Introduction
Osteosarcoma (OS) is the most common primary bone
malignancy in children and adolescents, and accounts for ~20% of
all primary bone cancer worldwide (1,2). Although
there have been improvements in surgical and multi-agent
chemotherapeutics, the long-term survival rate for patients with
non-metastatic OS remains at ~60% (3), whereas, currently, a 5-year survival
rate of 30% is observed in patients with metastatic OS (1,4).
Therefore, it is essential to analyze the biological evidence at
the molecular level of OS to identify novel prognostic markers and
determine the underlying molecular mechanism of OS metastasis.
p62, also known as sequestosome 1, is a
multifunctional scaffold protein that regulates cell proliferation,
apoptosis, cell survival, inflammation and tumorigenesis through
the catabolism of molecules (5,6). The p62
protein is one of the selective substrates of autophagy, localized
on the membranes of autophagosomes (7). It has been identified that the
accumulation of the p62 protein is variably associated with
different types of tumor, including cancer of the prostate, breast,
lung, colon and endometrium (8–12). An
excessive expression of p62 has been identified to be a cause of
poor prognosis in certain tumor types, including prostate and
endometrial cancer (11,12). Furthermore, it has been demonstrated
that the abnormal expression of p62 may be associated with the
activation of oncogenic signaling pathways, including the mammalian
target of rapamycin (mTOR), mitogen-activated protein kinase,
nuclear factor κB (NF-κB), Wnt/β-catenin and potentially other
signaling pathways (7,13). Therefore, it is suggested that p62 may
function as an oncogene and is involved in poor prognosis in human
cancer.
However, the function and clinical significance of
p62 has not yet been completely elucidated in OS. In the present
study, the expression of p62 was examined and its association with
clinicopathological characteristics was investigated in patients
with OS.
Materials and methods
Patients and tumor specimens
The present study was approved by the Ethics
Committee of Xi'an HongHui Hospital (Xi'an, China). Either the
patients or their guardians provided written informed consent for
participation in the study. Formalin-fixed paraffin-embedded
tissues were obtained from 85 patients with OS who underwent tumor
resection at Xi'an HongHui Hospital between March 2007 and November
2009. Patients did not receive preoperative chemotherapy or
radiotherapy. However, patients in Enneking stages I, II or III of
the disease (14) received
postoperative adjuvant chemotherapy [six courses of ifosfamide (2
g/m2 for 5 days/course), methotrexate (8 g/m2
for 1 day/course], and Adriamycin (50 mg/m2 for 1
day/course). All patients were followed up for ≥3 years. However,
15 patients were unreachable (17.7%), and therefore failed to
complete follow-up. Consequently, the data presented were for a
total of 70 patients (82.3%) with a median follow-up of 42 months
(range, 2–68 months). Of the 70 patients, 22 were female and 48
were male, with a median age of 19 years (range, 8–65 years). A
total of 10 osteochondroma specimens were used as normal controls,
including 7 male and 3 female patients with a median age of 32
years (range, 15–54 years). All samples were evaluated for
diagnosis by two experienced pathologists in Hong Hui Hospital,
Xi'an Jiaotong University College of Medicine, (Xi'an, China).
Materials
RPMI-1640 medium was purchased from HyClone (GE
Healthcare, Logan, UT, USA). Fetal bovine serum (FBS), penicillin,
streptomycin and glutamine were purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Immobilon Western horseradish
peroxidase chemiluminescence kit and polyvinylidene fluoride
membrane were from EMD Millipore (Billerica, MA, USA). Polyclonal
antibodies against p62 were purchased from Cell Signaling
Technology Inc. (1:2,000; cat. no. 5114, Danvers, MA, USA).
Horseradish peroxidase (HRP)-conjugated goat anti-rabbit
immunoglobulin and monoclonal antibodies against β-actin were
purchased from Bioworld Technology, Inc. (1:5,000; cat. no. AP0060;
St. Louis Park, MN, USA).
Cell culture
Human OS cell sub-lines F4 and F5M2 were established
and maintained in the laboratory of Tangdu Hospital (The Fourth
Military Medical University, Xi'an, China) as described previously
(15). The cells were cultured in
RPMI-1640 medium supplemented with 10% FBS, penicillin (100 U/ml),
streptomycin (100 µg/ml) and glutamine (2 mM) at 37°C in a
humidified incubator containing 5% CO2. The human
osteoblast hFOB1.19 cell line was purchased from the American Type
Culture Collection (Manassas, VA, USA) and cultured similarly in
Dulbecco's modified Eagle's medium (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany).
Immunohistochemical analysis
Immunohistochemistry was performed as described in
our previous study (16). The
anti-p62 antibody was used at a dilution of 1:100. Images were
observed at magnification, ×400 under a fluorescence microscope.
