Introduction
Inflammation is known to contribute to cancer
progression (1,2), and several inflammatory markers, such as
the neutrophil-to-lymphocyte ratio (NLR), the
lymphocyte-to-monocyte ratio (LMR) and the Glasgow prognostic score
(GPS) have been reported to be associated with clinical outcomes in
patients with various types of cancer, including colorectal cancer
(3–8).
Recently, a new inflammatory marker, the systemic inflammatory
score (SIS), based on the combination of the LMR and the serum
albumin concentration has been reported to be a useful prognostic
marker in patients with clear-cell renal cell carcinoma, colorectal
cancer and oral cavity squamous cell carcinoma (9–11).
However, there are only a few reports on the SIS,
and the prognostic value of the SIS in patients with unresectable
metastatic colorectal cancer (mCRC) remains unclear. In addition,
the optimum cut-off value may change depending on the type of
cancer and stage and merits further study.
This study aimed to evaluate the prognostic value of
the SIS and to determine its optimum cut-off value in patients with
unresectable mCRC who underwent chemotherapy.
Materials and methods
Patients
This retrospective cohort study included 160
patients who underwent combination chemotherapy for unresectable
mCRC at the Department of Surgical Oncology of Osaka City
University (Osaka, Japan) between January 2008 and December
2016.
Methods
Blood samples were collected within one week prior
to the initiation of chemotherapy. We analyzed the differential
white blood cell count using an XE-5000 hematology analyzer
(Sysmex, Kobe, Japan) based on the manufacturer's protocol. The LMR
was calculated by dividing the absolute number of circulating
lymphocytes by the absolute number of circulating monocytes. We
assessed the serum albumin concentrations by a chemiluminescent
immunoassay (Wako, Osaka, Japan) according to the manufacturer's
protocol. The SIS was defined according to the methods of a
previous report (9), using the
combination of the LMR and the serum albumin concentration:
patients with LMR >4.44 and serum albumin level >4.0 g/dl
were given a score of 0; patients with LMR ≤4.44 or serum albumin
level ≤4.0 g/dl were given a score of 1; patients with LMR ≤4.4 and
4 serum albumin level ≤4.0 g/dl were given a score of 2. The
location of the primary tumor was defined as follows. The oral side
of the splenic flexure was termed ‘the right side’ and the anal
side of splenic flexure was termed ‘the left side’. Furthermore, we
defined synchronous and metachronous metastases as follows.
Synchronous metastases were defined as metastatic lesions that were
already confirmed at the time of the diagnosis of the primary
lesion; metachronous metastases were defined as metastatic lesions
that developed after the excision of the primary tumor, regardless
of the period.
Ethical considerations
All patients were informed of the investigational
nature of this study and provided their written informed consent
for the retrospective analysis of their data. Full ethical approval
was granted by the Ethics Committee of Osaka City University
(approval no. 926).
Statistical analysis
The significance of the correlations between the SIS
and the clinicopathological characteristics were analyzed using the
Chi-squared test. Survival curves were constructed using the
Kaplan-Meier method and were compared using the log-rank test. A
multivariate analysis was performed using a Cox proportional
hazards model. All of the statistical analyses were performed using
the SPSS software program (version 19.0; IBM, Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patients' baseline
characteristics
The characteristics of the 160 patients in the
present study are summarized in Table
I. The study population included 86 male patients and 74 female
patients. The median age of the patients was 65 years (range: 18 to
89). According to the definition of the Eastern Cooperative
Oncology Group performance status (PS), 140 patients were
classified as having a PS of 0, 17 were classified as having a PS
of 1, and 3 were classified as having a PS of 2. A total of 39
patients had primary tumors located on the right side, and 121 had
primary tumors located on the left side. One hundred and seven
patients had single-organ metastasis, and 53 multiple organs
affected by metastases. All of the patients underwent combination
chemotherapy with oxaliplatin, irinotecan plus
5-fluorouracil/leucovorin, or a prodrug of 5-fluorouracil as
first-line chemotherapy. The regimens used for all of the patients
in this study were considered to have the same efficacy (12–14).
Seventy-five patients received FOLFOX, 53 received CapeOX, 25
received FOLFIRI, and 7 SOX. A total of 103 patients underwent
chemotherapy combined with molecular-targeted therapy. The median
follow-up period for the surviving patients was 21.8 months (range:
1.2 to 94.0 months). A total of 113 patients died during the
follow-up period.
 | Table I.The patients' baseline
characteristics. |
Table I.
