Introduction
Oral cancer is a global public health problem
standing among the 10 primary causes of cancer-associated mortality
globally (1). The incidence of oral
cancer remains high in Asian and Western countries (2). Oral cancer affects a notable number of
patients <40 years of age (3). In
Taiwan, oral cancer is the fourth most common cancer type in males
based on a report in 2011 from the Department of Health, which
indicated that 7.9/100,000 individuals succumb annually to oral
cancer (4). It has been reported that
oral cancer result in high rates of mortality and markedly affects
patient's quality of life (5). The
World Health Organization has recognized the importance of health
education, prevention, tracking, early diagnosis and treatment in
dealing with oral cancer (6).
Scientific investigators have thus far focused on the compounds
from natural products for treatment of patients with oral
cancer.
The major treatment option for patients with oral
cancer with stage I and II disease (7) is surgery and radiotherapy, which results
in a 5-year survival rate of >70% (8,9). The
application of concurrent chemoradiation presents an attractive
alternative to traditional surgical management in advanced squamous
cell carcinoma of head and neck, as it has been identified to
improve loco-regional control, disease free survival and overall
survival (10,11). Quercetin
[2-(3,4-dihydroxy-phenyl)-3,5, 7-trihydroxy-4H-1-benzopyran-4-one]
is a polyphenolic flavonoid present in plants including fruits,
vegetables and several other human dietary sources including seeds
and nuts (12,13), and has been identified to exert
various beneficial health effects, including the suppression of
inflammation, promotion of immune function and anti-aging effects
(14,15). Quercetin has been demonstrated to
induce cytotoxic effects on human breast (16), lung (17) and prostate (18) cancer cell lines through the induction
of apoptosis. Furthermore, it was identified that quercetin
suppresses the proliferation of gastric (19), colon (20), liver (21) and cervical (22) cancer cells.
Although numerous studies have identified that
quercetin has an impact on a number of human cancer cell lines,
including oral cancer cells lines SCC-9 (23), SCC-1483, SCC-25, SCC-QLL1 (24) and HSC-3 (25), potential actions of quercetin on the
oral cancer cells SAS have been poorly explored. The underlying
molecular mechanism is not fully understood. Therefore, in the
present study, the effect of quercetin on the human oral cancer SAS
cell line was investigated. It was identified that quercetin
induced apoptosis of SAS cells in vitro via endoplasmic
reticulum (ER) stress- and mitochondria-signaling pathways.
Materials and methods
Chemicals and reagents
Quercetin (cat. no. Q4951; ≥95%), propidium iodide
(PI), Trypsin-EDTA, L-glutamine and penicillin-streptomycin were
obtained from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum
(FBS) were purchased from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Fluo-3/AM, dihexyloxacarbocyanine iodide
(DiOC6) and dichloro-dihydro-fluorescein diacetate
(H2DCF-DA) were obtained by Invitrogen; Thermo Fisher
Scientific, Inc.
Cell culture
Human oral cancer cells SAS cells were purchased
from the Food Industry Research and Development Institute (Hsinchu,
Taiwan). These cells were maintained in DMEM supplemented with 10%
FBS, 100 U/ml penicillin, 100 µg/ml streptomycin and 2 mM
glutamine, and were cultured at 37°C in a humidified incubator in
an atmosphere containing 5% CO2 (26,27).
Cell morphology and viability
assays
SAS cells (1×105 cells/well) were placed
in 12-well plates with DMEM for 24 h then quercetin (40 µM) or 1%
dimethyl sulfoxide as a vehicle control was added to each well for
0, 12, 24 and 48 h. In order to examine morphological changes,
cells in each well were examined and images were captured using
contrast phase microscopy at a magnification, ×400. To measure the
percentage of viable cells, cells were collected from each
treatment well, counted and stained with PI (5 µg/ml) at room
temperature in the dark then immediately analyzed using a Flow
Cytometry system (BD Biosciences, San Jose, CA, USA) assay as
previously described (26,28).
Annexin V/PI staining
Cell apoptosis was measured using an Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit (BD
Biosciences) as described previously (29,30).
Briefly, SAS cells (5×104 cells/ml) in 12-well culture
plates were treated with quercetin (40 µM) for 24 and 48 h or 1%
DMSO as a vehicle control. Cells were harvested and then
re-suspended in Annexin V binding buffer, followed by incubation
with Annexin V-FITC/PI in the dark for 15 min according to the
manufacturer's protocol for labeling of apoptotic cells (29,30). In
each experiment, 1×104 cells were analyzed using Cell
Quest™ program (Version 5.2.1; BD Biosciences).
Experiments were performed in triplicate.
Measurement of reactive oxygen species
(ROS), intracellular Ca2+ and mitochondrial membrane
potential (ΔΨm)
Flow cytometry was used to measure the levels of
ROS, Ca2+ and MMP in SAS cells following exposure to
quercetin. SAS cells (1×105 cells/well) were placed in
12-well plates and were treated with 40 µM quercetin or 1% DMSO as
a vehicle control for various time periods (1, 3, 6, 9, 12, 24 and
48 h). Cells were isolated and re-suspended in 500 µl
H2DCF-DA (10 µM), then kept in darkness for 30 min at
37°C to measure the levels of ROS (H2O2);
re-suspended in 500 µl DiOC6 (4 µmol/l) and maintained
in darkness for 30 min at 37°C to measure the levels of MMP; or
re-suspended in 500 µl of Fluo-3/AM (2.5 µg/ml) and kept in
darkness for 30 min at 37°C for intracellular Ca2+
concentrations measurement. After 30 min of incubation, all samples
were analyzed using flow cytometry as described previously
(30,31).
Caspase-3, caspase-8 and caspase-9
activities assay
Flow cytometry was used to measure the activities of
caspase-3, caspase-8 and caspase-9. SAS cells (1×105
cells/well) were incubated with 40 µM quercetin or 1% DMSO as a
vehicle control for 6, 24 and 48 h, harvested, washed with 1X PBS
and re-suspended in 25 µl 10 µM substrate solution sourced from
caspase activity assay kits (PhiPhiLux-G1D1 for caspase-3,
CaspaLux8-L1D2 for caspase-8 and CaspaLux9-M1D2 for caspase-9,
OncoImmunin, Inc., Gaithersburg, MD, USA) prior to incubation at
37°C for 60 min. Following incubation, cells were washed with PBS
and were immediately analyzed using flow cytometry as described
previously (29,30).
Western blotting analysis
SAS cells (1.5×106 cells/dish) were
incubated in a 10-cm dish for 24 h at 37°C, then incubated with 40
µM quercetin for 6, 12, 24 and 48 h or 1% DMSO as a vehicle
control. Cells were collected then lysed and denatured using
ice-cold lysis buffer for 1 h (10 mM Tris-HCl (pH 7.4), 150 mM
NaCl, 1 mM EGTA, 0.3 mM PMSF, 0.2 mM sodium orthovanadate, 0.1%
SDS, 1 mM EDTA, 1% NP-40, 10 mg/ml leupeptin and 10 mg/ml
aprotinin). Total protein quantity was determined using a Bio-Rad
protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
as described previously (29). Equal
amounts of total protein (40 µg) were separated using SDS-PAGE (12%
v/v) and then transferred onto polyvinylidene difluoride membranes.
Following blocking with 5% non-fat dried milk in PBS with Tween-20
for 1 h at room temperature, membranes were immunoblotted with
specific primary antibodies [Apoptosis-inducing factor (AIF; cat.
no. sc-13116), activating transcription factor-6α (ATF-6α; cat. no.
sc-22799), ATF-6β (cat. no. sc-30597), cytochrome c (Cyto c;
cat. no. sc-13560), endonuclease G (Endo G; cat. no. sc-365359),
gastrin-releasing peptide-78 (GRP-78; cat. no. sc-13968),
iron-responsive element-1α (IRE-1α; cat. no. sc-20790), X-box
binding protein 1 (XBP-1; cat. no. sc-7160), TNF-related
apoptosis-inducing ligand (TRAIL; cat. no. sc-80393) antibodies
were supplied by Santa-Cruz Biotechnology, Inc. (Dallas, TX, USA;
all dilution, 1:1,000); apoptotic protease activating factor 1
(Apaf-1; cat. no. 8723), Bcl-2-associated death promoter (Bad; cat.
no. 9239), Bcl-2 homologous antagonist killer (Bak; cat. no.
