Introduction
Retinoblastoma (RB) is a common cancer in
ophthalmology caused by the mutation of the RB1 gene, and it
has been widely investigated in recent years (1). RB is more common in children, and the
incidence rate is 7–25%. Approximately 70% of the patients develop
a unilateral eye tumor. It is the first tumor found to have a
genetic basis (2), and approximately
40% of RB is hereditary (3). Although
the survival rate of RB patients is very high, its mortality cannot
be ignored, because RB is easily complicated with other malignant
tumors. Therefore, radiotherapy is often avoided in the treatment
(4).
Drug treatment is a good direction. Docetaxel (DTX)
is a second-line therapy for some tumors, and it can effectively
prolong the survival period of patients, with less side effects
(5). Platinum drugs, such as
carboplatin, can destroy cancer cells by inducing double-stranded
deoxyribonucleic acid (DNA) breaks (6).
In this study, effects of DTX combined with
carboplatin treatment on the survival of RB mice were explored by
establishing RB mouse models.
Materials and methods
Research objects
ICR mice, grade CL were purchased from Better
Biotechnology Co., Ltd. (Nanjing, China). Shuke and Beita rat feed
of specific-pathogen-free (SPF) grade was purchased from Jiangsu
Xietong Organism Co., Ltd. (Nanjing, China) for feeding. ICR mice
were aged 9–11 weeks and weighing 15–25 g. The animals had free
access to food and water at room temperature of 21±2°C and humidity
of 30–70%, with fluorescent lighting; the feeding box was replaced
weekly 1–2 times, and the bottle was replaced weekly 1–2 times. DTX
was purchased from Shanghai Shifeng Biological Technology Co., Ltd.
(Shanghai, China); carboplatin was purchased from Shenzhen
Simeiquan Biotechnology Co., Ltd. (Shenzhen, China); and
retinoblastoma Y-79 cell lines were purchased from the Institute of
Basic Medicine, Chinese Academy of Medical Sciences.
Establishment of ICR mouse models
Establishment of ICR mouse models referred to the
modeling methods of Corson et al (7). ICR mice received intraperitoneal
anesthesia with 50 mg/kg pentobarbital sodium; retinoblastoma Y-79
cell lines were prepared into a cell suspension at a concentration
of 4.0×107/ml and injected into the vitreous body of the
right eyes of ICR mice to establish mouse models of RB xenografts;
lincomycin hydrochloride and erythromycin were used to reduce
inflammation after operation; and observation was conducted for 7
days. The intraocular conditions of mice were recorded daily, and
the success of models was determined through pathological
diagnosis.
Treatment methods
A total of 120 RB ICR mouse models were successfully
established and divided into four groups, A, B, C and D (n=30) by
random number table method. Group A received DTX combined with
carboplatin treatment, group B received DTX alone treatment, group
C received carboplatin alone treatment, and group D was given
normal saline. DTX, carboplatin and normal saline, all with a dose
of 10 mg/kg, were injected into the caudal vein, once a day for one
week.
Observation indexes
Among 150 ICR mice, a total of 120 ICR mice were
pathologically diagnosed with positive RB. The survival time of ICR
mice was observed. After a 1-week administration, the ICR mice were
sacrificed with carbon dioxide (CO2), both eyes were
collected, and the size and weight of tumor were observed.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
19.0 Asia Analytics (formerly SPSS China) was used for statistical
analysis. The χ2 test was used for comparison of the
rates. Measurement data were expressed as mean ± SD, and t-test was
used for pairwise comparisons. Analysis of variance and Dunnett's
post-hoc test was used for comparisons among multiple groups.
P<0.05 indicated that the difference was statistically
significant.
Results
General data
The selected mice were healthy ICR mice of grade CL,
and they were uniformly fed with Shuke and Beita rat feed of SPF
grade. Among the 150 ICR mice, 120 mouse models were successfully
established, the mortality rate was 20%. The 120 ICR mice with
successful models had no difference in body weight (F=1.225,
P=0.48) before and after the modeling. The average age of ICR mice
was 10.5±0.4 weeks (Table I).
 | Table I.Basic conditions of the four groups of
mice. |
Table I.
