Introduction
Retinoic acid (RA), an active metabolite of vitamin
A, is a critical signaling molecule that is involved in the
differentiation, proliferation and apoptosis of a wide variety of
cell types (1). While essentially all
cell types express nuclear RA receptors (RA nuclear receptors and
retinoid-X-receptors) and can potentially respond to RA to
positively or negatively regulate expression of RA target genes.
Disruption of retinoid signaling through mutations in these nuclear
receptors has been found in certain types of tumor cells (1). In addition, insufficient RA signaling
such as in vitamin A deficiency (VAD) has been linked to increased
susceptibility to carcinogenesis (2–8). It is
generally believed that cellular responsiveness is primarily
determined by intracellular RA levels, rather than by the serum
concentration of RA (2). The
availability of RA for the cell is regulated by a coordinated
balance between vitamin A nutritional status, and RA biosynthesis
and catabolism. Thus, it is hypothesized that the concept of
‘cellular RA bioavailability’ is critical to understand the
pathogenesis of diseases that are caused by RA insufficiency
(1,2).
To examine the impact of RA depletion, a state of RA
deficiency was induced by RA metabolism in cells. Previous studies
demonstrated that the expression levels of the RA-metabolizing
enzyme cytochrome P450 26A1 (CYP26A1) were elevated in various
tumor types, while reduced cellular RA bioavailability caused by
CYP26A1 expression served a stimulatory oncogenic effect on
carcinogenesis (2). Additionally, it
was demonstrated that the cells expressing CYP26A1 gain significant
resistance to differently acting apoptogenic factors due to the
state of RA insufficiency caused by metabolic inactivation of RA
(1). Several studies have also
demonstrated that various metabolites of vitamin A are present in
cancer patients, as well as that RA metabolism and CYP26A1
expression levels are enhanced in various types of cancer (1–7). These
observations are consistent with accumulating evidence suggesting
the association of VAD with increased susceptibility to
carcinogenesis and elevated risk for a number of human cancer types
(8).
The relevance of elevated CYP26A1 expression in
human cancer has yet to be elucidated in vivo. In order to
address the oncogenic role of CYP26A1, transgenic mice that
ubiquitously overexpressed CYP26A1 under the control of the
cytomegalovirus (CMV) promoter were generated in the present study.
A two-stage skin carcinogenesis mouse model was employed, using
7,12-dimethylbenz[a]anthracene (DMBA) as a carcinogen and
12-O-tetradecanoylphorbol-13-acetate (TPA) as a chemical
tumor promoter, which is a widely used model in the study of
epithelial carcinogenesis (9,10). In the present study, it was observed
that enhanced expression of CYP26A1 increased the susceptibility of
skin carcinogenesis initiated by DMBA.
Materials and methods
Mouse room conditions
Animals were kept in the environmental conditions to
reduce mouse stress. Mice were maintained in a 12/12 h light/dark
cycle to minimize noises, vibrations and odors. Technicians in the
animal facility did not enter the mouse room during the dark cycle.
The animals were kept in temperatures of 22–24°C with 40–50%
humidity. Food and clean water were freely accessible at all times.
Transgenic CYP26A1 mice were maintained for up to 15 months to
observe phenotypes in the natural course, for example, in the
absence of DMBA/TPA treatment. The maintenance and handling of
animals were conducted using protocols approved by the Animal Care
Committee of Sapporo Medical University School of Medicine
(approval no. #12-040).
Generation of CYP26A1 transgenic
mice
Generation of CYP26A1 transgenic mice was outsourced
to a commercial company (Oriental Yeast Co., Ltd., Kyoto, Japan).
Briefly, the mice were generated by microinjection of a linearized
plasmid into the pronucleus of a single-cell embryo isolated for
super-ovulated JAX C57BL/6J mice (Charles River Laboratories Japan,
Inc., Yokohama, Japan), according to the manufacturer's protocol.
The plasmid used was a green fluorescent protein (GFP)-tagged open
reading frame clone of Mus musculus CYP26A1 (NM_007811) in a
GFP expression vector under control of the CMV promoter (OriGene
Technologies, Inc., Rockville, MD, USA). Embryos were implanted
into pseudopregnant female: CD-1 ICR mice (Charles River
Laboratories Japan, Inc.) and allowed to develop to term. A total
of 5 transgenic founder mice were finally identified by evaluating
tail DNA samples. Founder mice were back-crossed with JAX C57BL/6J
mice for successive generations and their offspring were used in
further experiments.
