores among others. Finally, patients undergoing
surgery on the spine exhibited a shorter median survival time when
compared with those undergoing surgery on the long bone and soft
tissue. In addition, studies have demonstrated that the presence or
absence of extramedullary lesions in MM patients is closely
correlated with the prognosis (26,27). The
present study revealed that the prognosis of MM patients with
extramedullary lesions was worse in comparison with that of
patients without extramedullary lesions, which may also explain why
the soft tissue cases had a shorter survival time. Other important
considerations, including an advanced tumor stage, health condition
of the patients, preoperative duration of symptoms, other
accompanying diseases and interruption of other treatments, such as
chemotherapy, should also be analyzed.
The surgical outcome of lytic bone lesions in MM is
frequently compared with that of bone metastases. In earlier
reports, the overall survival time in metastatic bone disease
ranged between 6 and 22 months (28,29),
depending on the type of primary tumor. Recent studies concluded
that the median survival time of MM patients is longer as compared
with that of patients with bone metastases (24,30). This
explains the fact that, in myeloma patients requiring orthopedic
surgery, a treatment decision should be made comprising a stable
reconstruction of the bone defects. Recently, minimally invasive
stabilization using bone cement, such as the PKP and percutaneous
vertebroplasty (PVP) methods, have been demonstrated to be an
effective and safe strategy for vertebral body pathologic fractures
in MM patients (31,32). Pain relief was apparent in the early
stages following PKP/PVP treatment (20). In the present study, 30 out of the 33
patients (90.9%, 30/33) in group A exhibited improved neurological
impairment subsequent to surgery. However, in a previous study,
only 14 out of 49 patients (29%) exhibited improved neurological
function after surgery, and 10 of them were treated by dorsal
decompression and stabilization (24). Other authors have reported that up to
81% of patients with spinal neoplasm experienced neurological
improvement following surgery combining anterior-posterior
approaches (33,34). The prognosis for neurological recovery
is adversely affected by the degree and duration of canal
narrowing, demonstrating that patients may benefit from earlier
decompression regardless of the selected surgical procedure
(35). The surgical sites of the
majority of cases included into the present study were in the spine
or in the long bone/soft tissue, and patients benefited
significantly from surgery. The post-surgical complication rate was
low (9.1% in group A vs. 3.1% in group B). A study by
Pascal-Moussellard et al (36)
reported a complication rate of 19% (17/145) following vertebral
metastasis surgery. The complication rates in groups A and B in the
current study were lower compared with that reported following
surgery in patients with metastases. Refractures in operated limbs
were not identified in the present study.
A study including 84 MM patients who were surgically
treated reported a recurrence rate of 6% (24). In the study by Hannisdal et al
(25), the total local recurrence
rate was 11.1%, which was similar to the recurrence rate of 6–22%
reported in spinal metastases (37–39). In
the current study, local recurrence was not reported in any of the
65 patients to date. This may be contributed to the destruction of
the MM microenvironment during the surgical procedure and the
effect of adjuvant treatment, as well as the limited length of
follow-up. Terpos et al (40)
reported that, although MRI is superior to positron emission
tomography (PET)/CT in the detection of marrow involvement, the
PET/CT examination was regarded as the best technique for the
follow-up of patients with MM. PET/CT was also proven to be an
independent prognostic value at diagnosis and subsequent to
treatment. However, in the patients of the present study, only
X-ray plain film examination was performed during follow-up and
out-patient review due to the financial ability of patients, which
should be taken into account. Certain other unknown reasons must
also be considered.
Albumin and serum LDH were regarded as markers of
the tumor burden and aggressive disease biology, respectively, in
the revised ISS classification (41).
LDH may be regarded as one of the adjuvant indexes to reflect the
prognostic and tumor burden of MM patients (42). In the present study, albumin and LDH
were identified as two of the prognostic factors via multivariate
Cox regression analysis. The advanced age, site of lytic bone
lesions and D-S stage III were indicated as negative prognostic
factors for survival in an earlier study (43). However, no significant difference in
these three factors was identified for all the patients and between
the two groups in the current study. The selection bias of MM
patients and grouping of patients should be considered for this.
