Introduction
Testicular germ cell cancer in a metastatic state is
curable with a cisplatin-based first line chemotherapy (1). It is assumed that the fast response upon
chemotherapy is mediated by apoptosis (2). Notwithstanding 10–15% of the patients
are refractory to first line chemotherapy and are hereby left with
palliative options (3,4). The high toxicity of the cisplatin-based
first line chemotherapy and the presence of non-responders with
potentially lethal clinical courses show the need of alternative
treatment strategies. Immunotherapies apply their antitumor effect
through mechanisms apart from standard cytotoxic agents. The
adaptive immune system has a number of mechanisms to detect and
eliminate cells with foreign antigens and has shown to be important
for tumor supressive activity. Cancer cells can escape this immune
response through active interference with the antigen-antibody
recognition system (5). Programmed
death receptor ligand 1 (PD-L1) is expressed on various cancers and
has been reported to play an important role in mediating and
promoting immunosuppression (6). The
interaction between PD-L1 and programmed cell death protein 1
(PD-1), which is expressed on immune cells including T-cells
attenuates lymphocytes activation and impairs anti-cancer T-cell
immune reaction (7). PD-1 and PD-L1
Inhibitors are already used as treatment of different cancers like
melanoma, lung cancer, kidney or bladder cancer (8–11). In some
cancers the level of PD-L1 expression is important for the response
of the checkpoint inhibitor (12,13).
An essential hallmark of cancer growth and
metastasis is marked pathologic angiogenesis (14). The deficient, hypoxic tumor
vasculature can also create an abnormal microenvironment, which
alters the proliferation, function and differentiation of immune
cells (15,16).
Vascular endothelial growth factor (VEGF) is the
most important angiogenic stimulation factor. Vascular endothelial
growth factor receptor 2 (VEGFR2) mediates vascular endothelial
growth. Several Studies have reported an important role of VEGFR2
expression in the pathogenesis and progression of testicular cancer
(17,18).
Still tyrosin kinase inhibitors (TKIs) with VEGFR2
as specific target (sunitinib, carbozantinib) have not been
investigated clinically in TGCT patients.
The association between PD-L1 expression and
clinical outcomes to vascular endothelial growth-factor targeted
therapies have been evaluated in metastatic clear cell renal cell
carcinoma (19). The aim of our study
was to get first evidence for the potential molecular context of
angiogenesis and immune checkpoints in the development and
progression of testicular cancers. Therefore, we performed a tissue
micro array based analysis with immunohistochemistry of PD-1, PD-L1
and VEGFR2 of testicular cancer and corresponding normal appearing
testis tissue.
Materials and methods
Patient selection and
clinico-pathologic analysis
Orchiectomy specimens of 84 testicular cancer
patients (41 seminoma patients and 43 non-seminoma patients) from
the Goethe University Hospital Frankfurt, Germany from 2002 to 2011
were evaluated. Following histological subtypes were seen:
Seminoma, teratocarcinoma, mature teratoma, immature teratoma,
embryonal carcinoma, chorion carcinoma, yolk sac carcinoma. All
patients provided written informed consent for the use of their
tissues, and the study was approved by the ethics committee of the
Goethe University, Frankfurt/Main, Germany. All patients revealed
adequate clinical follow up data.
The International Germ Cell Cancer Collaborative
Group (IGCCCG) defined a prognostic factor-based staging system for
metastatic testis tumors based on identification of clinically
independent adverse factors. This staging system has been
incorporated into the TNM Classification and uses histology,
location of the primary tumor, location of metastases and
pre-chemotherapy marker levels in serum samples as prognostic
factors to categorise patients into ‘good’, ‘intermediate’ or
‘poor’ prognosis [(20,21)EAU]. All patients with metastatic
disease were grouped in one of these categories.
Tissue microarray (TMA)
All tissue samples were retrieved from orchiectomy
specimens, fixed in 10% buffered formalin and embedded in paraffin
at time of surgery. For TMA construction H&E-stained slides of
human testicular germ cell tumors (TGCTs) were reviewed by a
certified pathologist. The most representative areas of each tumor
sample and assigned corresponding biopsies of the unaffected sites
of the orchiectomy specimens were marked. Cores (3 mm in diameter)
were punched out from the chosen areas of the formalin-fixed
paraffin-embedded (FFPE) blocks. The samples were assembled in the
array. The TMA location number was linked to the database including
the clinico-pathologic data.
Immunohistochemistry
An automated immunostainer (Ventana, Strasbourg,
France) using standard protocols was used for immunohistochemical
stainings. Briefly, 4 µm thick slides were heated to 100°C,
incubated with Inhibitor D (Ventana) and then incubated with the
primary antibody [PD-L1: Cell Signaling rabbit monoclonal antibody
(no. 13684; Cell Signaling Technology, Inc., Leiden, Netherlands);
PD-1: Abcam mouse monoclonal antibody (no. ab52587; Abcam
Cambridge, UK)]. VEGFR2 (no. 55B11): Cell Signaling rabbit
monoclonal antibody (no. 2479S; Cell Signaling Technology, Inc.)].
The secondary antibody solution was incubated after rinsing,
followed by sequential incubation with Blocker D (Ventana) and
SA-HRP D (Ventana). Visualization was accomplished using DAB D
(diaminobenzidine) and DAB H2O2 D (Ventana).
Finally, the slides were counterstained with Hemalaun and mounted.
The detailed immunohistochemical staining procedure was described
previously (22).
Staining quality and specificity were assured using
established protocols and antibodies (23–30),
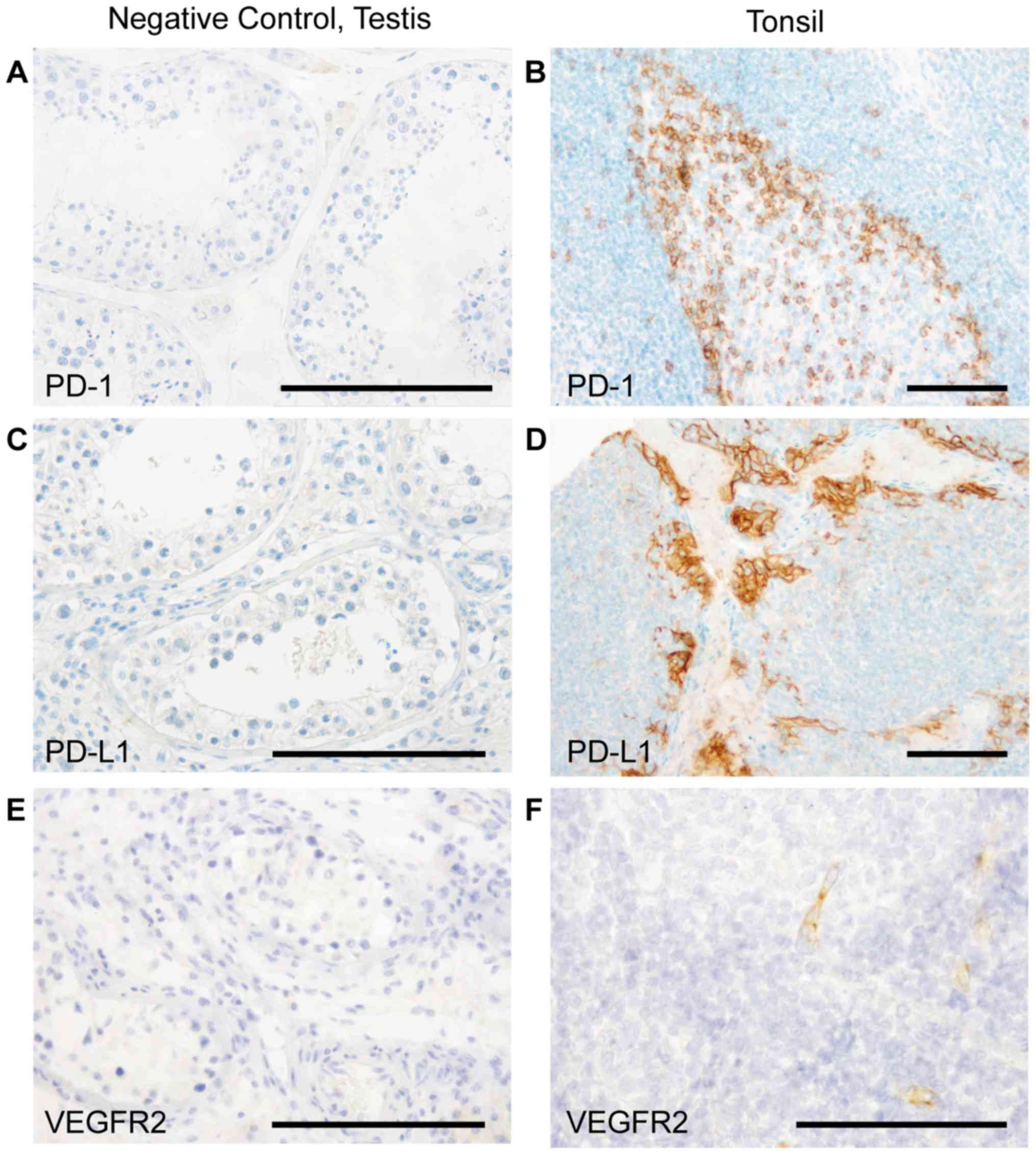
negative controls prior to staining as well as on-slide positive
controls on each tissue micro array slide (Fig. 1). We used tonsil material on every
single tissue micro array as a positive control as described in
literature before (31–38).