Genotyping of transgenic mice
Genotyping of transgenic animals was performed by
polymerase chain reaction (PCR) analysis of tail DNA. The DNA was
incubated with 2X GoTaq Green Master Mix (Promega Corp.,
Madison, WI, USA) to amplify the mouse CYP26A1 and
GFP sequences in the genomic DNA, using two independent
primer sets to confirm the genomic integration of introduced
cassettes. The PCR primers used were as follows: CYP26A1 primer set
1, 5′-TTCGGGTTGCTCTGAAGACT-3′ (forward) and
5′-TCCTCCAAATGGAATGAAGC-3′ (reverse); CYP26A1 primer set 2,
5′-CGGTTCAGCTTCATTCCATT-3′ (forward) and 5′-CAGTGGGGCTTGTCTTCATT-3′
(reverse); GFP primer set 1, 5′-TGATGGGCTACGGCTTCTAC-3′ (forward)
and 5′-GTGATGGGCTACGGCTTCTA-3′ (reverse); and GFP primer set 2,
5′-GCTGCCATCCAGATCGTTAT-3′ (forward) and 5′-CTTGAAGTGCATGTGGCTGT-3′
(reverse). The cycling conditions were as follows: Preheating at
96°C for 3 min, 40 cycles of 30 sec at 96°C, 30 sec at 58°C and 3
min at 72°C, followed by a final elongation of 7 min at 72°C.
Constitutive CYP26A1 mRNA expression
examination
To examine the sufficient amount of constitutive
CYP26A1 mRNA expression in the target tissues, reverse
transcription-quantitative PCR (RT-qPCR) analysis was performed as
described previously (2). Briefly,
total RNA was extracted using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) from the mouse tail
samples 3 weeks after birth, and reverse transcription was
subsequently performed using the Superscript II Reverse
Transcriptase kit (Invitrogen; Thermo Fisher Scientific, Inc.).
Samples were incubated at 42°C for 50 min, and the cDNA was then
incubated with 2X GoTaq Green Master Mix (Promega Corp.) and
the appropriate primers (CYP26A1 primer sets 1 and 2, as well as
GFP primer sets 1 and 2) to amplify the genes of interest. The
cycling conditions were as follows: 20–40 cycles of 30 sec at 96°C,
30 sec at 58°C and 1 min at 72°C, followed by a final elongation of
7 min at 72°C. The expression of each gene of interest was analyzed
using cycling parameters that were optimized previously for the
detection of linearity, allowing for semiquantitative analysis of
signal intensities, as described previously (2). PCR experiments were repeated in
triplicate independent reactions to ensure elevated expression of
CYP26A1 in mice.
Skin carcinogenesis model
The chemical carcinogen DMBA (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was dissolved in acetone at a
concentration of 5 mg/ml. The chemical tumor promoter TPA
(Sigma-Aldrich; Merck KGaA) was dissolved in ethanol at a
concentration of 1 mg/ml.
In order to establish the two-stage skin cancer
model, transgenic male (n=17) and female mice (n=11) from five
different founder lines, as well as control mice (JAX C57BL/6J mice
purchased from Charles River Laboratories Japan, Inc.; male, n=5;
female, n=4), received a single application of 100 µg DMBA or
vehicle (20 µl acetone) only by subcutaneous injection in the
dorsal skin at 8–9 weeks of age. Subsequently, the mice were
administered 1 µg TPA at 1 week after the single initiation by
DMBA. Mice received TPA topically by subcutaneous injection in the
dorsal skin twice a week within 20 weeks after administration of
DMBA. In the second experiment to verify the observations of the
first experiment, the same experiment was repeated according to the
same protocol, using transgenic male (n=11) and female mice (n=14),
as well as control mice (male, n=4; female, n=4).
In addition, a one-stage skin cancer model was
further established in the present study. Briefly, transgenic male
(n=9) and female mice (n=11) from the five founder lines, as well
as control mice (JAX C57BL/6J mice purchased form Charles River
Laboratories Japan, Inc.; male, n=2; female, n=2) were administered
100 µg DMBA in the dorsal skin without subsequent treatment with
TPA. In the second line of experiment to verify our observations in
the first experiment that was similar design in the two-stage skin
cancer model, we separately repeated same experiment in a same
protocol, using transgenic male (n=11) and female mice (n=14), as
well as control mice (male, n=2; female, n=2).
Examination
The humane endpoint of the present study was to
observe the tumor formation and development within 20 weeks after
the initiation with DMBA. This is a standard observation period
reported to be required for squamous neoplasia such as papilloma
formation in a two-stage skin carcinogenesis model in mice, and to
be appropriate by taking action in escaping from the potential pain
and distress (9–11).