The final scores of expression of p62 were calculated as described
previously (16). Each specimen was
categorized as follows: 0, (0); 1+, (1–3); 2+,
(4–8);
and 3+, (9–12). Scores of 0–3 and 4–12 were designated
low and high expression, respectively.
Short hairpin RNA (shRNA) targeting
p62
The lentiviruses with shRNA targeting p62
(5′-TCGAGGAAATGGGTCCACCAGGAATTCAAGA-3′) and shRNA negative control
were purchased from Hanbio Biotechnology Co., Ltd. (Shanghai,
China). F5M2 and F4 cells were transfected with the lentiviruses at
a multiplicity of infection of 100 using TransDux transfection
reagent (System Biosciences, Palo Alto, CA, USA). Stable
p62-knockdown OS cells (F5M2-shp62 cells or F4-shp62 cells) were
selected using 3 mg/ml puromycin (Hanbio Biotechnology Co., Ltd.)
and confirmed using the reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blotting. These
stable knockdown cells and controls (Green Fluorescent Protein
(GFP)-F5M2 cells or GFP-F4 cells) were used for subsequent
experiments following transfection for 48 h.
RT-qPCR
F5M2 and F4 cells, as well as OS adjacent healthy
tissue, were harvested and lysed with TRIzol (Invitrogen; Thermo
Fisher Scientific, Inc.). All samples were sent to UcallM Biotech
Company (Wuxi, China) for subsequent experimental procedures. The
Revert Aid First-Strand RT-PCR kit (Invitrogen: Thermo Fisher
Scientific, Inc.) was used to synthesize cDNA from 500 ng total
RNA. Then 2 µl cDNA was amplified in 20 µl reaction mixture
containing 10 µl of SYBR Green PCR mix (Invitrogen: Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
following thermocycling conditions were used: 5 min at 95°C,
followed by 40 cycles of 10 sec at 95°C and 30 sec at 60°C. The p62
gene expression was calculated using the 2−ΔΔCq method
(17). The primers used for
amplification were as follows: Human p62 forward,
5′-TGAGGAACAGATGGAGTCGG-3′ and reverse, 5′-GAGATGTGGGTACAAGGCAG-3′;
human GAPDH forward, 5′-ACCCACTCCTCCACCTTTG-3′ and reverse,
5′-CACCACCCTGTTGCTGTAG-3′. All experiments were performed in
triplicate and repeated at least three times independently.
Cell counting kit-8 (CCK-8) assay
F5M2-shp62, GFP-F5M2, F4-shp62 and GFP-F4 cells were
seeded into 96-well plates at a density of 2,000 cells/well. After
24 h of incubation at 37°C in 5% CO2, 10 µl/well CCK-8
reagent (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was
added to one plate, which was incubated for 2 h at 37°C and
measured using a microplate reader (Thermo Fisher Scientific, Inc.)
at 490 nm. Each experiment was repeated three times
independently.
Transwell migration and invasion
assays
The Transwell migration and invasion assays were
performed as in our previous study (18). The migratory and invasive potential of
cells were assessed using a Transwell (Corning Inc., Corning, NY,
USA) inserts only or using inserts pre-coated with 50 µl of BD
Matrigel™ Basement Membrane Matrix (1:3 dilution, BD
Biosciences, Franklin Lakes, NJ, USA). The upper chambers were
seeded with 1×104 cells suspended in serum-free
RPMI-1640 medium, and the lower chambers were filled with complete
RPMI-1640 medium with 10% FBS (0.6 ml/well). The invasion was
evaluated after 16 h of incubation at 37°C. The migration was
assessed after 24 h of incubation. Invasion and migration were
examined under a light microscope (Nikon Eclipse 80i; Nikon
Corporation, Tokyo, Japan) at a magnification, ×200 in five
randomized fields. All experiments were independently repeated
three times.
Western blot analysis
Cellular lysates were prepared in chilled
radioimmunoprecipitation assay lysis buffer (Cell Signaling
Technology, Inc.), and the protein concentrations were determined
using a BCA protein assay kit (Pierce; Thermo Fisher Scientific,
Inc.). Proteins (10 µl) were loaded and separated by
electrophoresis on 10% SDS-polyacrylamide gels at 110 V for 2 h.