The patients' baseline
characteristics.
| Characteristics | No. of patients |
|---|
| Median age, years
(range) | 65 (18–89) |
| Sex |
|
| Male | 86 |
|
Female | 74 |
| Performance
status |
|
| 0 | 140 |
| 1 | 17 |
| 2 | 3 |
| Location of primary
tumor |
|
| Right
side | 39 |
| Left
side | 121 |
| Histological
type |
|
| Well,
moderately | 143 |
| Poorly,
mucinous | 17 |
| RAS status |
|
| Wild
type | 64 |
| Mutant
type | 54 |
|
Unknown | 42 |
| Detection of
unresectable tumor |
|
|
Synchronous | 106 |
|
Metachronous | 54 |
| Number of organs
affected by metastasis |
|
| One
organ | 107 |
|
Multiple organs | 53 |
| Peritoneal
dissemination |
|
|
Negative | 125 |
|
Positive | 35 |
| First-line
chemotherapy regimen |
|
|
FOLFOX | 75 |
|
CapeOX | 53 |
|
FOLFIRI | 25 |
|
SOX | 7 |
| Molecular-targeted
therapy |
|
|
Bevacizumab | 85 |
|
Cetuximab | 11 |
|
Panitumumab | 7 |
|
None | 57 |
|
Lymphocyte-to-monocyte ratio |
|
| Median
(range) | 4.53
(1.25–14.06) |
| Serum albumin
concentration |
|
| Median
(range) | 3.9 (2.5–4.9) |
| Systemic
inflammatory score |
|
| 0 | 46 |
| 1 | 68 |
| 2 | 46 |
Correlations between the SIS and
clinicopathological factors
The correlations between the SIS and
clinicopathological factors are shown in Table II. The SIS and clinicopathological
factors did not differ to a statistically significant extent.
 | Table II.The correlations between the SIS and
the clinicopathological factors. |
Table II.
The correlations between the SIS and
the clinicopathological factors.
|
| SIS |
|
|---|
|
|
|
|
|---|
| Clinicopathological
factor | 0 (n=46) | 1 (n=68) | 2 (n=46) | P-value |
|---|
| Age |
|
|
| 0.385 |
| <65
years | 27 | 31 | 24 |
|
| ≥65
years | 19 | 37 | 22 |
|
| Sex |
|
|
| 0.718 |
|
Male | 23 | 39 | 24 |
|
|
Female | 23 | 29 | 22 |
|
| Location of primary
tumor |
|
|
| 0.416 |
| Right
side | 13 | 18 | 8 |
|
| Left
side | 33 | 50 | 38 |
|
| Performance
status |
|
|
| 0.630 |
| 0 | 42 | 58 | 40 |
|
| ≥1 | 4 | 10 | 6 |
|
| Histological
type |
|
|
| 0.335 |
| Well,
moderately | 43 | 58 | 42 |
|
| Poorly,
mucinous | 3 | 10 | 6 |
|
| RAS status |
|
|
| 0.310 |
| Wild
type | 25 | 22 | 17 |
|
| Mutant
type | 16 | 26 | 12 |