Potential pain and distress may be intrinsic in the
experimental animals since 2 different types of carcinogenesis
model were employed. Even in these cases, however, efforts were
made to avoid and minimize potential pain and distress of animals.
The mice were examined twice a week by palpitation in order to
determine skin tumor formation (e.g., tumor size and its number)
within 20 weeks after administration of the carcinogen DMBA.
Following the final dose of TPA, animals were sacrificed at 20
weeks after DMBA treatment, or indicated time points in the course
of the tumor formation experiments, to evaluate tumors
histologically. Tumors exceeding a maximum diameter >1 mm were
designated as tumor formation in the protocol and measured weekly.
Mice were euthanized if skin ulceration of the primary tumor
occurred. Formalin-fixed, paraffin-embedded tissue specimens were
prepared and stained with hematoxylin and eosin according to a
standard protocol, as described previously (2,5,6). The histology of all tumors was examined
under a light microscope (Olympus, Tokyo, Japan) independently by a
number of trained surgical pathologists, who were officially
certified by Japanese Society of Pathology.
Statistical analyses were not conducted during the
course of the present study because the goal was to demonstrate the
differences in histology of tumor and tumor growth (e.g., onset of
tumor formation and development) that were observed in control and
CYP26A1 transgenic mice.
Results
Generation of CYP26A1 transgenic
mice
The results of our previous study suggested that
enhanced CYP26A1 expression may potentially lead to neoplastic
transformation of keratinocytes during skin carcinogenesis
(5). Thus, the present study assessed
the oncogenic role of CYP26A1 by examining the effect of
CYP26A1 overexpression in the skin tissues of transgenic mice.
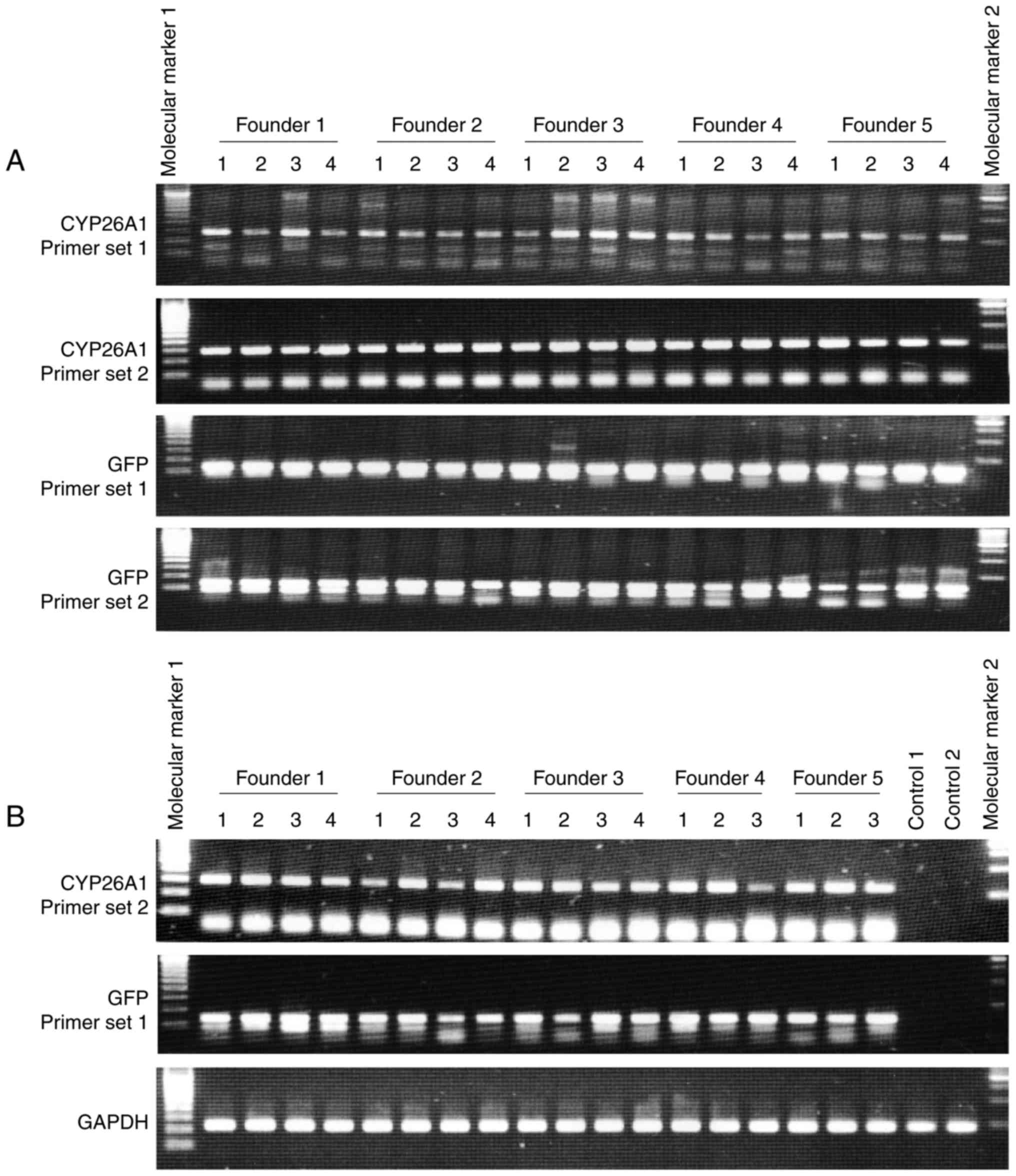
Genomic PCR analyses revealed genomic integration of
the introduced cDNA by detecting sequences of the CYP26A1