Following electrophoresis, the SDS-PAGE gels were transferred
electronically to polyvinylidene difluoride (PVDF) membranes
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). PVDF membranes
were blocked using a solution containing 5% skimmed milk and
incubated overnight at 4°C with the following antibodies: Anti-p62
and anti-β-actin (1:1,000). Following washing with Tris-buffered
saline with Tween-20, the membranes were incubated for 1 h at room
temperature with HRP-conjugated goat anti-rabbit immunoglobulin and
monoclonal antibodies against β-actin. Reactive proteins were
visualized using an Immobilon western horseradish peroxidase
chemiluminescence kit (EMD Millipore, Billerica, MA, USA). Relative
p62 expression levels as analyzed using ImageJ software version
1.47 (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The statistical analyses were carried out using
GraphPad Prism software (version 5.0; GraphPad Software Inc., La
Jolla, CA, USA). The immunohistochemistry data were analyzed using
the χ2 test. Survival data were analyzed using the
Kaplan-Meier with log-rank tests estimator method. The RT-qPCR,
western blot analysis, inhibitor migration assay and inhibitor
invasion assay results were analyzed by one-way analysis of
variance. All other data were analyzed using a two-sided Student's
t-test. The data are presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinicopathological features and
prognostic implications of p62 expression
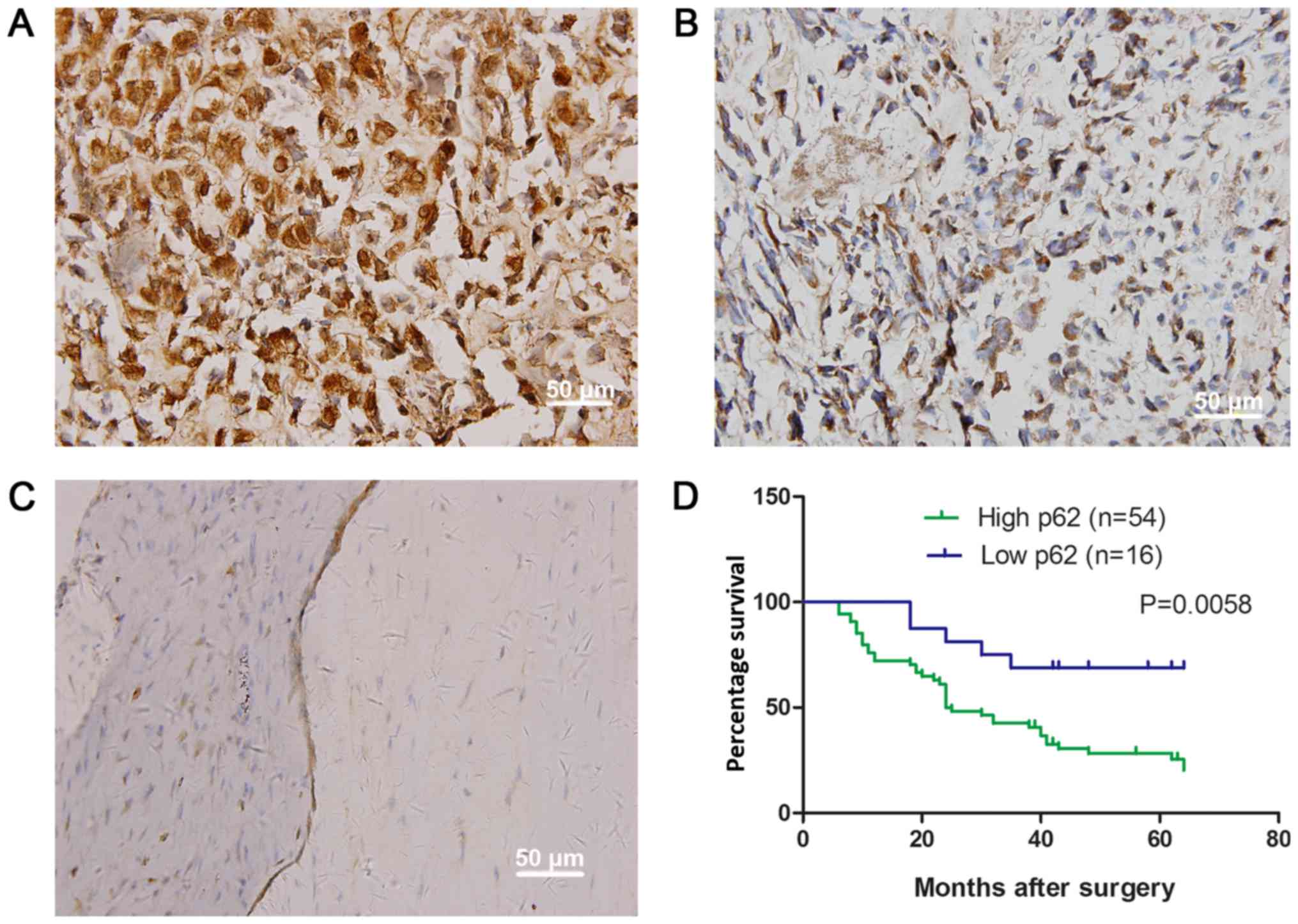
Using immunohistochemistry, it was identified that
the p62 was distributed in the cytoplasm and perinuclear membrane
of the OS cells (Fig. 1A and B).
