Introduction
Colon cancer is a common malignant tumor of the
gastrointestinal tract (1). Domestic
studies following colorectal surgery suggest that the annual
survival rate of patients with high risk stage II and stage III
colon cancer is ~20–30% (2). The
primary cause of mortality in patients with colon cancer is
recurrence and metastasis (2). In
recent years, the morbidity of colon cancer has increased (3). The main subtypes of colon cancer include
adenocarcinoma, mucinous adenocarcinoma and undifferentiated
carcinoma (3). The primary
therapeutic method for treating colon cancer is surgical resection,
which is accompanied by chemotherapy, immunotherapy and traditional
Chinese medicine (TCM) (3).
Metastasis is a common feature of aggressive colon cancer, with
these metastases primarily affecting the liver (4); ~30% of patients exhibit pre- or
postoperative liver metastasis (3).
However, a minority of patients are suitable for excision and are
susceptible to postoperative recurrence (5).
Distant metastasis of colon cancer is a topic that
has been well researched in recent years (6). Although there are a large number of
novel chemotherapeutics available, the toxic and adverse effects,
as well as the tolerance generated by these therapies, mean that
they are not completely satisfactory (7). Prior studies have demonstrated that
patients with cancer succumb to drug-resistant disease induced by
chemotherapeutics, which invalidates treatment (7). There is a lack of novel effective
chemotherapeutics that can overcome this resistance (8), as the molecular mechanisms of
chemotherapy resistance and the associated genetic changes are
complicated and comprise multiple aspects, including drug
transport, supersession, repair and regulation of apoptosis
(8).
Apoptosis is regulated by a number of factors,
including nuclear transcription factor-κB (NF-κB) (9), which combines with the fixed nucleotide
sequences of certain gene promoters. NF-κB is activated by multiple
stimuli, including cell adhesive attraction (10). The activation of NF-κB regulates cell
apoptosis and inflammation (10).
However, suppression of NF-κB activity is able to increase the
occurrence of spontaneous apoptosis in cancer cells or increase
apoptosis induced by cytotoxic drugs (9). On the basis of these data, it has been
speculated that cell adhesion may regulate apoptosis genes and
changes the sensitivity of cancer cells to drugs by activating
NF-κB, to induce multi-cell drug resistance (10).
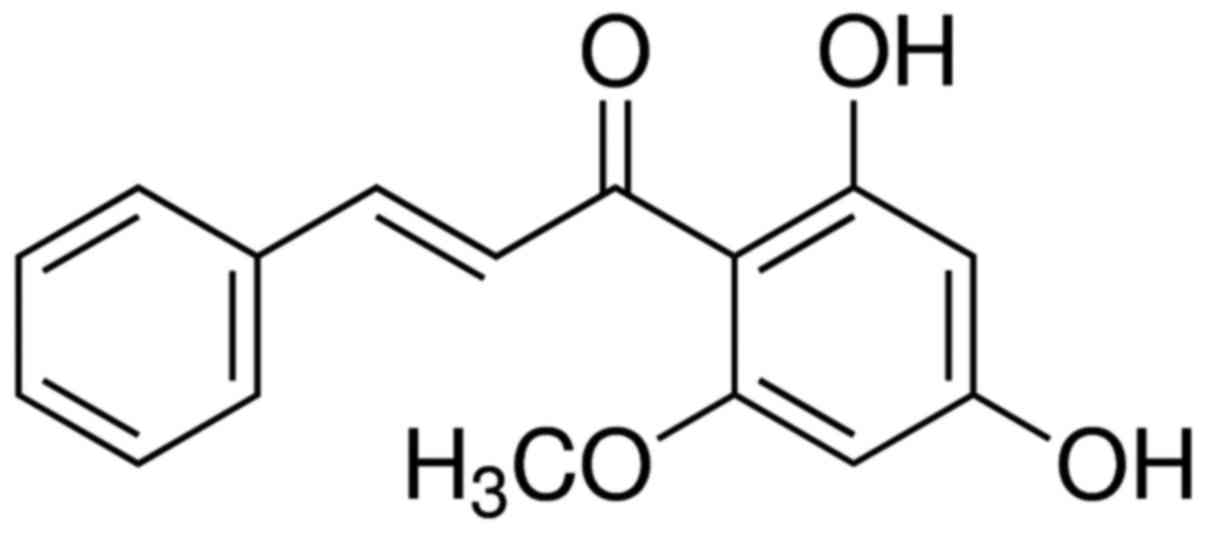
Cardamonin was originally extracted and separated
from black cardamom, of the family Zingiberaceae (the fruit of
Amomum subulatum; Fig. 1) in
1976 and is a monomeric alkaloid (11). A previous study revealed that
cardamonin serves an important role in cell proliferation by
regulating various signal transduction pathways (12). Cardamonin activates the mechanistic
target of rapamycin complex 1 downstream target p70 ribosomal S6
kinase and eukaryotic initiation factor 4E binding protein 1 in
smooth muscle cells to reverse insulin resistance (13). Furthermore, cardamonin is able to
activate mitogen-activated protein kinase and NF-κB signal pathways
in mononuclear cells, regulating inflammatory responses mediated by
lipopolysaccharides (14). The
monomer regulates cell surface receptors and Wnt/β-catenin signal
pathways in the inflammatory response (14). The aim of the present study was to
investigate the effect of cardamonin on chemotherapy-resistant
colon cancer cell and its possible mechanism.
