Introduction
Ovarian serous carcinoma is a common cause of cancer
deaths in females worldwide (1,2). Patients
are generally diagnosed in an advanced stage of disease and have a
high mortality rate (3). The standard
treatment is maximum debulking surgery and platinum-based
chemotherapy (4). Despite a high
response rate for chemotherapy, the majority of patients will be
resistant to first-line agents and these patients have a
particularly poor prognosis (5).
Platinum agents are key drugs in primary chemotherapy for patients
with ovarian carcinoma, however, there are no clinical biomarkers
that predict platinum sensitivity. At recurrence, the possibility
of response to re-treatment with platinum-based chemotherapy
depends on the platinum-free interval, which is calculated from the
time of last platinum administration to the time of cancer
recurrence (6). If the ovarian
carcinoma recurs within 6 months from the last platinum
administration it is considered to be ‘platinum resistant’, whereas
if recurrence occurs more than 6 months after the last platinum
administration it is considered to be ‘platinum sensitive’
(7). The sensitivity to
platinum-based chemotherapy is an independent prognostic factor for
overall and progression-free survival of patients with ovarian
carcinoma (8). It is difficult to
predict the sensitivity to platinum-based chemotherapy before the
first recurrence therefore ‘platinum-resistant’ patients are
identified retrospectively after recurrence of their cancer or
failure to respond to initial platinum-based chemotherapy.
Understanding the predictors of response to platinum-based
chemotherapy will help us select sensitive patients for
chemotherapy and spare resistant patients from the toxicity of
platinum-based chemotherapy, and will also allow customization of
treatments and clinical stratification of patients with ovarian
carcinoma.
Currently, there are no reliable methods to
determine or predict platinum sensitivity. To improve the prognosis
of platinum-resistant ovarian serous carcinoma, the aim of this
study was to find new biomarkers with prognostic and predictive
potential and search for new therapeutic targets.
Reactive oxygen species (ROS) generated by
chemotherapeutic agents that are able to evade antioxidant defenses
cause cell damage and death (9–11).
Uncoupling proteins (UCPs) are part of the superfamily of
mitochondrial anion transporters (12,13). There
are five known types of UCP (UPC1 to UPC5) with varied
characteristics and different tissue distribution (14). The superoxide from the mitochondrial
inner membrane activates UCP2 and UCP3, and it can reduce ROS
generation (15). UCP2 is broadly
expressed in cancer cells and is able to suppress mitochondrial ROS
production, in turn mitigating oxidative stress (16). Loss of UCP2 function can increase the
production of ROS, while its overexpression may promote
cytoprotection by mitigating oxidative stress (17,18).
Additionally, UCP2 advances carcinogenesis and chemoresistance
(19–21). UCP2 is associated with human colon
carcinogenesis (22,23). Mitochondrial uncoupling by UCP2
induces resistance to gemcitabine in pancreatic cancer cells
(19) and inhibition of UCP2 with
genipin sensitizes cancer cells to chemotherapeutic agents
(19–21). This evidence suggests that UCP2 is a
potential target for cancer treatment with chemotherapeutic agents
that promote oxidative stress. The expression of UCP2 and its
association with sensitivity to platinum-based chemotherapy for
ovarian serous carcinoma was investigated in this study.
Materials and methods
Patients and samples
The present study included 54 patients with ovarian
serous carcinoma (FIGO stages III and IV). All patients were
treated at Osaka City University Hospital (Osaka, Japan) from
January 2005 to December 2012. Patients were divided into two
groups based on recurrence of cancer within 6 months from the last
platinum administration. Both groups underwent maximum debulking
surgery followed by platinum-based chemotherapy. In the first group
(platinum-sensitive group), disease did not recur within 6 months
from the last platinum administration whereas in the second group
(platinum resistant group), disease recurred within 6 months.
Written informed consent was obtained from each patients prior to
surgery. The study proposal was approved by the Institutional
Review Board (IRB) of Osaka City University Hospital (IRB no.
3525).
Immunohistochemical staining
Immunohistochemical analysis was performed to
examine UCP2 expression in paraffin-embedded sections using an
anti-UCP2 antibody (cat. no. ab116263; Abcam, Cambridge, UK) and a
Dako LSAB2 Peroxidase kit (cat. no. K0675; Agilent Technologies,
Inc., Santa Clara, CA, USA). The paraffin-embedded sections (4
µm-thick) were de-paraffinized, hydrated, and immersed in 3%
hydrogen peroxide for 10 min at room temperature to block
endogenous peroxidase activity. For antigen retrieval, sections
were immersed in 10 mM citrate buffer (pH 6.0) and heated in an
autoclave at 110°C for 20 min. Then tissue sections were washed in
PBS and incubated overnight at 4°C with a 1:100 dilution of the
aforementioned rabbit polyclonal antibody for UCP2. Next, sections
were washed in PBS for 15 min and incubated with biotinylated goat
anti-rabbit immunoglobulin G (Dako; Agilent Technologies, Inc.) for
10 min. After washed with PBS, sections were incubated with a
streptavidin-peroxidase solution and 3,3′-diaminobenzidine was used
as the chromogenic reagent. Finally, sections were counterstained
with hematoxylin. The specificity of the immunohistochemical
reactions was confirmed by omitting the primary antibody.