12105), B-cell lymphoma 2 (Bcl-2; cat. no. 2870), Caspase-3 (cat.
no. 9665s), Caspase-8 (cat. no. 9746), Caspase-9 (cat. no. 9508)
antibodies were supplied by Cell Signaling Technology (Danvers, MA,
USA; all dilution, 1:1,000); Caspase-6 (cat. no. AB10512; dilution,
1:2,000), BH3 interacting-domain death antagonist (Bid; cat. no.
AB1730; dilution, 1:500), Fas-associated protein with death domain
(FADD; cat. no. 05-486; dilution, 1:1,000), Fas ligand (Fas-L; cat.
no. 05-571; dilution, 1:2,000) antibodies were supplies by EMD
Millipore (Billerica, MA, USA); Caspase-4 (cat. no. 556459;
dilution, 1:500) and Fas (cat. no. 610198, dilution, 1:5,000)
antibodies were obtained from BD Biosciences (Bedford, MA, USA);
poly(ADP-ribose) polymerase (PARP; cat. no. ab6079-1; dilution,
1:400) and Caspase-7 (cat. no. ab181579; dilution, 1:5,000)
antibodies were obtained from Abcam (Cambridge, MA, USA); Bcl-extra
large (Bcl-x; cat. no. B9304; dilution, 1:2,000), Caspase-2 (cat.
no. C7349; dilution, 1:200) and β-actin (cat. no. A5316; dilution,
1:10,000) antibodies were supplied by Sigma-Aldrich; Merck KGaA]
for 1 h at room temperature (25°C). Subsequently, the membranes
were incubated with secondary antibodies [horseradish peroxidase
(HRP)-conjugated mouse immunoglobulin G (IgG; cat. no. GTX213112)
and rabbit HRP-conjugated IgG secondary antibodies (cat. no.
GTX213110) at dilution, 1:5,000 (GeneTex, Inc., Irvine, CA, USA)]
for 1 h at room temperature. Chemiluminescence signals were
enhanced using enhanced chemiluminescence reagent (EMD Millipore)
(27,29,30), and
images were captured using the MultiGel-21 Image system (TOP BIO
CO., Taipei, Taiwan).
Confocal laser scanning microscopy
assay
SAS cells (1×105 cells/well) were
maintained on 6-well chamber slides with or without 40 µM quercetin
or 1% DMSO as a vehicle control for 48 h and then fixed in 4%
formaldehyde in PBS for 15 min at room temperature. Triton X-100 in
PBS (0.1%) was added to the cells for 15 min at room temperature,
followed by 2% bovine serum albumin (Sigma-Aldrich; Merck KGaA) for
1 h at room temperature to block non-specific binding sites. Cells
were then incubated overnight with primary antibodies, including
anti-Cyto c (cat. no. sc-13560; 1:250 dilution), AIF (cat. no.
sc-13116; 1:250 dilution) and anti-Endo G (cat. no. sc-365359;
1:250 dilution) (all in green fluorescence) at 4°C, and then
followed by incubation with a secondary antibody (FITC-conjugated
goat anti-mouse IgG; cat. no. 115-545-003; 1:100 dilution; Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 1 h at
room temperature, and PI (red fluorescence) staining for
examination as described previously (30). Slides were mounted, examined with oil
immersion and images (magnification, ×630) were captured using a
Leica TCS SP2 Confocal Spectral Microscope (Leica Microsystems
GmbH, Mannheim, Germany).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Differences between groups were analyzed using one-way
analysis of variance followed by the Dunnett's method. P<0.05
was considered to indicate a statistically significant difference.
Statistical analysis was performed using SigmaPlot for Windows
(version 12.0; Systat Software, Inc., San Jose, CA).
Results
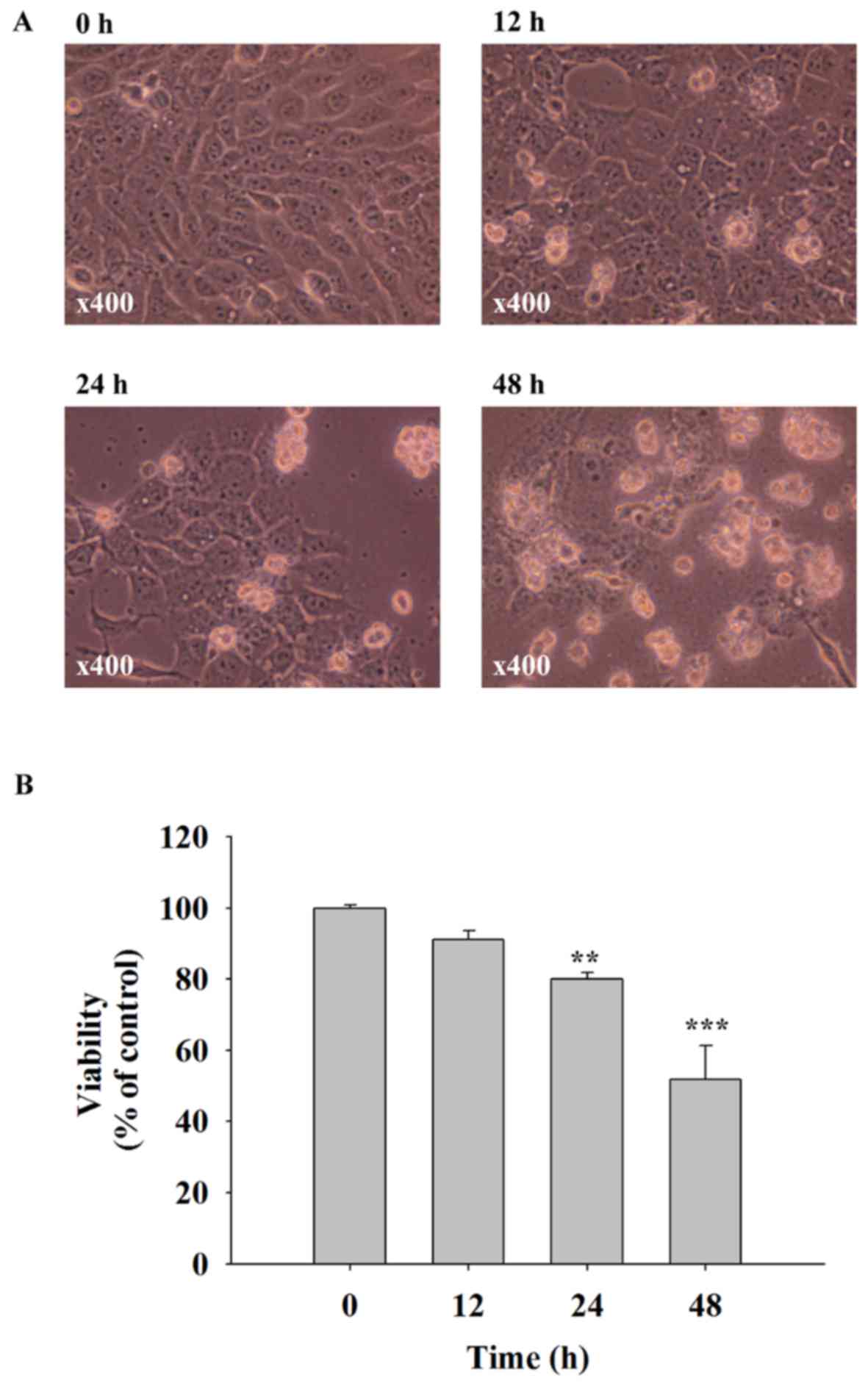
Quercetin induces cell morphological
changes and decreases the cell viability of SAS cells
SAS cells were treated with 40 µM of quercetin for
0, 12, 24 and 48 h. Cells were examined for morphological changes
and the percentage of viable cells. The results indicated that
quercetin induced cell morphological changes including cell
shrinkage and cell floating, and significantly decreased the
quantity of viable cells (Fig. 1).