Materials and methods
Cell lines and cell culture
The human colon cancer HCT116 cell line was
purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan,
China) and cultured for 24 h in Roswell Park Memorial Institute
(RPMI)-1640 medium containing 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin (all from Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C in an atmosphere
containing 5% CO2. Fluorouracil (5-FU) was purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). 5-FU-resistant
HCT116 cells were generated by continuous exposure to increasing
concentrations (0, 1.5, 3.25, 6.5, 12.5, 25 µmol/l) of 5-FU for 6–8
months.
Cell viability assay
HCT116 cells (5×103 cells/well) were
cultured in a 96-well plate and treated with cardamonin (0, 10, 20,
40 and 80 µM, Sigma-Aldrich; Merck KGaA) for 24, 48 and 72 h, as
described previously (15). A total
of 20 µl MTT (Sigma-Aldrich; Merck KGaA; 0.5 mg/ml) was added to
each well for 4 h and the medium was removed. Next, 150 µl DMSO was
added per well for 20 min to dissolve formazan crystals. Cell
viability was measured using an ELISA reader at 492 nm.
Cell apoptosis analysis
HCT116 cells (2×106 cells/well) were
cultured in a 6-well plate and treated with cardamonin (0, 20, 40
and 80 µM) for 72 h. Apoptosis was measured using an Annexin
V-FITC/PI Cell Apoptosis Double Dye kit (BD Biosciences, Franklin
Lakes, NJ, USA). Cells were resuspended with 1X binding buffer (BD
Biosciences, Franklin Lakes, NJ, USA). The cell suspension was
incubated with 5 µl Annexin V-FITC and 5 µl PI at room temperature
for 15 min in the dark. Cell apoptosis was measured using a flow
cytometer (C6; BD Biosciences) and analyzed using FlowJo software
(version 7.6.1; FlowJo LLC, Ashland, OR, USA).
Caspase activity assay
HCT116 cells (2×106 cells/well) were
cultured overnight in a 6-well plate and treated with cardamonin
(0, 20, 40 and 80 µM) for 72 h. Cell lysates were prepared by the
addition of 100 µl lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) for 15 min. Protein content was
determined using a bicinchoninic acid assay. A total of 5–10 µg
protein was incubated with caspase-3 colorimetric DEVD-pNA
substrate (Beyotime Institute of Biotechnology) and caspase-9
colorimetric LEHD-pNA (Beyotime Institute of Biotechnology) at 37°C
for 1 h. Caspase activity was measured using an ELISA reader at 405
nm.
Western blotting
HCT116 cells (2×106 cells/well) were
cultured overnight in a 6-well plate and treated with cardamonin
(0, 20, 40 and 80 µM) for 72 h. Cell lysates were prepared by the
addition of 100 µl lysis buffer for 15 min. Protein content was
determined using the BCA method. A total of 50 µg protein per lane
was separated by 8–10% SDS-PAGE and electrotransferred onto
polyvinylidene difluoride membranes. The membranes were blocked
with TBST containing 5% non-fat dried milk at 37°C for 1 h and
incubated with specific primary antibodies targeted at B-cell
lymphoma-associated X (Bax, sc-6236, 1:1,000), Myc proto-oncogene
protein (c-MYC, sc-789, 1:1,000), octamer-binding transcription
factor 4 (Oct4, sc-9081, 1:1,000), and cyclin E (sc-481, 1:1,000)
(all from Santa Cruz Biotechnology, Inc.), testes-specific protease
50 (TSP50, ab181993, 1:1,000; Abcam), NF-κB (sc-101749, 1:1,000)
and GAPDH (sc-25778, 1:1,000) (both from Santa Cruz Biotechnology,
Inc.) overnight at 4°C. Following three washes with TBST, membranes
were incubated with anti-rabbit or mouse horseradish
peroxidase-conjugated secondary antibodies (sc-2004 or sc-2005,
1:5,000; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. Signals were detected by enhanced chemiluminescence
(ECL Plus detection system) and band density was analyzed using
Carestream MI software (Carestream Health, Inc., Rochester, NY,
USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed using SPSS software (version
19.0; IBM Corp., Armonk, NY, USA). Statistical analysis was
performed using one-way analysis of variance analysis, with
post-hoc Student-Newman-Keuls test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of cardamonin on 5-FU-resistant
colon cancer cell growth
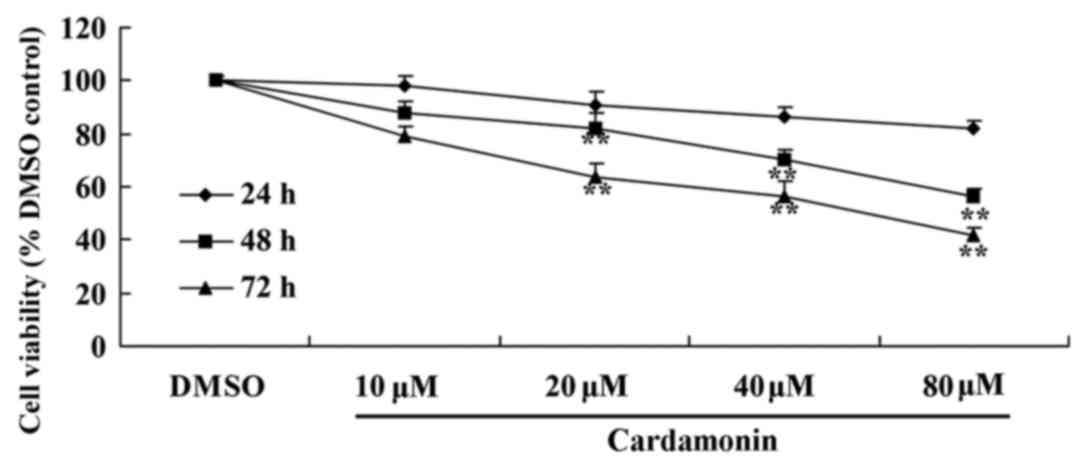
To determine the anticancer effect of cardamonin on
chemotherapy-resistant colon cancer cell growth, an MTT assay was
used to measure the viability of HCT166 cells. As presented in
Fig. 2, cardamonin reduced the
viability of 5-FU-resistant HCT166 cells in dose- and
time-dependent manner. Particularly, 20–80 µM of cardamonin
significantly reduced the viability of 5-FU-resistant HCT116 cells
following treatment for 24, 48 and 72 h (Fig. 2; P<0.01).