The immunohistochemical expression of UCP2 were
assessed quantitatively according to the weighted score method of
Sinicrope et al (24).
Staining intensity was categorized into three classes: 1+, weak;
2+, moderate; and 3+, intense. The mean percentage of stained tumor
cells was classified as follows: 0, ≤5%; 1, 5< and <25%; 2,
25< and <50%; 3, 50< and <75%; 4, >75%. The weighted
score was determined by multiplying the score of staining intensity
for each tissue specimen by that of percentage of stained tumor
cells.
Cell culture
The human ovarian serous carcinoma cell line OVSAHO
(no. JCRB1046; National Institutes of Biomedical Innovation, Health
and Nutrition, Osaka, Japan) was cultured in RPMI medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.). Cells
were cultured in a humidified 5% CO2 atomosphere and at
37°C.
Chemosensitivity assay
The sensitivity of cells to carboplatin was examined
using a Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies,
Inc., Kumamoto, Japan). Cells were collected and seeded into a
96-well tissue culture plate at a density of approximately
2×103 cells/ml. After 24 h the culture medium was
replaced with 100 µl of fresh medium per well and 10 µl dimethyl
sulfoxide (DMSO) alone or containing 50 µM genipin (cat. no.
G-4796; Sigma Aldrich, Missouri, USA) was added to each well. Cells
were then treated with carboplatin (10–1,000 µM) for 24 h. At the
end of treatment 10 µl CCK-8 was added and the plates were
incubated for 2 h before measurement of the absorbance at 450 nm
with a microplate reader (Corona Electric Co., Ltd., Ibaraki,
Japan). Dose-response graphs were constructed as the percentage of
viable cells compared with the control cells.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction
Total RNA was extracted from the human ovarian
serous carcinoma cell line OVSAHO using a RNeasy Mini kit (Qiagen
GmbH, Hilden, Germany) and reverse transcribed into cDNA using a
High Capacity cDNA Reverse Transcription Kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Gene expression of UCP2 was
determined using a TaqMan Gene Expression Assay (Applied
Biosystems; Thermo Fisher Scientific, Inc.) in the Applied
Biosystems 7500 Fast Real-Time PCR System. For quantification, gene
expression was normalized to that of GAPDH.
Statistical analysis
Statistical analyses were performed using SPSS
software version 21.0 (IBM SPSS, Armonk, NY, USA). Data are
presented as the mean ± standard error in Figures and as the mean ±
standard deviation in Tables. Kaplan-Meier and log-rank analyses
were performed to assess prognosis. Mann-Whitney U test was
performed to compare the weighted scores. The differences between
the means of two groups were assessed using Student's t-test, and
associations of the categorical variables in two groups were
assessed using χ2 tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
A total of 54 patients with ovarian serous carcinoma
were divided into the platinum-sensitive group (n=27) and the
platinum-resistant group (n=27). Table
I shows age, FIGO stage, tumor marker, and post-surgery
observations for the study patients. There were no significant
differences in these parameters between the two groups other than
postoperative residual disease.
 | Table I.Characteristics of patients in the
platinum-sensitive and -resistant groups. |
Table I.
Characteristics of patients in the
platinum-sensitive and -resistant groups.