Basic conditions of the four groups of
mice.
|
| Group A | Group B | Group C | Group D | F value | P-value |
|---|
| Age (weeks old) |
8.1±0.4 |
8.5±0.2 |
8.4±0.3 |
8.6±0.1 | 0.632 | 0.899 |
| Body weight before
the modeling (g) |
19.4±1.3 |
16.5±1.8 |
14.2±1.5 |
17.1±1.2 | 0.512 | 0.645 |
| Body weight after the
modeling (g) |
29.8±1.2 |
22.4±1.3 |
27.6±2.5 |
26.1±1.6 | 0.589 | 0.785 |
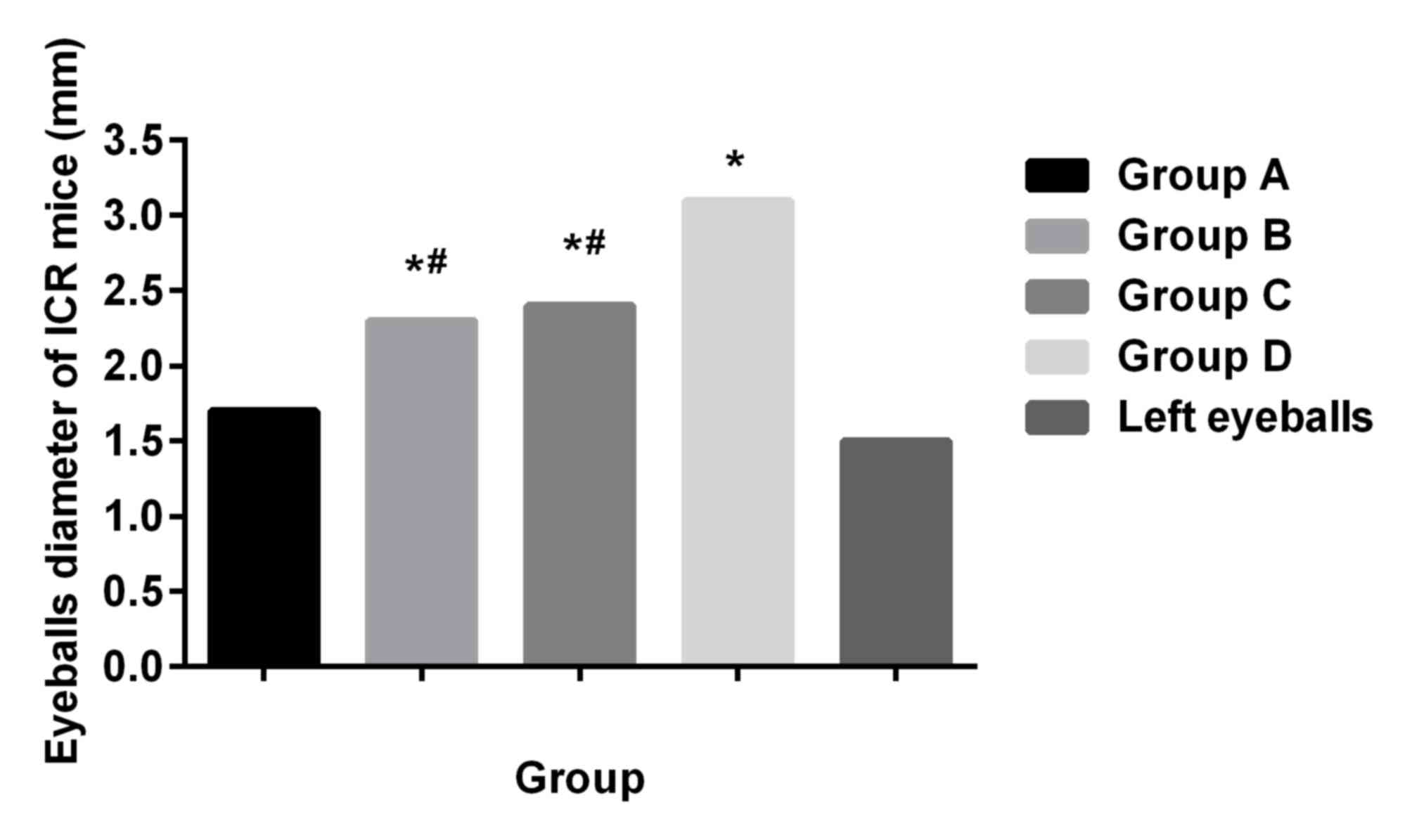
Examination of eyeball diameter of ICR
mice
After injection of retinoblastoma-79 cells for 2
days, the right eyeballs of ICR mice were slightly protuberant
compared with the left eyeballs; 4 days after the modeling, the
right eyeballs of ICR mice became more protuberant; and 7 days
later, the right eyeballs were protruding outside the eye sockets.