|
|
Unknown | 5 | 20 | 17 |
|
| Detection of
unresectable tumor |
|
|
| 0.119 |
|
Synchronous | 29 | 41 | 36 |
|
|
Metachronous | 17 | 27 | 10 |
|
| Number of organs
affected by metastasis |
|
|
| 0.960 |
| One
organ | 30 | 46 | 31 |
|
|
Multiple organs | 16 | 22 | 15 |
|
| Peritoneal
dissemination |
|
|
| 0.101 |
|
Negative | 34 | 50 | 41 |
|
|
Positive | 12 | 18 | 5 |
|
| Molecular-targeted
therapy |
|
|
| 0.312 |
|
Absent | 13 | 24 | 20 |
|
|
Present | 33 | 44 | 26 |
|
Prognostic significance of the SIS
according to the original cut-off values defined in the previous
report
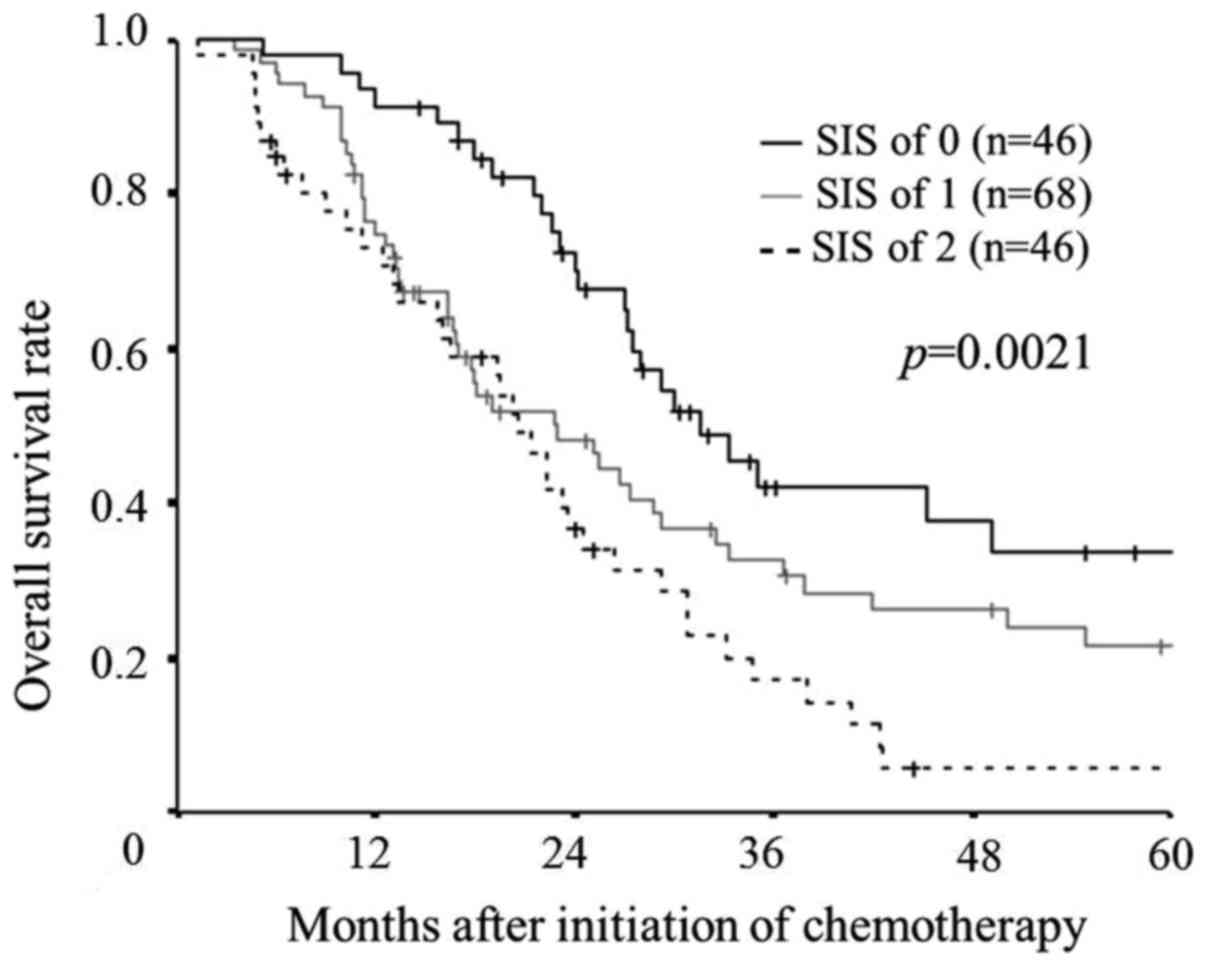
The median overall survival time was 31.6 months in
those with a SIS of 0, 22.9 months in those with a SIS of 1, and
20.6 months in those with a SIS of 2. A log-rank test demonstrated
significant differences in the overall survival among the three
groups (P=0.0021). However, there were no significant differences
in the overall survival between the patients with a SIS of 1 and
those with a SIS of 2, although the overall survival rate tended to
be worse in patients with a SIS of 2 than in those with a SIS of 1
(P=0.0810; Fig. 1).
Univariate and multivariate analyses
of the risk factors for overall survival
In the univariate analysis, the PS, the location of
the primary tumor, the RAS status and the SIS were associated with
overall survival. Furthermore, a multivariate analysis demonstrated
that gender, the location of the primary tumor and the SIS were
independent prognostic factors for survival (Table III).
 | Table III.Univariate and multivariate analyses
of risk factors for overall survival. |
Table III.
Univariate and multivariate analyses
of risk factors for overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
|
<65 | Reference |
|
| Reference |
|
|
|
≥65 | 1.318 | 0.908–1.912 | 0.146 | 1.309 | 0.825–2.077 | 0.254 |
| Sex |
|
|
|
|
|
|
|
Male | Reference |
|
| Reference |
|
|
|
Female | 1.256 | 0.868–1.817 | 0.227 | 1.771 | 1.097–2.859 | 0.019 |
| Performance
status |
|
|
|
|
|
|
| 0 | Reference |
|
| Reference |
|
|
| ≥1 | 1.824 | 1.085–3.065 | 0.023 | 1.448 | 0.664–3.154 | 0.352 |
| Location of primary
tumor |
|
|