| Characteristics | Platinum sensitive
(n) | Platinum resistant
(n) | P-value |
|---|
| No. of patients | 27 | 27 |
|
| Age (years) |
|
| 0.725a |
| Mean ±
SD | 61.0±12.2 | 60.0±10.0 |
|
| FIGO stage |
|
| 0.277b |
| IIIA | 1 | 0 |
|
| IIIB | 3 | 1 |
|
| IIIC | 21 | 19 |
|
| IVA | 1 | 4 |
|
| IVB | 1 | 3 |
|
| Tumor marker |
|
| 0.374a |
| CA125,
U/ml (mean) | 3,343.3 | 2,180.1 |
|
| Postoperative
residual disease |
|
| 0.004b |
| None | 5 | 0 |
|
| <1
cm | 10 | 4 |
|
| >1
cm | 12 | 23 |
UCP2 expression in ovarian serous
carcinoma tissue
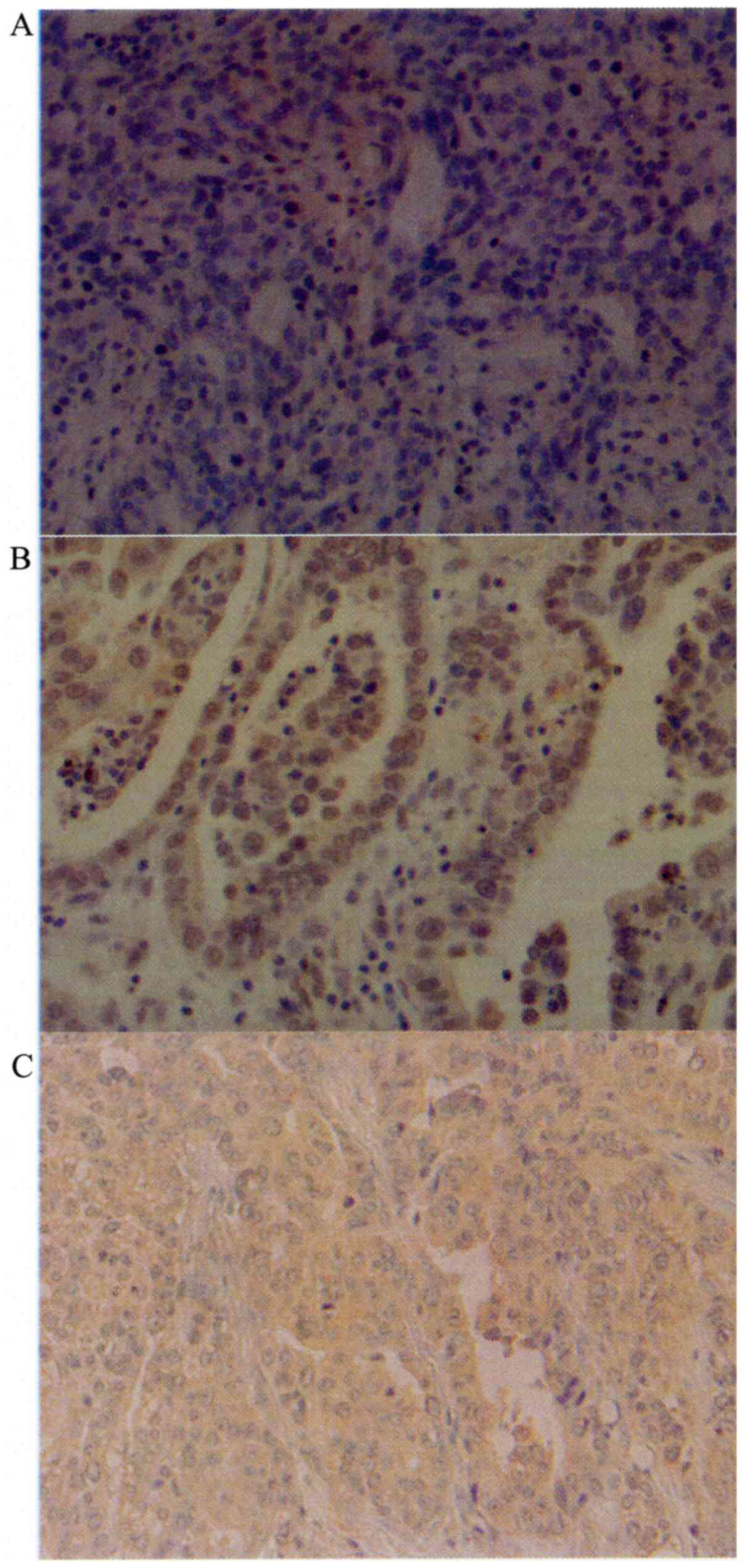
Cytoplasmic expression of UCP2 was observed in tumor
cells (Fig. 1). Table II shows the UCP2 weighted scores in
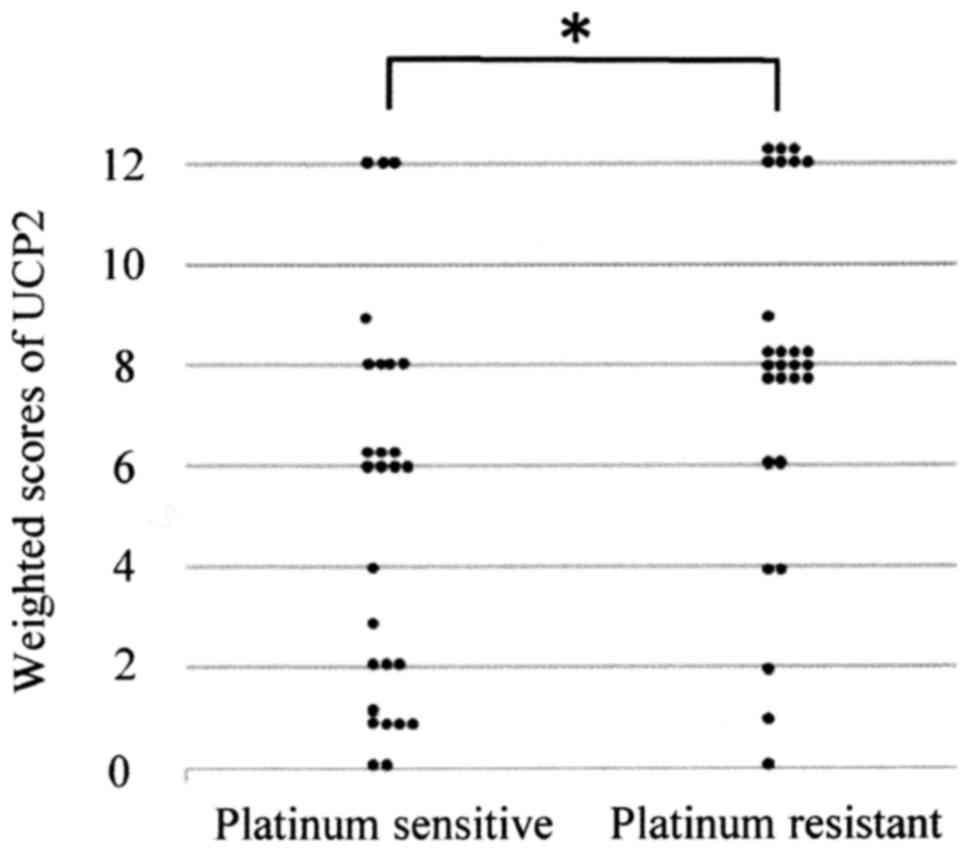
tissues of the two patient groups. The mean weighted score for UCP2
expression was significantly lower in the platinum-sensitive group
compared with the platinum-resistant group (5.1 and 7.9,
respectively, P=0.005; Table II and
Fig. 2).
 | Table II.Weighted scores for uncoupling protein
2 expression in the platinum-sensitive and -resistant groups. |
Table II.
Weighted scores for uncoupling protein
2 expression in the platinum-sensitive and -resistant groups.
|
| No. of patients |
|---|
|
|
|
|---|
| Weighted score | Platinum
sensitive | Platinum
resistant |
|---|
| 0 | 2 | 1 |
| 1 | 5 | 1 |
| 2 | 3 | 1 |
| 3 | 1 | 0 |
| 4 | 1 | 2 |
| 6 | 7 | 2 |
| 8 | 4 | 12 |
| 9 | 1 | 1 |
| 12 | 3 | 7 |
| Total | 27 | 27 |
| Mean |
5.1 |
7.9 |
In continuation, cases were classified into two
groups according to their UCP2 expression levels: the low UCP2
expression group (weighted score, 0–6) and the high UCP2 expression
