Introduction
The mortality of cancer is an important problem
worldwide. Although many therapeutic strategies have been
developed, they have not been sufficient for overcoming cancer. In
particular, recurrence and treatment resistance make it difficult
to treat cancer. Over the past decade, cancer stem-like cells
(CSCs), which possess self-renewal and multipotency, are regarded
as the cause of tumor formation, recurrence, metastasis, and drug
resistance (1). Therefore, it is
important to understand the properties of CSCs.
The several markers of CSCs have been reported, and
Oct4 and Nanog play important roles in stem cells (2–4). Oct4, the
homeodomain transcription factor of the POU family, is a key
transcription factor for maintaining the self-renewal and
pluripotency of embryonic stem cells (ESCs) (2). In addition, it has been reported that
Oct4 plays a crucial role in the maintenance of dedifferentiation
in CSCs (3). Similarly, Nanog, the
homeobox domain transcription factor, is an essential regulator of
ESCs (5). Both Oct4 and Nanog are
essential to maintain stem cell properties (5), and Nanog appears to be one of the
markers of CSCs (4).
CSCs are also known to possess a high efflux system,
and express several adenosine triphosphate-binding cassette (ABC)
transporters, such as P-glycoprotein (P-gp), breast cancer
resistance protein (BCRP). Therefore, high expression of ABC
transporters were common features of CSCs (6).
Recently, it has been reported that S100 proteins
play crucial roles in maintaining cancer stem-like properties
(7,8).
S100 is a family of calcium binding proteins of the EF-hand type
that are involved in tumor development and progression, such as
cell growth, migration invasion, tumor microenvironment, and stem
properties (7,8).
S100A16 is a novel protein of the S100 family that
is up-regulated in various malignant tumors (9). It had been reported that S100A16 was
associated with epithelial mesenchymal transition (EMT) and p53
tumor suppressor function (10,11). The
EMT plays crucial roles in the formation and differentiation of
tissues. Recent studies suggest that EMT is involved in cancer
metastasis, progression, and CSC properties (7,12,13). These previous studies suggest that
S100A16 plays an important role in CSCs. However, the role of
S100A16 in cancer cells remains to be fully elucidated. In
addition, the roles of S100A16 in CSCs also remain to be fully
elucidated.
Therefore, we focused on S100A16 and investigated
the roles of S100A16 in cancer cells by sphere formation assay.
Materials and methods
Cell culture
Yumoto, a human cervical squamous carcinoma cell
line and has a wild-type p53 gene, was maintained in RPMI 1640
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with
10% fetal bovine serum, 100 U/ml penicillin, 100 µg/ml
streptomycin, and 0.25 µmg/ml amphotericin B at 37°C in 5%
CO2.
Sphere formation
Single-cell suspensions of Yumoto cells were seeded
in serum-free Dulbecco's modified Eagle's medium/Nutrient Mix F-12
medium (1:1), containing GlutaMAX-I (DMEM/F12 + GlutaMAX;
Invitrogen; Thermo Fisher Scientific, Inc.) medium with 20 ng/ml
human recombinant epidermal growth factor (EGF) and 20 ng/ml human
recombinant basic fibroblast growth factor (bFGF), cultured at a
density of 1×106 cells/5 ml/dish in 60 mm ultra-low
attachment dishes (Corning Incorporated, Corning, NY, USA) at 37°C
in 5% CO2 for 3 days.
RNA interference
S100A16 and negative control small interfering RNA
(siRNA) duplexes were purchased from Qiagen GmbH, (Hilden,
Germany). Yumoto cells were transfected using the Lipofectamine
RNAiMAX™ reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. After the cells had
incubated 24 h at 37°C in 5% CO2, they were used in the
following experiments.
Proteasome inhibition experiment
After the cells were cultured under the conditions
of the sphere formation for 3 days, these cells were treated with
10 µM lactacystin (Merck KGaA, Darmstadt, Germany) under the
conditions of the sphere formation for 24 h.
Isolation of RNA and reverse
transcription and polymerase chain reaction (RT-PCR) method
Total RNA was extracted using the TRIzol reagent
according to the manufacturer's instructions (Thermo Fisher
Scientific, Inc.). RT-PCR was performed with the SuperScript
One-Step RT-PCR system (Thermo Fisher Scientific, Inc.) and
gene-specific primers according to the manufacturer's instructions.
Reaction mixtures contained total RNA (100 ng of each), 0.2 mM
dNTPs, 0.2 µM of sense and antisense primers, an enzyme mixture
including SuperScript II RT, Platinum Taq DNA polymerase, and 1×
buffer with 1.2 mM MgSO4. The reaction was performed at
50°C for 20 min, 94°C for 2 min, followed by 26–30 cycles of 94°C
for 15 sec, 55°C for 30 sec, and 70°C for 30 sec. These sequences
used the following primers: Oct4, 5′-(GTGGAGAGCAACTCCGATGGG)-3′,
and 5′-(CTCCACCCACTTCTGCAGCAA)-3′; Nanog,
5′-(TCCAACATCCTGAACCTCAGC)-3′, and 5′-(CTGGAACTGCATGCAGGACTG)-3′;
P-gp, 5′-(TACAGCACGGAAGGCCTAATG)-3′, and
5′-(TGTTCTCAGCAATGCTGCAGT)-3′; BCRP, 5′-(TCAGGAAGACTTATGTTCCAC)-3′,
and 5′-(AGCTCTGTTCTGGATTCCAGT)-3′; multidrug resistance-associated
protein 1 (MRP1), 5′-(GACACAGTGGACTCCATGATC)-3′, and
5′-(CCACCAAGCCAGCACTGAGGC)-3′; p53,
5′-(CGCTGCTCAGATAGCGATGGTCTGG)-3′, and
5′-(GATTCTCTTCCTCTGTGCGCCGGTC)-3′; S100A16,
5′-(ACTGCTACACGGAGCTGGAGA)-3′, and 5′-(GCAAGGGTCAGAGGAAGGTCT)-3′;
GAPDH, 5′-(GCTCACTGGCATGGCCTTCCGTGTC)-3′, and
5′-(CTCCTTGGAGGCCATGTGGGCCATG)-3′.