and reporter gene GFP in skin tissues of offspring mice
(Fig. 1A). There was no positive
control sample because, to the best of our knowledge, this is the
first report to establish CYP26A1 transgenic animals. In addition,
elevated CYP26A1 expression was observed in skin tissues
from different founder lines (Fig.
1B). The expression of CYP26A1 mRNA varied in the
animals; however, mice were not selected for the subsequent
experiments according to the levels of CYP26A1 expression,
in order to exclude possible selection bias for a specific
phenotype. It has been previously reported that constitutive
expression of CYP26A1 was weak but present in basal keratinocytes
in human skin (5). However, in the
present study, CYP26A1 was not detectable in control animals by
RT-qPCR analysis using skin tissue samples (Fig. 1B).
Lack of spontaneous tumor formation in
CYP26A1 transgenic mice
CYP26A1 transgenic animals displayed no evident
abnormalities for ≤15 months, while male and female mice were
viable and fertile. In addition, no macroscopic tumors were
detected by palpitation every month. Furthermore, histological
evaluations of various organs, including the skin, mammary glands,
head and neck tissues, lung, gastrointestinal tract, liver and
central nervous system, of the transgenic mice also presented no
evidence of abnormalities, such as hyperplasia, dysplasia and
neoplasia, or active inflammation (data not shown).
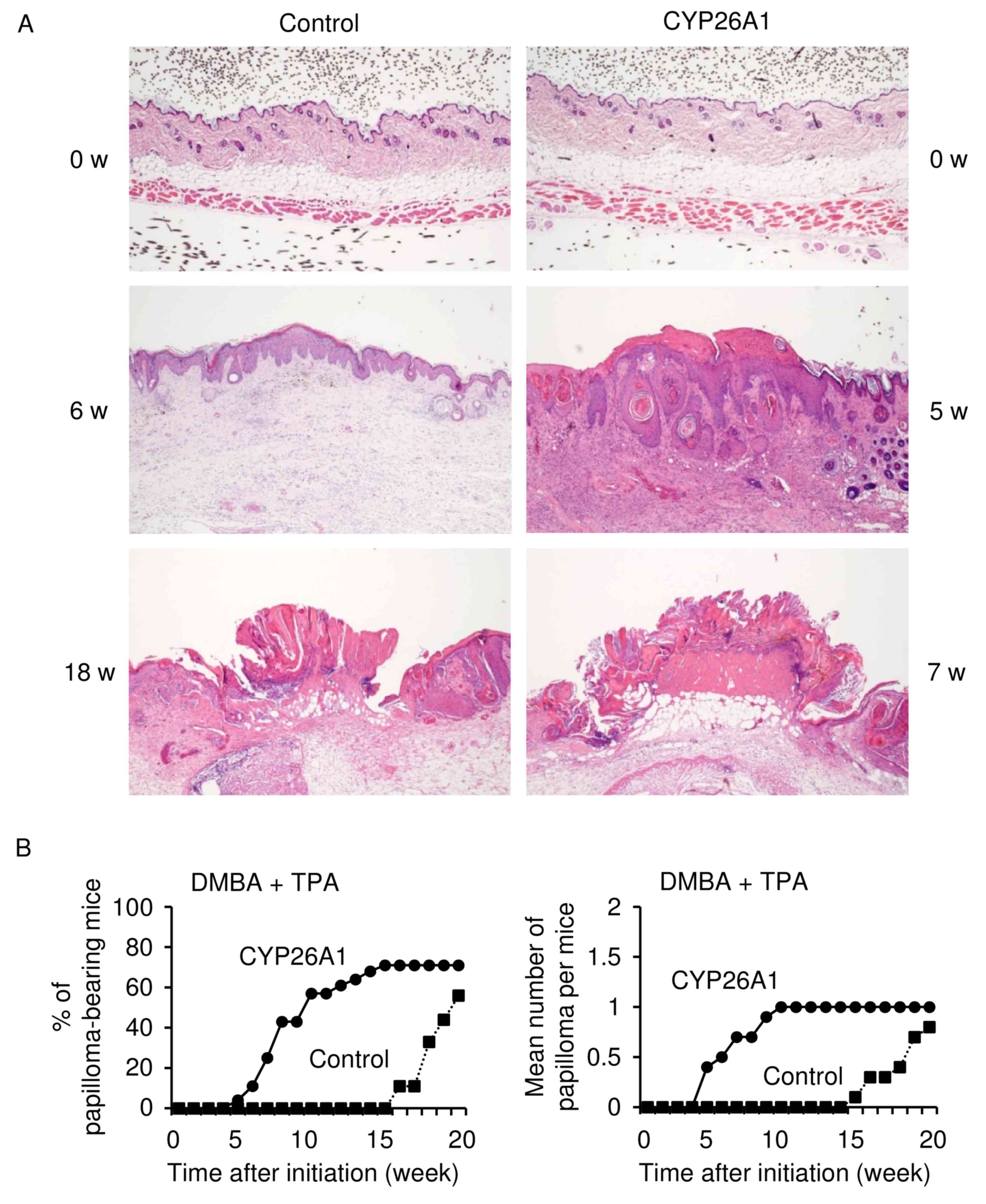
Two-stage skin cancer model
A two-stage skin cancer model was used in the
present study to examine the role of CYP26A1 in enhancing tumor
formation and development in combination with exposure to a
carcinogenic agent (Fig. 2). The
chemical carcinogen DMBA was administered to CYP26A1 transgenic
mice, followed by treatment with the chemical tumor promoter TPA.
The histology of skin tissues in the control and CYP26A1 transgenic
mice (8–9 weeks of age) was normal before the treatment (Fig. 2A). By contrast, the skin treated by