Besides, although no significant difference in the indication of
prognosis was detected for the preoperative duration of symptoms in
the present study, this factor serves an important role in
improving the quality of life of patients and decreasing
complications, such as bone disease, anemia and renal failure in MM
(44). General practitioners
decision-making aids and public education campaigns are required to
reduce the time-to-diagnosis (45).
Furthermore, it was observed herein that the VAS score decreased
gradually in the two groups between the time prior to surgery and
at 1 or 6 months following surgery. Notably, a statistically
significant difference was observed in the postoperative VAS score
at 1 and 6 months after surgery between groups A and B (both
P<0.001). Thus, it is suggested that the MM patients should be
treated individually subsequent to accepting surgery, particularly
regarding the postoperative analgesic use. The postoperative pain
in MM patients could be controlled effectively by using the
appropriate dose of analgesic drugs.
In conclusion, based on the literature and the
current findings, it is suggested that surgical treatment is an
effective method in MM patients whether the lesion is located in
the spine or in the long bone and soft tissue. Preoperative pain,
PTA, albumin, urine protein and postoperative chemotherapy are
associated with the patient prognosis. Postoperative analgesic use
should be individualized according to the different surgical sites
and postoperative periods. Finally, studies depicting the outcomes
of MM patients with different surgical sites are limited, thus,
further investigation need to be undertaken in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and analyzed in the present
study are included in this published article.
Authors' contributions
All these authors contributed equally to this work.
JTS and XRD conceived and designed the study. LXZ, HL, ZYX and XRD
acquired, analyzed and interpreted the information. JTS and XRD
wrote, reviewed and/or revised the manuscript. XRD, ZYX and JTS
proofread and formatted the manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from the subjects for
participation into the present study. Ethical approval was obtained
from the The College Research Ethics Committee, Beijing Chao-yang
Hospital, Capital Medical University (Beijing, China).
Consent for publication
Consent for publication of this article has been
obtained from all patients included in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Terpos E, Berenson J, Raje N and Roodman
GD: Management of bone disease in multiple myeloma. Expert Rev
Hematol. 7:113–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Edwards CM, Edwards JR, Lwin ST, Esparza
J, Oyajobi BO, McCluskey B, Munoz S, Grubbs B and Mundy GR:
Increasing Wnt signaling in the bone marrow microenvironment
inhibits the development of myeloma bone disease and reduces tumor
burden in bone in vivo. Blood. 111:2833–2842. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raje N and Roodman GD: Advances in the
biology and treatment of bone disease in multiple myeloma. Clin
Cancer Res. 17:1278–1286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Terpos E and Dimopoulos MA: Myeloma bone
disease: Pathophysiology and management. Ann Oncol. 16:1223–1231.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vallet S and Anderson KC: CCR1 as a target
for multiple myeloma. Expert Opin Ther Targets. 15:1037–1047. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coleman RE: Bisphosphonates: Clinical
experience. Oncologist. 9 Suppl 4:S14–S27. 2004. View Article : Google Scholar
|
|
7
|
Terpos E, Cibeira MT, Blade J and Ludwig
H: Management of complications in multiple myeloma. Semin Hematol.
46:176–189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adamietz IA, Schöber C, Schulte RW, Peest
D and Renner K: Palliative radiotherapy in plasma cell myeloma.
Radiother Oncol. 20:111–116. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Greipp PR, Miguel San J, Durie BG, Crowley
JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H,
Kyle RA, et al: International staging system for multiple myeloma.
J Clin Oncol. 23:3412–3420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rajkumar SV, Dimopoulos MA, Palumbo A,
Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E,
Richardson P, et al: International Myeloma Working Group updated
criteria for the diagnosis of multiple myeloma. Lancet Oncol.
15:e538–e548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dimopoulos MA and Terpos E: Multiple
myeloma. Ann Eur Soc Med Oncol. 21 Suppl 7:vii143–vii150. 2010.
|
|
12
|
He J, Yang L, Han X, Zheng G, Zheng W, Wei
G, Wu W, Ye X, Shi J, Xie W, et al: The choice of regimens based on
bortezomib for patients with newly diagnosed multiple myeloma. PLoS
One. 9:e991742014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Wang L, Lu Y, Chen X, Geng Q, Wang
W and Xia Z: Long-term outcomes of different bortezomib-based
regimens in Chinese myeloma patients. Onco Targets Ther. 9:587–595.