Effect of cardamonin on 5-FU-resistant
colon cancer cell death
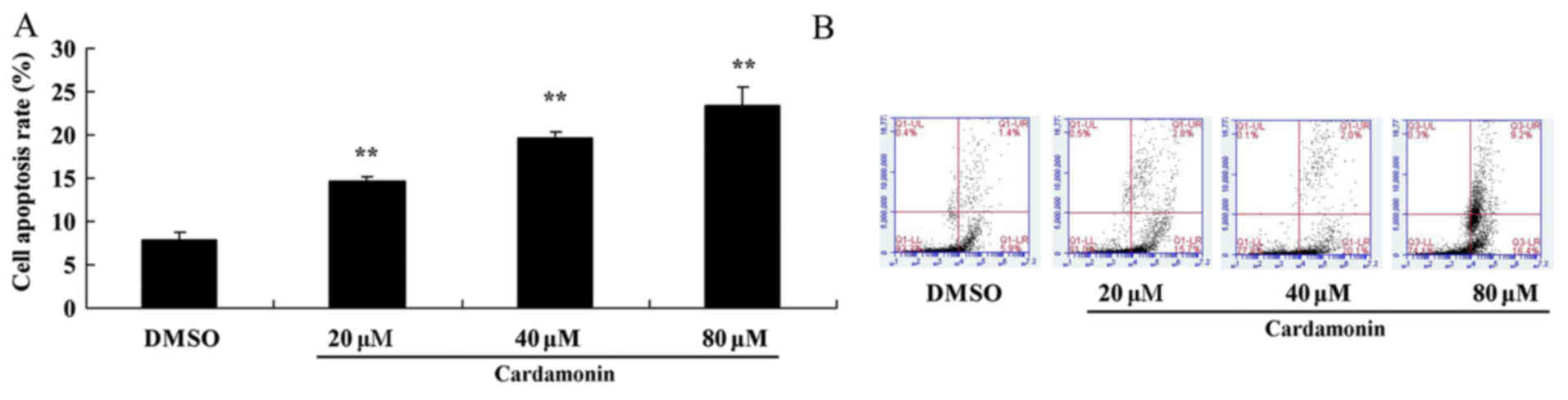
The apoptosis rate in 5-FU-resistant HCT116 cells
was assessed using flow cytometry. Increasing doses of cardamonin
was demonstrated to promote 5-FU-resistant HCT166 cell apoptosis in
a dose-dependent manner, with 40–80 µM of cardamonin significantly
increasing 5-FU-resistant HCT166 cell apoptosis at 72 h (Fig. 3; P<0.01).
The effect of cardamonin on
caspase-3/9 activities levels in 5-FU -colon cancer cell
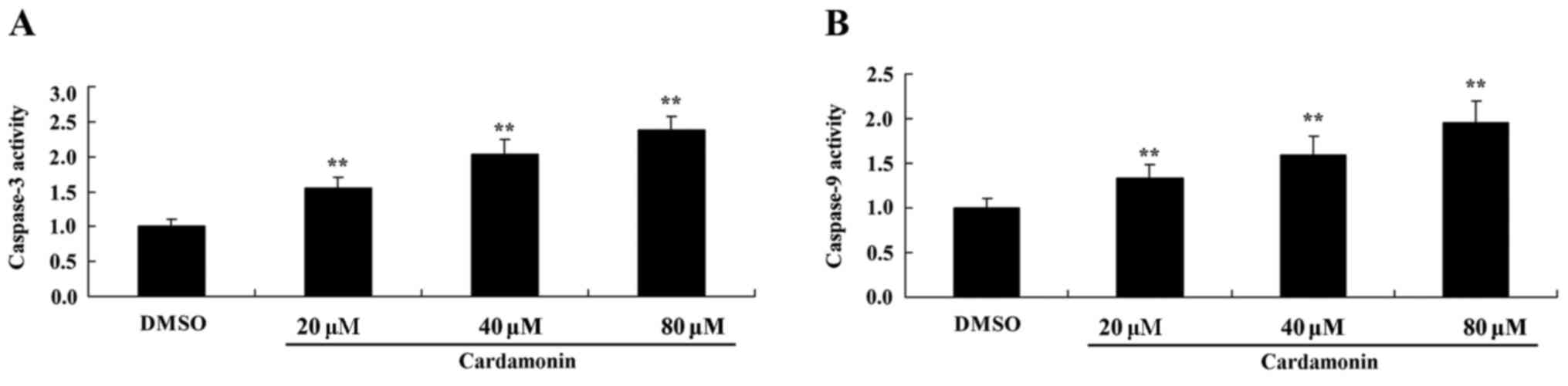
The apoptotic mechanism of 5-FU-resistant HCT166
cells treated with cardamonin was analyzed. The activity of
caspase-3 and −9 was measured using commercial kits. Caspase-3/9
activity was significantly increased in 5-FU-resistant HCT166 cells
treated with 40–80 µM of cardamonin compared with the control group
(Fig. 4; P<0.01).