Cell extracts and western
blotting
Cells were lysed with RIPA buffer (50 mM Tris-HCl,
pH 8.0, 150 mM NaCl, 1% Nonidet P-40 (NP-40), 0.1% SDS, 0.5% sodium
deoxycholate) containing protease inhibitor (complete EDTA-free;
Roche Diagnostics, Indianapolis, IN, USA) and centrifuged at 12,000
rpm for 10 min at 4°C. The total cell extracts (50 µg each) were
separated by 15% SDS-polyacrylamide gel electrophoresis and
transferred to polyvinylidene difluoride membrane (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) for S100A16, Oct4, Nanog,
p53, and GAPDH detection. The membranes were washed in
Tris-buffered saline with Tween 20 (TBS-T, composed of 10 mM
Tris-HCl, pH 8, 150 mM NaCl, and 0.05% Tween 20), blocked for 1 h
at room temperature with 5% nonfat milk in TBS-T, and probed
overnight at 4°C with anti-S100A16 (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), anti-Oct4 (Bioworld Technology, Inc., St.
Louis Park, MN, USA), anti-Nanog (Abcam, Cambridge, UK), anti-p53
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and anti-GAPDH
(Cell Signaling Technology, Inc., Danvers, MA, USA) antibodies.
After incubation for 1 h at room temperature with horseradish
peroxide-conjugated secondary antibody, immunoreactive proteins
were detected by the ECL detection system (GE Healthcare Life
Sciences, Little Chalfont, UK).
Measurement of spheroid size in sphere
formation assay
The spheroid size at day 3 in the sphere formation
assay was measured by microscopy and calibration slide. The
spheroid size was defined as the average spheroid diameter of 100
spheroids.
Statistical analysis
Differences between groups were tested by Student's
t-test. Data are presented as means ± SD. Differences were
considered significant at P<0.01.
Results
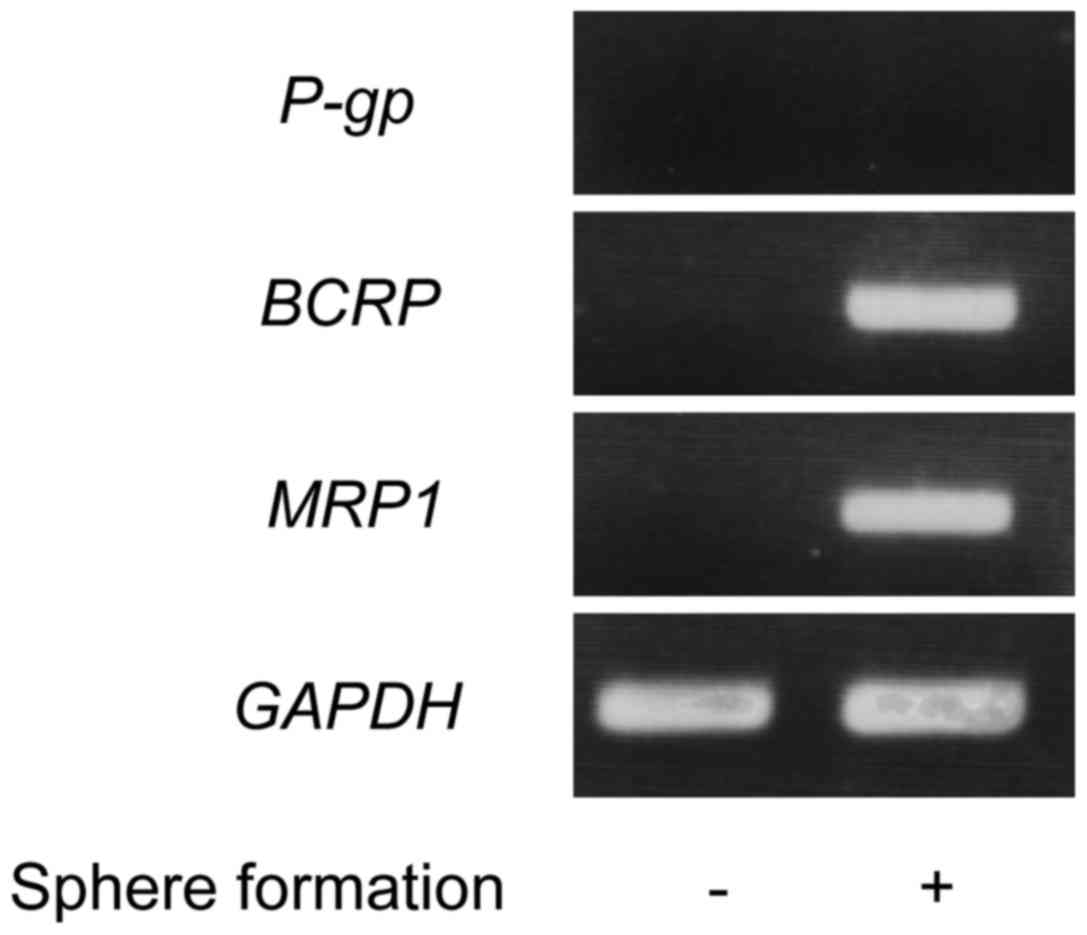
Expression levels of Oct4, Nanog, ABC
transporter, and S100A16 in sphere formation of Yumoto cells
To confirm the properties of the sphere formation of
Yumoto cells, the expression levels of Oct4, Nanog, ABC
transporter, and S100A16 were examined. As shown in Figs. 1 and 2,
the expression levels of Oct4, Nanog, BCRP, MRP1, and S100A16
increased in the sphere formation of Yumoto cells compared with the
monolayer cultured cells. These data show that the expression of
S100A16 up-regulates in the sphere formation of Yumoto cells.