Compared with osteochondroma samples, the expression rate of p62
was significantly increased in OS (Table
I; Fig. 1C). The increased
expression of p62 protein was observed in 54/70 samples (77.14%)
and was identified to be significantly associated with tumor size
(P=0.001), metastasis (P=0.036) and clinical staging (P=0.003)
(Table II). These patients were also
significantly associated with a decreased overall survival time
(P=0.0058; Fig. 1D).
 | Table I.Expression levels of p62 in
osteosarcoma and osteochondroma specimens. |
Table I.
Expression levels of p62 in
osteosarcoma and osteochondroma specimens.
|
|
| p62 expression |
|
|---|
|
|
|
|
|
|---|
| Group | n | ++/+++ | −/+ | P-value |
|---|
| Osteosarcoma | 70 | 54 (77.1%) | 16 (22.9%) | <0.01 |
| Osteochondroma | 20 | 6 (30.0%) | 14 (70.0%) |
|
 | Table II.Association between p62 expression
and clinicopathological data in patients with osteosarcoma. |
Table II.
Association between p62 expression
and clinicopathological data in patients with osteosarcoma.
|
|
| p62 expression |
|
|---|
|
|
|
|
|
|---|
| Variable | n | High | Low | P-value |
|---|
| Total | 70 | 54 | 16 |
|
| Sex |
|
|
| 0.551 |
|
Male | 48 | 38 | 10 |
|
|
Female | 22 | 16 | 6 |
|
| Age, years |
|
|
| 0.816 |
|
≥20 | 16 | 12 | 4 |
|
|
<20 | 54 | 42 | 12 |
|
| Tumor size,
cm2 |
|
|
| 0.001a |
|
<50 | 20 | 16 | 12 |
|
|
≥50 | 27 | 38 | 4 |
|
| Histology |
|
|
| 0.355 |
|
Osteoblastic | 31 | 25 | 6 |
|
|
Chondroblastic | 8 | 4 | 4 |
|
|
Fibroblastic | 10 | 8 | 2 |
|
|
Telangiectatic | 15 | 12 | 3 |
|
|
Mixed | 6 | 5 | 1 |
|
| Metastasis |
|
|
| 0.036a |
|
Yes | 29 | 26 | 3 |
|
| No | 41 | 28 | 13 |
|
| Clinical stage |
|
|
| 0.003a |
|
I/IIA | 17 | 9 | 8 |
|
|
IIB | 24 | 19 | 5 |
|
|
III | 29 | 26 | 3 |
|
Human OS cell lines F5M2 and F4
express p62
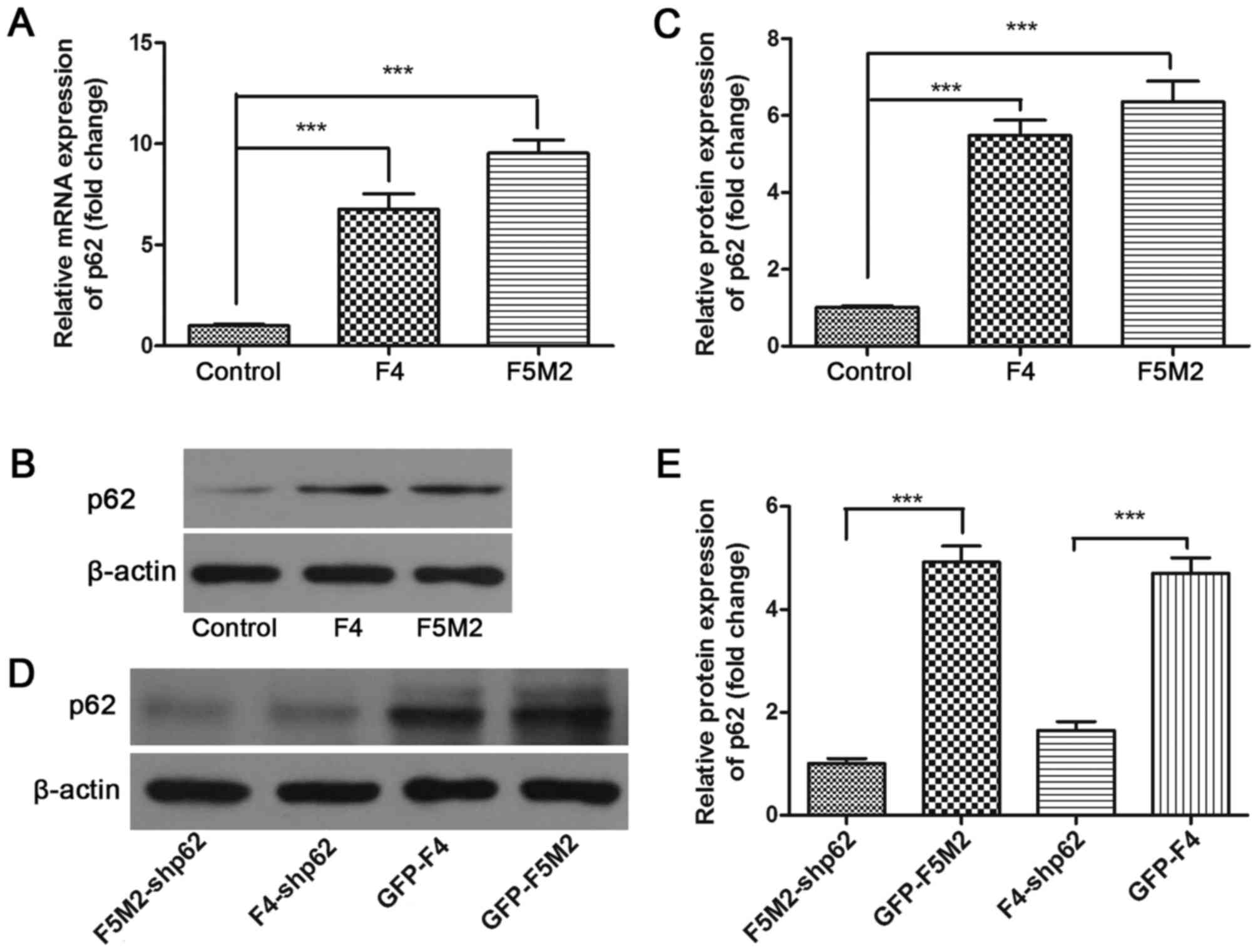
RT-qPCR results identified that the expression level
of p62 mRNA was highest in the human OS cell lines F5M2 and F4 and
lowest in the human osteoblastic cell line hFOB1.19 (P<0.001;
Fig. 2A). The expression level of p62
protein in these cell lines was then assessed by western blotting.
The results demonstrated that OS cells (F5M2 and F4) expressed
increased levels of p62 protein compared with osteoblastic cells
(hFOB1.19) (Fig. 2B), which was
identified to be a significant difference (Fig. 2C).
p62 knockdown decreases the
proliferative capacity of F5M2 and F4 cells