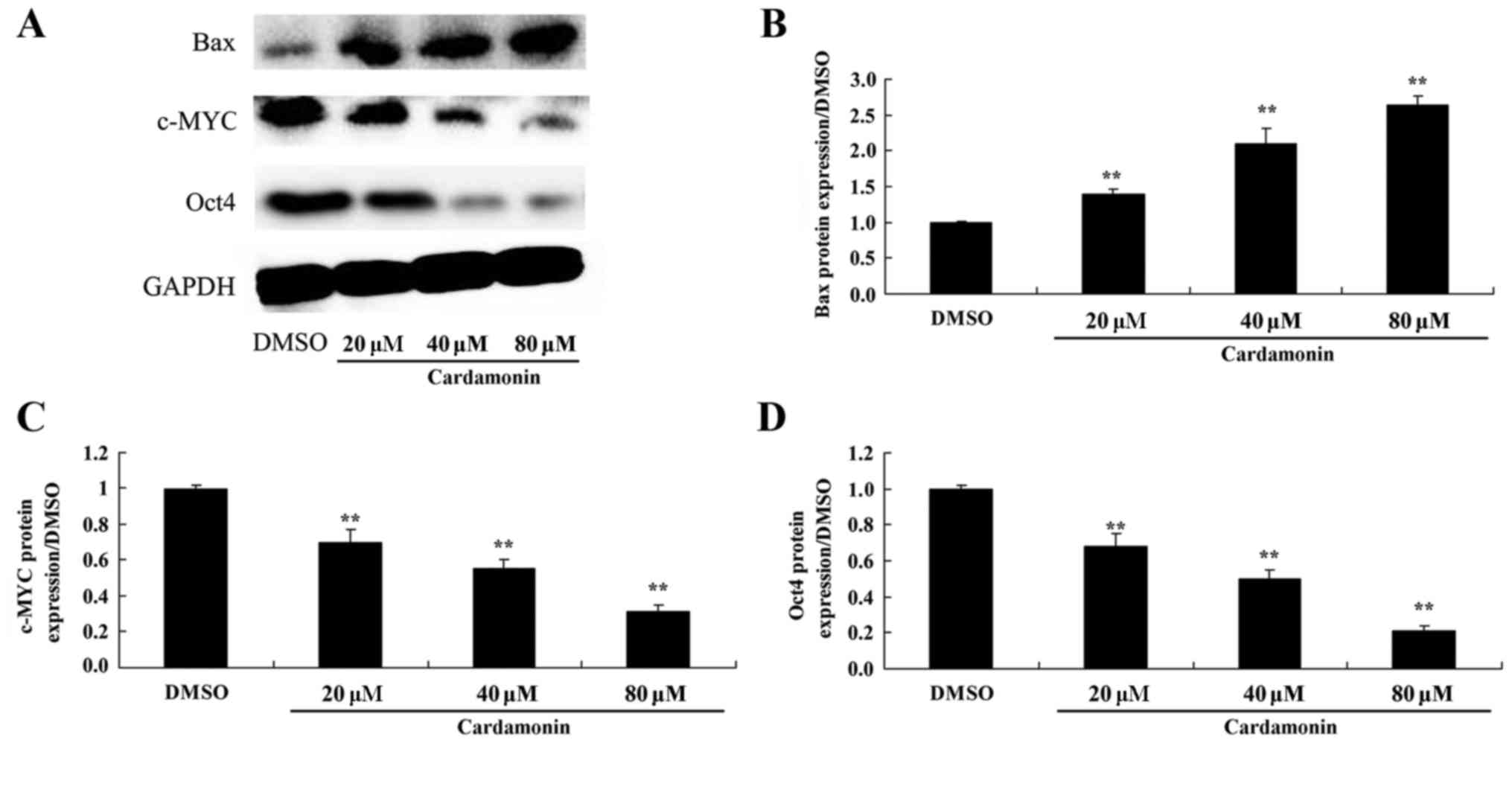
The effect of cardamonin on Bax, c-MYC
and Oct4 protein expression in 5-FU-resistant colon cancer
cells
The influence of cardamonin on Bax, c-MYC and Oct4
protein expression in chemotherapy-resistant HCT116 cells was
assessed. Compared with the control, 40–80 µM of cardamonin
significantly increased Bax protein expression and suppressed that
of c-MYC and Oct4 in 5-FU-resistant HCT166 cells (Fig. 5; P<0.01).