DMBA and TPA in control and CYP26A1 transgenic animals demonstrated
a varying degree of hyperplastic change, which was characterized by
the proliferation of keratinocytes. However, a wide range of
aberrant keratinization, dyskeratosis and a significant increase of
melanocytes were not observed. Certain tumors arising in the
CYP26A1 transgenic mice were composed by thick, interwoven tracts
of pigmented basaloid and squamous epidermal cells with formation
of pseudo horn cysts, resembling acanthotic type seborrheic
keratosis, which is usually common in sunlight-damaged human skin
of older individuals.
In CYP26A1 transgenic mice, it was observed that
constitutive expression of CYP26A1 induced squamous hyperplasia
followed by papilloma formation within 7 weeks after treatment with
DMBA (Fig. 2B). Tumor growth was
significantly accelerated as compared with the onset of tumor
formation following DMBA/TPA treatment in the control mice. In the
control mice, papillomas developed at 15–17 weeks after DMBA/TPA
treatment, which is consistent with the time reported to be
required for papilloma formation in a two-stage skin carcinogenesis
model in C57BL/6 mice (11).
Microscopic appearance of papillomas is characterized by a
papillary and acanthotic growth of well-differentiated squamous
cells with hyperkeratinization. Squamous papillomas of the skin
that developed in transgenic and control mice were
indistinguishable morphologically, although the maximum sizes of
tumors detected in these animals were different (Fig. 2A). Mild chronic inflammation was
present beneath the epithelium in CYP26A1 transgenic and control
mice in the presence of DMBA and TPA (data not shown). An increased
incidence of tumor formation was observed in all of the five
founder transgenic lines. Despite the clear evidence that CYP26A1
expression leads to early tumor formation, CYP26A1 expression was
not sufficient to increase the mean number of tumors (Fig. 2B). These data suggested that CYP26A1
expression promotes skin tumorigenesis induced by DMBA.