Of note, not every core of the TMA was evaluable for
each protein due to technical reasons, resulting in variations of
numbers of the analyzed tissue specimens. This fact can be
considered a possible limitation of the study.
Scoring
The stained TMAs were evaluated with an Olympus BX50
light microscope. For semi-quantitative evaluation of PD-L1
immunohistochemistry, a multi-score of staining frequency and
intensity was applied. Staining frequency was assessed as follows:
0, 0–1%; 1, 1–10%; 2, 10–25%; 3, 25–50%; and 4, >50%. The
staining intensity was rated as follows: 0, no staining; 1, weak;
2, moderate; and 3, strong. This scoring system was described
previously (39). PD-1
immunohistochemistry tissue micro array cores were evaluated if
PD-1 positive infiltrating cells were present in the tumor (=1) or
not (=0). VEGFR2 expression was categorized into the following
expression intensities: 0, no staining; 1, weak; 2, moderate; and
3, strong.
Statistical analysis
The semi-quantitative scores were assigned as
ordinal scale response variable and analyzed together with nominal
variables (tissue/tumor type). Non-parametric Wilcoxon test was
used for statistical analyses regarding histology scores.
Chi-square test was performed analyzing contingency tables.
Significance level of α=0.05 was selected for all tests.
Statistical analysis was performed using JMP 11.0.0 software (SAS
Institute, Inc., Cary, NC, USA).
Results
PD-L1 is significantly upregulated in
testicular tumor and PD-1 positive cells significantly infiltrate
the testicular tumor compared to normal testicular tissue
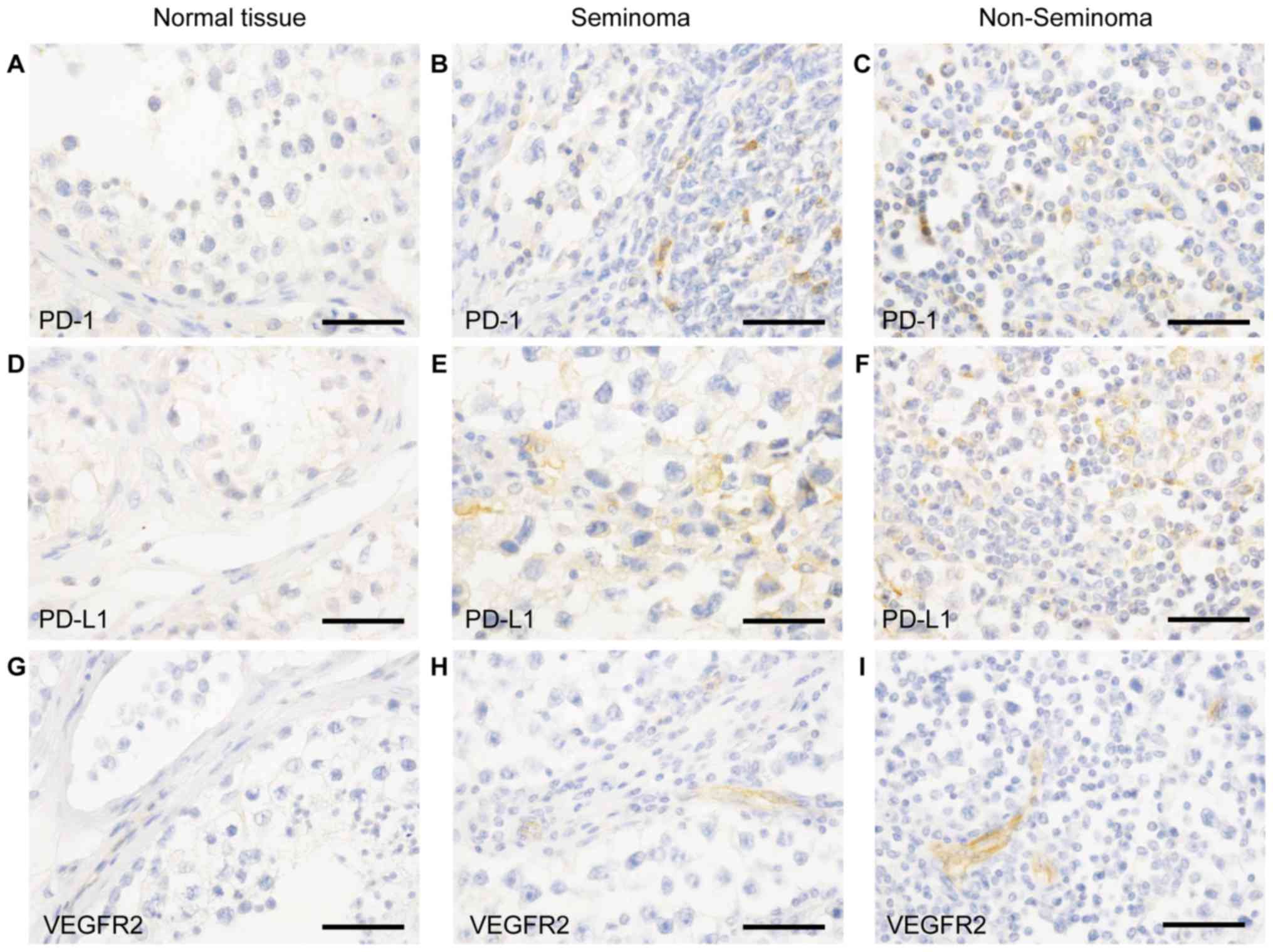
We did not detect PD-1 expression on cells
morphologically identifiable as tumor cells in any of the tumor
samples or in the normal testicular tissue (Fig. 2A-C).
Of note, the PD-1 positive infiltrating cells both
in seminoma (Fig. 2B) and
non-seminoma (Fig. 2C) show a higher
count than in normal testicular tissue. Here we could not detect
PD-1 positive infiltrates (Fig.
2A).