Seven days after the modeling, ICR mice were sacrificed with
CO2, and both eyes were collected. Diameters of the
right eyeballs in ICR mice were significantly larger than those of
the left eyeballs (P<0.05), diameters of the affected eyeballs
in group A were significantly shorter than those in the other three
groups (P<0.05), and there was no significant difference
compared with normal left eyeballs (P>0.05). Diameters in group
B and group C were not different from each other (P>0.05), but
shorter than those in group D (P<0.05) (Fig. 1).
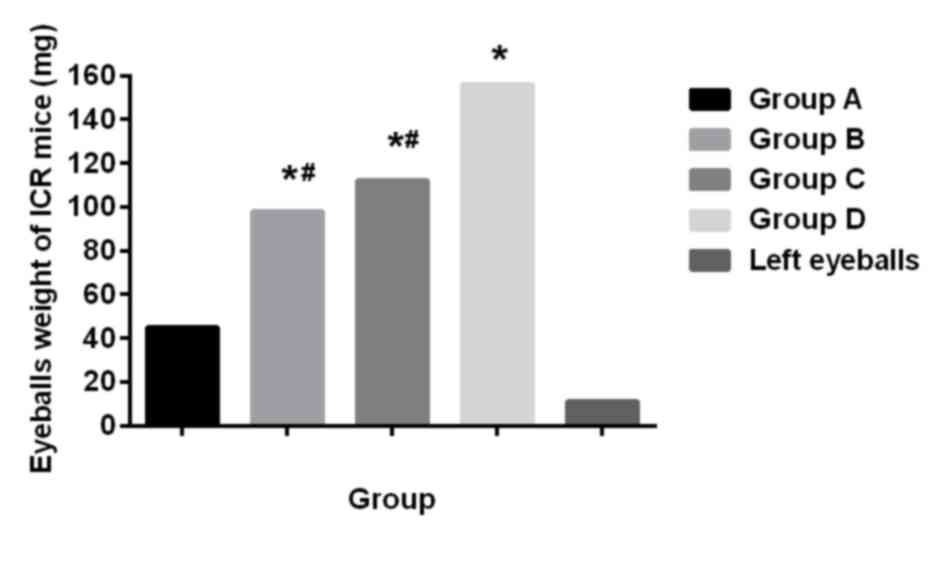
Comparison of eyeball weight of ICR
mice
The weight of the right eyeballs of ICR mice was
significantly heavier than that of the left eyeballs (P<0.05).
The weight of the affected eyes of ICR mice the groups A, B, C and
D was different, the weight in group A was lighter than that in the
other three groups (P<0.05), and there was no significant
difference compared with normal eyeball weight (P>0.05); there
was no difference in weight between group B and group C
(P>0.05), but the weight was lighter compared with that in group
D (P<0.05) (Fig. 2).
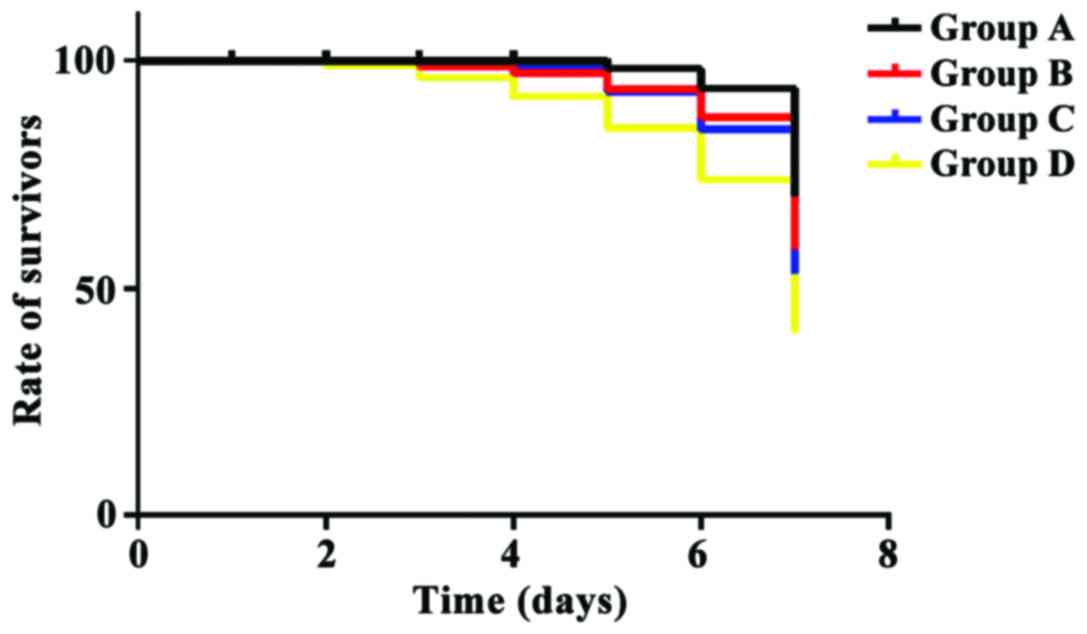
Comparison of the survival time in ICR
mice after treatment
The survival time of ICR mice in groups A, B and C
was significantly longer than that in group D (P<0.05); the
survival time of ICR mice in group A was significantly longer than
that in group B and group C (P<0.05). There was no significant
difference in the survival time of mice between groups B and C
(P>0.05) (Fig. 3).