These effects were time-dependent. A 40 µM dose of quercetin
decreased cell viability to ~50% when compared with the control
group (0 h) that was selected for all experiments.
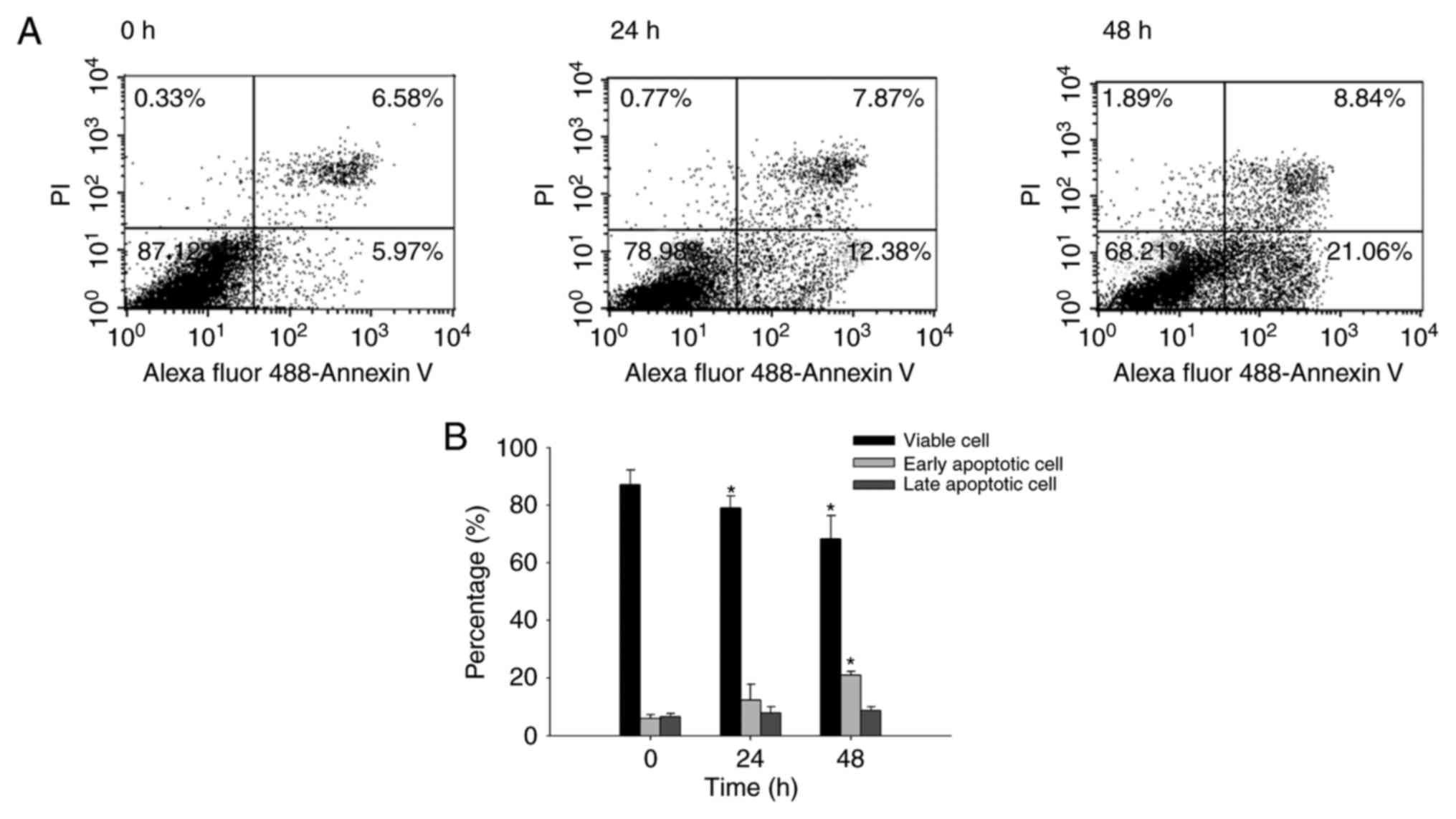
Quercetin induces apoptosis in SAS
cells
In order to investigate the effects of quercetin on
apoptosis in SAS cells, Annexin V/PI-double staining was performed.
The results of dot plots (Fig. 2A)
indicated that quercetin induced apoptosis of cells, with a
increase in the percentage of early apoptotic cells detected at 24
h (5.97%) and 48 h (21.06%). The percentage of apoptotic cells at
different times are presented in Fig.
2B; the results indicate that quercetin significantly induced
early-stage apoptosis in a time-dependent manner.
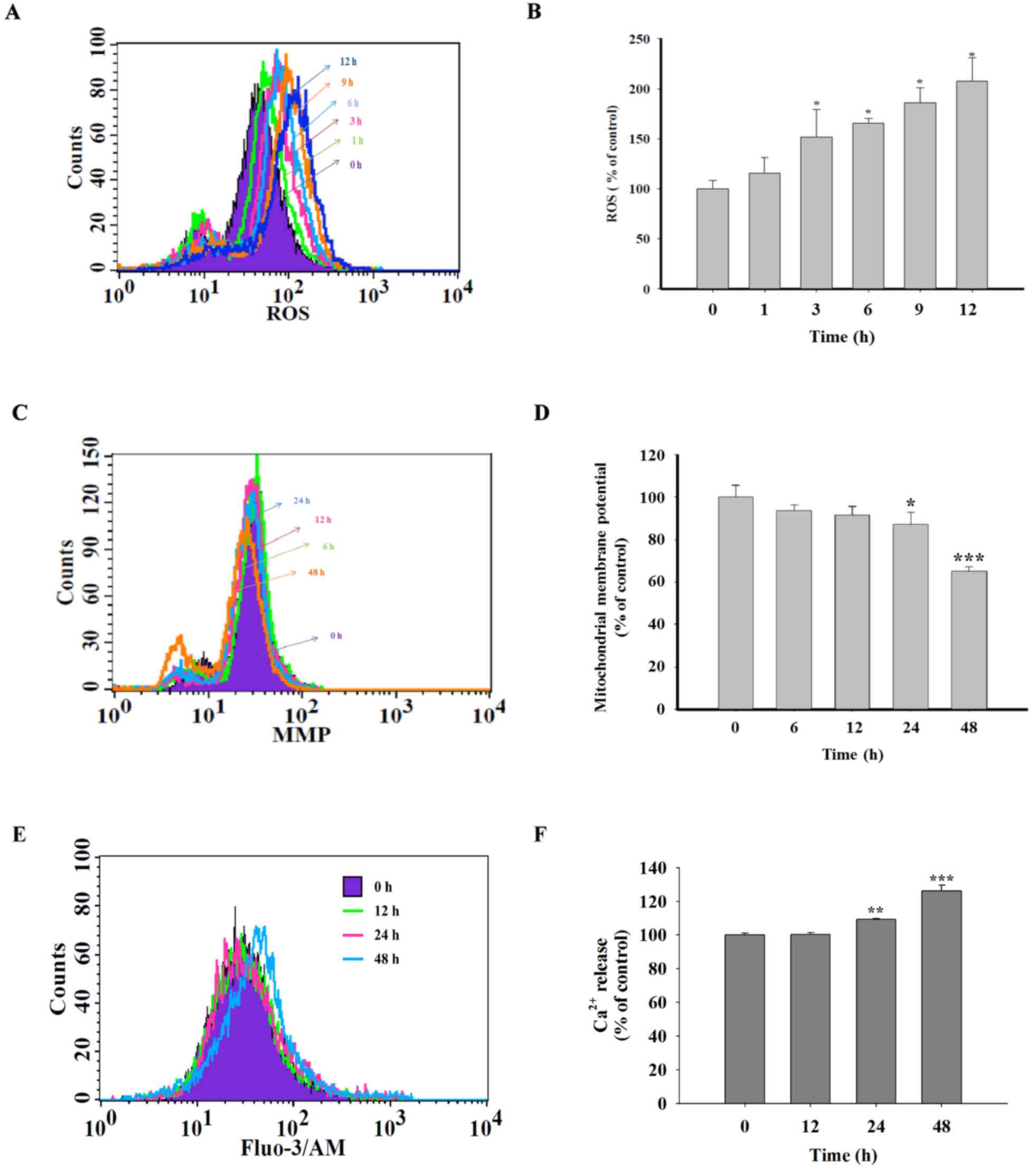
Quercetin induces ROS and
Ca2+ production and decreases the levels of
ΔΨm in SAS cells
In order to investigate whether the cell apoptosis
induced by quercetin in SAS cells involve the production of ROS and
Ca2+ or dysfunction of mitochondrial, cells were treated
with quercetin for various time periods and then analyzed using a
flow cytometric assay. Fig. 3A and B
identified that quercetin significantly increased ROS production
following 3 h treatment. Fig. 3C and
D showed that quercetin treatment significantly decreased the
levels of ΔΨm following 24 h treatment. In addition, it
was demonstrated that quercetin significantly promoted
Ca2+ production following 24 h treatment (Fig. 3E and F).