Immunohistochemical analyses for PD-L1 revealed that
the checkpoint protein was mainly detected within the parenchyma of
the tumor of seminomas (Fig. 2E) and
non-seminomas (Fig. 1F) in comparison
to the normal tissue (Fig. 2D).
These findings were confirmed by statistical
analysis. We found general significant differences in
PD-L1-expression between normal tissue vs. tumor (Fig. 3B) with P<0.0001 and also in PD-1
positive infiltrative cells in the neoplastic testis (Fig. 3A) with P=0.018. Seminoma and
non-seminoma both show on separate analysis to the normal tissue
significant expression of PD-L1 (Fig. 4C
and D) and significant amount of PD-1 positive infiltrating
cells (Fig. 4A and B).
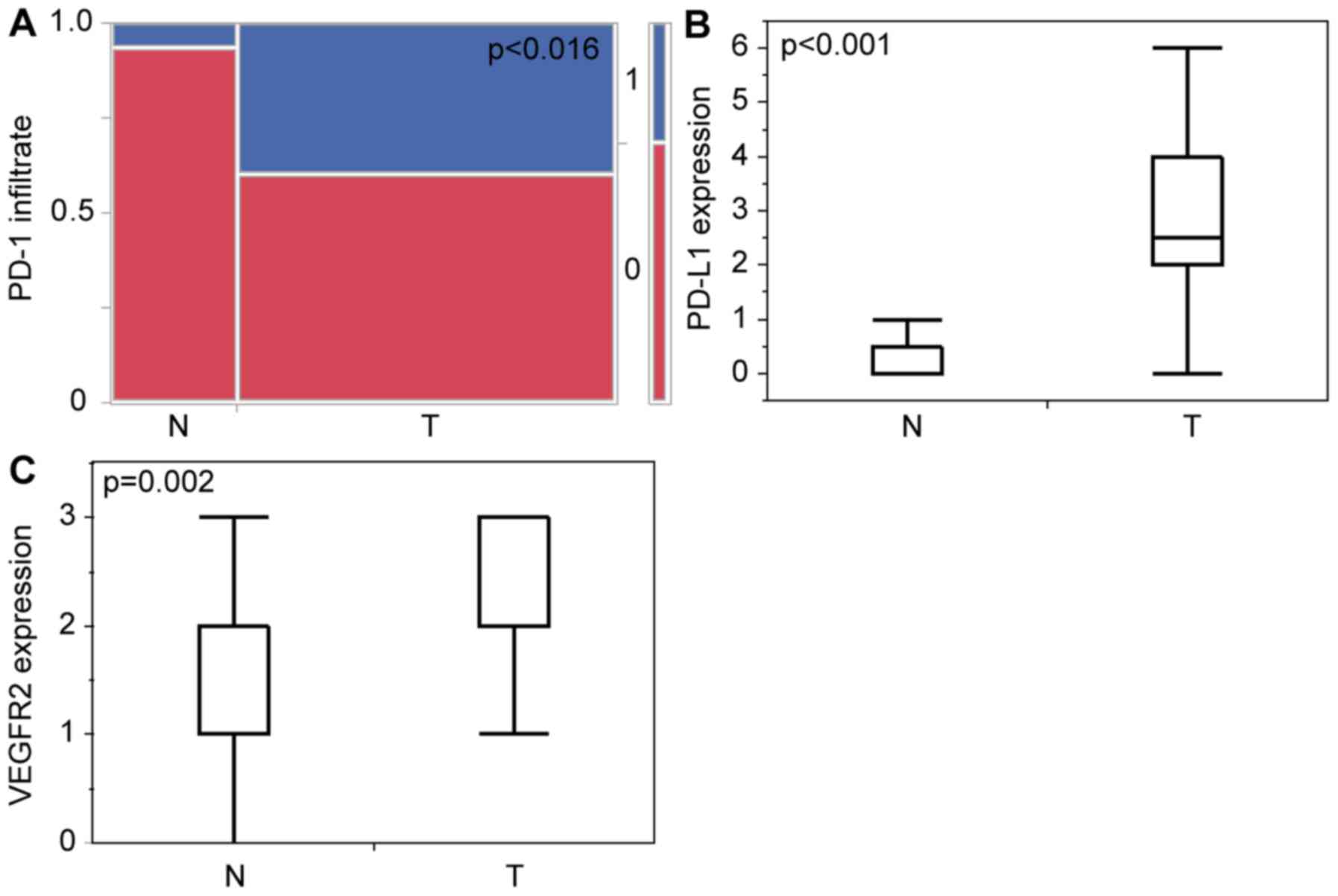 | Figure 3.Statistical analysis of
immunohistochemical staining of tumor vs. normal tissues. (A)
Contingency table of the presence of PD-1-immunopositive
infiltrating cells (0=no, 1=yes) of normal appearing testicular
tissue (n=15; no, 14/93.3%; yes, 1/6.67%) and testicular cancer
(n=45; no, 27/60%; yes, 18/40%) P=0.0162. (B) Histogram of PD-L1
expression score of normal appearing testicular tissue (n=17; min:
0; max: 3; mean: 0) and testicular cancer (n=46; min, 0; max, 6;
mean, 2.5), P<0.001. (C) Histogram of VEGFR2 expression score of
normal appearing testicular tissue (n=14; min, 0; max, 3; mean, 1)
and testicular cancer (n=67; min, 0; max, 3; mean, 2), P=0.002.
PD-1, programmed cell death protein 1; PD-L1, programmed cell death
ligand 1; VEGFR2, vascular endothelial growth factor receptor 2; N,
normal testicular tissues; T, testicular cancer tissues. |
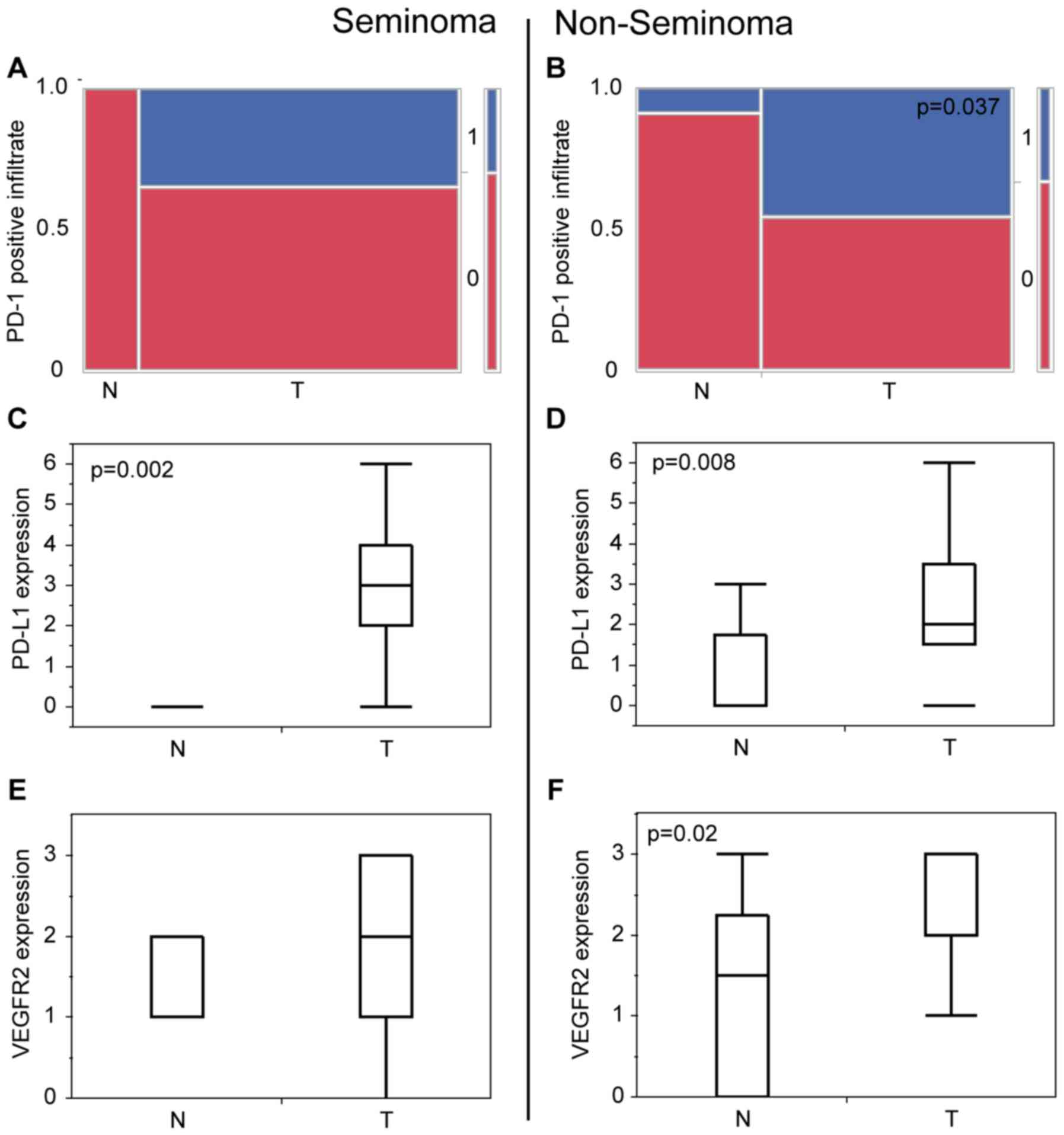 | Figure 4.Statistical analysis of
immunohistochemical staining of tumor vs. normal tissues grouped in
Seminoma and Non-Seminoma. (A) Contingency table of the presence of
PD-1-immunopositive infiltrating cells (0=no, 1=yes) in seminoma of
normal appearing testicular tissue (n=4; no, 4/100%; yes,/0%) and
testicular cancer (n=23; no, 15/65.2%; yes, 8/34.8%) P=0.160. (B)
Contingency table of the presence of PD-1-immunopositive
infiltrating cells (0, no; 1, yes) in non-seminoma of normal
appearing testicular tissue (n=11; no, 10/90.9%; yes, 1/9.1%) and
testicular cancer (n=22; no, 12/54.5%; yes, 10/45.5%) P=0.037. (C)
Histogram of the PD-L1 expression score of normal appearing
testicular tissue of patients with seminomas (n=9; min: 0; max: 1;
mean: 0) and seminoma tissue (n=25; min, 0; max, 6; mean, 3),