Discussion
RB is a very common primary malignant tumor among
minors in ophthalmology (8). With the
extension of the course of disease, the risks of bone tumors, soft
tissue sarcoma and melanoma are also increasing (9,10). RB also
becomes more harmful, so how to control and even cure RB is a very
important issue.
In this study, RB ICR mouse models were established
to explore the effects of DTX combined with carboplatin in the
treatment of RB. All the mice selected in this study were of grade
CL. Its advantages are that it avoids ethical disputes, has a wide
source of materials, and avoids drug damage to patients.
In this study, ICR mice were given DTX treatment,
carboplatin treatment, and combined treatment of the two,
respectively. It was found that both DTX and carboplatin could
prolong the survival time of ICR mice; however, DTX combined with
carboplatin had a better effect on the prolongation of the survival
time of mice. DTX has a significant antitumor activity. It can
inhibit expression of cyclin dependent kinase 4 (CDK4), cyclin D1
and cyclin E1, induce low phosphorylation of RB, and block the
transformation of cells in G0/G1 to S phase (11). Carboplatin is a common
second-generation platinum chemotherapy drugs, and it is also a
non-specific antitumor drug applied by injection. It destroys the
cytotoxicity of DNA and hinders the development of tumors (12). Our results show that the weight and
diameters of the affected eyes were all improved after treatment
with DTX and carboplatin, which was more obvious in the mice
treated by DTX combined with carboplatin, indicating that the
efficacy of DTX, carboplatin and the combination of the two in RB
are confirmed.
In recent years, there are some reports on the
therapeutic effects of DTX in RB. The study of DTX in non-small
cell lung (13), breast (14) and prostate cancer (15) is widely reported, similarly to
Carboplatin (16–18). In a study by Li and his colleagues
(19), it was found that although DTX
combined with cisplatin is more effective than monotherapy in the
treatment of non-small cell lung cancer, combination therapy
produces more frequent side effects, such as anemia,
thrombocytopenia, nausea and vomiting. This problem was also found
in patients with prostate cancer in the treatment with DTX combined
with carboplatin by Bouman-Wammes and his colleagues (20). Changes in blood parameters of mice
were not collected in this study, so they could not be compared. It
will be the direction of our research in the next study, and we
also need large clinical data to support us. Therefore, although
DTX combined with carboplatin is more effective in the treatment of
RB, its safety remains to be studied.
The therapeutic effects of DTX, carboplatin and the
combination of the two in the treatment of RB are worthy of
recognition. They can effectively prolong the survival time of
mice, but their safety remains to be studied.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CS conceived and designed the study, and drafted the
manuscript. CS and QZ established ICR mouse models, and analyzed
and interpreted observation indexes. Both authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Weihai Municipal Hospital (Shandong, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Villanueva: Tumorigenesis: Establishing
the origin of retinoblastoma. Nat Rev Cancer. 14:706–707. 2014.
|
|
2
|
Thériault BL, Dimaras H, Gallie BL and
Corson TW: The genomic landscape of retinoblastoma: A review. Clin
Exp Ophthalmol. 42:33–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamihara J, Bourdeaut F, Foulkes WD,
Molenaar JJ, Mossé YP, Nakagawara A, Parareda A, Scollon SR,
Schneider KW, Skalet AH, et al: Retinoblastoma and neuroblastoma
predisposition and surveillance. Clin Cancer Res. 23:e98–e106.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sethi RV, Shih HA, Yeap BY, Mouw KW,
Petersen R, Kim DY, Munzenrider JE, Grabowski E, Rodriguez-Galindo
C, Yock TI, et al: Second nonocular tumors among survivors of
retinoblastoma treated with contemporary photon and proton
radiotherapy. Cancer. 120:126–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sikov WM, Berry DA, Perou CM, Singh B,
Cirrincione CT, Tolaney SM, Kuzma CS, Pluard TJ, Somlo G, Port ER,
et al: Impact of the addition of carboplatin and/or bevacizumab to
neoadjuvant once-per-week paclitaxel followed by dose-dense
doxorubicin and cyclophosphamide on pathologic complete response
rates in stage II to III triple-negative breast cancer: CALGB 40603
(Alliance). J Clin Oncol. 33:13–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Corson TW, Samuels BC, Wenzel AA, Geary
AJ, Riley AA, McCarthy BP, Hanenberg H, Bailey BJ, Rogers PI,
Pollok KE, et al: Multimodality imaging methods for assessing
retinoblastoma orthotopic xenograft growth and development. PLoS
One. 9:e990362014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wong JR, Morton LM, Tucker MA, Abramson
DH, Seddon JM, Sampson JN and Kleinerman RA: Risk of subsequent
malignant neoplasms in long-term hereditary retinoblastoma
survivors after chemotherapy and radiotherapy. J Clin Oncol.