group (weighted score, 8–12). Table
III shows the characteristics of the high and low expression
groups, with analyses revealing no significant differences between
the two groups other than postoperative residual disease.
 | Table III.Characteristics of patients in the
low and high UCP2 expression groups. |
Table III.
Characteristics of patients in the
low and high UCP2 expression groups.
|
| No. of
patients |
|
|---|
|
|
|
|
|---|
|
Characteristics | Low UCP2 expression
(score ≤6) | High UCP2
expression (score ≥8) | P-value |
|---|
| No. of
patients | 26 | 28 |
|
| Age (years) |
|
| 0.610a |
| Mean ±
SD | 61.3±11.1 | 59.8±11.2 |
|
| FIGO stage |
|
| 0.694b |
|
IIIA | 0 | 1 |
|
|
IIIB | 3 | 1 |
|
|
IIIC | 19 | 21 |
|
|
IVA | 2 | 3 |
|
|
IVB | 2 | 2 |
|
| Tumor marker |
|
| 0.566a |
| CA125,
U/ml (mean) | 3,156.7 | 2,395.0 |
|
| Tumor size
(mm) |
|
| 0.144a |
| Mean ±
SD | 46.9±17.2 | 53.7±14.9 |
|
| Postoperative
residual disease |
|
| 0.005b |
|
None | 2 | 3 |
|
| <1
cm | 12 | 2 |
|
| >1
cm | 12 | 23 |
|
Correlation of platinum sensitivity
with UCP2 expression
Within the low UCP2 expression group, 19 cases
(73.1%) belonged to the platinum-sensitive group while 7 (26.9%)
belonged to the platinum-resistant group. In the high UCP2
expression group, 8 cases (28.6%) belonged to the
platinum-sensitive group and 20 (71.4%) belonged to the
platinum-resistant group. The low UCP2 expression group was
significantly more sensitive to platinum-based chemotherapy than
the high UCP2 expression group (P=0.001; Table IV).
 | Table IV.Number of patients with low and high
uncoupling protein 2 expression in the platinum-sensitive and
-resistant groups. |
Table IV.