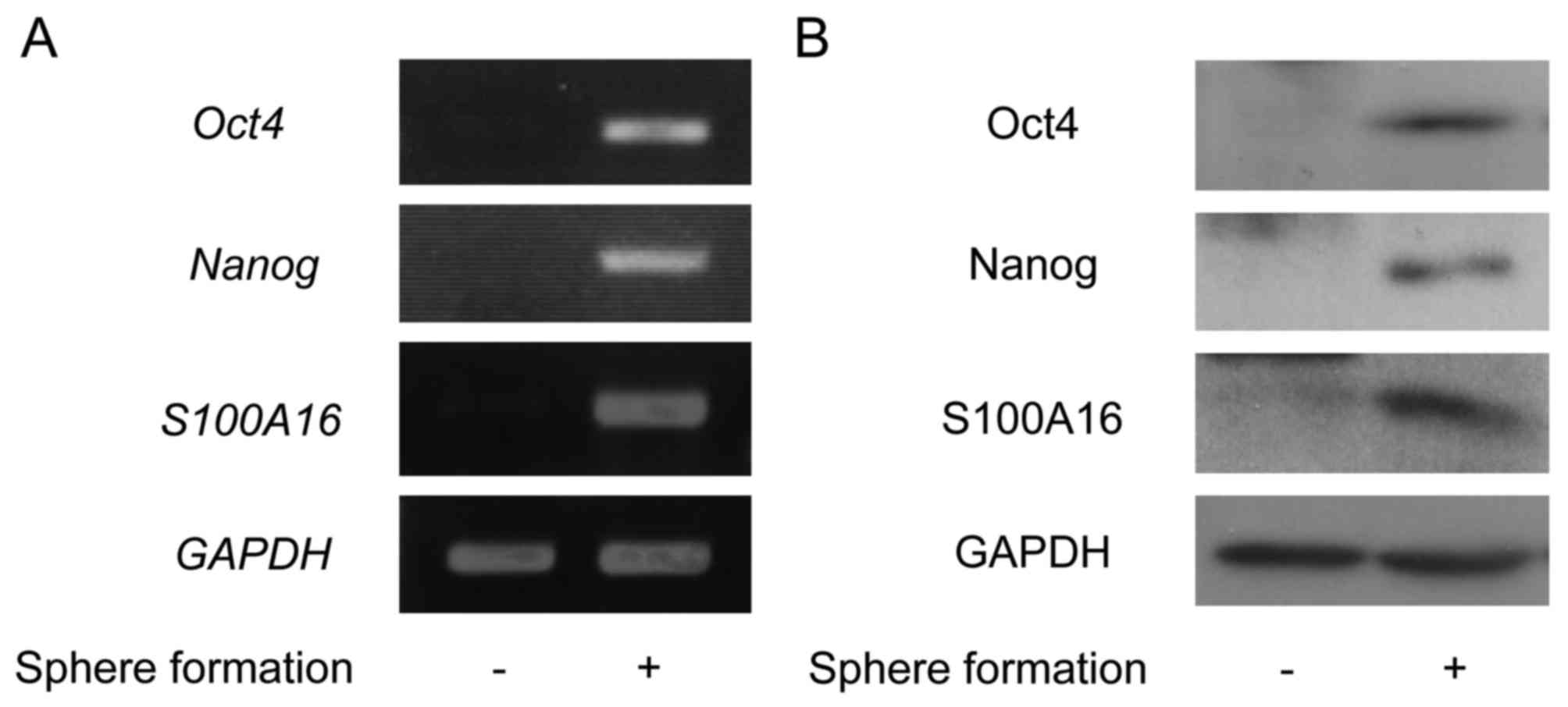 | Figure 1.The expression levels of Oct4, Nanog,
and S100A16 in the sphere formation of Yumoto cells. The cells were
cultured under the non-adherence and serum-free culture conditions
of the sphere formation assay for 3 days. (A) Total RNA was
extracted, and the expressions of Oct4, Nanog, and
S100A16 were evaluated by reverse transcription-polymerase
chain reaction. (B) The cell lysates were prepared from these
cells, and the expressions of Oct4, Nanog, and S100A16 were
detected by immunoblotting using antibodies against Oct4, Nanog,
and S100A16. Oct4, octamer-binding transcription factor 4; Nanog,
homeobox protein NANOG. |
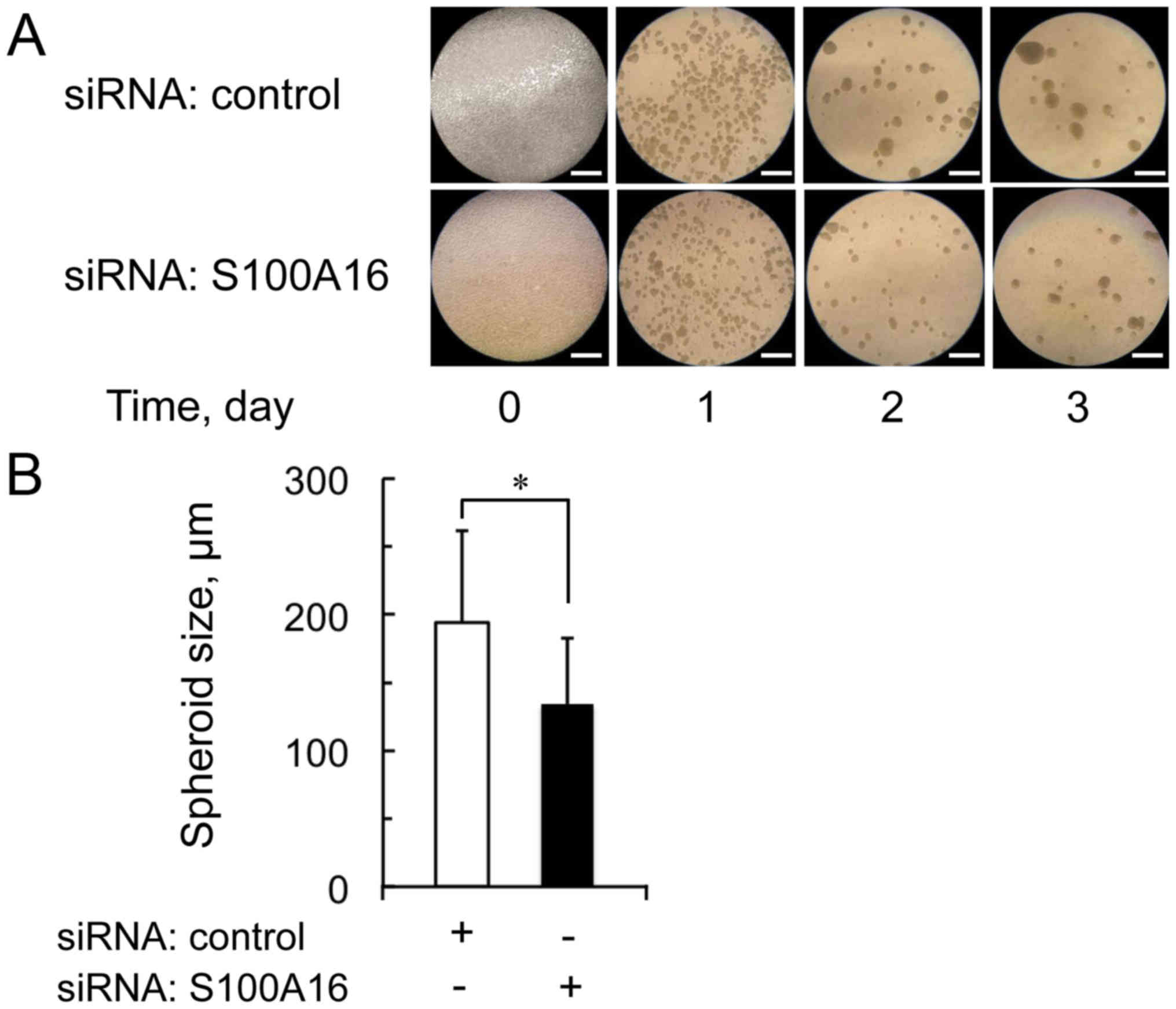
Effect of S100A16-targeting siRNA on
spheroid forming in sphere formation of Yumoto cells
Only CSCs formed spheroids and survived in the
non-adherence and serum-free culture conditions of the sphere
formation assay. To examine whether S100A16 regulates the
spheroid-like body formation of Yumoto cells, the S100A16 or
control siRNA treated cells were cultured under the non-adherence
and serum-free culture conditions of the sphere formation assay for
3 days. Fig. 3 shows the effect of
S100A16 siRNA on spheroid forming in the sphere formation of Yumoto
cells over the 3 days. The spheroid size was significantly
decreased in S100A16 siRNA treated cells compared with control
siRNA treated cells. These data indicate that S100A16 is involved
in spheroid forming in the sphere formation of Yumoto cells.
Effect of S100A16-targeting siRNA on
expression of Oct4, Nanog, and p53 in sphere formation of Yumoto
cells
Fig. 4 shows the
effect of S100A16-targeting siRNA on the expression levels of Oct4,
Nanog, and p53 in the sphere formation of Yumoto cells evaluated by
RT-PCR and immunoblotting. In the sphere formation of Yumoto cells,
the expression levels of Oct4 and Nanog were decreased in S100A16
siRNA treated cells compared with control siRNA treated cells.
These data suggest that S100A16 up-regulates Oct4 and Nanog
expression in the CSCs of Yumoto cells.