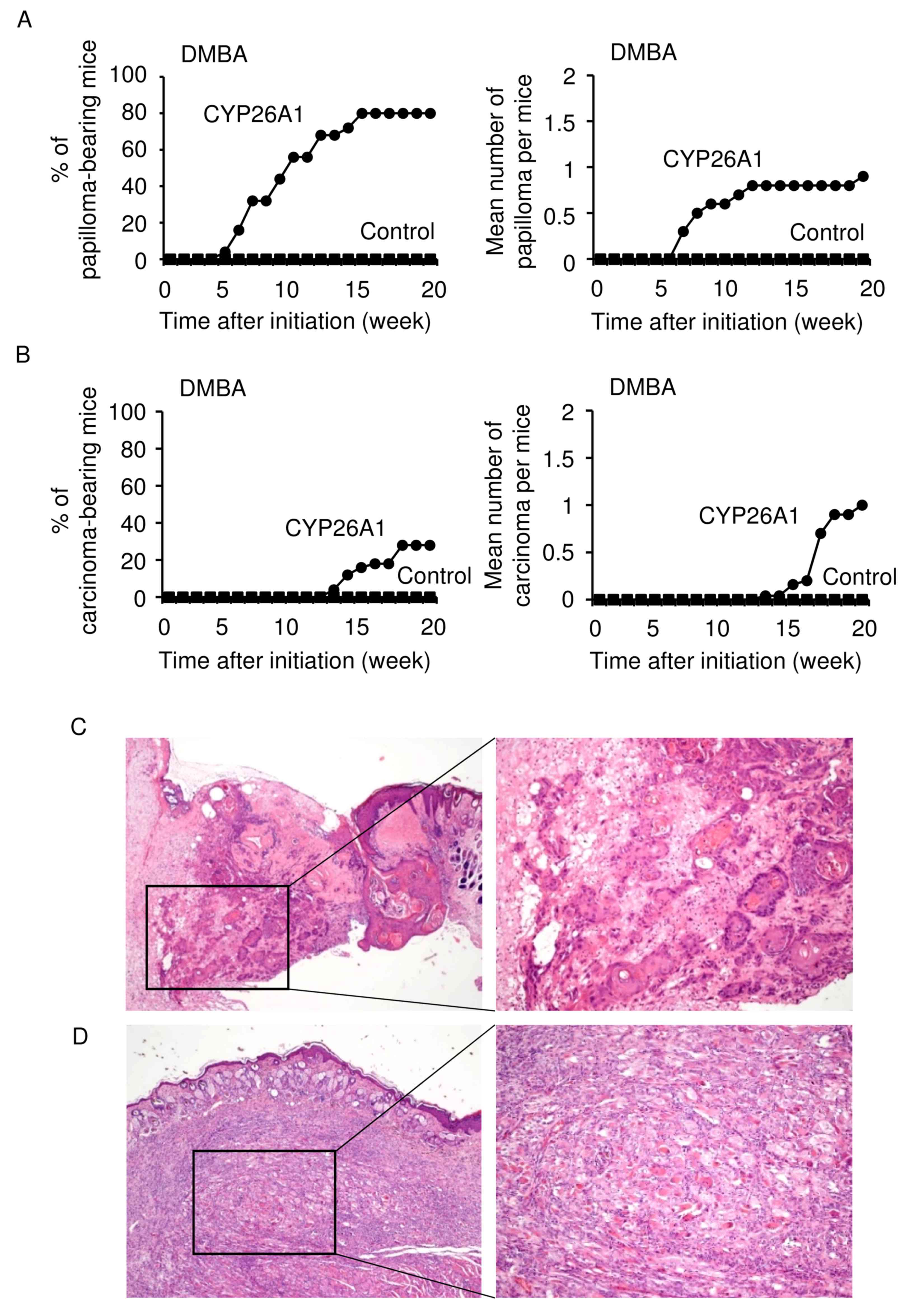
One-stage skin cancer model
The present study next examined whether CYP26A1
expression had any effect on the DMBA-induced skin carcinogenesis
in the absence of the tumor promoter TPA (Fig. 3). CYP26A1 expression increased the
susceptibility of these mice to the induction of papilloma
formation by DMBA treatment alone (Fig.
3A). In CYP26A1 transgenic mice, papillomas appeared at 5–8
weeks, and the increase in the incidence of papillomas was similar
to that observed in the two-stage skin carcinogenesis model
(Fig. 2B; DMBA/TPA model).