|
|
|
|
| Left
side | Reference |
|
| Reference |
|
|
| Right
side | 1.982 | 1.273–3.086 | 0.002 | 2.179 | 1.192–3.984 | 0.011 |
| Histological
type |
|
|
|
|
|
|
| Well,
moderately | Reference |
|
| Reference |
|
|
| Poorly,
mucinous | 0.685 | 0.355–1.321 | 0.259 | 0.552 | 0.257–1.185 | 0.127 |
| RAS status |
|
|
|
|
|
|
| Wild
type | Reference |
|
| Reference |
|
|
| Mutant
type | 1.699 | 1.100–2.625 | 0.017 | 1.533 | 0.957–2.456 | 0.075 |
| Detection of
unresectable tumor |
|
|
|
|
|
|
|
Synchronous | Reference |
|
| Reference |
|
|
|
Metachronous | 1.107 | 0.740–1.656 | 0.621 | 1.171 | 0.642–2.133 | 0.607 |
| The number of
organs affected by metastasis |
|
|
|
|
|
|
| 1 | Reference |
|
| Reference |
|
|
| ≥2 | 1.119 | 0.756–1.656 | 0.573 | 0.650 | 0.360–1.175 | 0.154 |
| Peritoneal
dissemination |
|
|
|
|
|
|
|
Negative | Reference |
|
| Reference |
|
|
|
Positive | 1.145 | 0.726–1.805 | 0.560 | 1.739 | 0.850–3.559 | 0.130 |
| Molecular targeted
therapy |
|
|
|
|
|
|
|
Present | Reference |
|
| Reference |
|
|
|
Absent | 1.116 | 0.764–1.630 | 0.571 | 0.960 | 0.601–1.534 | 0.866 |
| SIS |
|
|
|
|
|
|
| 0 | Reference |
|
| Reference |
|
|
| 1 | 1.567 | 0.975–2.516 | 0.063 | 1.873 | 1.075–3.263 | 0.027 |
| 2 | 2.386 | 1.451–3.921 | 0.001 | 4.138 | 2.163–7.916 | <0.001 |
Setting new cut-off values for the LMR
and the serum albumin concentration
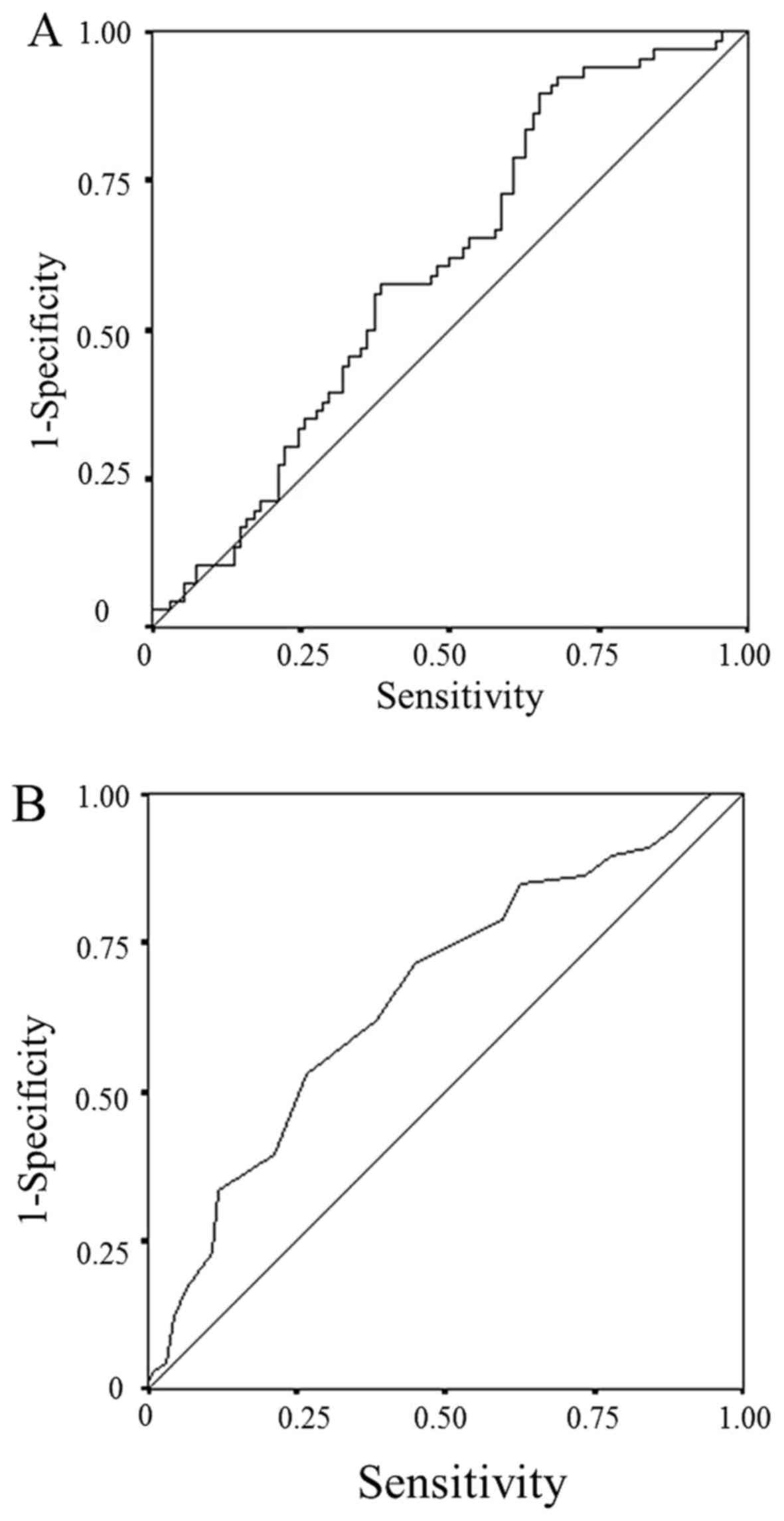
A receiver operating characteristic (ROC) curve
analysis was used to determine the optimal cut-off LMR and serum
albumin level. We used the LMR and serum albumin level, continuous
variables, as the test variable and the 24.4-month survival (median
survival time: 24.4 months) as the state variable. The optimum
cut-off values were selected based on the highest Youden index; the
optimum cut-off LMR was 2.96 (sensitivity: 89.4%; specificity:
35.1%), while the optimum cut-off serum albumin level was 4.0
(sensitivity: 53.0%; specificity: 73.4%) (Fig. 2). The patients were classified into
the high-LMR (n=120) and low-LMR (n=40) groups based on the new
cut-off LMR. In the same way, the patients were classified into the
high-ALB (n=60) and low-ALB (n=100) groups.