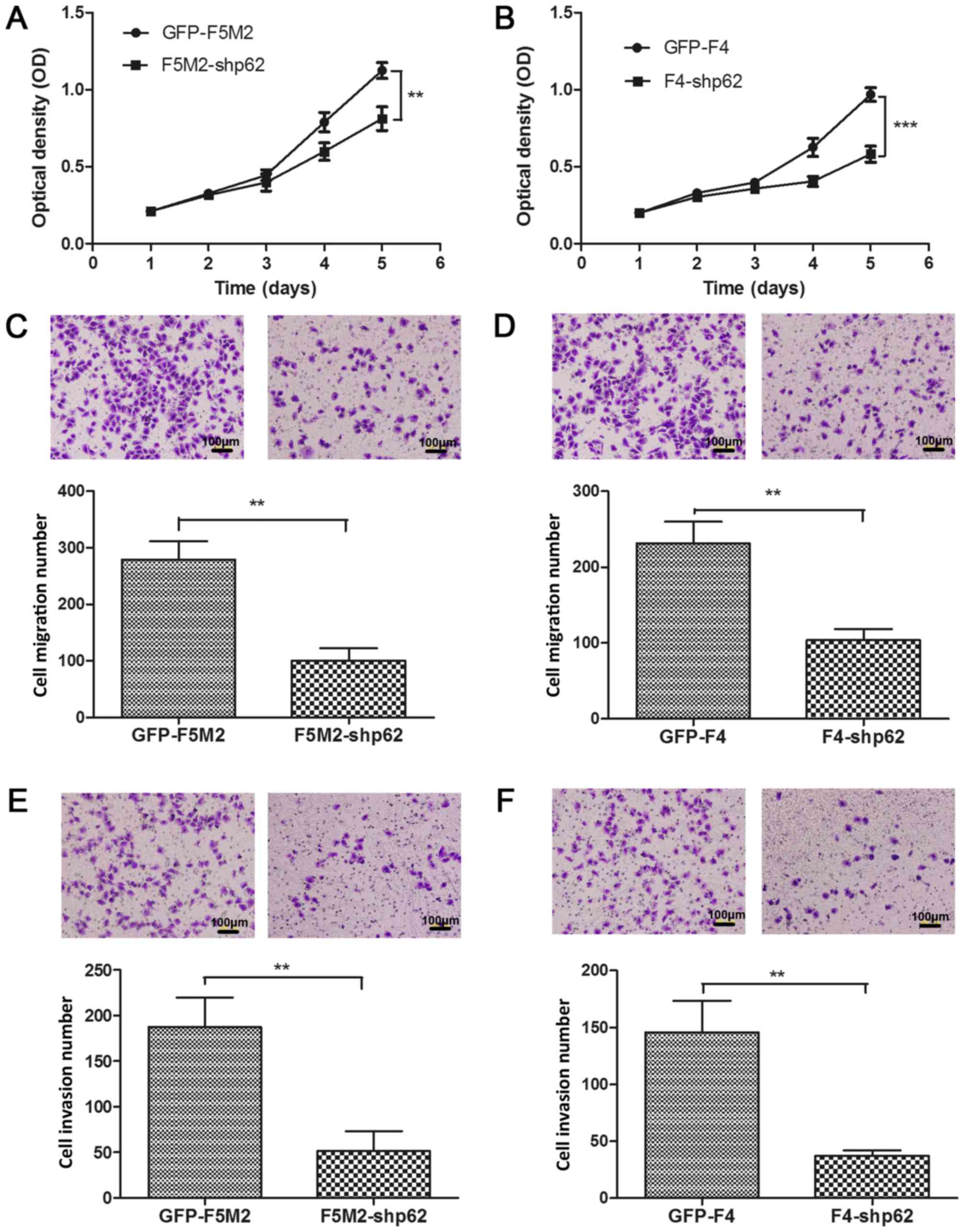
A CCK-8 cell proliferation assay was used to examine
the effect of p62 inhibition on the in vitro proliferative
capacity of F5M2 and F4 cells. p62 expression was stably
downregulated by lentiviral-mediated shRNA. Compared with the
controls (GFP-F5M2 and GFP-F4), the p62 expression was efficiently
decreased in the stable p62-inhibited cells (F5M2-shp62 and
F4-shp62) (Fig. 2D), which was
identified to be a significant difference (Fig. 2E). Subsequently, the CCK-8 cell
proliferation assay demonstrated that stable p62 knockdown
decreased the proliferation of F5M2 (P<0.01; Fig. 3A) and F4 cells (P<0.001; Fig. 3B) compared with their respective
controls.
p62 knockdown decreases the migration
and invasion of F5M2 and F4 cells
The migration assay results demonstrated that p62
inhibition significantly decreased the migratory ability of F5M2
cells (P<0.01; Fig. 3C) and F4
cells (P<0.01; Fig. 3D) when
compared with the corresponding control groups. The invasion assay
results revealed that p62 inhibition significantly decreased the
invasive ability of F5M2 (P<0.01; Fig.
3E) and F4 cells (P<0.01; Fig.
3F).
Discussion
The results of the present study demonstrated that
the increased expression of p62 protein was significantly
associated with tumor size, metastasis and clinical staging in
human OS, and may be used as a prognostic factor. However, patients
with increased expression of p62 protein were identified to be
significantly associated with decreased overall survival times
compared with those with low p62 expression. To the best of our
knowledge, this present study is the first to investigate the
clinical significance of p62 expression in OS.
The abnormal expression of p62 detected in a variety
of tumors, including breast, prostate, lung, liver and oral cancer,
serves an active function in tumorigenesis and metastasis (12,19,20). To
determine the p62 protein levels in prostate cancer, Burdelski
et al (11) used
immunohistochemistry to assess 7,822 prostate cancers and found
that p62 stained in 73% (5,716/7,822). In addition, the protein was
markedly associated with high Gleason grade, positive nodal status,
advanced pathological tumor stage and positive resection margin
(12). Therefore, the accumulation of
p62 may be considered as a predictor of the detrimental prognostic
behavior of prostate cancer (12).
The expression of p62 is substantially weak in normal gastric