32:3284–3290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kleinerman RA, Yu CL, Little MP, Li Y,
Abramson D, Seddon J and Tucker MA: Variation of second cancer risk
by family history of retinoblastoma among long-term survivors. J
Clin Oncol. 30:950–957. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kleinerman RA, Schonfeld SJ and Tucker MA:
Sarcomas in hereditary retinoblastoma. Clin Sarcoma Res. 2:152012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh SK, Banerjee S, Acosta EP, Lillard
JW and Singh R: Resveratrol induces cell cycle arrest and apoptosis
with docetaxel in prostate cancer cells via a p53/p21WAF1/CIP1 and
p27KIP1 pathway. Oncotarget. 8:17216–17228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang KB, Francis JH and Abramson DH: What
do we know about intraocular carboplatin? J Ocul Pharmacol Ther.
30:688–690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Swain SM, Kim SB, Cortés J, Ro J,
Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A,
Knott A, et al: Pertuzumab, trastuzumab, and docetaxel for
HER2-positive metastatic breast cancer (CLEOPATRA study): Overall
survival results from a randomised, double-blind,
placebo-controlled, phase 3 study. Lancet Oncol. 14:461–471. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kwon ED, Drake CG, Scher HI, Fizazi K,
Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R,
Mahammedi H, et al: CA184-043 Investigators: Ipilimumab versus
placebo after radiotherapy in patients with metastatic
castration-resistant prostate cancer that had progressed after
docetaxel chemotherapy (CA184-043): A multicentre, randomised,
double-blind, phase 3 trial. Lancet Oncol. 15:700–712. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Langer CJ, Gadgeel SM, Borghaei H,
Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins
RG, Stevenson JP, Jalal SI, et al: KEYNOTE-021 investigators:
Carboplatin and pemetrexed with or without pembrolizumab for
advanced, non-squamous non-small-cell lung cancer: A randomised,
phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol.
17:1497–1508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O'Shaughnessy J, Schwartzberg L, Danso MA,
Miller KD, Rugo HS, Neubauer M, Robert N, Hellerstedt B, Saleh M,
Richards P, et al: Phase III study of iniparib plus gemcitabine and
carboplatin versus gemcitabine and carboplatin in patients with
metastatic triple-negative breast cancer. J Clin Oncol.
32:3840–3847. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Katsumata N, Yasuda M, Isonishi S,
Takahashi F, Michimae H, Kimura E, Aoki D, Jobo T, Kodama S,
Terauchi F, et al: Japanese Gynecologic Oncology Group: Long-term
results of dose-dense paclitaxel and carboplatin versus
conventional paclitaxel and carboplatin for treatment of advanced
epithelial ovarian, fallopian tube, or primary peritoneal cancer
(JGOG 3016): A randomised, controlled, open-label trial. Lancet
Oncol. 14:1020–1026. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li A, Wei ZJ, Ding H, Tang HS, Zhou HX,
Yao X and Feng SQ: Docetaxel versus docetaxel plus cisplatin for
non-small-cell lung cancer: A meta-analysis of randomized clinical
trials. Oncotarget. 8:57365–57378. 2017.PubMed/NCBI
|
|
20
|
Bouman-Wammes EW, de Munck L, van den Berg
HP, Beeker A, Smorenburg CH, Verheul HMW, Gerritsen WR and van den
Eertwegh AJM: A randomized phase II trial of docetaxel plus
carboplatin versus docetaxel in patients with castration-resistant
prostate cancer who have progressed after response to prior
docetaxel chemotherapy: The RECARDO trial. Eur J Cancer. 90:1–9.
2018. View Article : Google Scholar : PubMed/NCBI
|