2016.PubMed/NCBI
|
|
14
|
Zham H, Moradi A, Rakhshan A, Zali A,
Rahbari A, Raee M, Ashrafi F, Ahadi M, Larijani L, Baikpour M and
Khayamzadeh M: Does Histologic Subtype Influence the Post-Operative
Outcome in Spinal Meningioma? Iran J Cancer Prev.
9:e38382016.PubMed/NCBI
|
|
15
|
Yasuda T, Kawaguchi Y, Suzuki K, Nakano M,
Seki S, Watabnabe K, Kanamori M and Kimura T: Five-year follow up
results of posterior decompression and fixation surgery for delayed
neural disorder associated with osteoporotic vertebral fracture.
Medicine (Baltimore). 96:e93952017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weinstein JN and McLain RF: Primary tumors
of the spine. Spine (Phila Pa 1976). 12:843–851. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ha KY, Min CK, Seo JY, Kim YH, Ahn JH,
Hyun NM and Kim YC: Bone cement augmentation procedures for spinal
pathologic fractures by multiple myeloma. J Korean Med Sci.
30:88–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rades D, Huttenlocher S, Dunst J, Bajrovic
A, Karstens JH, Rudat V and Schild SE: Matched pair analysis
comparing surgery followed by radiotherapy and radiotherapy alone
for metastatic spinal cord compression. J Clin Oncol. 28:3597–3604.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morgan GJ, Child JA, Gregory WM, Szubert
AJ, Cocks K, Bell SE, Navarro-Coy N, Drayson MT, Owen RG, Feyler S,
et al: Effects of zoledronic acid versus clodronic acid on skeletal
morbidity in patients with newly diagnosed multiple myeloma (MRC
Myeloma IX): Secondary outcomes from a randomised controlled trial.
Lancet Oncol. 12:743–752. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khan OA, Brinjikji W and Kallmes DF:
Vertebral augmentation in patients with multiple myeloma: A pooled
analysis of published case series. AJNR Am J Neuroradiol.
35:207–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edwards CM, Zhuang J and Mundy GR: The
pathogenesis of the bone disease of multiple myeloma. Bone.
42:1007–1013. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weber MH, Burch S, Buckley J, Schmidt MH,
Fehlings MG, Vrionis FD and Fisher CG: Instability and impending
instability of the thoracolumbar spine in patients with spinal
metastases: A systematic review. Int J Oncol. 38:5–12.
2011.PubMed/NCBI
|
|
23
|
Fisher CG, DiPaola CP, Ryken TC, Bilsky
MH, Shaffrey CI, Berven SH, Harrop JS, Fehlings MG, Boriani S, Chou
D, et al: A novel classification system for spinal instability in
neoplastic disease: An evidence-based approach and expert consensus
from the Spine Oncology Study Group. Spine (Phila Pa 1976).
35:E1221–E1229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zeifang F, Zahlten-Hinguranage A,
Goldschmidt H, Cremer F, Bernd L and Sabo D: Long-term survival
after surgical intervention for bone disease in multiple myeloma.
Ann Oncol J Eur Soc Med Oncol. 16:222–227. 2005. View Article : Google Scholar
|
|
25
|
Hannisdal E, Kildahl-Andersen O, Grøttum
KA and Lamvik J: Prognostic factors in multiple myeloma in a
population-based trial. Eur J Haematol. 45:198–202. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cerny J, Fadare O, Hutchinson L and Wang
SA: Clinicopathological features of extramedullary
recurrence/relapse of multiple myeloma. Eur J Haematol. 81:65–69.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Köse M, Buraniqi E, Akpinar TS, Kayacan SM
and Tükek T: Relapse of multiple myeloma presenting as
extramedullary plasmacytomas in multiple organs. Case Rep Hematol.