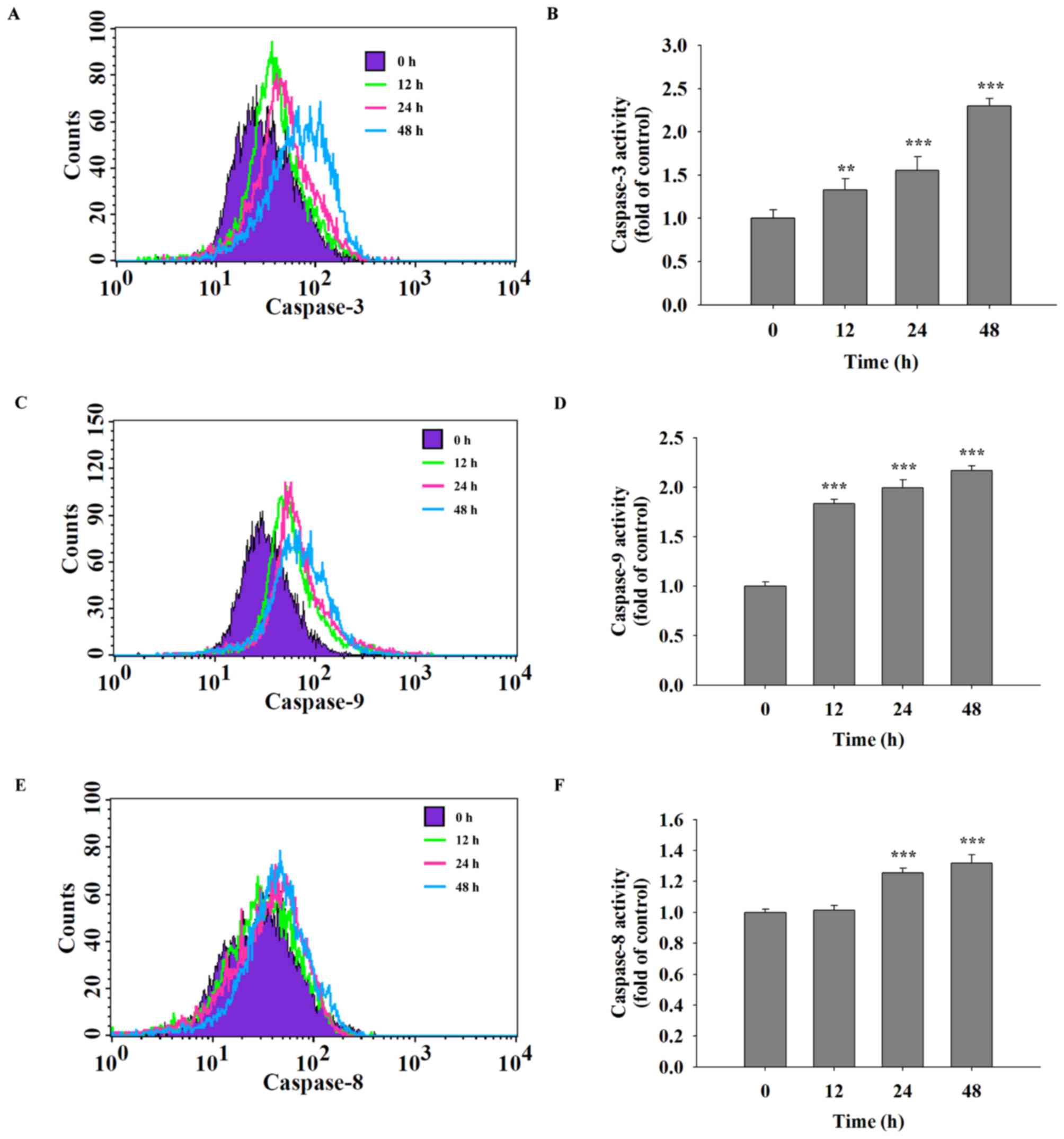
Quercetin increases the activities of
caspase-3, caspase-8 and caspase-9 in SAS cells
It was hypothesized that quercetin was able to
induce cell apoptosis in SAS cells through the activation of
caspases. Following treatment of SAS cells with quercetin (40 µM)
for various time periods, caspase-3, −9 and −8 activities were
assayed using flow cytometric analysis. The results indicated that
quercetin significantly increased the activities of caspase-3
(Fig. 4A and B), caspase-9 (Fig. 4C and D) and caspase-8 (Fig. 4E and F) in a time-dependent
manner.
Quercetin alters apoptosis-associated
protein expression in SAS cells
In order to understand whether quercetin induced the
apoptosis of SAS cells through the effects of apoptosis-associated
proteins, SAS cells were treated with quercetin (40 µM) for various
time periods and then the levels of apoptosis-associated proteins
were measured using western blotting. The results demonstrated that
quercetin markedly increased the expression of caspase-2, Bak, Bid
and Bad (Fig. 5A), Cyto c, Apaf-1,
Endo G, AIF and PARP (Fig. 5B),
active form of caspase-9, caspase-3, caspase-6 and −7 (Fig. 5C), TRAIL, Fas-L, Fas, FADD, and active
form of caspase-8 (Fig. 5D), ATF-6α,
ATF-6β, XBP-1, IRE-1α, caspase-4 and GRP-78 (Fig. 5E), but inhibited the expression of
Bcl-2 and Bcl-x (Fig. 5A),
pro-caspase-3 (Fig. 5C). These
results suggested that quercetin induced apoptosis of SAS cells via
cell surface receptor (Fas-L and Fas) and mitochondria-dependent
pathways.