Spontaneous development of invasive squamous cell
carcinoma was observed in ~25% of the CYP26A1 transgenic animals at
13 weeks following single treatment with DMBA (Fig. 3B). Squamous cell carcinomas arising in
the CYP26A1 mice consisted of nests and strands of atypical
epithelial cells with central keratinization, which have abundant
eosinophilic cytoplasm and a large round atypical nucleus (Fig. 3C). These cells infiltrated into the
subcutaneous fibrous and adipose tissue, while there was a mild
chronic inflammatory cell infiltrate at the periphery of the
tumors. In addition, scattered squamous intraepidermal neoplasia of
the skin was observed in a surrounding area of squamous cell
carcinoma, presenting acanthosis consisting of variable atypia of
keratinocytes and focal parakeratosis with a loss of cell polarity
(data not shown). Although there has been an accumulated body of
evidence demonstrating that DMBA promotes the initiation and
development of epithelial neoplasia (9,10), the
current study also observed the formation of non-epithelial
malignancy of the skin, i.e., sarcoma detected in the subcutaneous
tissue lining with the intact epidermis (Fig. 3D). These tumors did not demonstrate
specific differentiation phenotypes of tumor cells upon
immunohistochemical analysis. By contrast, control mice did not
develop palpable tumors, or present any definite histological
abnormalities in skin tissues within 20 weeks. These observations
suggested that CYP26A1 expression predisposes mice to skin
carcinogenesis, supporting a stimulatory oncogenic role of CYP26A1
in skin neoplasia.
Discussion
In the present study, it was demonstrated that
enhanced expression of CYP26A1 increases the susceptibility to skin
carcinogenesis initiated by DMBA. The skin tissue of CYP26A1
transgenic mice had increased sensitivity to DMBA-mediated
carcinogenesis, even when compared with control animals that were
subjected to combined treatment with DMBA and TPA. CYP26A1
transgenic animals did not develop spontaneous tumors, or
demonstrate any histological abnormalities over the course of the
experiments. A possible explanation for these observations is that
CYP26A1 overexpression alone was not sufficient for the initiation
of epidermal keratinocytes that would lead to the development of a
skin tumor. This hypothesis is consistent with our previous results
indicating that forced expression of CYP26A1 in non-transformed
culture cells did not cause anchorage-independent growth in soft
agar (2). However, spontaneous
development of cutaneous squamous cell carcinoma in CYP26A1
transgenic animals was observed following single treatment of DMBA
in the absence of a tumor promoter. These findings suggested that
CYP26A1 expression serves a fundamental role in determining the
cellular commitment to carcinogenesis. These data are in agreement
with previous observations, suggesting that enhanced expression of
CYP26A1 has a stimulatory oncogenic function in neoplasia (2,5–7).
Previous studies unveiled a functional association
between CYP26A1-mediated cellular RA insufficiency and enhanced
tumorigenicity, implicating CYP26A1 as a possible candidate
oncogene (1,2,5–7). Accumulated evidence suggested that
CYP26A1 overexpression may contribute to skin carcinogenesis by
causing a state of functional VAD. This hypothesis is based on a
mechanistic link between VAD and increased risk for various types
of cancer (8). Consistently, a number
of published studies have identified that individuals with VAD
accumulate DNA damage at a higher frequency, eventually resulting
in increased risk and incidence of cancer (12,13).
Conversely, there has been increased interest in the use of RA and
synthetic derivatives (known as retinoids) in cancer therapy and
dermatology. Several models have been used to demonstrate the
effectiveness of retinoids in suppressing carcinogenesis in a
variety of tissues, including in the skin, oral cavity, liver,
mammary epithelia, bladder and prostate (14,15). These
previous studies suggested that the use of several types of RA and
synthetic derivatives may be utilized in the treatment of
premalignant lesions, as well as in the prevention of secondary
tumors and the recurrence of cancer.
It has been demonstrated that CYP26A1 modulates a
wide variety of genes to favor cell survival, which results in a
selective growth advantage for the cells (1–7).
Furthermore, CYP26A1 upregulation alters the gene expression
profile already present in tumor cells to potentially generate a
wide variety of specific and potent pro-survival signals that
render cells resistant to apoptosis. Given the pleiotropic activity
of RA, it is plausible that CYP26A1 expression affects a vast array
of physiological pathways in regulating various independent
signaling molecules associated with tumorigenic events.