P=0.002. (D) Histogram of the PD-L1 expression score of normal
appearing testicular tissue of patients with non-seminomas (n=8;
min, 0; max, 3; mean, 0) and non-seminoma tissue (n=21, min, 0;
max, 6; mean, 2), P=0.008. (E) Histogram of the PD-1 expression
score of normal appearing testicular tissue of patients with
seminomas (n=8; min, 1; max, 2; mean, 1) and seminoma tissue (n=35;
min, 0; max, 3; mean, 2), P=0.051. (F) Histogram of the PD-1
expression score of normal appearing testicular tissue of patients
with non-seminomas (n=6; min, 0; max, 3; mean, 1.5) and
non-seminoma tissue (n=32; min, 1; max, 3; mean, 3), P=0.002. PD-1,
programmed cell death protein 1; PD-L1, programmed cell death
ligand 1; VEGFR2, vascular endothelial growth factor receptor 2; N,
normal testicular tissues; T, testicular cancer tissues. |
On comparison of seminoma vs. non seminoma there is
no significant difference of infiltration of PD-1 positive
infiltrating cells (Fig. 5A) and
PD-L1 expression (Fig. 5B).
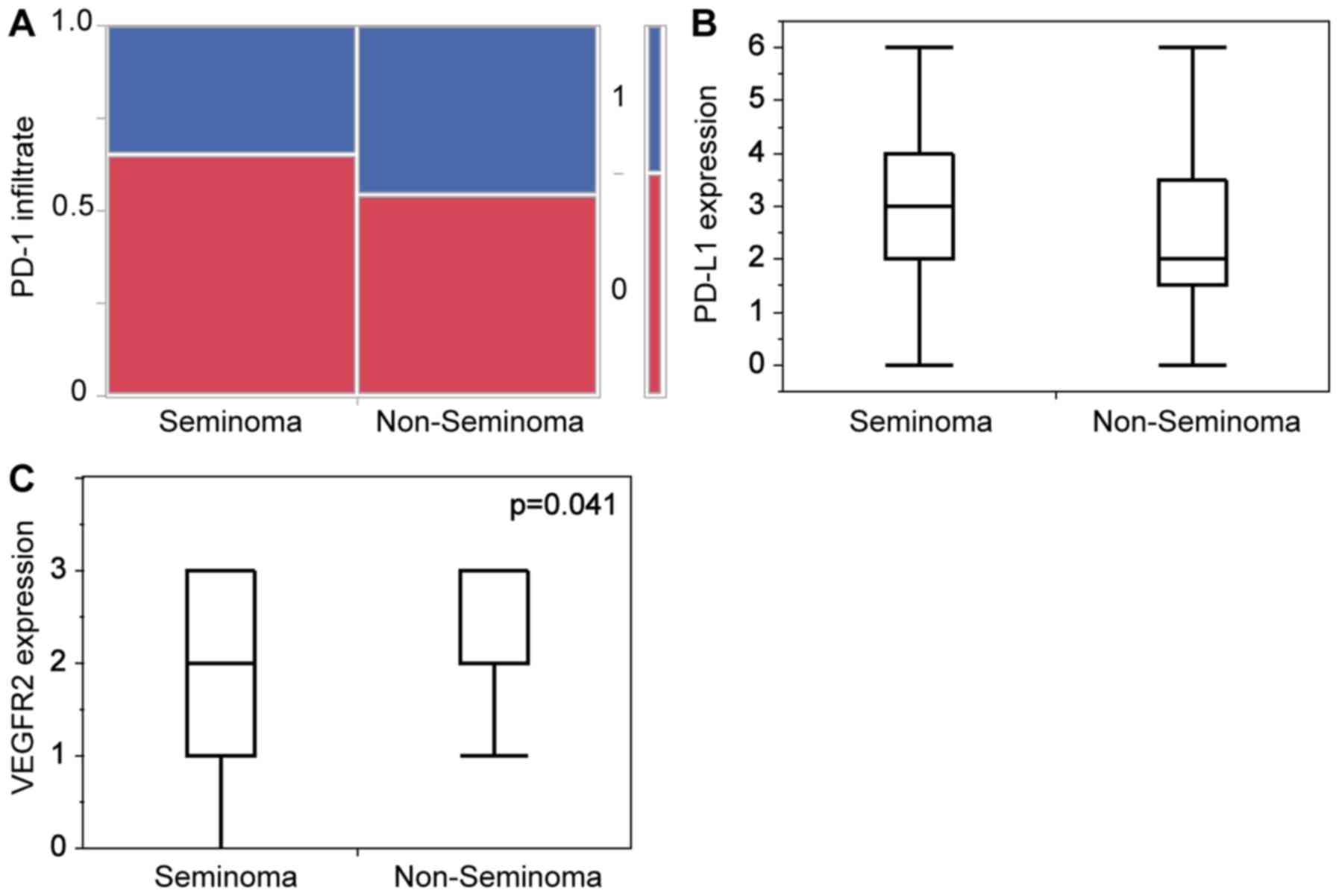 | Figure 5.Statistical analysis of
immunohistochemical staining of Seminoma vs. Non-Seminoma. (A)
Contingency table of the presence of PD-1-immunopositive
infiltrating cells (0, no; 1, yes) of seminoma (n=23; no, 15/65.2%;
yes, 8/34.8%) and non-seminoma (n=22; no, 12/54.5%; yes, 10/45.5%)
P=0.465. (B) Histogram of the PD-L1 expression score of seminomas
(n=25; min, 0; max, 6; mean, 3) and non-seminomas (n=21; min, 0;
max, 6; mean, 2), P=0.507. (C) Histogram of the VEGFR2 expression
score of seminomas (n=35; min, 0; max, 3; mean, 2) and
non-seminomas (n=32; min: 1; max: 3; mean: 3), P=0.041. PD-1,
programmed cell death protein 1; PD-L1, programmed cell death
ligand 1; VEGFR2, vascular endothelial growth factor receptor
2. |
VEGFR2 is significantly upregulated in
testicular tumor
In general, staining scores were significantly
higher for VEGFR2 in the tumor tissue (Fig. 3C) in comparison to the normal testis
(P=0.002). Here we see a distinct loco-regional pattern with higher
scores in areas with a higher vessel density. But on a separate
immunohistochemical analysis only non-seminoma (Fig. 2I) show a higher expression in
comparison to normal tissue (Fig. 3G)
with P=0.02 (Fig. 4F). VEGFR2
expression in seminoma (Fig. 2H)
compared to normal tissue is not significantly different (Fig. 4E). Whereas non-seminoma has a
significantly higher expression of VEGFR2 compared to seminoma
(Fig. 5C).