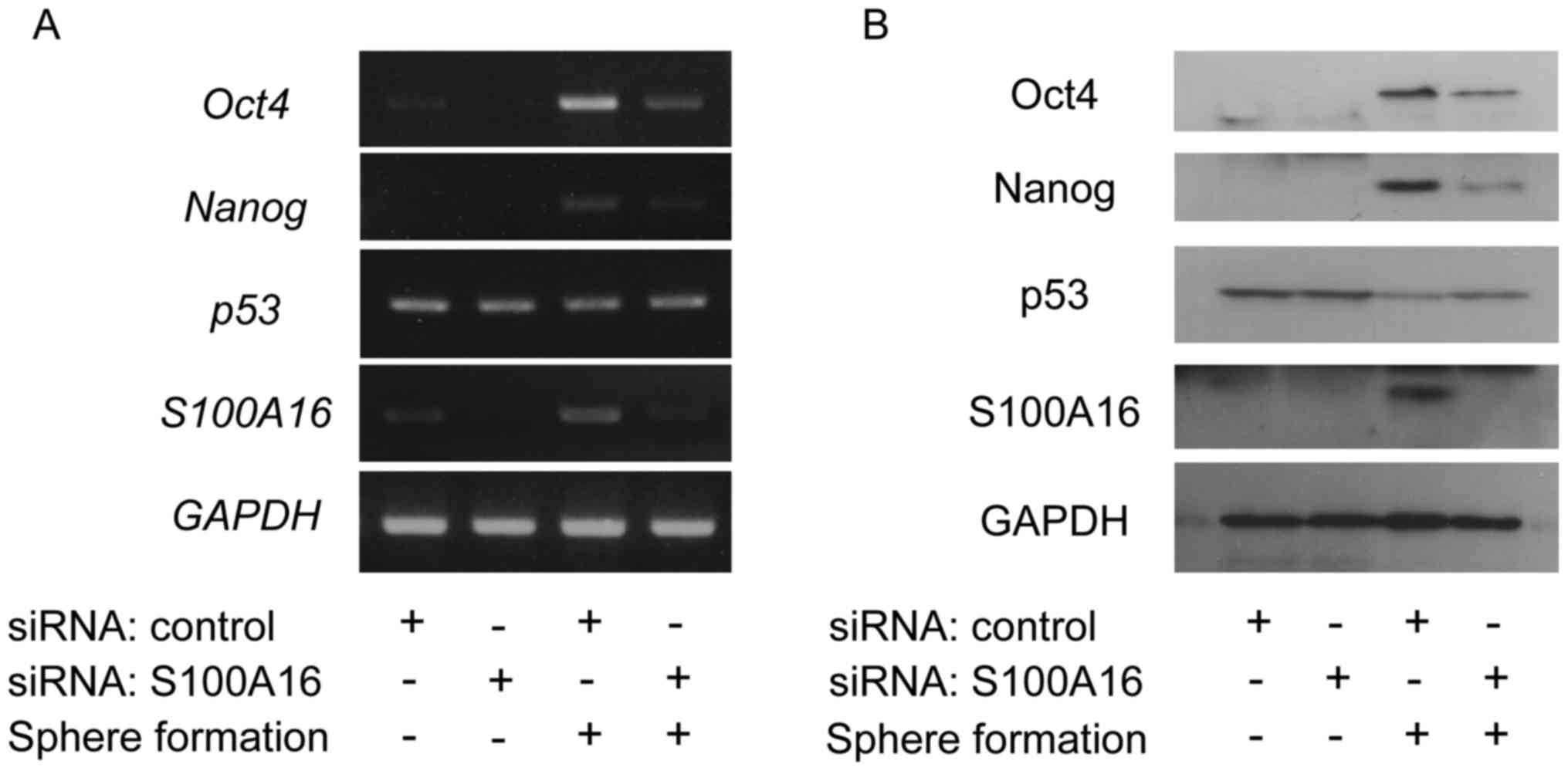 | Figure 4.Effect of S100A16-targeting siRNA on
the expression of Oct4, Nanog, and p53 in the sphere formation of
Yumoto cells. The cells were transfected with S100A16-targeting or
control siRNA. After these cells were incubated for 24 h, these
cells were cultured under the conditions of the sphere formation
assay for 3 days. (A) Total RNA was extracted, and the expression
levels of Oct4, Nanog, p53, and S100A16 were
evaluated by reverse transcription-polymerase chain reaction. (B)
The cell lysates were prepared from these cells, and the expression
levels of Oct4 Nanog, p53, and S100A16 were detected by
immunoblotting using antibodies against Oct4, Nanog, p53, and
S100A16. Oct4, octamer-binding transcription factor 4; Nanog,
homeobox protein NANOG; siRNA, short interfering RNA. |
It has been reported that the expressions of Oct4
and Nanog are regulated by p53 and that S100A16 interacts with p53
(11,14). In order to confirm whether p53 was
involved in the up-regulation of Oct4 and Nanog by S100A16, the
expression level of p53 was examined in the sphere formation of
Yumoto cells after transfection with S100A16 siRNA. As shown in
Fig. 4, there was no change in the
mRNA expression level of p53, whereas the protein expression
level of p53, which was decreased in the sphere formation, was
recovered by S100A16 knockdown. Therefore, the up-regulation of
Oct4 and Nanog by S100A16 seems to be mediated by suppressing p53
in the sphere formation of Yumoto cells.
Effect of proteasome inhibition on
expression of Oct4, Nanog, p53, and S100A16 in sphere formation of
Yumoto cells
It has been reported that some S100 family members
interact with p53 and promote proteasome-dependent degradation of
p53 (7,8,15,16). In order to confirm whether S100A16 was
involved in p53 proteasome degradation, proteasome inhibition by
lactacystin was examined in the sphere formation of Yumoto cells.