Number of patients with low and high
uncoupling protein 2 expression in the platinum-sensitive and
-resistant groups.
| UCP2
expression | Platinum sensitive,
number (%) | Platinum resistant,
number (%) | P-value |
|---|
| Low expression
(score ≤6) | 19 (73.1) | 7 (26.9) | 0.001a |
| High expression
(score ≥8) | 8 (28.6) | 20 (71.4) |
|
Survival
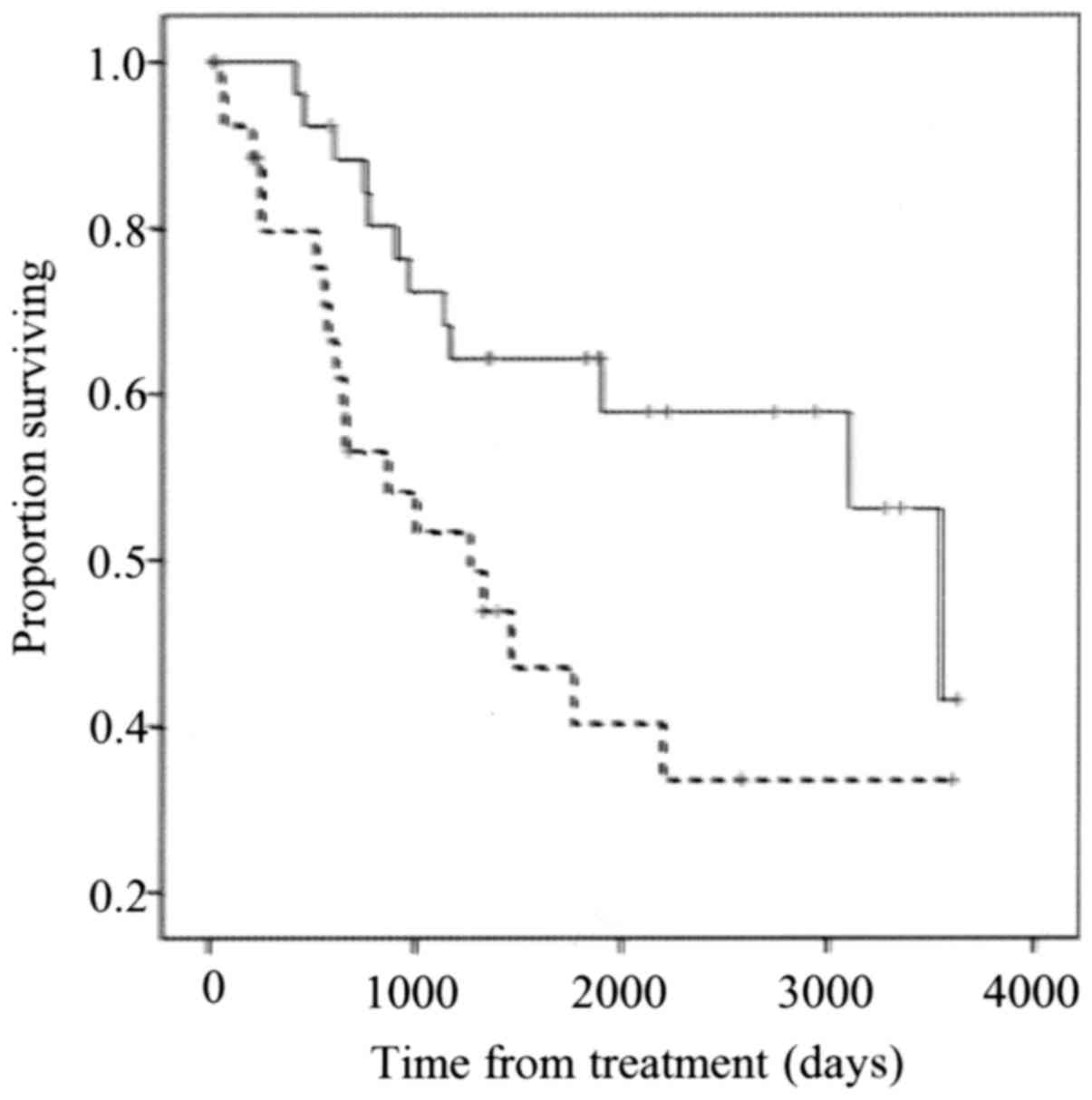
The low UCP2 expression group showed significantly
better overall survival compared with the high UCP2 expression
group (P=0.006; Fig. 3).