Prognostic value of the LMR and the
serum albumin concentration
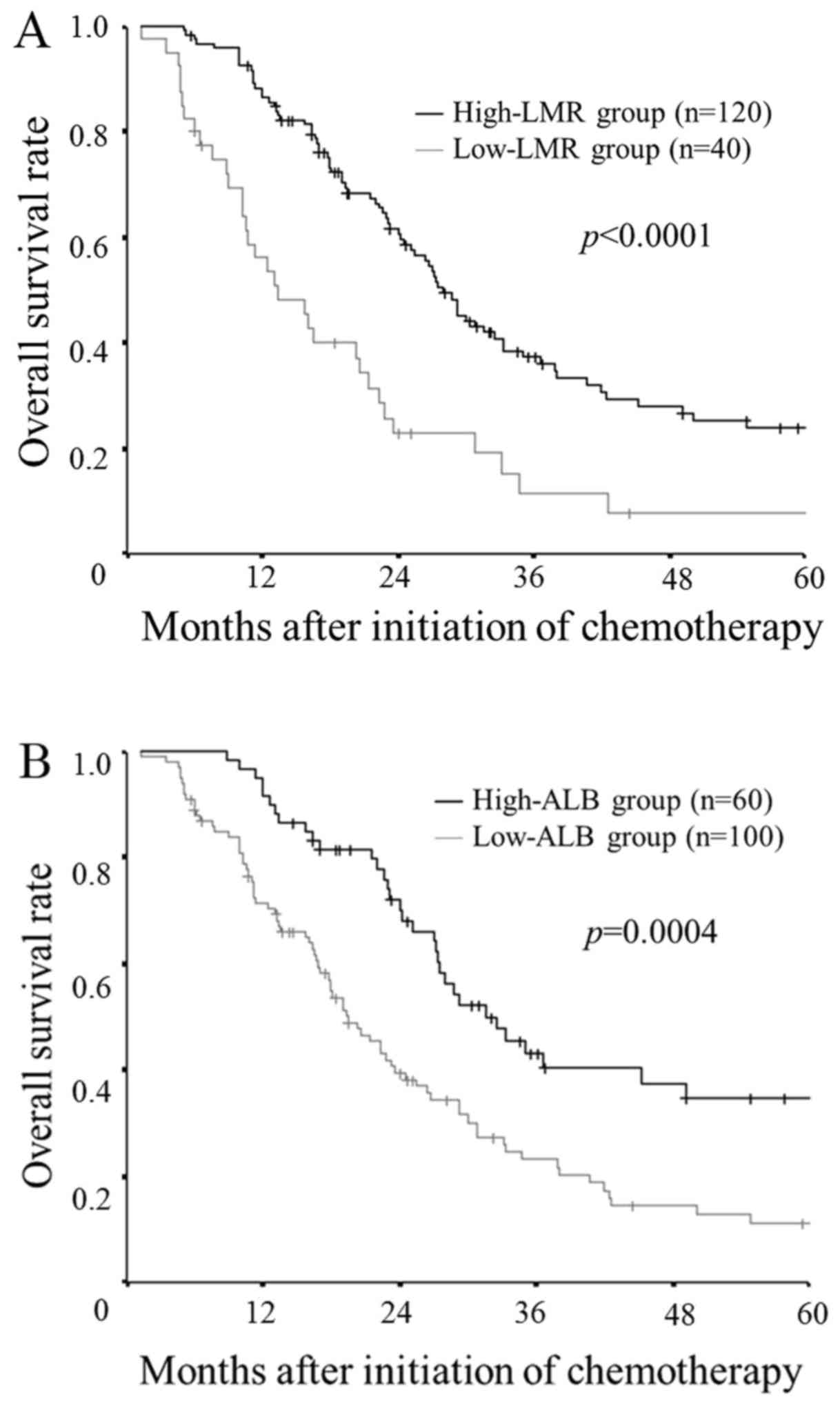
The patients in the low-LMR group had a
significantly worse overall survival rate in comparison to the
patients in the high-LMR group (P<0.0001; Fig. 3A). Similarly, the patients in the
low-ALB group had significantly worse overall survival in
comparison to the high-ALB group (P=0.0004; Fig. 3B).