tissue samples, whereas increased cytoplasmic and nuclear levels
are detected in gastric cancer (21).
The immunohistochemical analysis of the present study indicated
increased expression p62 protein in 77.14% (54/70) of the analyzed
OS samples.
Previous studies have demonstrated that the
autophagy system constantly degraded the p62 protein (22,23),
whereas other studies indicated that p62 expression is regulated at
the transcriptional level (24,25).
However, the specific function of p62 in tumors remains unclear,
and it has been demonstrated that increased p62 mRNA expression is
associated with increased p62 protein expression in the oral
squamous cell carcinoma (19). In the
present study, it was demonstrated that the p62 mRNA and protein
levels were upregulated in human F5M2 and F4 OS cells, suggesting
that the protein regulation occurred at the transcriptional and
translational levels.
In order to investigate the function of p62 in tumor
cell proliferation, migration and invasion, stable p62 knockdown
cell lines were established using specific shRNA. Using a CCK-8
assay, p62 knockdown was determined to suppress the proliferation
of F5M2 and F4 OS cells. Furthermore, the migration and invasion
assays demonstrated that p62 knockdown decreased the migration and
invasion of F5M2 and F4 cells in vitro. Thus, increased p62
expression increased the proliferation, migration and invasion of
OS cells in vitro. Previous studies have also established
that the p62 protein functions as a hub for various signal
transduction pathways for cell survival and cell death (20). The overexpression of p62 has been
identified to promote tumor cell survival via impairment of the
NF-κB signaling pathway (26). The
NRF2-Keap1 pathway consisted of the transcription factor nuclear
factor erythroid 2-related factor 2 (NRF2), and the cytoplasmic
contact protein Keap1. NRF2 is a central regulator of the cellular
antioxidant stress reaction. Furthermore, NRF2 is able to regulate
the expression of several detoxification enzymes and a series of
antioxidative protein genes, thereby serving a major function in
antitumor mechanisms (6). Under
normal conditions, NRF2 was degraded by the ubiquitin-proteasome
pathway through interaction with Keap1. When exposed to the
pro-electronic reagent, reactive oxygen species and nitric oxide,
Keap1 is activated, leading to NRF2 heterodimerization and
translocation to the nucleus (6).