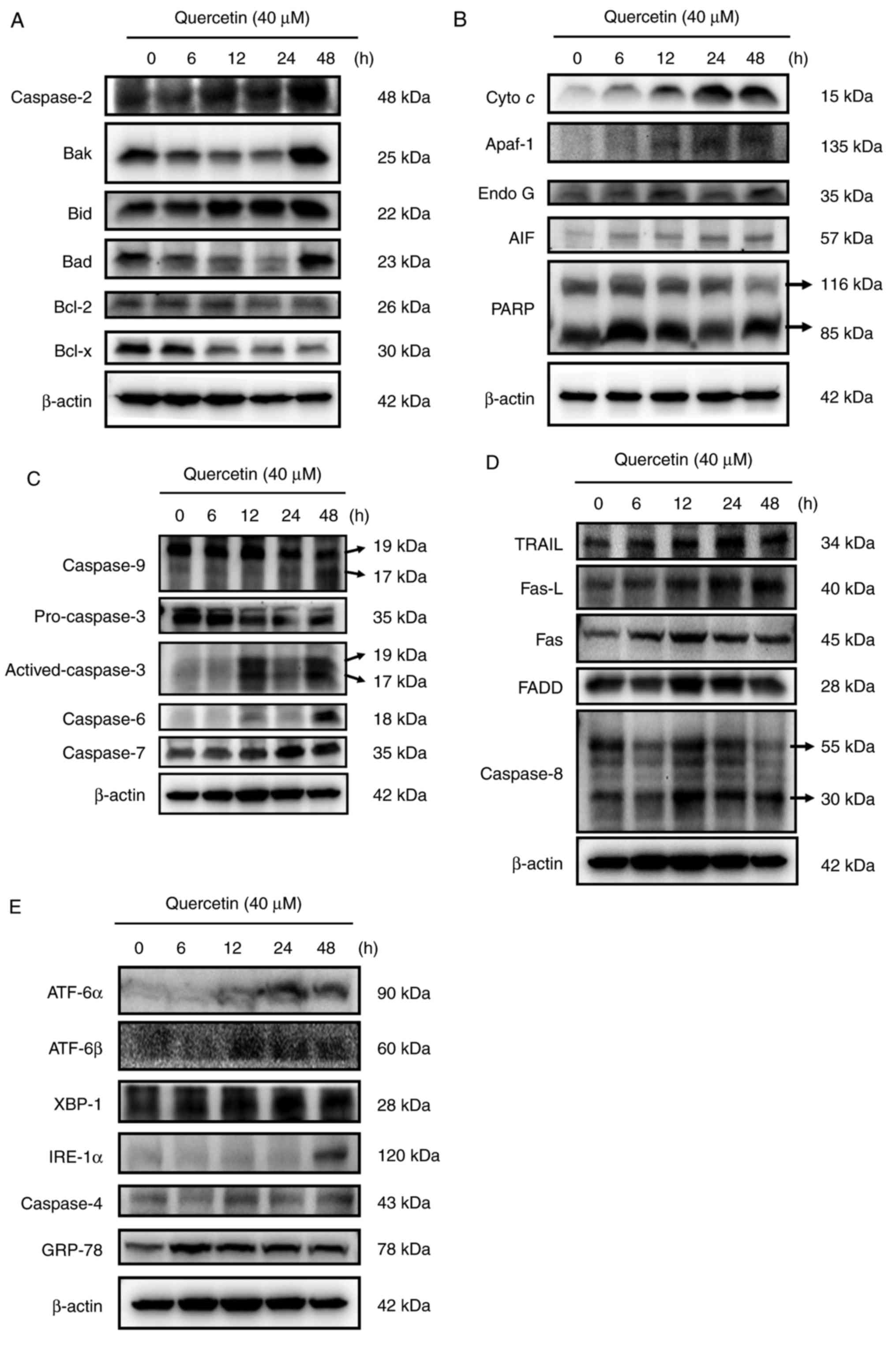 | Figure 5.Quercetin affected the levels of
apoptosis-associated proteins of SAS cells. Cells were treated with
quercetin and the total protein levels were determined and used for
SDS page gel electrophoresis. The levels of (A) caspase-2, Bak,
Bid, Bad, Bcl-2 and Bcl-x; (B) cytochrome c, Apaf-1, Endo G, AIF
and PARP; (C) caspase-9, pro-caspase-3, active-caspase-3, caspase-6
and caspase-7; (D) TRAIL, Fas-L, Fas, FADD and caspase-8; (E)
ATF-6α, ATF-6β, XBP-1, IRE-1α and GRP-78 were examined. Bak, Bcl-2
homologous antagonist killer; Bid, BH3 interacting-domain death
antagonist; Bad, Bcl-2-associated death promoter, Bcl-2, B cell
lymphoma 2; Bcl-xl, Bcl-extra large; Apaf-1, apoptotic protease
activating factor; Endo G, endonuclease G; AIF, apoptosis-inducing
factor; PARP, poly(ADP-ribose) polymerase; TRAIL, TNF-related
apoptosis-inducing ligand; Fas-L, Fas ligand; FADD, fas-associated
protein with death domain; ATF-6β, activating transcription
factor-6β; XBP-1, X-box binding protein 1; IRE-1α, iron responsive
element-1α; GRP-78, gastrin releasing peptide-78. |
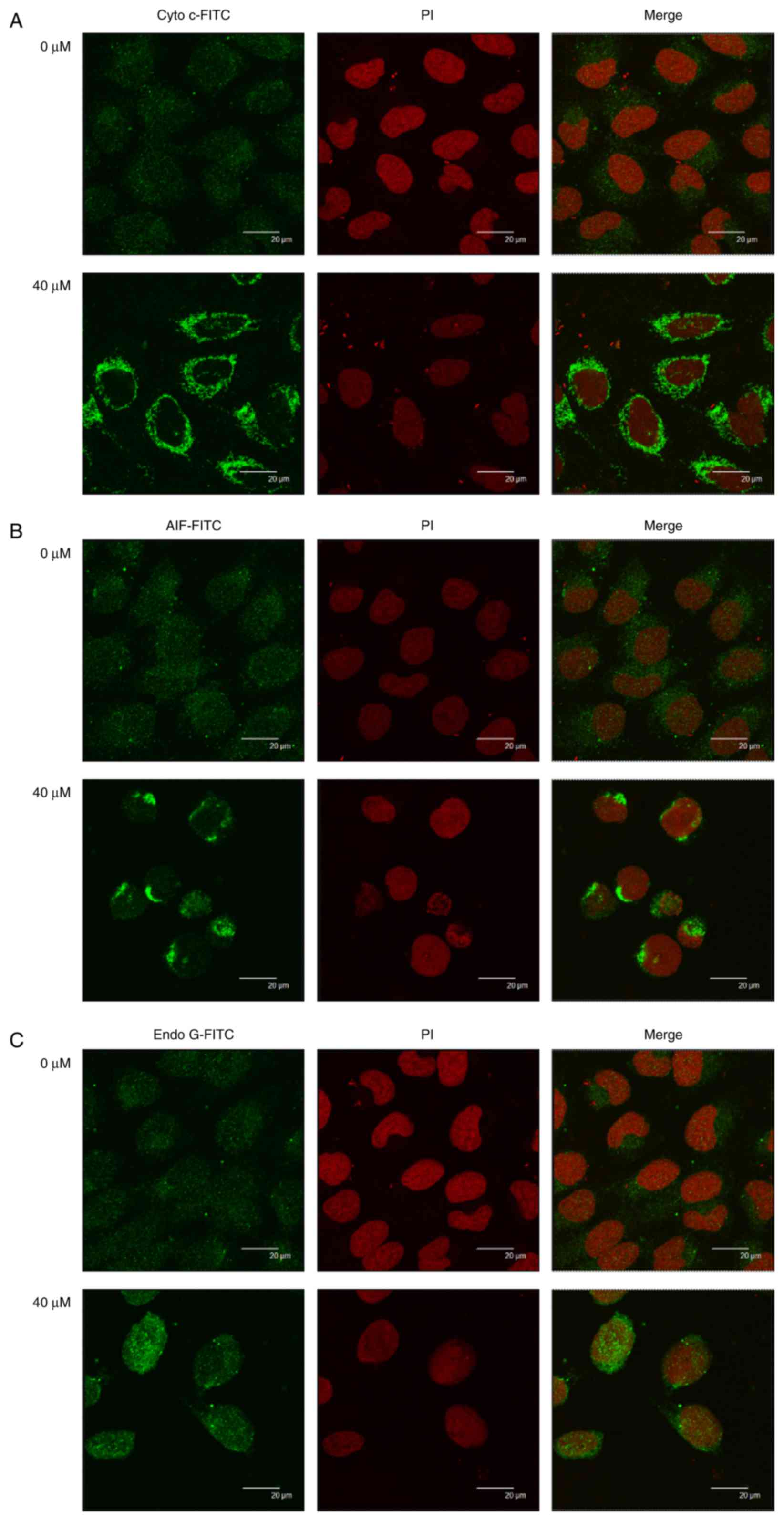
Quercetin alters the translocation of
apoptotic-associated proteins in SAS cells
In order to investigate the effect of quercetin on
the release and translocation of Cyto c, AIF, and Endo G during
apoptosis of SAS cells, cells were treated with or without 40 µM of
quercetin for 48 h, then stained by anti-Cyto c, -AIF and -Endo G.