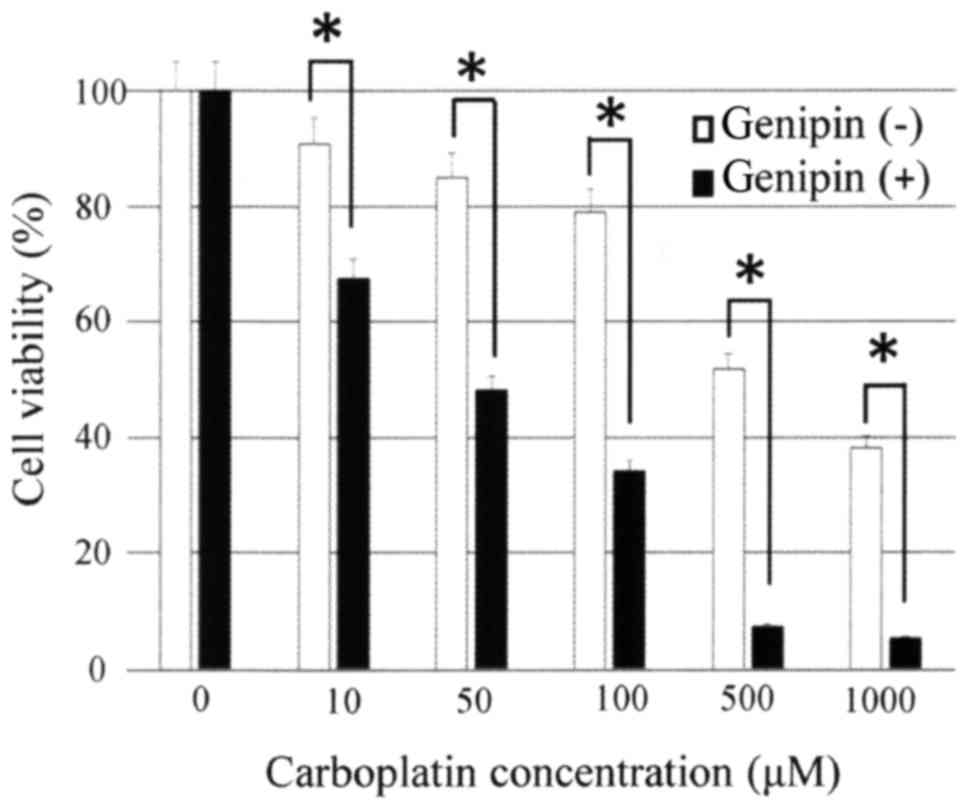
Inhibition of UCP2 by genipin enhances
the sensitivity of ovarian carcinoma cells to carboplatin
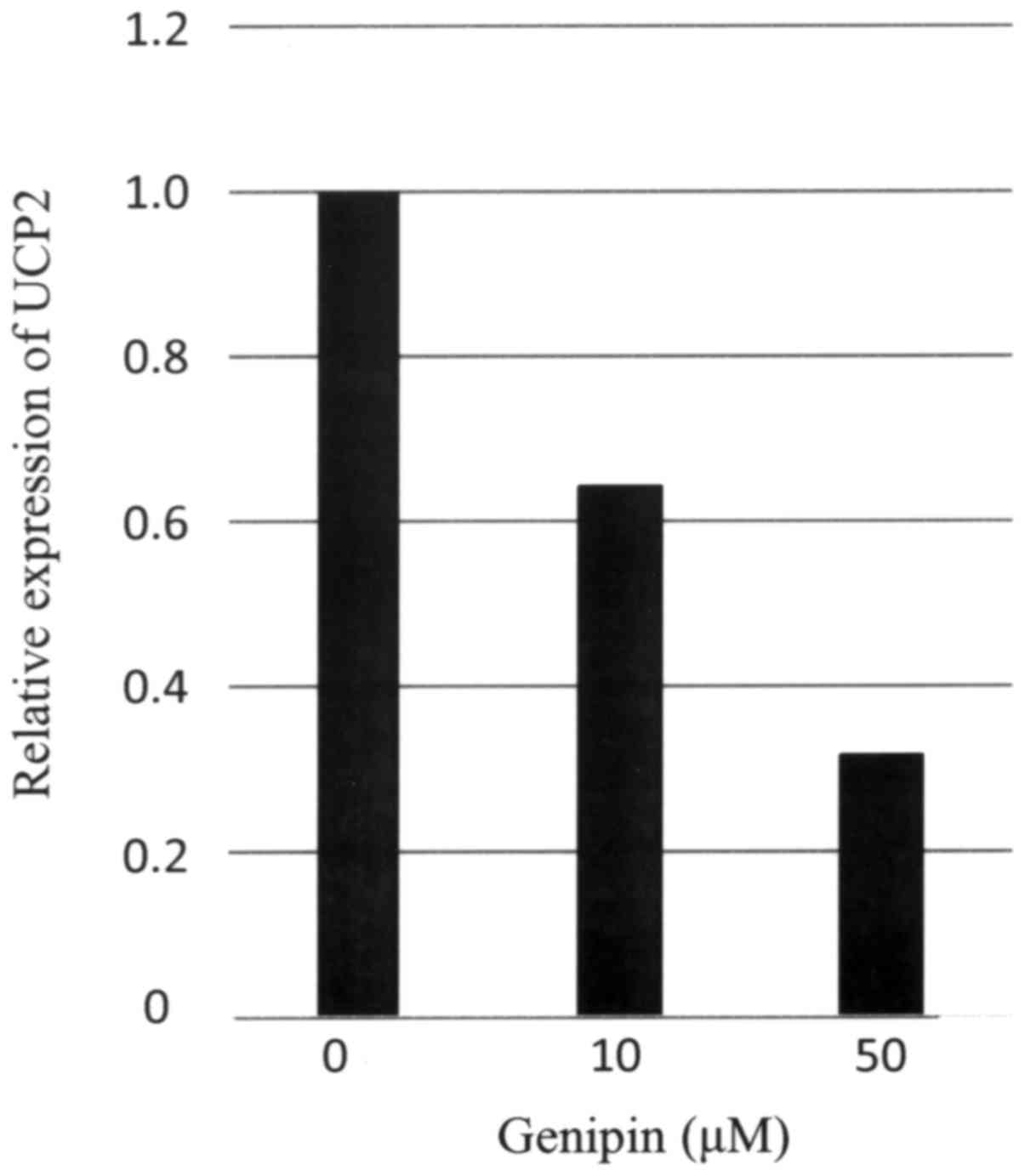
Expression of UCP2 mRNA in the ovarian serous
carcinoma cell line OVSAHO was confirmed by real-time PCR.