As shown in Fig. 5A, the protein
expression level of p53 was increased by lactacystin. The protein
expression levels of Oct4 and Nanog, which were increased in the
sphere formation, were decreased by lactacystin, whereas no
difference was observed in the S100A16 protein levels between the
presence or absence of lactacystin. The spheroid size was not
affected by the addition of lactacystin. The results suggested that
S100A16 may up-regulate Oct4 and Nanog expression to promote p53
degradation in the CSCs of Yumoto cells (Fig. 5B).
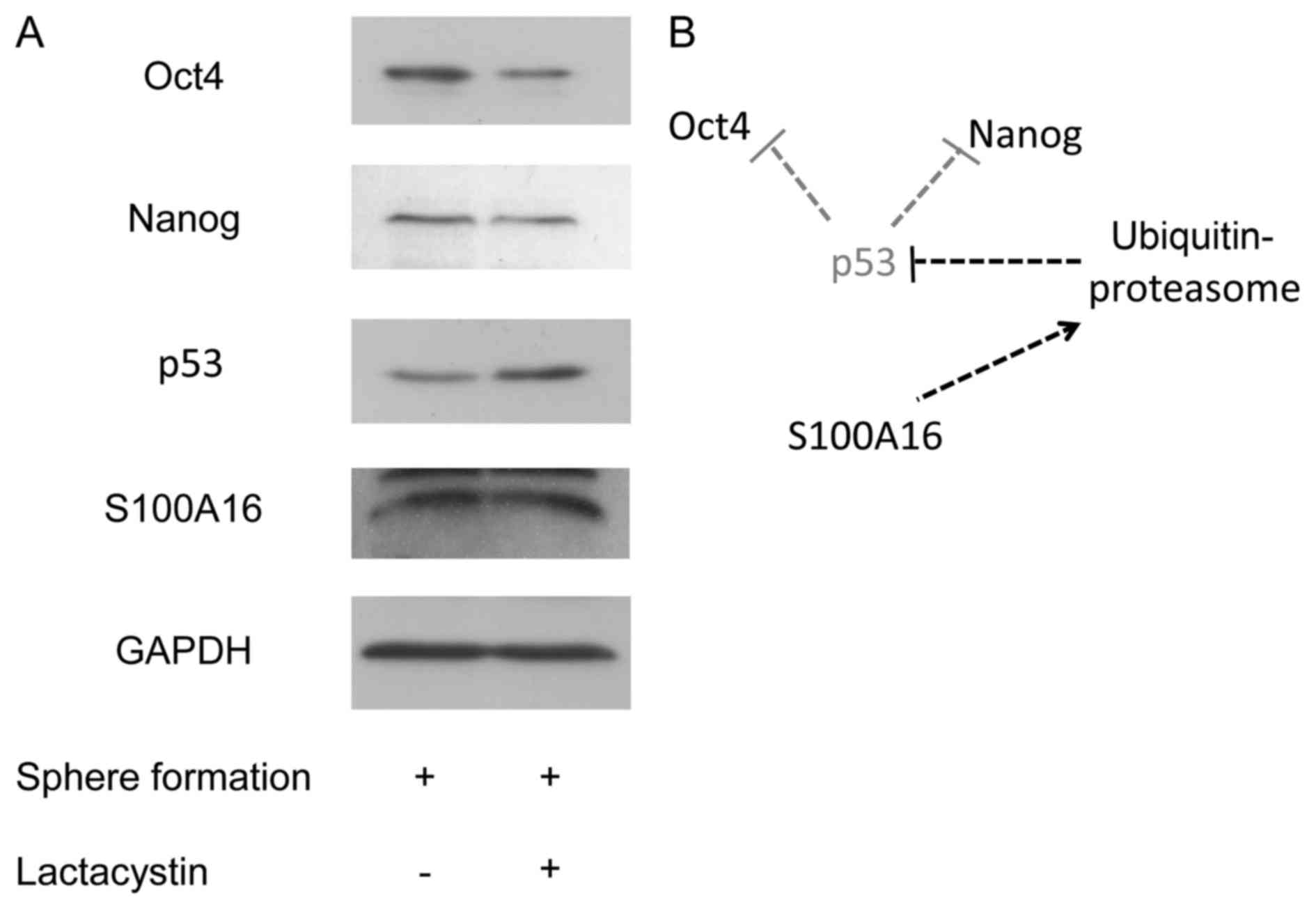 | Figure 5.(A) Effect of proteasome inhibition on
the expressions of Oct4, Nanog, p53, and S100A16 in the sphere
formation of Yumoto cells. After the cells were cultured under the
conditions of the sphere formation assay for 3 days, these cells
were treated with proteasome inhibitor lactacystin (10 µM) under
the conditions of the sphere formation assay for 24 h. The cell
lysates were prepared from these cells, and the expression levels
of Oct4, Nanog, p53, and S100A16 were detected by immunoblotting
using antibodies against Oct4, Nanog, p53, and S100A16. (B)
Potential schematic diagram of the effect of S100A16 on the
expression of Oct4 and Nanog expression in CSCs of Yumoto human
cervical carcinoma cells. Oct4, octamer-binding transcription
factor 4; Nanog, homeobox protein NANOG. |
Discussion
We have demonstrated in this study that the S100A16
gene positively regulated the expressions of Oct4 and Nanog in the
sphere formation of Yumoto cells. This is the first report that
S100A16 is involved in the expression levels of Oct4 and Nanog in
CSCs.