Images were captured using a confocal laser microscopic system. The
results revealed that quercetin markedly increased cytochrome
c (Fig. 6A), AIF (Fig. 6B) and Endo G (Fig. 6C) release from mitochondria to the
cytoplasm in SAS cells compared with the corresponding control
group.
Discussion
Numerous studies have demonstrated that quercetin is
able to induce cell death through cell cycle arrest and the
induction of apoptosis in a number of human cancer cell lines
(19–25). However, there remains a lack of
available information to demonstrate quercetin-induced cell
apoptosis of human oral cancer cells; thus, in the present study,
the cytotoxic effects of quercetin on human oral cancer SAS cells
were investigated in vitro. Notably, induction of cancer
cell apoptosis has been recognized to be an optimal strategy of
anti-cancer drugs (32,33). In the present study it was identified
that quercetin: i) induced cell morphological changes and decreased
total viable cell numbers; ii) induced apoptotic cell death; iii)
increased ROS and Ca2+ levels, but decreased the
ΔΨm; iv) increased the activities of caspase-3,
caspase-9 and caspase-8; v) increased the levels of pro-apoptotic
proteins, including Bak, Bid and Bad and cell surface receptors,
including Fas-L and Fas, but decreased anti-apoptotic proteins
including Bcl-2 and Bcl-x; and vi) induced Cyto c, AIF and Endo G
release from mitochondria to cytoplasm which was confirmed by
confocal laser microscopy.
The cell viability assay results indicated that
quercetin induced cytotoxic effects based on the morphological
changes observed and decreased the total number of viable cells.
Furthermore, Annexin V/PI staining demonstrated that quercetin
induced cell apoptosis. These results demonstrated that quercetin
may have decreased cell numbers partially through the induction of
cell apoptosis. Numerous studies have identified that DNA
fragmentation is one of the characteristics of apoptotic cell death
(33–35). Annexin V/PI staining was used to
confirm that quercetin induced apoptosis of SAS cells. Annexin V/PI
staining is recognized as a protocol for measuring and quantifying
the percentage of apoptotic cells (36,37).
It was hypothesized that quercetin-induced apoptotic
cell death involved ROS and Ca2+ production, and
decreased the levels of ΔΨm in SAS cells. It was
identified that quercetin increased the production of ROS and
Ca2+. It has been reported that ROS production is
involved in agent-induced cancer cell apoptosis (38–40). It
has also been reported that agent-induced ER stress leads to
Ca2+ release from the endoplasm leading to cell
apoptosis (41). Based on the data of
the present study, it is hypothesized that quercetin-induced
apoptotic cell death involves ROS and Ca2+ production in
SAS cells. Numerous studies have identified that agent-induced cell
apoptosis may be due to a dysfunction in mitochondria or decreased
levels of ΔΨm (13,42,43).
The results in the current study also identified that quercetin
decreased the levels of ΔΨm in SAS cells. The results
from western blotting revealed that quercetin increased the protein
expression of TRAIL, Fas-L, Fas, FADD and caspase-8, which
indicated that quercetin induced apoptosis of SAS cells through
cell surface death receptors and mitochondria-dependent pathways.
It has also been reported that quercetin treatment resulted in
apoptosis in human oral cancer HSC-3 and TW206 cells through Fas,
and caspase-3 activation (25). It
was also identified that quercetin increased the active form of
caspase-3 and caspase-9 in SAS cells in the current study.
It is well documented that the balance between pro-
and anti-apoptotic proteins regulate cell viability (44), and drug-induced apoptotic pathways
modulate pro- and anti-apoptotic protein expression (45). Western blotting was used for
additional examination of the effects of apoptosis-associated
protein expression, with results indicating that quercetin induced
upregulation of major pro-apoptotic proteins including Bak, Bid and
Bad downregulation of major anti-apoptotic proteins including Bcl-2
and Bcl-x in SAS cells. The results of the present study indicated
that the anti-apoptotic/pro-apoptotic protein ratio was markedly
decreased at 48 h post-treatment in SAS cells. It has been reported
that decreased levels of ΔΨm during apoptosis may be
associated with drug-induced Bcl-2/Bax imbalance (46). The results of the present study
demonstrate that quercetin decreased the levels of ΔΨm
in SAS cells at 24–48 h treatment. The western blotting results
indicated that quercetin increased the expression of Cyto c, AIF
and Endo G, which was confirmed using confocal laser microscopy.
These data suggest that Cyto c, AIF and Endo G expression levels
are markedly increased in SAS cancer cells. Based on the
aforementioned results, it is hypothesized that quercetin-induced
cell apoptosis occurs partially via mitochondria-mediated signaling
pathways in SAS cells.
In conclusion, quercetin affects the ratio of
anti-/pro-apoptotic proteins, which may lead to dysfunction of
mitochondria (decreased levels of ΔΨm) followed by the
release of Cyto c, AIF and Endo G from mitochondria, inducing
cell-destruction by triggering apoptosis (Fig. 7). An understanding of the underlying
molecular mechanism of the action of quercetin in human oral SAS
cells in vitro may provide valuable information for its
potential application in oral cancer prevention and therapy in the
future.