PD-1, PD-L1 and VEGFR2 show no
difference in expression in IGCCCG stages of non-seminoma
The IGCCCG defines a prognostic factor-based staging
system for metastatic testis tumour based on identification of some
clinical independent adverse factors (EAU).
In our analysis only non-seminoma patients with
known IGCCG stage were included. Here we have patient distribution
in all 3 stages (good, intermediate and poor). When comparing the
level of tumor expression of PD-L1 (Fig.
6A) and VEGFR2 (Fig. 6B) and the
PD-1 positive infiltrate cells (Fig.
6C) in regards of these three clinical stages there is no
significant difference, respectively.
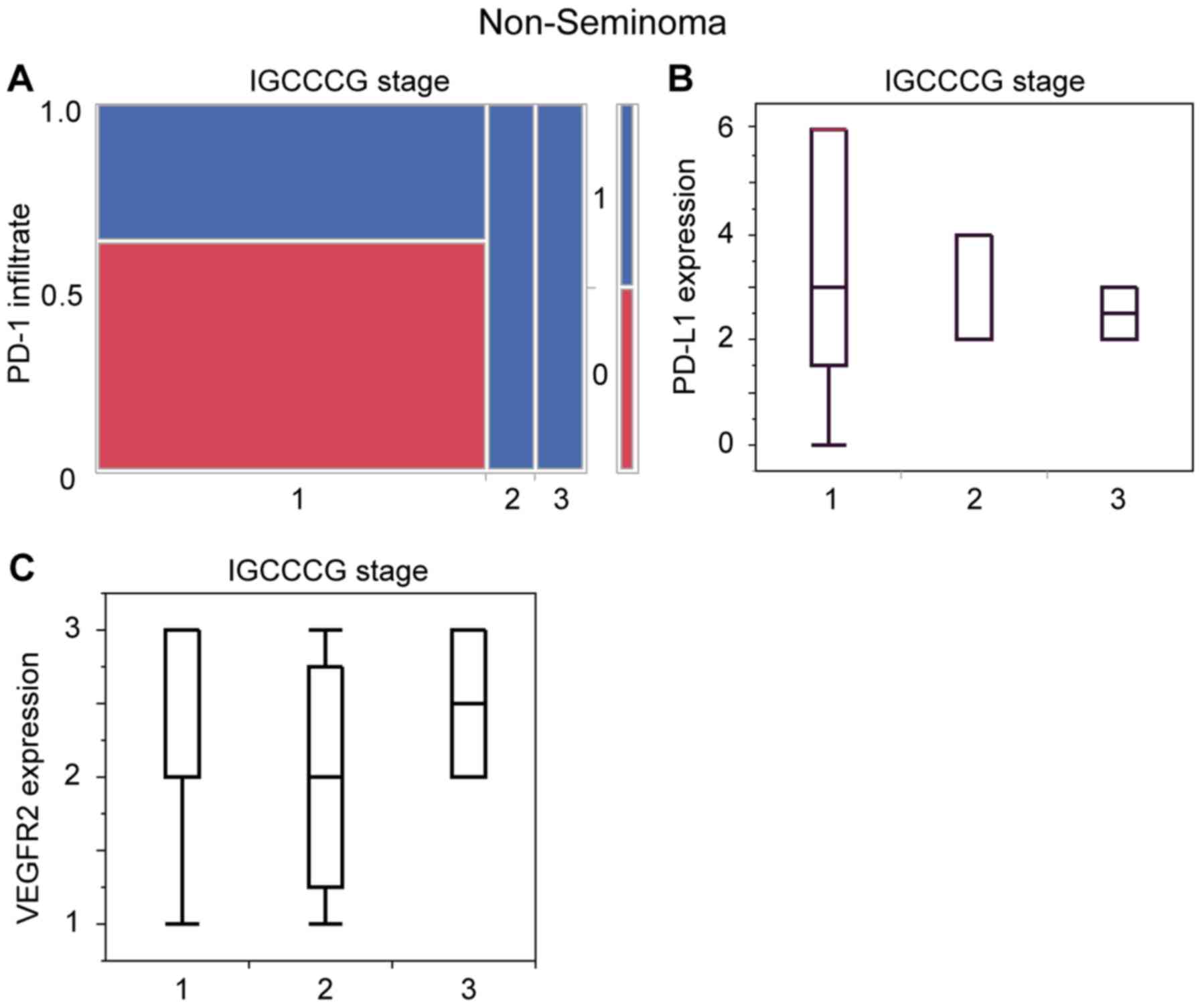 | Figure 6.Statistical analysis of
immunohistochemical staining of the biomarkers in dependence of
IGCCCG stage. (A) Contingency table of the presence of
PD-1-immunopositive infiltrating cells (0=no, 1=yes) in the
non-seminoma of different IGCCCG stages (stage 1: n=16; no,
10/62.5%; yes, 6/37.5%; stage 2: n=2; no, 0/0%; yes, 2/100%; stage
3: n=2; no, 0/0%; yes, 2/100%) P=0.82. (B) Histogram of the PD-L1
expression score of IGCCCG good prognosis (stage 1: n=13; min, 0;
max, 6; mean, 3), IGCCCG intermediate prognosis (stage 2: n=3; min,
2; max, 4; mean, 2) and IGCCCG poor prognosis (stage 3: n=2; min,
2; max, 3; mean, 2.5) P=0.968. (C) Histogram of VEGFR2 expression
score of IGCCCG good prognosis (1: n=22, min: 1; max: 3; mean: 3),
IGCCCG intermediate prognosis (stage 2: n=4; min, 1; max, 3; mean,
2) and IGCCCG poor prognosis (stage 3: n=2; min, 2; max, 3; mean,
2.5) P=0.248. IGCCCG, International Germ Cell Cancer Collaborative
Group; PD-1, programmed cell death protein 1; PD-L1, programmed
cell death ligand 1; VEGFR2, vascular endothelial growth factor
receptor 2. |
In IGCCCG of seminoma there is only good and
intermediate prognosis per se. But in our case we have only
seminoma patients with known IGCCG good prognosis. Thus no further
statistical evaluation was possible.
Discussion
In our study we investigated the expression profiles
of PD-L1, PD-1 and VEGFR2 in different types of testicular cancer
and normal appearing testis tissue to gain a first insight into a
potential in vivo correlation of tumor immune-checkpoint
status and angiogenesis.