In the present study, we used the sphere formation
assay to evaluate CSCs. The sphere formation assay is based on the
mechanism by which only CSCs survive, by forming spheroids, under
non-adherence and serum-free culture conditions (17). The sphere formation assay is
particularity useful to enrich the potential CSC subpopulations.
Furthermore, numerous studies have demonstrated purported CSCs by
the sphere formation assay (17–19).
It has been reported that Oct4 expression maintained
cancer stem-like properties, and treatment with Oct4 siRNA resulted
in decreased spheroid size in the sphere formation (3,20). The
CSCs survived by forming spheroids in the culture conditions of the
sphere formation assay, whereas non CSCs did not survive and did
not form spheroids. Therefore, the suppression of the Oct4
expression after treatment with S100A16 siRNA affects the cancer
stem cell-like properties and reduces the number of viable cells in
the culture conditions of the sphere formation of Yumoto cells.
The tumor suppressor protein, p53, is an important
factor for the cell cycle and apoptosis, and its dysfunction is
associated with increased tumor development (21,22). It
has been reported that Oct4 and Nanog were negatively regulated by
p53 (14), and p53 was negatively
regulated by the interaction of S100A16 with p53 (11). It has been reported that S100 protein
family members interact with p53 and affect p53 function (7–9,15). In particular, S100A4 interacts with
p53 and promotes p53 proteasome degradation (16). In the present study, the protein
expression levels of Oct4 and Nanog, which were increased in the
sphere formation, were decreased by lactacystin, whereas no
difference was observed in the S100A16 protein levels between the
presence or absence of lactacystin. Although p53 is the frequently
mutated tumor suppressor gene in human cancer (21,22), the
Yumoto cell line has a wild-type p53 gene (23). Hence, the results suggested that
S100A16 may up-regulate Oct4 and Nanog to promote p53 degradation
in the CSCs of Yumoto cells (Fig.
5B). It has been reported that a number of S100 proteins affect
p53 function through several mechanisms, such as inhibition of p53
phosphorylation, modulation of the p53 oligomerization state, and
p53 degradation (2,3). Further studies by other methods are
needed to elucidate the mechanism for the regulation of p53
function by S100A16.
Regulation of Oct4 and Nanog by the
ubiquitin-proteasome system and Oct4 and Nanog up-regulation by
proteasome inhibition in human embryonic stem cells has been
reported (24,25). These previous reports were opposite to
our result that Oct4 and Nanog were down-regulated by the
proteasome inhibitor. However, it has been reported that proteasome
activity is down-regulated in CSCs (26). Thus, the regulation of Oct4 and Nanog
expression may be more affected by p53 than the
ubiquitin-proteasome system in the CSCs of Yumoto cells.
In the present study, Yumoto human cervical
carcinoma cells are used for experiments. There are no reports on
the expression of S100A16 in cervical carcinoma. It has been
reported that a high S100A16 expression level is correlated to poor
prognosis in breast cancer and lung adenocarcinoma (27,28),
whereas a low S100A16 expression level is correlated with reduced
survival and poor tumor differentiation in oral squamous cell
carcinoma (29). Further studies are
needed to clarify the function of S100A16 and its clinical
significance in the various cancer types.
In conclusion, our data indicates that S100A16 is a
positive regulator of Oct4 and Nanog in the CSCs of Yumoto human
cervical carcinoma cells and that S100A16 may play important roles
in the CSCs of Yumoto human cervical carcinoma cells.
Acknowledgements
The authors would like to thank Chizuko Nakamura for
their support and advice at Gyokusui-Kai Hospital.
Funding
The present study was supported by JSHP KAKENHI
(grant no. 26460201).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NT designed the study and wrote the initial draft of
the manuscript. RI contributed to the analysis and interpretation
of the data, and assisted in the preparation of the manuscript. YN,
SM, YuT and YaT contributed to data collection and interpretation
and critically reviewed the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CSCs
|
cancer stem-like cells
|
|
EMT
|
epithelial mesenchymal transition
|
|
siRNA
|
small interfering RNA
|
|
RT-PCR
|
reverse transcription and polymerase
chain reaction
|
|
ESCs
|
embryonic stem cells
|
References
|
1
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosner MH, Vigano MA, Ozato K, Timmons PM,
Poirier F, Rigby PW and Staudt LM: A POU-domain transcription
factor in early stem cells and germ cells of the mammalian embryo.
Nature. 345:686–692. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kumar SM, Liu S, Lu H, Zhang H, Zhang PJ,
Gimotty PA, Guerra M, Guo W and Xu X: Acquired cancer stem cell
phenotypes through Oct4-mediated dedifferentiation. Oncogene.
31:4898–4911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jeter CR, Yang T, Wang J, Chao HP and Tang
DG: Concise review: NANOG in cancer stem cells and tumor
development: An update and outstanding questions. Stem Cells.
33:2381–2390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chambers I, Colby D, Robertson M, Nichols
J, Lee S, Tweedie S and Smith A: Functional expression cloning of
Nanog, a pluripotency sustaining factor in embryonic stem cells.
Cell. 113:643–655. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Savage P: Chemotherapy curable
malignancies and cancer stem cells: A biological review and
hypothesis. BMC Cancer. 16:9062016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen H, Xu C, Jin Q and Liu Z: S100
protein family in human cancer. Am J Cancer Res. 4:89–115.