UCP2 expression in OVSAHO cells was suppressed following 24
h of incubation with 10 or 50 µM genipin (Fig. 4). Then, we examined whether the
sensitivity of ovarian serous carcinoma cells to carboplatin was
affected by treatment with genipin. Genipin-mediated inhibition of
UCP2 expression in OVSAHO cells significantly enhanced their
sensitivity to carboplatin (Fig.
5).
Discussion
UCP2 is widely expressed in cancer cells, and the
expression of UCP2 is linked with ROS levels in various types of
tissue (19,20). ROS production in cancer cells is
inhibited through the expression of UCP2; therefore, high
expression of UCP2 can protect cells from oxidative stresses and
cell damage (22,23). UCP2 enhances both chemoresistance and
carcinogenesis, and downregulation of UCP2 leads to increased cell
death due to chemotherapy (25,26).
This study shows a significant correlation between
UCP2 expression and platinum sensitivity in patients with ovarian
serous carcinoma. Patients with low UCP2 expression tended to be
sensitive to platinum-based chemotherapy, and low UCP2 expression
group showed significantly longer overall survival time than the
high UCP2 expression group.
The present study demonstrated that the
proliferation of OVSAHO cells was attenuated by the addition of
genipin following administration of platinum agent. This is
consistent with former reports using other cancer cells (19,25,26).
Moreover, these findings indicate that genipin can sensitize cancer
cells to chemotherapeutic agents. The clinical application of
genipin as a potential drug-sensitizing agent warrants further
study.
These results suggest that UCP2 expression levels in
patients with ovarian serous carcinoma are associated with the
effectiveness of platinum-based chemotherapy. Therefore, UCP2
stands for a potential predictive marker of whether platinum based
chemotherapy is likely to be effective in patients with ovarian
serous carcinoma. Understanding the predictors of response to
platinum-based chemotherapy can help us select patients sensitive
to chemotherapy while sparing resistant patients from unnecessary
toxicity of platinum-based chemotherapy, and also allows
customization of treatments and clinical stratification of patients
with ovarian cancer.
In summary, UCP2 expression may be a predictive
marker of the efficacy of platinum-based chemotherapy in patients
with ovarian serous carcinoma. The present study is the first to
demonstrate a correlation between UCP2 expression and platinum
sensitivity. This knowledge can be great help to improve the
prognosis of patients with ovarian serous carcinoma. We are
planning further investigation regarding molecular mechanism.
Acknowledgements
The authors would like to thank Dr Mary Derry for
editing a draft of this manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Borley J, Wilhelm-Benartzi C, Brown R and
Ghaem-Maghami S: Does tumour biology determine surgical success in
the treatment of epithelial ovarian cancer? A systematic literature
review. Br J Cancer. 107:1069–1074. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
du Bois A, Quinn M, Thigpen T, Vermorken
J, Avall-Lundqvist E, Bookman M, Bowtell D, Brady M, Casado A,
Cervantes A, et al: 2004 consensus statements on the management of
ovarian cancer: Final document of the 3rd international gynecologic
cancer intergroup ovarian cancer consensus conference (GCIG OCCC
2004). Ann Oncol. 16:VII7–VI12. 2005. View Article : Google Scholar
|
|
4
|
Japan society of gynecologic oncology:
Formulation committee of the treatment guidelines for ovarian.
https://jsgo.or.jp/guideline/ransou2015.htmlSeptember
1–2017
|
|
5
|
Ozols RF, Bundy BN, Greer BE, Fowler JM,
Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM
and Baergen R: Gynecologic Oncology Group: Phase III trial of
carboplatin and paclitaxel compared with cisplatin and paclitaxel
in patients with optimally resected stage III ovarian cancer: A
gynecologic oncology group study. J Clin Oncol. 21:3194–3200. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Friedlander M, Trimble E, Tinker A,
Alberts D, Avall-Lundqvist E, Brady M, Harter P, Pignata S,
Pujade-Lauraine E, Sehouli J, et al: Int J Gynecol Cancer.