Prognostic value of the SIS according
to the new cut-off value derived in our data set
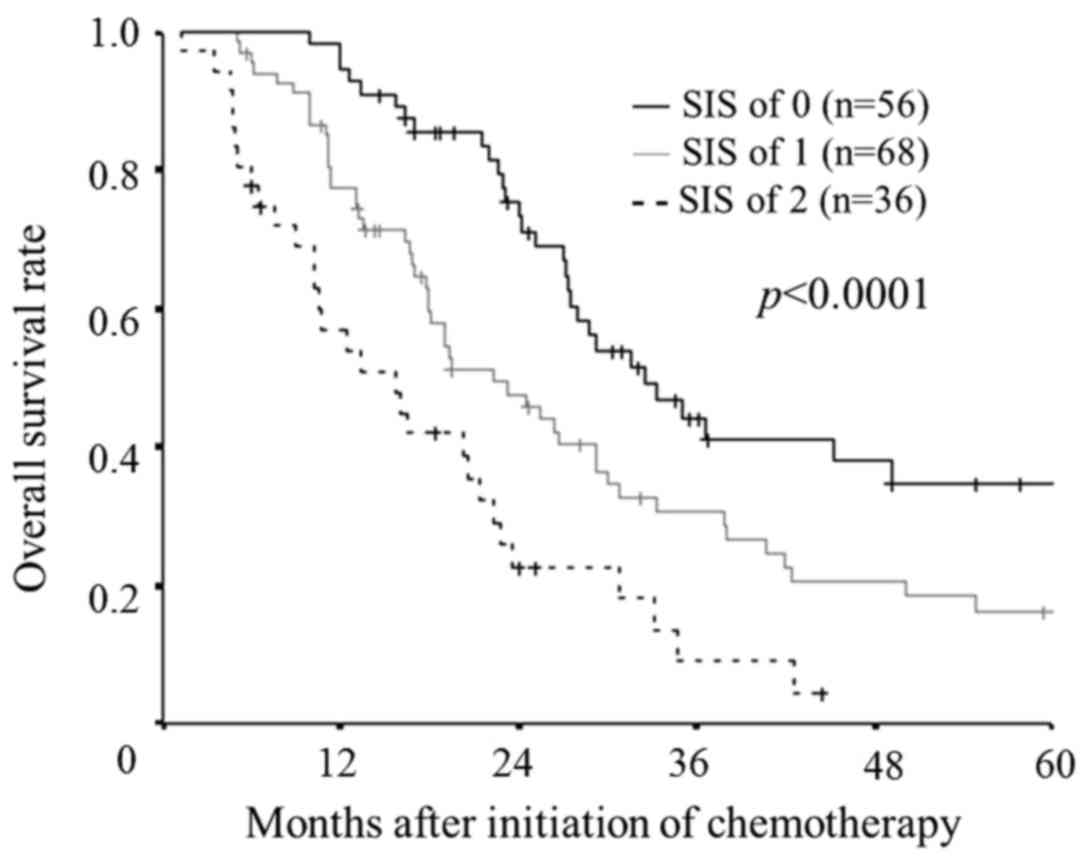
According to the new cut-off value (LMR: 2.96, serum
albumin level: 4.0) as well as the original cut-off value, the SIS
was significantly associated with the overall survival rates
(P<0.0001; Fig. 4). Furthermore,
there were significant differences between the each subgroup.
Discussion
The results obtained in this study suggested that
the SIS was significantly associated with the survival outcomes and
may be useful as a prognostic biomarker in patients with
unresectable mCRC. To our knowledge, this is the first study to
assess the prognostic value of the SIS in patients with
unresectable mCRC.
Albumin is a protein synthesized in the liver. Under
conditions of systemic inflammation, the ability to synthesize
albumin decreases, resulting in hypoalbuminemia (15). Therefore, a low serum albumin
concentration is associated with ongoing systemic inflammation. Due
to the fact that continuous systemic inflammation promotes cancer
progression (1,2), hypoalbuminemia is associated with a poor
survival (16).
The LMR reflects the balance between the immune
status of the host and the degree of tumor burden. Lymphocytes play
a key role in anticancer immunity (1,17), and a
decreasing number of lymphocytes has been reported to be associated
with a poor prognosis (18,19). In contrast, monocytes contribute to
cancer progression (1,20,21).
Circulating monocytes differentiate into macrophages in the cancer
microenvironment (22,23). Most macrophages in the cancer
microenvironment have an M2-like phenotype and promote tumor
growth, angiogenesis and metastasis (20,24). Thus
an increasing number of monocytes has been reported to be
associated with a poor prognosis (5,18,25). For these reasons, a low LMR is
associated with a poor prognosis.
The serum albumin concentration and white blood cell
count are inexpensive to measure and are routinely applied in
clinical practice. The combination of these two inflammatory
markers based on different mechanisms may enable a more accurate
prognostic prediction.
Both the serum albumin concentration and the LMR,
which are components of the SIS, are markers related to
inflammation, but their severity is not always correlated with each
other (11). Therefore, the
combination of these two markers enables a more detailed
stratification. The SIS can be used to classify patients into three
risk subgroups, whereas most inflammatory markers reported as
prognostic markers in previous reports are only able to divide
patients into two groups. The GPS as well as the SIS can classify
patients into three risk subgroups. However, according to the GPS,
most patients (80–90%) are classified into the low-risk group
(10,26,27), and
the distribution of the GPS score is not well-balanced. In
contrast, the distribution of the SIS score is relatively
well-balanced. These results suggest that the SIS may have higher
clinical utility than other inflammatory markers.