Subsequently, the nuclear NRF2 is able to induce a series of
transcription cell protective genes (6). The p62 protein is able to directly
interact with Keap1 of NRF2, and the excessive expression of p62 is
able to continuously stimulate NRF2, which is hypothesized to lead
to tumor progression (27). Aberrant
mitosis often leads to tumor occurrence (28). The active association of
cyclin-dependent kinase (CDKs) with cell cycle proteins is the key
to an orderly cell cycle (28). The
lack of cell cycle proteins or the presence of CDK inhibitor leads
to cell cycle arrest and cell death (29). The study of Linares et al
(30) elucidated the expression of
p62 protein in different cells at various stages of the cell cycle,
and identified that the protein had been phosphorylated in the
early stage of mitosis. Following inhibition of the activity of
CDKs, the p62 protein was phosphorylated at Thr269 and
Ser272, suggesting that it may be involved in the
mitotic division of cells (30). In
addition, it has been demonstrated that p62 is able to activate the
mTOR signaling pathway to regulate cell growth and autophagy
(31).
There were several limitations to the present study.
First, the study was primarily limited by the number of patient
samples. Secondly, despite osteochondroma as the control group, the
study lacked normal tissue as the control group. Finally, the
regulatory mechanism of p62 in OS was not elucidated. Further
studies with larger patient populations involving normal tissue,
mechanism of p62 in OS, and animal experiment are essential to
assess p62 more accurately as a therapeutic target for OS.
In summary, the results of the present study
demonstrated that p62 was upregulated in human OS. Furthermore, the
overexpression of p62 protein is associated with poor survival
rates in patients with OS. Additionally, p62 may be involved in
osteosarcoma progression; however, further studies are a
prerequisite for understanding the significance of increased
expression of p62 in order to develop specific and comprehensive
treatments.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Project of
Science and Technology Department of Shaanxi Province (grant nos.
2015SF116, 2015SF110 and 2013K14-02-12).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HX, ML and KZ analyzed and interpreted the patient
data. LS and BH performed the immunohistochemical analysis, YL, QW
and YZ performed RT-qPCR, western blot, CCK-8 assay and Transwell
and were major contributors in writing the manuscript. CR and ND
performed cell transfection. HL and CZ performed statistical
analysis. ZL and TM designed the study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Xi'an HongHui Hospital (Xi'an, China). Either the
patients or their guardians provided written informed consent for
participation in the study.
Consent for publication
The patient or their guardian provided written
informed consent for the publication of any associated data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Duchman KR, Gao Y and Miller BJ:
Prognostic factors for survival in patients with high-grade
osteosarcoma using the surveillance, epidemiology, and end results
(SEER) program database. Cancer Epidemiol. 39:593–599. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maugg D, Rothenaigner I, Schorpp K,
Potukuchi HK, Korsching E, Baumhoer D, Hadian K, Smida J and
Nathrath M: New small molecules targeting apoptosis and cell
viability in osteosarcoma. PLoS One. 10:e01290582015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferguson WS and Goorin AM: Current
treatment of osteosarcoma. Cancer Invest. 19:292–315. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Manley S, Williams JA and Ding WX: Role of
p62/SQSTM1 in liver physiology and pathogenesis. Exp Biol Med
(Maywood). 238:525–538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moscat J and Diaz-Meco MT: p62 at the
crossroads of autophagy, apoptosis, and cancer. Cell.
137:1001–1004. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Inoue D, Suzuki T, Mitsuishi Y, Miki Y,
Suzuki S, Sugawara S, Watanabe M, Sakurada A, Endo C, Uruno A, et
al: Accumulation of p62/SQSTM1 is associated with poor prognosis in
patients with lung adenocarcinoma. Cancer Sci. 103:760–766. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo RZ, Yuan ZY, Li M, Xi SY, Fu J and He
J: Accumulation of p62 is associated with poor prognosis in
patients with triple-negative breast cancer. Onco Targets Ther.