2014.PubMed/NCBI
|
|
8
|
Bresnick AR, Weber DJ and Zimmer DB: S100
proteins in cancer. Nat Rev Cancer. 15:96–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marenholz I and Heizmann CW: S100A16, a
ubiquitously expressed EF-hand protein which is up-regulated in
tumors. Biochem Biophys Res Commun. 313:237–244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou W, Pan H, Xia T, Xue J, Cheng L, Fan
P, Zhang Y, Zhu W, Xue Y, Liu X, et al: Up-regulation of S100A16
expression promotes epithelial-mesenchymal transition via Notch1
pathway in breast cancer. J Biomed Sci. 21:972014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Zhang R, Xin J, Sun Y, Li J, Wei D
and Zhao AZ: Identification of S100A16 as a novel adipogenesis
promoting factor in 3T3-L1 cells. Endocrinology. 152:903–911. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiou SH, Wang ML, Chou YT, Chen CJ, Hong
CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS and Wu CW:
Coexpression of Oct4 and Nanog enhances malignancy in lung
adenocarcinoma by inducing cancer stem cell-like properties and
epithelial-mesenchymal transdifferentiation. Cancer Res.
70:10433–10444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abdelalim EM and Tooyama I: Knockdown of
p53 suppresses Nanog expression in embryonic stem cells. Biochem
Biophys Res Commun. 443:652–657. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin J, Yang Q, Wilder PT, Carrier F and
Weber DJ: The calcium-binding protein S100B down-regulates p53 and
apoptosis in malignant melanoma. J Biol Chem. 285:27487–27498.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Orre LM, Panizza E, Kaminskyy VO, Vernet
E, Gräslund T, Zhivotovsky B and Lehtiö J: S100A4 interacts with
p53 in the nucleus and promotes p53 degradation. Oncogene.
32:5531–5540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pastrana E, Silva-Vargas V and Doetsch F:
Eyes wide open: A critical review of sphere-formation as an assay
for stem cells. Cell Stem Cell. 8:486–498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei B, Han XY, Qi CL, Zhang S, Zheng ZH,
Huang Y, Chen TF and Wei HB: Coaction of spheroid-derived stem-like
cells and endothelial progenitor cells promotes development of
colon cancer. PLoS One. 7:e390692012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujii H, Honoki K, Tsujiuchi T, Kido A,
Yoshitani K and Takakura Y: Sphere-forming stem-like cell
populations with drug resistance in human sarcoma cell lines. Int J
Oncol. 34:1381–1386. 2009.PubMed/NCBI
|
|
20
|
Chen YC, Hsu HS, Chen YW, Tsai TH, How CK,
Wang CY, Hung SC, Chang YL, Tsai ML, Lee YY, et al: Oct-4
expression maintained cancer stem-like properties in lung
cancer-derived CD133-positive cells. PLoS One. 3:e26372008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hollstein M, Sidransky D, Vogelstein B and
Harris CC: p53 mutations in human cancers. Science. 253:49–53.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Levine AJ, Momand J and Finlay CA: The p53
tumour suppressor gene. Nature. 351:453–456. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Naniwa J, Kigawa J, Akeshima R, Kanamori
Y, Itamochi H, Oishi T, Iba T and Terakawa N: Leptomycin B enhances
CDDP-sensitivity via nuclear accumulation of p53 protein in
HPV-positive cells. Cancer Sci. 94:1099–1103. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu HM, Liao B, Zhang QJ, Wang BB, Li H,
Zhong XM, Sheng HZ, Zhao YX, Zhao YM and Jin Y: Wwp2, an E3
ubiquitin ligase that targets transcription factor Oct-4 for
ubiquitination. J Biol Chem. 279:23495–23503. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Floyd ZE, Staszkiewicz J, Power RA, Kilroy
G, Kirk-Ballard H, Barnes CW, Strickler KL, Rim JS, Harkins LL, Gao
R, et al: Prolonged proteasome inhibition cyclically upregulates
Oct3/4 and Nanog gene expression, but reduces induced pluripotent
stem cell colony formation. Cell Reprogram. 17:95–105. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vlashi E, Kim K, Lagadec C, Donna LD,
McDonald JT, Eghbali M, Sayre JW, Stefani E, McBride W and Pajonk
F: In vivo imaging, tracking, and targeting of cancer stem cells. J
Natl Cancer Inst. 101:350–359. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanaka M, Ichikawa-Tomikaw N, Shishito N,
Nishiura K, Miura T, Hozumi A, Chiba H, Yoshida S, Ohtake T and
Sugino T: Co-expression of S100A14 and S100A16 correlates with a
poor prognosis in human breast cancer and promotes cancer cell
invasion. BMC Cancer. 13:532015. View Article : Google Scholar
|
|
28
|
Saito K, Kobayashi M, Nagashio R, Ryuge S,
Katono K, Nakashima H, Tsuchiya B, Jiang SX, Saegusa M, Satoh Y, et
al: S100A16 is a prognostic marker for lung adenocarcinomas. Asian
Pac J Cancer Prev. 16:7039–7044. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sapkota D, Bruland O, Parajuli H, Osman
TA, Teh MT, Johannessen AC and Costea DE: S100A16 promotes
differentiation and contributes to a less aggressive tumor
phenotype in oral squamous cell carcinoma. BMC Cancer. 15:6312015.
View Article : Google Scholar : PubMed/NCBI
|