In the last decade multiple approaches were
undertaken in order to team up with the immune system in the fight
against cancer. Excitement was great upon the introduction of e.g.,
vaccines or monoclonal antibodies.
In a great number of recent studies immune
checkpoint inhibitors seem to be promising options in urogenital
malignancies in different therapeutic settings and stages (8,9,40). Immune checkpoints like PD-L1 are
pivotal to prevent autoimmunity. Through a complex system of
excitatory and inhibitory signals, circulating PD-1-receptor
carrying immune cells, like activated CD4+ T cells, CD8+ T cells,
natural killer cells, monocytes and B cells, can be activated or
inhibited (41). With this mechanism
malignant cells can be immunologically identified. But multiple
tumors express immune checkpoints to escape lethal immune cell
attacks. From an immunologic point of view it is known that the
testis tissue has a naturally suppressed immune response (42) and is considered a privileged site
(43). It can tolerate autoantigens
from developing germ cells. The immunologic homeostasis involves
multiple mechanisms such as physiological anatomical structure,
systemic immune tolerance, and active local immunosuppression
(44,45). Cheng et al discuss that
PD-1/PD-L1 contribute to the immune response of the testis
(42), which is conflicting to the
findings of multiple studies (25,46)
including ours with little or no PD-L1 expression in normal
testicular tissue as mentioned above.
In our study we see that PD-L1 is significantly
upregulated in testicular cancer in comparison to the normal
tissue. The phenomenon of elevated PD-L1 expression in testicular
germ cell cancer has been described before (25,46).
Contrary to the findings of Cierna et al (25) we see no significant difference in the
PD-L1 expression of seminoma and non-seminoma.
The percentage values of Fankhauser et al may
also imply a not statistical difference of PD-L1 expression between
seminoma and non-seminomatous tumors (46).
The comparison of seminoma and non-seminomatous
(Non-seminomatous germ cell tumors, NSGCT) tumors is complicated.
The NSGCTs are a very heterogeneous group containing
chorioncarcinoma, yolk sac tumors, embryonal carcinoma and
teratoma. Separate positive PD-L1 expression analysis showed values
between 13% (teratoma) and 80% (chorioncarcinoma) (46).
In general the direct comparison of studies is
especially difficult because of inconsistent scoring and divergent
used antibodies.
Studies of other genitourinary cancers have shown
that the PD-L1 expression status may correlate with the therapeutic
response and outcome of PD-L1 and PD-1 immunotherapy (47). This may also be the case in testicular
cancer. But as mentioned above a standardized scoring system is
needed in order to answer this question.
Another important pillar of the physiologic immune
(suppressing) testicular system is the Blood-Testis-Barrier (BTB).
The BTB can provide an adequate microenvironment for
spermatogenesis, by effectively preventing immunological component
in the blood from entering the seminiferous tubules and by
sequestering the autoantigenic germ cell from access to the immune
system (44). In case of malignant
lesions this barrier will be destroyed. Pathologic vessels run
through the neoplasm.
Previous studies suggested that VEGF and its
receptors VEGFR have an important role in the development and
progression of TCGT (48). Adam et
al described increased expression of VEGF and VEGFR2 in
patients with TGCT especially in non-seminoma (49). Nitzsche et al showed that
blocking VEGFR2 with the antiangiogenic compound HP-14 inhibited
growth of platinum sensible and -resistant TGCT cells and
suppressed tumor angiogenesis (18).
In line with these results sunitinib, an orally
applicable VEGFR2 inhibitor has been evaluated in a phase II study
in platinum refractory advanced germ cell tumor patients (50). We also see that VEGFR2 is
significantly upregulated in testicular tumor as compared to normal
tissue. But in the separate tumor analysis VEGFR2 shows a higher
expression in non-seminoma tumor than in seminoma. Jones et
al have shown that non-seminomatous tumours have a higher
microvesicular density and higher expression of VEGF (51). Nevertheless it is known that
seminomatous tumors, with their homogeneous sections, are
crisscrossed with tiny unnatural venule like tumor vessels
(52).
Our findings imply that immune cells are able to
infiltrate the usually immune-privileged testicular tissue because
of the vascular structural alteration resulting from malignant
transformation. The damaged BTB cannot maintain the immune
suppressive environment.
We see a significant infiltration of PD-1 positive
immune cells in both subtypes of testicular tumor compared to
normal tissue with no significant difference between the two types
of tumor. The presence of PD-1 presenting tumor infiltrating
lymphocytes (TILs) may have strong prognostic and predictive
features in different types of tumors (53–56).
Contrary to our findings, a recent study showed more PD-1
expressing TILs in seminoma (87%) vs. non-seminoma (42,9%) in
direct comparison (6). In the case of
seminoma a study of Sakai et al described TIL recruitment
into tumor tissue through aforementioned tiny venule like tumor
vessels (52). Chovanec et al
stated that PD-1 expressing TILs did neither have prognostic value
nor a correlation with clinico-pathologic characteristics (24). Interestingly, same has been shown in
brain metastases, which also grow in an immune-privileged
micromillieu (23).
We also see no significant difference in PD-1
expressing TILs in the different clinical IGCCCCG stages (Fig. 6A, B).
A major limitation of the study is the retrospective
nature and the absence of extragonadal tumor or metastatic tumor
tissue, which behavior during treatment finally defines the success
of therapy and the clinical result. Despite of these facts the
findings of the study should be regarded as hypothesis
generating.
Our investigation shows the presence of potential
PD-1 expressing cytotoxic immune cells in the tumor
microenvironment, hypothetically migrated through a pathologic
vascular system with an impaired BTB. The morbid tumor vascularity
usually creates a more immune suppressive microenvironment in
different types of cancer (57–59). But
in testicular cancer, with an already physiologic suppressed
immunologic microenvironment, there is a paradox effect with an
influx of immune competent cells.
However these cells cannot exert their antitumor and
cytotoxic effects because they are exposed to inhibition through
the PD-L1 immune escape of the tumor. Neither of these
loco-regional molecular findings of VEGFR2 expression, PD-L1
expression nor PD-1 expressing TILs seem to correlate with
clinico-pathologic characteristics in our patient cohort (Fig. 6A-C).
Our data suggests that both the anti-PD-1/PD-L1
immunotherapy and the anti-angiogenic therapy might be a promising
option in the treatment of testicular cancer. It needs to be
addressed, after further evaluation of PD-1, PDL-1 and VEGFR2
function in testicular cancer, whether these treatment options
could be possibly administered individually, sequentially or in
combination. In this matter evaluation of the effect of knocking
down or elevating the expression of PD-1 on vascularization e.g.,
in an animal model would be decisive.
It seems feasible that PD-1 expressing cytotoxic
cells need tumor vessels in order to migrate into the tumor. Here
an anti-angiogenic treatment might obstruct the path into the tumor
for the immune competent cells and thus diminish the effect of the
PD-L1/PD-1 inhibition. But an alternative hypothesis states that
anti-angiogenic agents can also transiently ‘normalize’ the
abnormal structure and function of tumor vasculature to make it
more efficient for oxygen and (Anti-PD-L1/PD-1) drug delivery.
Clinical efficiency of the mentioned treatment
modalities and the importance of PD-L1-expression and/or PD-1
expressing TILs need further investigation in clinical trials.
Acknowledgements
MM would like to thank the Luxembourg National
Research Fond (FNR) for the support (FNR PEARL P16/BM/11192868
grant).
References
|
1
|
Hanna NH and Einhorn LH: Testicular
cancer-discoveries and updates. N Engl J Med. 371:2005–2016. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mayer F, Stoop H, Scheffer GL, Scheper R,
Oosterhuis JW, Looijenga LH and Bokemeyer C: Molecular determinants
of treatment response in human germ cell tumors. Clin Cancer Res.