6:883–888. 2013.PubMed/NCBI
|
|
10
|
Inui T, Chano T, Takikita-Suzuki M,
Nishikawa M, Yamamoto G and Okabe H: Association of p62/SQSTM1
excess and oral carcinogenesis. PLoS One. 8:e743982013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burdelski C, Reiswich V, Hube-Magg C,
Kluth M, Minner S, Koop C, Graefen M, Heinzer H, Tsourlakis MC,
Wittmer C, et al: Cytoplasmic accumulation of sequestosome 1 (p62)
is a predictor of biochemical recurrence, rapid tumor cell
proliferation, and genomic instability in prostate cancer. Clin
Cancer Res. 21:3471–3479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iwadate R, Inoue J, Tsuda H, Takano M,
Furuya K, Hirasawa A, Aoki D and Inazawa J: High expression of p62
protein is associated with poor prognosis and aggressive phenotypes
in endometrial cancer. Am J Pathol. 185:2523–2533. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Puissant A, Fenouille N and Auberger P:
When autophagy meets cancer through p62/SQSTM1. Am J Cancer Res.
2:397–413. 2012.PubMed/NCBI
|
|
14
|
Jawad MU and Scully SP: In brief:
Classifications in brief: Enneking classification: Benign and
malignant tumors of the musculoskeletal system. Clin Orthop Relat
Res. 468:2000–2002. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, Yang TT, Wang W, Sun HH, Ma BA, Li
CX, Ma Q, Yu Z and Fan QY: Establishment and characterization of
human osteosarcoma cell lines with different pulmonary metastatic
potentials. Cytotechnology. 61:37–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu Y, Guan GF, Chen J, Hu B, Sun C, Ma Q,
Wen YH, Qiu XC and Zhou Y: Aberrant CXCR4 and β-catenin expression
in osteosarcoma correlates with patient survival. Oncol Lett.
10:2123–2129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Ma Q, Liu T, Guan G, Zhang K,
Chen J, Jia N, Yan S, Chen G, Liu S, et al: Interleukin-6
suppression reduces tumour self-seeding by circulating tumour cells
in a human osteosarcoma nude mouse model. Oncotarget. 7:446–458.
2016.PubMed/NCBI
|
|
19
|
Liu JL, Chen FF, Lung J, Lo CH, Lee FH, Lu
YC and Hung CH: Prognostic significance of p62/SQSTM1 subcellular
localization and LC3B in oral squamous cell carcinoma. Br J Cancer.
111:944–954. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Yang Z and Dong J: P62: An
emerging oncotarget for osteolytic metastasis. J Bone Oncol.
5:30–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mohamed A, Ayman A, Deniece J, Wang T,
Kovach C, Siddiqui MT and Cohen C: P62/Ubiquitin IHC expression
correlated with clinicopathologic parameters and outcome in
gastrointestinal carcinomas. Front Oncol. 5:702015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khan MM, Strack S, Wild F, Hanashima A,
Gasch A, Brohm K, Reischl M, Carnio S, Labeit D, Sandri M, et al:
Role of autophagy, SQSTM1, SH3GLB1, and TRIM63 in the turnover of
nicotinic acetylcholine receptors. Autophagy. 10:123–136. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bitto A, Lerner CA, Nacarelli T, Crowe E,
Torres C and Sell C: P62/SQSTM1 at the interface of aging,
autophagy, and disease. Age (Dordr). 36:96262014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sahani MH, Itakura E and Mizushima N:
Expression of the autophagy substrate SQSTM1/p62 is restored during
prolonged starvation depending on transcriptional upregulation and
autophagy-derived amino acids. Autophagy. 10:431–441. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jain A, Lamark T, Sjøttem E, Larsen KB,
Awuh JA, Øvervatn A, McMahon M, Hayes JD and Johansen T: p62/SQSTM1
is a target gene for transcription factor NRF2 and creates a
positive feedback loop by inducing antioxidant response
element-driven gene transcription. J Biol Chem. 285:22576–22591.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mathew R, Karp CM, Beaudoin B, Vuong N,
Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, et al:
Autophagy suppresses tumorigenesis through elimination of p62.
Cell. 137:1062–1075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayes JD and McMahon M: NRF2 and KEAP1
mutations: Permanent activation of an adaptive response in cancer.
Trends Biochem Sci. 34:176–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hydbring P, Malumbres M and Sicinski P:
Non-canonical functions of cell cycle cyclins and cyclin-dependent
kinases. Nat Rev Mol Cell Biol. 17:280–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hirai H and Nakatsuru Y: Evaluating
chemical CDK inhibitors as cell death inducers. Methods Mol Biol.
1336:167–178. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Linares JF, Amanchy R, Greis K, Diaz-Meco
MT and Moscat J: Phosphorylation of p62 by cdk1 controls the timely
transit of cells through mitosis and tumor cell proliferation. Mol
Cell Biol. 31:105–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duran A, Amanchy R, Linares JF, Joshi J,
Abu-Baker S, Porollo A, Hansen M, Moscat J and Diaz-Meco MT: p62 is
a key regulator of nutrient sensing in the mTORC1 pathway. Mol
Cell. 44:134–146. 2011. View Article : Google Scholar : PubMed/NCBI
|