21:771–775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Markman M, Rothman R, Hakes T, Reichman B,
Hoskins W, Rubin S, Jones W, Almadrones L and Lewis JL Jr: J Clin
Oncol. 9:389–393. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kyrgiou M, Salanti G, Pavlidis N,
Paraskevaidis E and Ioannidis JP: Survival benefits with diverse
chemotherapy regimens for ovarian cancer: Meta-analysis of multiple
treatments. J Natl Cancer Inst. 98:1655–1663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pelicano H, Carney D and Huang P: ROS
stress in cancer cells and therapeutic implications. Drug Resist
Updat. 7:97–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alexandre J, Batteux F, Nicco C, Chéreau
C, Laurent A, Guillevin L, Weill B and Goldwasser F: Accumulation
of hydrogen peroxide is an early and crucial step for
paclitaxel-induced cancer cell death both in vitro and in vivo. Int
J Cancer. 119:41–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fruehauf J and Meyskens FL Jr: Reactive
oxygen species: A breath of life or death? Clin Cancer Res.
13:789–794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boss O, Muzzin P and Giacobino JP: The
uncoupling proteins, a review. Eur J Endocrinol. 139:1–9. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fleury C and Sanchis D: The mitochondrial
uncoupling protein-2: Current status. Int J Biochem Cell Biol.
31:1261–1278. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baffy G: Uncoupling protein-2 and cancer.
Mitochondrion. 10:243–252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Echtay KS, Murphy MP, Smith RA, Talbot DA
and Brand MD: Superoxide activates mitochondrial uncoupling protein
2 from the matrix side. Studies using targeted antioxidants. J Biol
Chem. 277:47129–47135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duval C, Nègre-Salvayre A, Dogilo A,
Salvayre R, Pénicaud L and Casteilla L: Increased reactive oxygen
species production with antisense oligonucleotides directed against
uncoupling protein 2 in murine endothelial cells. Biochem Cell
Biol. 80:757–764. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mattiasson G, Shamloo M, Gido G, Mathi K,
Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T,
Gonzalez-Zulueta M, et al: Uncoupling protein-2 prevents neuronal
death and diminishes brain dysfunction after stroke and brain
trauma. Nat Med. 9:1062–1068. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teshima Y, Akao M, Jones SP and Marban E:
Uncoupling protein-2 overexpression inhibits mitochondrial death
pathway in cardiomyocytes. Circ Res. 93:192–200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horimoto M, Resnick MB, Konkin TA,
Routhier J, Wands JR and Baffy G: Expression of uncoupling
protein-2 in human colon cancer. Clin Cancer Res. 10:6203–6207.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carretero MV, Torres L, Latasa U,
Garcia-Trevijano ER, Prieto J, Mato JM and Avila MA: Transformed
but not normal hepatocytes express UCP2. FEBS Lett. 439:55–58.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Imai K, Fukuda T, Wada T, Kawanishi M,
Tasaka R, Yasui T and Sumi T: UCP2 expression may represent a
predictive marker of neoadjuvant chemotherapy effectiveness for
locally advanced uterine cervical cancer. Oncol Lett. 14:951–957.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Derdák Z, Fülöp P, Sabo E, Tavares R,
Berthiaume EP, Resnick MB, Paragh G, Wands JR and Baffy G: Enhanced
colon tumor induction in uncoupling protein-2 deficient mice is
associated with NF-kappaB activation and oxidative stress.
Carcinogenesis. 27:956–961. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Collins P, Jones C, Choudhury S, Damelin L
and Hodgson H: Increased expression of uncoupling protein 2 in
HepG2 cells attenuates oxidative damage and apoptosis. Liver Int.
25:880–887. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sinicrope FA, Ruan SB, Cleary KR, Stephens
LC, Lee JJ and Levin B: Bcl-2 and p53 oncoprotein expression during
colorectal tumorigenesis. Cancer Res. 55:237–241. 1995.PubMed/NCBI
|
|
25
|
Pozza Dalla E, Fiorini C, Dando I,
Menegazzi M, Sgarbossa A, Costanzo C, Palmieri M and Donadelli M:
Role of mitochondrial uncoupling protein 2 in cancer cell
resistance to gemcitabine. Biochim Biophys Acta. 1823:1856–1863.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mailloux RJ, Adjeitey CN and Harper ME:
Genipin-induced inhibition of uncoupling protein-2 sensitizes
drug-resistant cancer cells to cytotoxic agents. PLoS One.
5:e132892010. View Article : Google Scholar : PubMed/NCBI
|