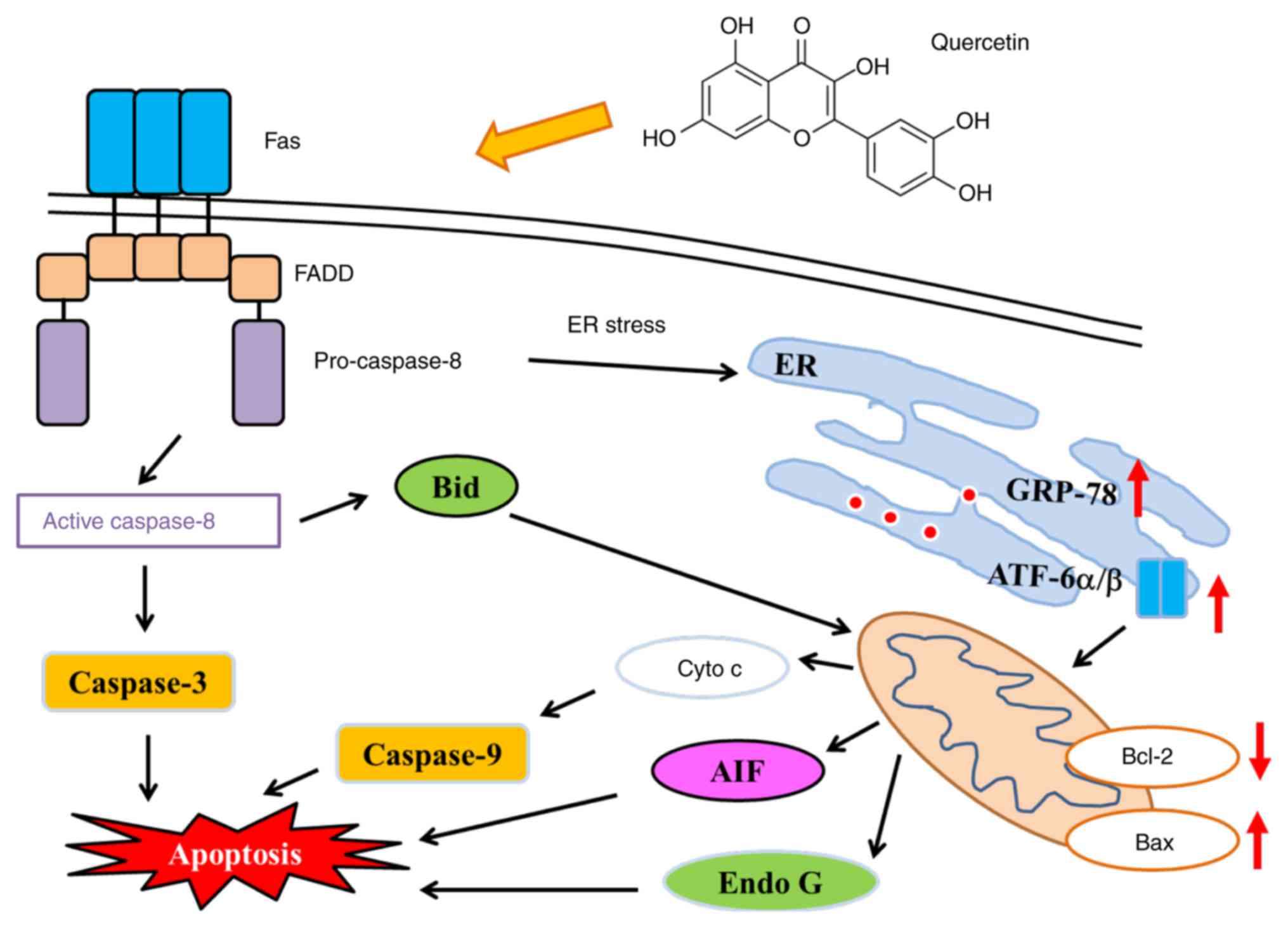 | Figure 7.Proposed signaling pathways for
quercetin induced apoptosis in SAS cells. FADD, fas-associated
protein with death domain; ER, endoplasmic reticulum; Cyto c,
cytochrome c; AIF, apoptosis-inducing factor; Endo G,
endonuclease G; Bcl-2, B cell lymphoma 2; Bax, Bcl-associated X
protein; Bid, BH3 interacting-domain death antagonist; GRP-78,
gastrin-releasing peptide-78; ATF-6α/β, activating transcription
factor-6α/β. |
Acknowledgements
Not applicable.
Funding
The present study was supported by the Asia
University (Taichung, Taiwan) (grant no. ASIA104-CMUH-05).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YSM, CNY, HCL, FSC and JGC conceived and designed
the experiments. YSM, CNY and HCL performed the experiments. YSM,
HCL, FSY, JJL, KWL and CLL analyzed the data and contributed in
reagents/materials/analysis tools. FSC and JGC wrote the paper.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maleki D, Ghojazadeh M, Mahmoudi SS,
Mahmoudi SM, Pournaghi-Azar F, Torab A, Piri R, Azami-Aghdash S and
Naghavi-Behzad M: Epidemiology of oral cancer in Iran: A systematic
review. Asian Pac J Cancer Prev. 16:5427–5432. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krishna A, Singh S, Kumar V and Pal US:
Molecular concept in human oral cancer. Natl J Maxillofac Surg.
6:9–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Halboub ES, Al-Anazi YM and Al-Mohaya MA:
Characterization of Yemeni patients treated for oral and pharyngeal
cancers in Saudi Arabia. Saudi Med J. 32:1177–1182. 2011.PubMed/NCBI
|
|
4
|
Chung TT, Pan MS, Kuo CL, Wong RH, Lin CW,
Chen MK and Yang SF: Impact of RECK gene polymorphisms and
environmental factors on oral cancer susceptibility and
clinicopathologic characteristics in Taiwan. Carcinogenesis.
32:1063–1068. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Galbiatti AL, Padovani-Junior JA, Maníglia
JV, Rodrigues CD, Pavarino ÉC and Goloni-Bertollo EM: Head and neck
cancer: Causes, prevention and treatment. Braz J Otorhinolaryngol.
79:239–247. 2013.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Langford R, Bonell CP, Jones HE, Pouliou
T, Murphy SM, Waters E, Komro KA, Gibbs LF, Magnus D and Campbell
R: The WHO Health Promoting School framework for improving the
health and well-being of students and their academic achievement.
Cochrane Database Syst Rev: CD008958. 2014. View Article : Google Scholar
|
|
7
|
Braun OM, Neumeister B, Neuhold N,
Siebenhandl A, Wimmer M, Holzner JH, Popp W, Strassl H, Dobrowsky W
and Gritzmann N: Histological grading of therapy induced regression
in squamous cell carcinomas of the oral cavity: A morphological and
immunohistochemical study. Pathol Res Pract. 185:368–372. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagao T, Chaturvedi P, Shaha A and
Sankaranarayanan R: Prevention and early detection of head and neck
squamous cell cancers. J Oncol. 2011:3181452011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Denaro N, Russi EG, Lefebvre JL and
Merlano MC: A systematic review of current and emerging approaches
in the field of larynx preservation. Radiother Oncol. 110:16–24.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El Deen DA, Toson EA and El Morsy SM:
Gemcitabine-based induction chemotherapy and concurrent with
radiation in advanced head and neck cancer. Med Oncol.
29:3367–3373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hertog MG, Feskens EJ, Hollman PC, Katan
MB and Kromhout D: Dietary flavonoids and cancer risk in the
Zutphen Elderly Study. Nutr Cancer. 22:175–184. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weisburger JH: Mechanisms of action of
antioxidants as exemplified in vegetables, tomatoes and tea. Food
Chem Toxicol. 37:943–948. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baowen Q, Yulin Z, Xin W, Wenjing X, Hao
Z, Zhizhi C, Xingmei D, Xia Z, Yuquan W and Lijuan C: A further
investigation concerning correlation between anti-fibrotic effect
of liposomal quercetin and inflammatory cytokines in pulmonary
fibrosis. Eur J Pharmacol. 642:134–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boots AW, Haenen GR and Bast A: Health
effects of quercetin: From antioxidant to nutraceutical. Eur J
Pharmacol. 585:325–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Staedler D, Idrizi E, Kenzaoui BH and
Juillerat-Jeanneret L: Drug combinations with quercetin:
Doxorubicin plus quercetin in human breast cancer cells. Cancer
Chemother Pharmacol. 68:1161–1172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cincin ZB, Unlu M, Kiran B, Bireller ES,
Baran Y and Cakmakoglu B: Molecular mechanisms of
quercitrin-induced apoptosis in non-small cell lung cancer. Arch
Med Res. 45:445–454. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bhat FA, Sharmila G, Balakrishnan S,
Arunkumar R, Elumalai P, Suganya S, Singh Raja P, Srinivasan N and
Arunakaran J: Quercetin reverses EGF-induced epithelial to
mesenchymal transition and invasiveness in prostate cancer (PC-3)
cell line via EGFR/PI3K/Akt pathway. J Nutr Biochem. 25:1132–1139.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu JL, Du J, Fan LL, Liu XY, Gu L and Ge
YB: Effects of quercetin on hyper-proliferation of gastric mucosal
cells in rats treated with chronic oral ethanol through the
reactive oxygen species-nitric oxide pathway. World J
Gastroenterol. 14:3242–3248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee DH and Lee YJ: Quercetin suppresses
hypoxia-induced accumulation of hypoxia-inducible factor-1alpha
(HIF-1alpha) through inhibiting protein synthesis. J Cell Biochem.