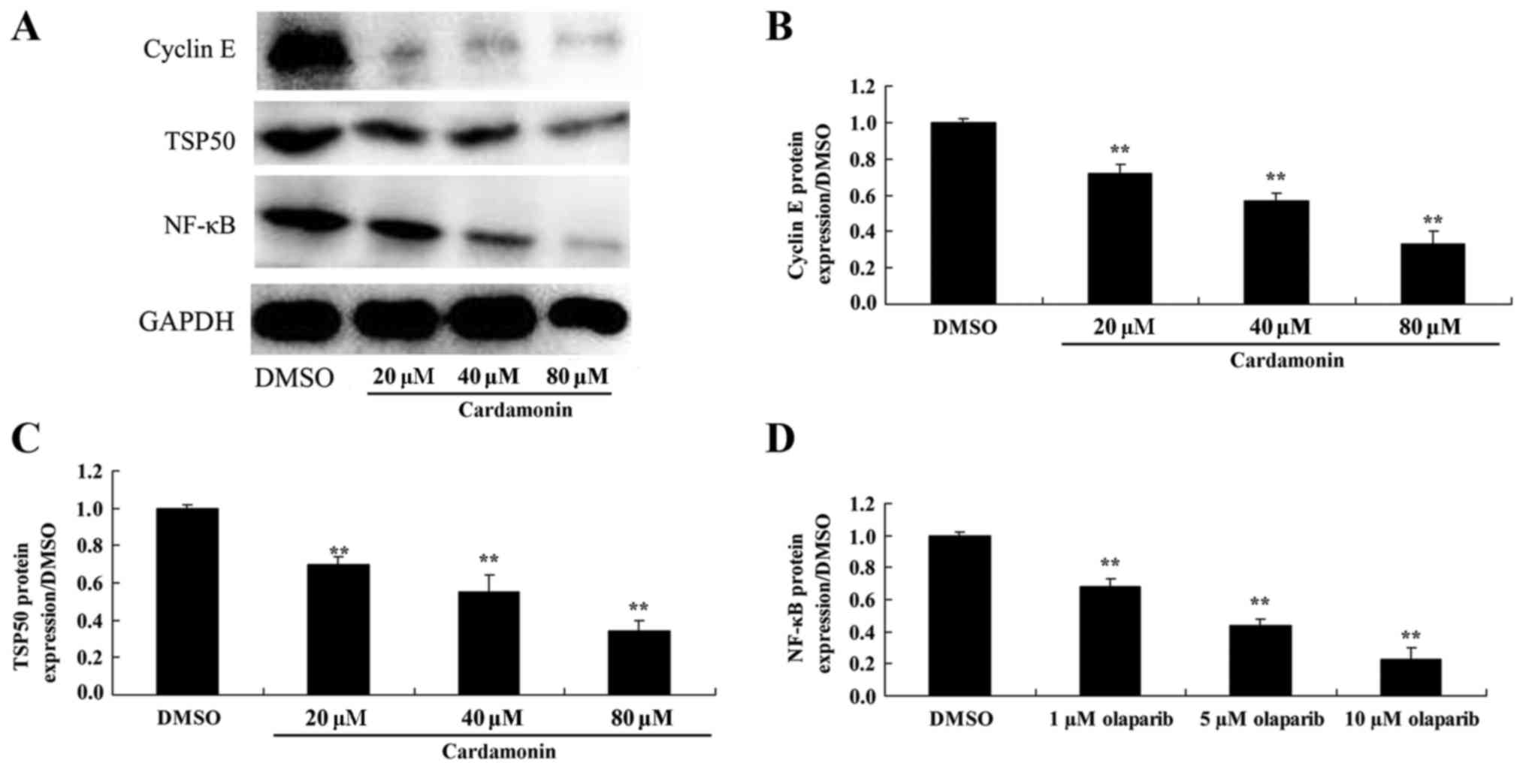
The effect of cardamonin on cyclin E,
TSP50 and NF-κB protein expression in 5-FU-resistant colon cancer
cells
Western blotting was used to investigate the
possible mechanism by which cardamonin exhibits its anticancer
effect. The results revealed that 40–80 µM of cardamonin
significantly suppressed of cyclin E, TSP50 and NF-κB protein
expression in 5-FU-HCT166 cell, compared with the control group
(Fig. 6; P<0.01).
Discussion
Chemotherapy is an important treatment modality for
patients with colon cancer; however, there are a number of
associated negative side effects, including myelosuppression,
gastrointestinal reaction and neurovirulence (16). As a result, the development of
effective treatments without these effects is a frequently studied
topic in the field of oncology (17).
It has been reported that TCM exhibits favorable effects in colon
cancer, improving symptoms and increasing the survival rate and
patient quality of life (17). In
addition, TCM avoids the side effects of chemotherapy by treating
them (18). In the present study,
cells were treated with 0, 10, 20, 40 and 80 µM of cardamonin for
24, 48 and 72 h, as previously described (15); the results indicated that cardamonin
significantly reduced cell viability and induced apoptosis in
5-FU-resistant HCT116 cells. Jia et al (14) reported that cardamonin reduced
chemotherapy-enriched breast cancer.
NF-κB serves an important role in the development
and progression of tumors (19).
NF-κB activation is associated with oncogenesis, angiogenesis,
distant metastasis, anti-apoptosis and chemotherapy resistance
(19). The association between NF-κB
activation and chemotherapy resistance has previously been
demonstrated (19). Cells
overexpressing NF-κB were insensitive to the chemotherapeutic
bleomycin in B-cell lymphoma (20).
In the present study, cardamonin significantly suppressed NF-κB
protein expression in 5-FU-resistant HCT166 cells.
Apoptosis is an important physiological and
pathological process (21). When
external stimulating factors act on pro-apoptotic proteins,
including caspase-3 and Bax, apoptotic pathways are activated
(22). When external stimulating
factors act on anti-apoptotic proteins, such as B-cell lymphoma 2,
apoptosis is restrained and cell survival increases (22). Apoptosis is regulated by multiple
factors, including NF-κB which is activated by a number of stimuli
and suppressed apoptosis by regulating the expression of pro- and
anti-apoptotic genes (23). The
results of the present study indicate that cardamonin significantly
increases caspase-3/9 activity and Bax expression in 5-FU-resistant
HCT116 cell.
c-MYC is a nuclear protein, and can bind chromosomal
DNA and regulates cell growth, differentiation and malignant
transformation (24). In a number of
human cancer cell lines, including myelogenous leukemia,
retinoblastoma, neuroblastoma, breast cancer and lung cancer, the
relevant sequences of c-MYC are amplified (25). Gene amplification of c-MYC is also
observed in cell lines of humans' colon cancer (25,26). These
findings also indicate that c-MYC has potential as an antitumor
target. The expression of c-MYC is associated with cell growth and
proliferation and is a potential inducible factor of apoptosis
(27). The results of the present
study demonstrate that cardamonin significantly suppresses c-MYC
protein expression in 5-FU-resistant HCT166 cells. Park et
al (28) demonstrated that
cardamonin suppresses the proliferation of colon cancer cells by
inhibiting the expression of cyclin D1 and c-MYC (28).