2015:4523052015.PubMed/NCBI
|
|
28
|
Lahtinen R, Laakso M, Palva I, Virkkunen P
and Elomaa I: Randomised, placebo-controlled multicentre trial of
clodronate in multiple myeloma. Finnish Leukaemia Group. Lancet
(London, England). 340:1049–1052. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tatsui H, Onomura T, Morishita S, Oketa M
and Inoue T: Survival rates of patients with metastatic spinal
cancer after scintigraphic detection of abnormal radioactive
accumulation. Spine. 21:2143–2148. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zadnik PL, Goodwin GC, Karami KJ, Mehta
AI, Amin AG, Groves ML, Wolinsky JP, Witham TF, Bydon A, Gokaslan
ZL and Sciubba DM: Outcomes following surgical intervention for
impending and gross instability caused by multiple myeloma in the
spinal column. J Neurosurg Spine. 22:301–309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zou J, Mei X, Gan M and Yang H:
Kyphoplasty for spinal fractures from multiple myeloma. J Surg
Oncol. 102:43–47. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kasperk C, Haas A, Hillengass J, Weiss C,
Neben K, Goldschmidt H, Sommer U, Nawroth P, Meeder PJ, Wiedenhöfer
B, et al: Kyphoplasty in patients with multiple myeloma a
retrospective comparative pilot study. J Surg Oncol. 105:679–686.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harrington KD: Anterior decompression and
stabilization of the spine as a treatment for vertebral collapse
and spinal cord compression from metastatic malignancy. Clin Orthop
Relat Res. 177–197. 1988.PubMed/NCBI
|
|
34
|
Kluger P, Korge A and Scharf HP: Strategy
for the treatment of patients with spinal neoplasms. Spinal Cord.
35:429–436. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Helweg-Larsen S: Clinical outcome in
metastatic spinal cord compression. A prospective study of 153
patients. Acta Neurol Scand. 94:269–275. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pascal-Moussellard H, Broc G, Pointillart
V, Siméon F, Vital JM and Sénégas J: Complications of vertebral
metastasis surgery. Eur Spine J. 7:438–444. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jansson KA and Bauer HC: Survival,
complications and outcome in 282 patients operated for neurological
deficit due to thoracic or lumbar spinal metastases. Eur Spine J.
15:196–202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weigel B, Maghsudi M, Neumann C,
Kretschmer R, Muller FJ and Nerlich M: Surgical management of
symptomatic spinal metastases. Postoperative outcome and quality of
life. Spine. 24:2240–2246. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lau D, Leach MR, La Marca F and Park P:
Independent predictors of survival and the impact of repeat surgery
in patients undergoing surgical treatment of spinal metastasis. J
Neurosurg Spine. 17:565–576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Terpos E, Dimopoulos MA and Moulopoulos
LA: The Role of Imaging in the treatment of patients with multiple
myeloma in 2016. Am Soc Clin Oncol Educ Book. Meeting.
35:e407–e417. 2016.
|
|
41
|
Rajkumar SV: Myeloma today: Disease
definitions and treatment advances. Am J Hematol. 91:90–100. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Long SF, Chen GA and Fang MS: Levels of
interleukin-16 in peripheral blood of 52 patients with multiple
myeloma and its clinical significance. Int J Clin Exp Med.
8:22520–22524. 2015.PubMed/NCBI
|
|
43
|
Bataille R, Boccadoro M, Klein B, Durie B
and Pileri A: C-reactive protein and beta-2 microglobulin produce a
simple and powerful myeloma staging system. Blood. 80:733–737.
1992.PubMed/NCBI
|
|
44
|
Kariyawasan CC, Hughes DA, Jayatillake MM
and Mehta AB: Multiple myeloma: Causes and consequences of delay in
diagnosis. QJM. 100:635–640. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Howell DA, Smith AG, Jack A, Patmore R,
Macleod U, Mironska E and Roman E: Time-to-diagnosis and symptoms
of myeloma, lymphomas and leukaemias: A report from the
Haematological Malignancy Research Network. BMC Hematol. 13:92013.
View Article : Google Scholar : PubMed/NCBI
|