According to the original cut-off values defined in
a previous report, the SIS was significantly associated with the
survival. However, the optimum cut-off values derived in our
dataset was different from those obtained in the previous report.
As cancer progresses, the degree of inflammation caused by the
response of the host to the cancer increases (3). In previous reports, the inflammatory
markers tended to increase as the stage progressed (3,10,28). Furthermore, even at the same stage,
the degree of inflammation may vary depending on the type of
cancer. The optimum cut-off values of the inflammatory markers used
in previous reports differed by type of cancer, even at the same
stage (29–31). Therefore, it is necessary to reset the
optimum cut-off value of the serum albumin concentration and the
LMR, which is most closely associated with the prognosis, depending
on the characteristics of the target, such as the cancer type and
stage. The optimum cut-off value of the SIS needs to be examined in
further studies, which include a large unified population of cancer
types, stages and treatments. The same may be true of the cut-off
for the GPS.
The AUC of the ROC curve for the LMR and the serum
albumin concentration were relatively low, despite both markers
having been reported to be useful prognostic markers in many
previous reports (5,6,16). We
thought that the small number of cases was the reason for the low
AUC. A large prospective study is therefore necessary to confirm
the usefulness of the SIS as a prognostic marker.
In conclusion, the SIS is considered to be a useful
biomarker for predicting the survival outcomes in patients with
unresectable mCRC cancer who undergo chemotherapy, although the
optimum cut-off value according to each patient's background needs
to be examined in further studies. Patients with high SIS scores
are expected to have a poor prognosis. Thus, an intensive
chemotherapy regimen aiming at cytoreduction-as opposed to disease
control-should be selected for patients with a high SIS score. The
SIS may contribute to decisions regarding the choice of therapeutic
strategies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MS and KM designed the study, performed the
statistical analysis and drafted the manuscript. HN, TF, SM, KK and
RA collected the clinical data and critically revised the
manuscript. KH and MO designed the study and critically reviewed
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All patients were informed of the investigational
nature of this study and provided their written informed consent.
Full ethical approval was granted by the ethics committee of Osaka
City University (approval number 926).
Consent for publication
All patients provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SIS
|
systemic inflammatory score
|
|
LMR
|
lymphocyte-to-monocyte ratio
|
|
mCRC
|
metastatic colorectal cancer
|
|
NLR
|
neutrophil-to-lymphocyte ratio
|
|
GPS
|
Glasgow prognostic score
|
|
PS
|
performance status
|
|
ROC curve
|
receiver operating characteristic
curve
|
|
CI
|
confidence interval
|
References
|
1
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shibutani M, Maeda K, Nagahara H, Noda E,
Ohtani H, Nishiguchi Y and Hirakawa K: A high preoperative
neutrophil-to-lymphocyte ratio is associated with poor survival in
patients with colorectal cancer. Anticancer Res. 33:3291–3294.
2013.PubMed/NCBI
|
|
4
|
Absenger G, Szkandera J, Stotz M,
Postlmayr U, Pichler M, Ress AL, Schaberl-Moser R, Loibner H,
Samonigg H and Gerger A: Preoperative neutrophil-to-lymphocyte
ratio predicts clinical outcome in patients with stage II and III
colon cancer. Anticancer Res. 33:4591–4594. 2013.PubMed/NCBI
|
|
5
|
Shibutani M, Maeda K, Nagahara H, Iseki Y,
Ikeya T and Hirakawa K: Prognostic significance of the preoperative
lymphocyte-to-monocyte ratio in patients with colorectal cancer.
Oncol Lett. 13:1000–1006. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo YH, Sun HF, Zhang YB, Liao ZJ, Zhao L,
Cui J, Wu T, Lu JR, Nan KJ and Wang SH: The clinical use of the
platelet/lymphocyte ratio and lymphocyte/monocyte ratio as
prognostic predictors in colorectal cancer: A meta-analysis.
Oncotarget. 8:20011–20024. 2017.PubMed/NCBI
|
|
7
|
Liu Y, He X, Pan J, Chen S and Wang L:
Prognostic role of Glasgow prognostic score in patients with
colorectal cancer: Evidence from population studies. Sci Rep.