9:767–773. 2003.PubMed/NCBI
|
|
3
|
Hussain SA, Ma YT, Palmer DH, Hutton P and
Cullen MH: Biology of testicular germ cell tumors. Expert Rev
Anticancer Ther. 8:1659–1673. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oechsle K, Honecker F, Cheng T, Mayer F,
Czaykowski P, Winquist E, Wood L, Fenner M, Glaesener S, Hartmann
JT, et al: Preclinical and clinical activity of sunitinib in
patients with cisplatin-refractory or multiply relapsed germ cell
tumors: A canadian urologic oncology group/german testicular cancer
study group cooperative study. Ann Oncol. 22:2654–2660. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Farkona S, Diamandis EP and Blasutig IM:
Cancer immunotherapy: The beginning of the end of cancer? BMC Med.
14:732016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sui X, Ma J, Han W, Wang X, Fang Y, Li D,
Pan H and Zhang L: The anticancer immune response of
anti-PD-1/PD-L1 and the genetic determinants of response to
anti-PD-1/PD-L1 antibodies in cancer patients. Oncotarget.
6:19393–19404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi L, Chen S, Yang L and Li Y: The role
of PD-1 and PD-L1 in T-cell immune suppression in patients with
hematological malignancies. J Hematol Oncol. 6:742013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Escudier B, Motzer RJ, Sharma P, Wagstaff
J, Plimack ER, Hammers HJ, Donskov F, Gurney H, Sosman JA, Zalewski
PG, et al: Treatment beyond progression in patients with advanced
renal cell carcinoma treated with nivolumab in checkmate 025. Eur
Urol. 72:368–376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mehta K, Patel K and Parikh RA:
Immunotherapy in genitourinary malignancies. J Hematol Oncol.
10:952017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gettinger SN, Horn L, Gandhi L, Spigel DR,
Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman
DM, et al: Overall survival and long-term safety of nivolumab
(anti-programmed death 1 antibody, BMS-936558, ONO-4538) in
patients with previously treated advanced non-small-cell lung
cancer. J Clin Oncol. 33:2004–2012. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weber JS, Gibney G, Sullivan RJ, Sosman
JA, Slingluff CL Jr, Lawrence DP, Logan TF, Schuchter LM, Nair S,
Fecher L, et al: Sequential administration of nivolumab and
ipilimumab with a planned switch in patients with advanced melanoma
(CheckMate 064): An open-label, randomised, phase 2 trial. Lancet
Oncol. 17:943–955. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patel SP and Kurzrock R: PD-L1 expression
as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther.
14:847–856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meng X, Huang Z, Teng F, Xing L and Yu J:
Predictive biomarkers in PD-1/PD-L1 checkpoint blockade
immunotherapy. Cancer Treat Rev. 41:868–876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rouhi P, Lee SL, Cao Z, Hedlund EM, Jensen
LD and Cao Y: Pathological angiogenesis facilitates tumor cell
dissemination and metastasis. Cell Cycle. 9:913–917. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Y, Yuan J, Righi E, Kamoun WS,
Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR,
Vianello F, et al: Vascular normalizing doses of antiangiogenic
treatment reprogram the immunosuppressive tumor microenvironment
and enhance immunotherapy. Proc Natl Acad Sci USA. 109:17561–17566.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmid MC and Varner JA: Myeloid cells in
the tumor microenvironment: Modulation of tumor angiogenesis and
tumor inflammation. J Oncol. 2010:2010262010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nitzsche B, Gloesenkamp C, Schrader M,
Hoffmann B, Zengerling F, Balabanov S, Honecker F and Höpfner M:
Anti-tumour activity of two novel compounds in cisplatin-resistant
testicular germ cell cancer. Br J Cancer. 107:1853–1863. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nitzsche B, Gloesenkamp C, Schrader M,
Ocker M, Preissner R, Lein M, Zakrzewicz A, Hoffmann B and Höpfner
M: Novel compounds with antiangiogenic and antiproliferative
potency for growth control of testicular germ cell tumours. Br J
Cancer. 103:18–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Einstein DJ and McDermott DF: Combined
blockade of vascular endothelial growth factor and programmed death
1 pathways in advanced kidney cancer. Clin Adv Hematol Oncol.
15:478–488. 2017.PubMed/NCBI
|
|
20
|
International germ cell consensus
classification: A prognostic factor-based staging system for
metastatic germ cell cancers. International germ cell cancer
collaborative group. J Clin Oncol. 15:594–603. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Albers P, Albrecht W, Algaba F, Bokemeyer
C, Cohn-Cedermark G, Fizazi K, Horwich A, Laguna MP, Nicolai N and
Oldenburg J: EAU Guidelines Testicular Cancer. European Association
of Urology. 2015.https://uroweb.org/wp-content/uploads/EAU-Guidelines-Testicular-Cancer-2016-1.pdf
|
|
22
|
Baumgarten P, Harter PN, Tönjes M, Capper
D, Blank AE, Sahm F, von Deimling A, Kolluru V, Schwamb B,
Rabenhorst U, et al: Loss of FUBP1 expression in gliomas predicts
FUBP1 mutation and is associated with oligodendroglial
differentiation, IDH1 mutation and 1p/19q loss of heterozygosity.
Neuropathol Appl Neurobiol. 40:205–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harter PN, Bernatz S, Scholz A, Zeiner PS,
Zinke J, Kiyose M, Blasel S, Beschorner R, Senft C, Bender B, et
al: Distribution and prognostic relevance of tumor-infiltrating
lymphocytes (TILs) and PD-1/PD-L1 immune checkpoints in human brain
metastases. Oncotarget. 6:40836–40849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chovanec M, Cierna Z, Miskovska V,
Machalekova K, Svetlovska D, Kalavska K, Rejlekova K, Spanik S,
Kajo K, Babal P, et al: Prognostic role of programmed-death ligand
1 (PD-L1) expressing tumor infiltrating lymphocytes in testicular
germ cell tumors. Oncotarget. 8:21794–21805. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cierna Z, Mego M, Miskovska V, Machalekova
K, Chovanec M, Svetlovska D, Hainova K, Rejlekova K, Macak D,
Spanik S, et al: Prognostic value of programmed-death-1 receptor
(PD-1) and its ligand 1 (PD-L1) in testicular germ cell tumors. Ann
Oncol. 27:300–305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Inaguma S, Wang Z, Lasota J,
Sarlomo-Rikala M, McCue PA, Ikeda H and Miettinen M: Comprehensive
immunohistochemical study of programmed cell death ligand 1
(PD-L1): Analysis in 5536 cases revealed consistent expression in
trophoblastic tumors. Am J Surg Pathol. 40:1133–1142. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mansfield AS, Aubry MC, Moser JC,
Harrington SM, Dronca RS, Park SS and Dong H: Temporal and spatial
discordance of programmed cell death-ligand 1 expression and
lymphocyte tumor infiltration between paired primary lesions and
brain metastases in lung cancer. Ann Oncol. 27:1953–1958. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo
CW, Khoo KH, Chang SS, Cha JH, Kim T, et al: Glycosylation and
stabilization of programmed death ligand-1 suppresses T-cell
activity. Nat Commun. 7:126322016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miettinen M, Rikala MS, Rys J, Lasota J
and Wang ZF: Vascular endothelial growth factor receptor 2 as a
marker for malignant vascular tumors and mesothelioma: An
immunohistochemical study of 262 vascular endothelial and 1640
nonvascular tumors. Am J Surg Pathol. 36:629–639. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Holzer TR, Fulford AD, Nedderman DM,
Umberger TS, Hozak RR, Joshi A, Melemed SA, Benjamin LE, Plowman
GD, Schade AE, et al: Tumor cell expression of vascular endothelial
growth factor receptor 2 is an adverse prognostic factor in
patients with squamous cell carcinoma of the lung. PLoS One.