Oct4 regulates the cellular function of human
embryonic stem cells (29). There are
epithelial and melanin stem cells positive for Oct4 expression in
human hair follicle tissues which are yet to differentiate into
multiple cells (30). A previous
study reported that Oct4 was abnormally expressed in malignant
colorectal, lung and breast cancers (30). Oct4 expression is associated with the
occurrence and development of tumors and the prognosis of patients
(30). A prior study reported that
Oct4 serves an important role in the drug resistance of malignant
tumors, including prostate and liver tumors (30). Oct4 mediates chemotherapy resistance
via the Oct4-AKT-ABCG2 pathway (31).
Together, these studies suggest that cardamonin suppresses Oct4
protein expression in 5-FU-resistant HCT166 cells.
The expression of TSP50 promotes tumor development
(32). TSP50 gene silencing in mouse
teratocarcinoma P19 cells has been reported to suppress tumor cell
proliferation, colony formation and migration, induce apoptosis and
enhance sensitivity to doxorubicin (33). The mechanism by which this occurs is
reported to be associated with the n E proteins in 5-FU-resistant
HCT116 cells. Mi et al (15)
reported that cardamonin inhibited cell viability via blocking the
activation of the TSP50-mediated NF-κB signaling pathway in cancer
cells (15). It is possible that
repression of the NF-κB signaling and cyclin E proteins in
5-FU-resistant HCT116 cells. Mi et al (15) reported that cardamonin inhibited cell
viability via blocking the activation of the TSP50-mediated NF-κB
signaling pathway in cancer cells (15). It is possible that repression of the
NF-κB signaling pathway is the mechanism by which cardamonin
elicits its anticancer effect in chemotherapy-resistant colon
cancer cells.
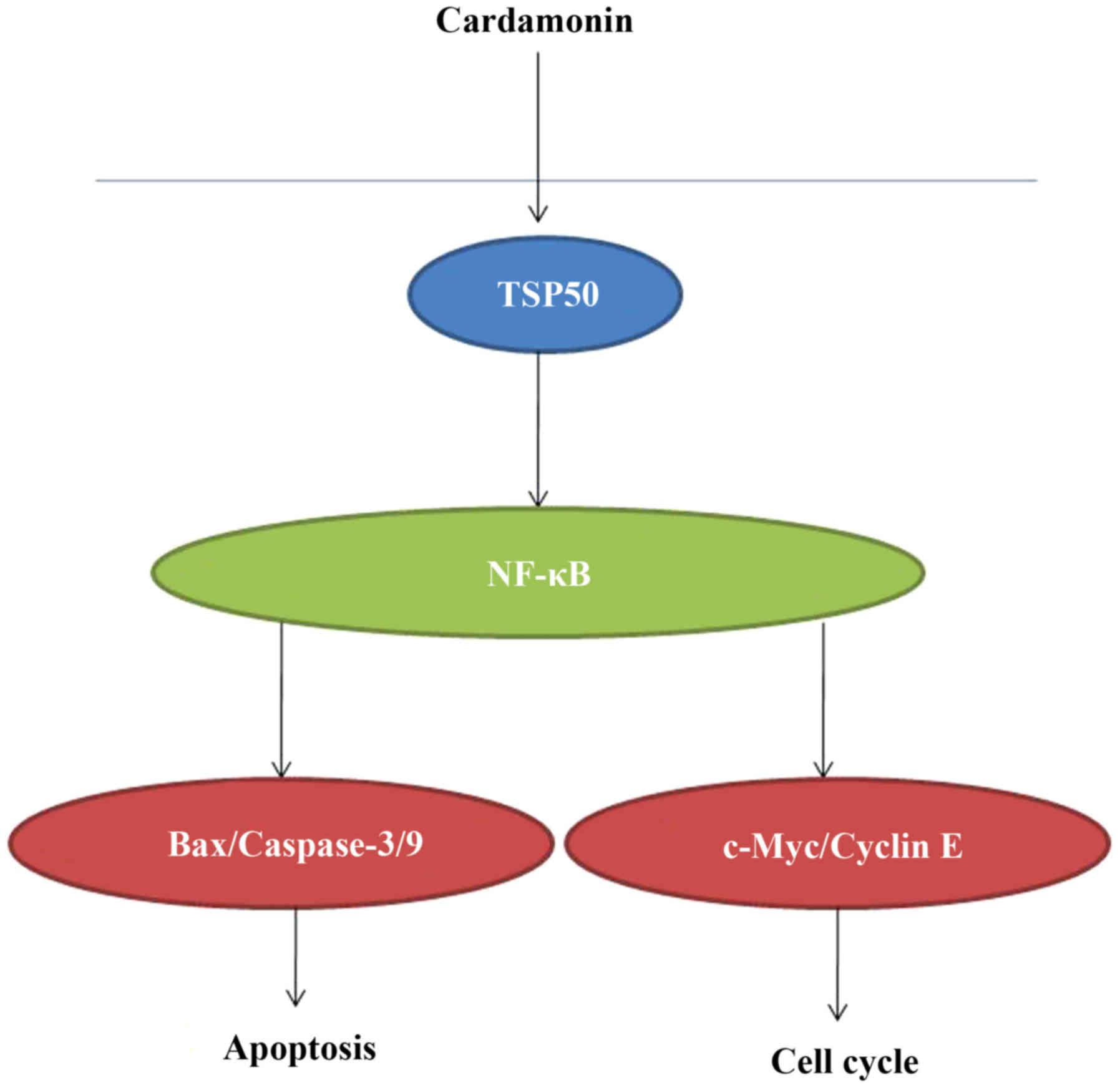
In conclusion, the results of the present study
demonstrated that cardamonin significantly suppresses
chemotherapy-resistant colon cancer cell growth, induces apoptosis
and promotes the activation of caspase-3/9 and Bax expression.