7:61442017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kishiki T, Masaki T, Matsuoka H, Kobayashi
T, Suzuki Y, Abe N, Mori T and Sugiyama M: Modified Glasgow
prognostic score in patients with incurable stage IV colorectal
cancer. Am J Surg. 206:234–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang Y, An H, Xu L, Zhu Y, Yang Y, Lin Z
and Xu J: Systemic inflammation score predicts postoperative
prognosis of patients with clear-cell renal cell carcinoma. Br J
Cancer. 113:626–633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki Y, Okabayashi K, Hasegawa H,
Tsuruta M, Shigeta K, Kondo T and Kitagawa Y: Comparison of
preoperative inflammation-based prognostic scores in patients with
colorectal cancer. Ann Surg. 267:527–531. 2018.PubMed/NCBI
|
|
11
|
Eltohami YI, Kao HK, Lao WW, Huang Y,
Abdelrahman M, Liao CT, Yen TC and Chang KP: The prediction value
of the systemic inflammation score for oral cavity squamous cell
carcinoma. Otolaryngol Head Neck Surg. Jan 1–2018.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cassidy J, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Randomized phase III study of capecitabine plus
oxaliplatin compared with fluorouracil/folinic acid plus
oxaliplatin as first-line therapy for metastatic colorectal cancer.
J Clin Oncol. 26:2006–2012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamada Y, Takahari D, Matsumoto H, Baba H,
Nakamura M, Yoshida K, Yoshida M, Iwamoto S, Shimada K, Komatsu Y,
et al: Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab
versus S-1 and oxaliplatin plus bevacizumab in patients with
metastatic colorectal cancer (SOFT): An open-label,
non-inferiority, randomised phase 3 trial. Lancet Oncol.
14:1278–1286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McMillan DC, Elahi MM, Sattar N, Angerson
WJ, Johnstone J and McArdle CS: Measurement of the systemic
inflammatory response predicts cancer-specific and non-cancer
survival in patients with cancer. Nutr Cancer. 41:64–69. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nazha B, Moussaly E, Zaarour M,
Weerasinghe C and Azab B: Hypoalbuminemia in colorectal cancer
prognosis: Nutritional marker or inflammatory surrogate? World J
Gastrointest Surg. 7:370–377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dunn GP, Old LJ and Schreiber RD: The
immunobiology of cancer immunosurveillance and immunoediting.
Immunity. 21:137–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shibutani M, Maeda K, Nagahara H, Ohtani
H, Sakurai K, Yamazoe S, Kimura K, Toyokawa T, Amano R, Tanaka H,
et al: Prognostic significance of the lymphocyte-to-monocyte ratio
in patients with metastatic colorectal cancer. World J
Gastroenterol. 21:9966–9973. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cézé N, Thibault G, Goujon G, Viguier J,
Watier H, Dorval E and Lecomte T: Pre-treatment lymphopenia as a
prognostic biomarker in colorectal cancer patients receiving
chemotherapy. Cancer Chemother Pharmacol. 68:1305–1313. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Condeelis J and Pollard JW: Macrophages:
Obligate partners for tumor cell migration, invasion, and
metastasis. Cell. 124:263–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leek RD and Harris AL: Tumor-associated
macrophages in breast cancer. J Mammary Gland Biol Neoplasia.
7:177–189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mantovani A, Bottazzi B, Colotta F,
Sozzani S and Ruco L: The origin and function of tumor-associated
macrophages. Immunol Today. 13:265–270. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paik KY, Lee IK, Lee YS, Sung NY and Kwon
TS: Clinical implications of systemic inflammatory response markers
as independent prognostic factors in colorectal cancer patients.
Cancer Res Treat. 46:65–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sugimoto K, Komiyama H, Kojima Y, Goto M,
Tomiki Y and Sakamoto K: Glasgow prognostic score as a prognostic
factor in patients undergoing curative surgery for colorectal
cancer. Dig Surg. 29:503–509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Furukawa K, Shiba H, Haruki K, Fujiwara Y,
Iida T, Mitsuyama Y, Ogawa M, Ishida Y, Misawa T and Yanaga K: The
Glasgow prognostic score is valuable for colorectal cancer with
both synchronous and metachronous unresectable liver metastases.
Oncol Lett. 4:324–328. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jia J, Zheng X, Chen Y, Wang L, Lin L, Ye
X, Chen Y, Chen D and Dettke M: Stage-dependent changes of
preoperative neutrophil to lymphocyte ratio and platelet to
lymphocyte ratio in colorectal cancer. Tumour Biol. 36:9319–9325.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shibutani M, Maeda K, Nagahara H, Ohtani
H, Sakurai K, Yamazoe A, Kimura K, Toyokawa T, Amano R, Kubo N, et
al: Significance of markers of systemic inflammation for predicting
survival and chemotherapeutic outcomes and monitoring tumor
progression in patients with unresectable metastatic colorectal
cancer. Anticancer Res. 35:5037–5046. 2015.PubMed/NCBI
|
|
30
|
Tanaka H, Muguruma K, Toyokawa T, Kubo N,
Ohira M and Hirakawa K: Differential impact of the
neutrophil-lymphocyte ratio on the survival of patients with stage
IV gastric cancer. Dig Surg. 31:327–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cedrés S, Torrejon D, Martínez A, Martinez
P, Navarro A, Zamora E, Mulet-Margalef N and Felip E: Neutrophil to
lymphocyte ratio (NLR) as an indicator of poor prognosis in stage
IV non-small cell lung cancer. Clin Transl Oncol. 14:864–869. 2012.
View Article : Google Scholar : PubMed/NCBI
|