8:e802922013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dellinger MT, Meadows SM, Wynne K, Cleaver
O and Brekken RA: Vascular endothelial growth factor receptor-2
promotes the development of the lymphatic vasculature. PLoS One.
8:e746862013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fanoni D, Tavecchio S, Recalcati S, Balice
Y, Venegoni L, Fiorani R, Crosti C and Berti E: New monoclonal
antibodies against B-cell antigens: Possible new strategies for
diagnosis of primary cutaneous B-cell lymphomas. Immunol Lett.
134:157–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ilie M, Khambata-Ford S, Copie-Bergman C,
Huang L, Juco J, Hofman V and Hofman P: Use of the 22C3 anti-PD-L1
antibody to determine PD-L1 expression in multiple automated
immunohistochemistry platforms. PLoS One. 12:e01830232017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li H and Pauza CD: CD25(+) Bcl6(low) T
follicular helper cells provide help to maturing B cells in
germinal centers of human tonsil. Eur J Immunol. 45:298–308. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Misawa Y, Misawa K, Kawasaki H, Imai A,
Mochizuki D, Ishikawa R, Endo S, Mima M, Kanazawa T, Iwashita T and
Mineta H: Evaluation of epigenetic inactivation of vascular
endothelial growth factor receptors in head and neck squamous cell
carcinoma. Tumour Biol. 39:10104283177116572017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Solinas C, Garaud S, De Silva P, Boisson
A, Van den Eynden G, de Wind A, Risso P, Vitória Rodrigues J,
Richard F, Migliori E, et al: Immune checkpoint molecules on
tumor-infiltrating lymphocytes and their association with tertiary
lymphoid structures in human breast cancer. Front Immunol.
8:14122017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Talay O, Shen CH, Chen L and Chen J: B7-H1
(PD-L1) on T cells is required for T-cell-mediated conditioning of
dendritic cell maturation. Proc Natl Acad Sci USA. 106:2741–2746.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nam-Cha SH, Roncador G, Sanchez-Verde L,
Montes-Moreno S, Acevedo A, Domínguez-Franjo P and Piris MA: PD-1,
a follicular T-cell marker useful for recognizing nodular
lymphocyte-predominant Hodgkin lymphoma. Am J Surg Pathol.
32:1252–1257. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Harter PN, Bunz B, Dietz K, Hoffmann K,
Meyermann R and Mittelbronn M: Spatio-temporal deleted in
colorectal cancer (DCC) and netrin-1 expression in human foetal
brain development. Neuropathol Appl Neurobiol. 36:623–635. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sharma P, Callahan MK, Bono P, Kim J,
Spiliopoulou P, Calvo E, Pillai RN, Ott PA, de Braud F, Morse M, et
al: Nivolumab monotherapy in recurrent metastatic urothelial
carcinoma (CheckMate 032): A multicentre, open-label, two-stage,
multi-arm, phase 1/2 trial. Lancet Oncol. 17:1590–1598. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Okazaki T and Honjo T: PD-1 and PD-1
ligands: From discovery to clinical application. Int Immunol.
19:813–824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng X, Dai H, Wan N, Moore Y,
Vankayalapati R and Dai Z: Interaction of programmed death-1 and
programmed death-1 ligand-1 contributes to testicular immune
privilege. Transplantation. 87:1778–1786. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang LL, Li ZH, Hu XH, Muyayalo KP, Zhang
YH and Liao AH: The roles of the PD-1/PD-L1 pathway at
immunologically privileged sites. Am J Reprod Immunol. doi:
10.1111/aji.12710.
|
|
44
|
Kaur G, Mital P and Dufour JM:
Testisimmune privilege-Assumptions versus facts. Anim Reprod.
10:3–15. 2013.PubMed/NCBI
|
|
45
|
Zhao S, Zhu W, Xue S and Han D: Testicular
defense systems: immune privilege and innate immunity. Cell Mol
Immunol. 11:428–437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fankhauser CD, Curioni-Fontecedro A,
Allmann V, Beyer J, Tischler V, Sulser T, Moch H and Bode PK:
Frequent PD-L1 expression in testicular germ cell tumors. Br J
Cancer. 113:411–413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang Y, Kang S, Shen J, He J, Jiang L,
Wang W, Guo Z, Peng G, Chen G, He J and Liang W: Prognostic
significance of programmed cell death 1 (PD-1) or PD-1 ligand 1
(PD-L1) expression in epithelial-originated cancer: A
meta-analysis. Medicine (Baltimore). 94:e5152015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bentas W, Beecken WD, Glienke W, Binder J
and Schuldes H: Serum levels of basic fibroblast growth factor
reflect disseminated disease in patients with testicular germ cell
tumors. Urol Res. 30:390–393. 2003.PubMed/NCBI
|
|
49
|
Adam M, Schmidt D, Wardelmann E, Wernert N
and Albers P: Angiogenetic protooncogene ets-1 induced
neovascularization is involved in the metastatic process of
testicular germ cell tumors. Eur Urol. 44:329–336. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Subbiah V, Meric-Bernstam F, Mills GB,
Shaw KR, Bailey AM, Rao P, Ward JF and Pagliaro LC: Next generation
sequencing analysis of platinum refractory advanced germ cell tumor
sensitive to Sunitinib (Sutent®) a
VEGFR2/PDGFRβ/c-kit/FLT3/RET/CSF1R inhibitor in a phase II trial. J
Hematol Oncol. 7:522014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jones A, Fujiyama C, Turner K, Fuggle S,
Cranston D, Turley H, Valtola R, Bicknell R and Harris AL:
Angiogenesis and lymphangiogenesis in stage 1 germ cell tumours of
the testis. BJU Int. 86:80–86. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sakai Y, Hoshino H, Kitazawa R and
Kobayashi M: High endothelial venule-like vessels and lymphocyte
recruitment in testicular seminoma. Andrology. 2:282–289. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Carbognin L, Pilotto S, Nortilli R,
Brunelli M, Nottegar A, Sperduti I, Giannarelli D, Bria E and
Tortora G: Predictive and prognostic role of tumor-infiltrating
lymphocytes for early breast cancer according to disease subtypes:
Sensitivity analysis of randomized trials in adjuvant and
neoadjuvant setting. Oncologist. 21:283–291. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Turksma AW, Coupé VM, Shamier MC, Lam KL,
de Weger VA, Belien JA, van den Eertwegh AJ, Meijer GA, Meijer CJ
and Hooijberg E: Extent and location of tumor-infiltrating
lymphocytes in microsatellite-stable colon cancer predict outcome
to adjuvant active specific immunotherapy. Clin Cancer Res.
22:346–356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yu X, Zhang Z, Wang Z, Wu P, Qiu F and
Huang J: Prognostic and predictive value of tumor-infiltrating
lymphocytes in breast cancer: A systematic review and
meta-analysis. Clin Transl Oncol. 18:497–506. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zeng DQ, Yu YF, Ou QY, Li XY, Zhong RZ,
Xie CM and Hu QG: Prognostic and predictive value of
tumor-infiltrating lymphocytes for clinical therapeutic research in
patients with non-small cell lung cancer. Oncotarget.
7:13765–13781. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Coussens LM, Zitvogel L and Palucka AK:
Neutralizing tumor-promoting chronic inflammation: A magic bullet?
Science. 339:286–291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Schlom J: Therapeutic cancer vaccines:
Current status and moving forward. J Natl Cancer Inst. 104:599–613.
2012. View Article : Google Scholar : PubMed/NCBI
|