105:546–553. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanigawa S, Fujii M and Hou DX:
Stabilization of p53 is involved in quercetin-induced cell cycle
arrest and apoptosis in HepG2 cells. Biosci Biotechnol Biochem.
72:797–804. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Priyadarsini Vidya R, Murugan Senthil R,
Maitreyi S, Ramalingam K, Karunagaran D and Nagini S: The flavonoid
quercetin induces cell cycle arrest and mitochondria-mediated
apoptosis in human cervical cancer (HeLa) cells through p53
induction and NF-κB inhibition. Eur J Pharmacol. 649:84–91. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haghiac M and Walle T: Quercetin induces
necrosis and apoptosis in SCC-9 oral cancer cells. Nutr Cancer.
53:220–231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang JW, Kim JH, Song K, Kim SH, Yoon JH
and Kim KS: Kaempferol and quercetin, components of Ginkgo biloba
extract (EGb 761), induce caspase-3-dependent apoptosis in oral
cavity cancer cells. Phytother Res. 24 Suppl 1:S77–S82. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang CY, Chan CY, Chou IT, Lien CH, Hung
HC and Lee MF: Quercetin induces growth arrest through activation
of FOXO1 transcription factor in EGFR-overexpressing oral cancer
cells. J Nutr Biochem. 24:1596–1603. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu KW, Chen JC, Lai TY, Yang JS, Weng SW,
Ma YS, Lin HY, Wu RS, Wu KC, Wood WG and Chung JG: Gypenosides
suppress growth of human oral cancer SAS cells in vitro and in a
murine xenograft model: The role of apoptosis mediated by
caspase-dependent and caspase-independent pathways. Integr Cancer
Ther. 11:129–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang YM, Velmurugan BK, Kuo WW, Chen YS,
Ho TJ, Tsai CT, Ye CX, Tsai CH, Tsai FJ and Huang CY: Inhibitory
effect of alpinate Oxyphyllae fructus extracts on Ang II-induced
cardiac pathological remodeling-related pathways in H9c2
cardiomyoblast cells. BioMed. 3:148–152. 2013. View Article : Google Scholar
|
|
28
|
Chiu CH, Chou YC, Lin JP, Kuo CL, Lu HF,
Huang YP, Yu CC, Lin ML and Chung JG: Chloroform extract of solanum
lyratum induced G0/G1 arrest via p21/p16 and induced apoptosis via
reactive oxygen species, caspases and mitochondrial pathways in
human oral cancer cell lines. Am J Chin Med. 43:1453–1469. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsieh WT, Lin HY, Chen JH, Kuo YH, Fan MJ,
Wu RS, Wu KC, Wood WG and Chung JG: Latex of Euphorbia antiquorum
induces apoptosis in human cervical cancer cells via c-jun
n-terminal kinase activation and reactive oxygen species
production. Nutr Cancer. 63:1339–1347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang SH, Wu LW, Huang AC, Yu CC, Lien JC,
Huang YP, Yang JS, Yang JH, Hsiao YP, Wood WG, et al: Benzyl
isothiocyanate (BITC) induces G2/M phase arrest and apoptosis in
human melanoma A375.S2 cells through reactive oxygen species (ROS)
and both mitochondria-dependent and death receptor-mediated
multiple signaling pathways. J Agric Food Chem. 60:665–675. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leung YM, Wong KL, Chen SW, Lu DY, Kuo CS,
Chen YR, Chen YW and Cheng TH: Down-regulation of voltage-gated
Ca2+ channels in Ca2+ store-depleted rat
insulinoma RINm5F cells. BioMed. 3:130–139. 2013. View Article : Google Scholar
|
|
32
|
Fesik SW: Promoting apoptosis as a
strategy for cancer drug discovery. Nat Rev Cancer. 5:876–885.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kerr JF, Winterford CM and Harmon BV:
Apoptosis. Its significance in cancer and cancer therapy. Cancer.
73:2013–2026. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iwai-Kanai E, Hasegawa K, Araki M, Kakita
T, Morimoto T and Sasayama S: alpha- and beta-adrenergic pathways
differentially regulate cell type-specific apoptosis in rat cardiac
myocytes. Circulation. 100:305–311. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li L, Zhu Z, Joshi B, Porter AT and Tang
DG: A novel hydroxamic acid compound, BMD188, demonstrates
anti-prostate cancer effects by inducing apoptosis. I: In vitro
studies. Anticancer Res. 19:51–60. 1999.PubMed/NCBI
|
|
36
|
Li H, Hui L, Xu W, Shen H, Chen Q, Long L
and Zhu X: Modulation of P-glycoprotein expression by triptolide in
adriamycin-resistant K562/A02 cells. Oncol Lett. 3:485–489. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sendrowski K, Rusak M, Sobaniec P, Iłendo
E, Dąbrowska M, Boćkowski L, Koput A and Sobaniec W: Study of the
protective effect of calcium channel blockers against neuronal
damage induced by glutamate in cultured hippocampal neurons.
Pharmacol Rep. 65:730–736. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Looi CY, Arya A, Cheah FK, Muharram B,
Leong KH, Mohamad K, Wong WF, Rai N and Mustafa MR: Induction of
apoptosis in human breast cancer cells via caspase pathway by
vernodalin isolated from Centratherum anthelminticum (L.) seeds.
PLoS One. 8:e566432013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Y, He QY, Sun RW, Che CM and Chiu JF:
GoldIII porphyrin 1a induced apoptosis by mitochondrial death
pathways related to reactive oxygen species. Cancer Res.
65:11553–11564. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang J, Li TZ, Xu GH, Luo BB, Chen YX and
Zhang T: Low-concentration capsaicin promotes colorectal cancer
metastasis by triggering ROS production and modulating Akt/mTOR and
STAT-3 pathways. Neoplasma. 60:364–372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Takenokuchi M, Miyamoto K, Saigo K and
Taniguchi T: Bortezomib causes ER stress-related death of acute
promyelocytic leukemia cells through excessive accumulation of
PML-RARA. Anticancer Res. 35:3307–3316. 2015.PubMed/NCBI
|
|
42
|
Harashima N, Minami T, Uemura H and Harada
M: Transfection of poly(I:C) can induce reactive oxygen
species-triggered apoptosis and interferon-β-mediated growth arrest
in human renal cell carcinoma cells via innate adjuvant receptors
and the 2–5A system. Mol Cancer. 13:2172014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hsu YC, Chiang JH, Yu CS, Hsia TC, Wu RS,
Lien JC, Lai KC, Yu FS and Chung JG: Antitumor effects of deguelin
on H460 human lung cancer cells in vitro and in vivo: Roles of
apoptotic cell death and H460 tumor xenografts model. Environ
Toxicol. 32:84–98. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Senft D, Weber A, Saathoff F, Berking C,
Heppt MV, Kammerbauer C, Rothenfusser S, Kellner S, Kurgyis Z,
Besch R and Häcker G: In non-transformed cells Bak activates upon
loss of anti-apoptotic Bcl-XL and Mcl-1 but in the absence of
active BH3-only proteins. Cell Death Dis. 6:e19962015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ahsan H, Reagan-Shaw S, Breur J and Ahmad
N: Sanguinarine induces apoptosis of human pancreatic carcinoma
AsPC-1 and BxPC-3 cells via modulations in Bcl-2 family proteins.
Cancer Lett. 249:198–208. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mao Q, Zhang PH, Wang Q and Li SL:
Ginsenoside F(2) induces apoptosis in humor gastric carcinoma cells
through reactive oxygen species-mitochondria pathway and modulation
of ASK-1/JNK signaling cascade in vitro and in vivo. Phytomedicine.
21:515–522. 2014. View Article : Google Scholar : PubMed/NCBI
|