These data suggest that the anticancer effect of cardamonin in
5-FU-resitant HCT116 cells may be mediated via TSP50/NF-κB protein
expression (Fig. 7). Cardamonin may
therefore be a potential treatment for chemotherapy-resistant colon
cancer and should be researched further.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kobayashi T, Masaki T, Kogawa K, Matsuoka
H and Sugiyama M: Efficacy of gum chewing on bowel movement after
open colectomy for left-sided colorectal cancer: A randomized
clinical trial. Dis Colon Rectum. 58:1058–1063. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karoui M, Rullier A, Luciani A, Bonnetain
F, Auriault ML, Sarran A, Monges G, Trillaud H, Le Malicot K, Leroy
K, et al: Neoadjuvant FOLFOX 4 versus FOLFOX 4 with Cetuximab
versus immediate surgery for high-risk stage II and III colon
cancers: A multicentre randomised controlled phase II trial-the
PRODIGE 22-ECKINOXE trial. BMC Cancer. 15:5112015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nuñez-Sánchez MA, García-Villalba R,
Monedero-Saiz T, García-Talavera NV, Gómez-Sánchez MB,
Sánchez-Álvarez C, García-Albert AM, Rodríguez-Gil FJ, Ruiz-Marín
M, Pastor-Quirante FA, et al: Targeted metabolic profiling of
pomegranate polyphenols and urolithins in plasma, urine and colon
tissues from colorectal cancer patients. Mol Nutr Food Res.
58:1199–1211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaufman HL, Kim DW, Kim-Schulze S,
DeRaffele G, Jagoda MC, Broucek JR and Zloza A: Results of a
randomized phase I gene therapy clinical trial of nononcolytic
fowlpox viruses encoding T cell costimulatory molecules. Hum Gene
Ther. 25:452–460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
André T, de Gramont A, Vernerey D,
Chibaudel B, Bonnetain F, Tijeras-Raballand A, Scriva A, Hickish T,
Tabernero J, Van Laethem JL, et al: Adjuvant fluorouracil,
leucovorin, and oxaliplatin in stage II to III colon cancer:
Updated 10-year survival and outcomes according to BRAF mutation
and mismatch repair status of the MOSAIC study. J Clin Oncol.
33:4176–4187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hussmann M, Janke K, Kranz P, Neumann F,
Mersch E, Baumann M, Goepelt K, Brockmeier U and Metzen E:
Depletion of the thiol oxidoreductase ERp57 in tumor cells inhibits
proliferation and increases sensitivity to ionizing radiation and
chemotherapeutics. Oncotarget. 6:39247–39261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Apostolou P, Toloudi M, Kourtidou E,
Mimikakou G, Vlachou I, Chatziioannou M and Papasotiriou I: Use of
the comet assay technique for quick and reliable prediction of in
vitro response to chemotherapeutics in breast and colon cancer. J
Biol Res (Thessalon). 21:142014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kozovska Z, Gabrisova V and Kucerova L:
Colon cancer: Cancer stem cells markers, drug resistance and
treatment. Biomed Pharmacother. 68:911–916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang L and Huang J: Oxymatrine inhibits
epithelial-mesenchymal transition through regulation of NF-kappaB
signaling in colorectal cancer cells. Oncol Rep. 36:1333–1338.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mladenova D, Pangon L, Currey N, Ng I,
Musgrove EA, Grey ST and Kohonen-Corish MR: Sulindac activates
NF-kappaB signaling in colon cancer cells. Cell Commun Signal.
11:732013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang S, Zhai C, Zhang Y, Yu Y, Zhang Y, Ma
L, Li S and Qiao Y: Cardamonin, a novel antagonist of hTRPA1 cation
channel, reveals therapeutic mechanism of pathological pain.
Molecules. 21:pii: E1145. 2016. View Article : Google Scholar
|
|
12
|
Niu PG, Zhang YX, Shi DH, Liu Y, Chen YY
and Deng J: Cardamonin inhibits metastasis of lewis lung carcinoma
cells by decreasing mTOR activity. PLoS One. 10:e01277782015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liao Q, Shi DH, Zheng W, Xu XJ and Yu YH:
Antiproliferation of cardamonin is involved in mTOR on aortic
smooth muscle cells in high fructose-induced insulin resistance
rats. Eur J Pharmacol. 641:179–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jia D, Tan Y, Liu H, Ooi S, Li L, Wright
K, Bennett S, Addison CL and Wang L: Cardamonin reduces
chemotherapy-enriched breast cancer stem-like cells in vitro and in
vivo. Oncotarget. 7:771–785. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mi XG, Song ZB, Sun LG, Bao YL, Yu CL, Wu
Y and Li YX: Cardamonin inhibited cell viability and tumorigenesis
partially through blockade of testes-specific protease 50-mediated
nuclear factor-kappaB signaling pathway activation. Int J Biochem
Cell Biol. 73:63–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Corvaisier M, Bauzone M, Corfiotti F,
Renaud F, El Amrani M, Monté D, Truant S, Leteurtre E, Formstecher
P, Van Seuningen I, et al: Regulation of cellular quiescence by
YAP/TAZ and cyclin E1 in colon cancer cells: Implication in
chemoresistance and cancer relapse. Oncotarget. 7:56699–56712.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ayeka PA, Bian Y, Mwitari PG, Chu X, Zhang
Y, Uzayisenga R and Otachi EO: Immunomodulatory and anticancer
potential of Gan cao (Glycyrrhiza uralensis Fisch.)
polysaccharides by CT-26 colon carcinoma cell growth inhibition and
cytokine IL-7 upregulation in vitro. BMC Complement Altern Med.