In conclusion, the present study clearly provided
evidence of the role of elevated CYP26A1 expression in promoting
cutaneous neoplasia induced by DMBA. However, the exact molecular
impact of CYP26A1 on carcinogenesis and the underlying regulatory
mechanism of CYP26A1 overexpression in human cancer remain to be
clarified. Therefore, future studies are warranted to better
understand the biological significance of CYP26A1 overexpression in
the neoplasia of human malignancies.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by grants
from the Grant-in-Aid for Scientific Research program from the
Japan Society for the Promotion of Science (JSPS KAKENHI; grant
nos. JP17K08697, JP16K08693, JP26460421, JP24390089 and
JP24790355).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MO designed and performed the present study,
participated in animal experiments and drafted the manuscript. AT
and KT participated in animal experiments and histochemical
evaluation of developed tumors in mice. MM and NS participated in
data analysis and interpretation, and helped to draft the
manuscript. All authors contributed to the manuscript discussion of
the article to be published and approved its final version.
Ethics approval and consent to
participate
The maintenance and handling of animals were
conducted using protocols approved by the Animal Care Committee of
Sapporo Medical University School of Medicine (approval no.
12-040).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Osanai M: Cellular retinoic acid
bioavailability in various pathologies and its therapeutic
implication. Pathol Int. 67:281–291. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Osanai M, Sawada N and Lee GH: Oncogenic
and cell survival properties of the retinoic acid metabolizing
enzyme, CYP26A1. Oncogene. 29:1135–1144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shelton DN, Sandoval IT, Eisinger A,
Chidester S, Ratnayake A, Ireland CM and Jones DA: Up-regulation of
CYP26A1 in adenomatous polyposis coli-deficient vertebrates via a
WNT-dependent mechanism: Implications for intestinal cell
differentiation and colon tumor development. Cancer Res.
66:7571–7577. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sonneveld E, van den Brink CE, van der
Leede BM, Schulkes RK, Petkovich M, van der Burg B and van der Saag
PT: Human retinoic acid (RA) 4-hydroxylase (CYP26) is highly
specific for all-trans-RA and can be induced through RA receptors
in human breast and colon carcinoma cells. Cell Growth Differ.
9:629–637. 1998.PubMed/NCBI
|
|
5
|
Osanai M and Lee GH: Enhanced expression
of retinoic acid metabolizing enzyme CYP26A1 in sunlight-damaged
human skin. Med Mol Morphol. 44:200–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Osanai M and Lee GH: Increased expression
of the retinoic acid-metabolizing enzyme CYP26A1 during the
progression of cervical squamous neoplasia and head and neck
cancer. BMC Res Notes. 7:6972014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Osanai M and Lee GH: The retinoic
acid-metabolizing enzyme CYP26A1 upregulates fascin and promotes
the malignant behavior of breast carcinoma cells. Oncol Rep.
34:850–858. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wolbach SB and Howe PR: Tissue changes
following deprivation of fat-soluble A vitamin. J Exp Med.
42:753–777. 1925. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abel EL, Angel JM, Kiguchi K and
DiGiovanni J: Multi-stage chemical carcinogenesis in mouse skin:
Fundamentals and applications. Nat Protoc. 4:1350–1362. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Neagu M, Caruntu C, Constantin C, Boda D,
Zurac S, Spandidos DA and Tsatsakis AM: Chemically induced skin
carcinogenesis: Updates in experimental models (Review). Oncol Rep.
35:2516–2528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sundberg JP, Sundberg BA and Beamer WG:
Comparison of chemical carcinogen skin tumor induction efficacy in
inbred, mutant, and hybrid strains of mice: Morphologic variations
of induced tumors and absence of a papillomavirus cocarcinogen. Mol
Carcinog. 20:19–32. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
French AL, Kirstein LM, Massad LS, Semba
RD, Minkoff H, Landesman S, Palefsky J, Young M, Anastos K and
Cohen MH: Association of vitamin A deficiency with cervical
squamous intraepithelial lesions in human immunodeficiency
virus-infected women. J Infect Dis. 182:1084–1089. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Z, Boudjelal M, Kang S, Voorhees JJ
and Fisher GJ: Ultraviolet irradiation of human skin causes
functional vitamin A deficiency, preventable by all-trans retinoic
acid pre-treatment. Nat Med. 5:418–422. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mohan R, Katiyar S, Khan S, Zaim M,
Mukhtar H and Agarwal R: Protective effect of all trans retinoic
acid against tumor promotion and progression in low- and high-risk
protocols of mouse skin chemical carcinogenesis. Int J Oncol.
8:1079–1088. 1996.PubMed/NCBI
|
|
15
|
Gressel KL, Duncan FJ, Oberyszyn TM, La
Perle KM and Everts HB: Endogenous retinoic acid required to
maintain the epidermis following ultraviolet light exposure in
SKH-1 hairless mice. Photochem Photobiol. 91:901–908. 2015.
View Article : Google Scholar : PubMed/NCBI
|