16:2062016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dahl O and Pfeffer F: Twenty-five years
with adjuvant chemotherapy for colon cancer-a continuous evolving
concept. Acta Oncol. 54:1–4. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang HW, Kim JM, Cha MY, Jung HC, Song IS
and Kim JS: Deguelin, an Akt inhibitor, down-regulates NF-κB
signaling and induces apoptosis in colon cancer cells and inhibits
tumor growth in mice. Dig Dis Sci. 57:2873–2882. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stark LA, Reid K, Sansom OJ, Din FV,
Guichard S, Mayer I, Jodrell DI, Clarke AR and Dunlop MG: Aspirin
activates the NF-kappaB signalling pathway and induces apoptosis in
intestinal neoplasia in two in vivo models of human colorectal
cancer. Carcinogenesis. 28:968–976. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Liu L, Qiu H, Zhang X, Guo W, Chen
W, Tian Y, Fu L, Shi D, Cheng J, et al: Ursolic acid simultaneously
targets multiple signaling pathways to suppress proliferation and
induce apoptosis in colon cancer cells. PLoS One. 8:e638722013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Radhakrishnan EK, Bava SV, Narayanan SS,
Nath LR, Thulasidasan AK, Soniya EV and Anto RJ: [6]-Gingerol
induces caspase-dependent apoptosis and prevents PMA-induced
proliferation in colon cancer cells by inhibiting MAPK/AP-1
signaling. PLoS One. 9:e1044012014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elkady AI, Hussein RA and El-Assouli SM:
Harmal extract induces apoptosis of HCT116 human colon cancer
cells, mediated by inhibition of nuclear factor-κB and activator
protein-1 signaling pathways and induction of cytoprotective genes.
Asian Pac J Cancer Prev. 17:1947–1959. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ding X, Zhou X, Jiang B, Zhao Q and Zhou
G: Triptolide suppresses proliferation, hypoxia-inducible factor-1α
and c-Myc expression in pancreatic cancer cells. Mol Med Rep.
12:4508–4513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thao NP, Luyen BT, Kim EJ, Kang JI, Kang
HK, Cuong NX, Nam NH, Kiem PV, Minh CV and Kim YH: Steroidal
constituents from the edible sea urchin Diadema savignyi
Michelin induce apoptosis in human cancer cells. J Med Food.
18:45–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takaoka Y, Shimizu Y, Hasegawa H, Ouchi Y,
Qiao S, Nagahara M, Ichihara M, Lee JD, Adachi K, Hamaguchi M and
Iwamoto T: Forced expression of miR-143 represses ERK5/c-Myc and
p68/p72 signaling in concert with miR-145 in gut tumors of Apc(Min)
mice. PLoS One. 7:e421372012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawasaki Y, Komiya M, Matsumura K, Negishi
L, Suda S, Okuno M, Yokota N, Osada T, Nagashima T, Hiyoshi M, et
al: MYU, a Target lncRNA for Wnt/c-Myc signaling, mediates
induction of CDK6 to promote cell cycle progression. Cell Rep.
16:2554–2564. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park S, Gwak J, Han SJ and Oh S:
Cardamonin suppresses the proliferation of colon cancer cells by
promoting β-catenin degradation. Biol Pharm Bull. 36:1040–1044.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Talebi A, Kianersi K and Beiraghdar M:
Comparison of gene expression of SOX2 and OCT4 in normal tissue,
polyps, and colon adenocarcinoma using immunohistochemical
staining. Adv Biomed Res. 4:2342015.PubMed/NCBI
|
|
30
|
Ng WL, Chen G, Wang M, Wang H, Story M,
Shay JW, Zhang X, Wang J, Amin AR, Hu B, et al: OCT4 as a target of
miR-34a stimulates p63 but inhibits p53 to promote human cell
transformation. Cell Death Dis. 5:e10242014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Amini S, Fathi F, Mobalegi J,
Sofimajidpour H and Ghadimi T: The expressions of stem cell
markers: Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Tcl1, Tbx3,
Dppa4, and Esrrb in bladder, colon, and prostate cancer, and
certain cancer cell lines. Anat Cell Biol. 47:1–11. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song ZB, Bao YL, Zhang Y, Mi XG, Wu P, Wu
Y, Yu CL, Sun Y, Zheng LH, Huang YX, et al: Testes-specific
protease 50 (TSP50) promotes cell proliferation through the
activation of the nuclear factor-κB (NF-κB) signalling pathway.
Biochem J. 436:457–467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu YL and Sun YN: Down-regulation of
testes-specific protease 50 induces apoptosis in human
laryngocarcinoma HEp2 cells in a NF-κB-mediated pathway. Mol Biol
Rep. 41:7743–7747. 2014. View Article : Google Scholar : PubMed/NCBI
|