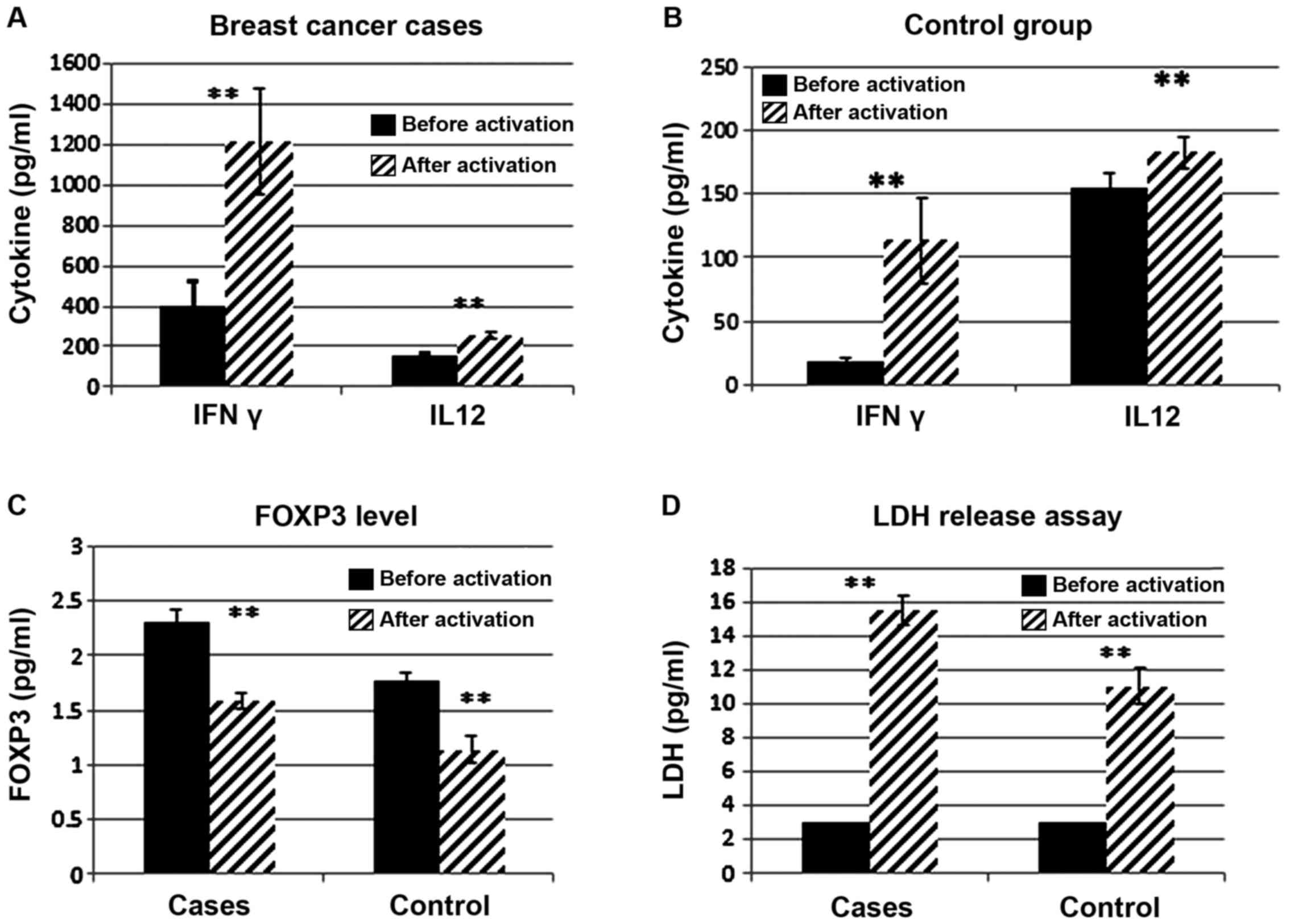
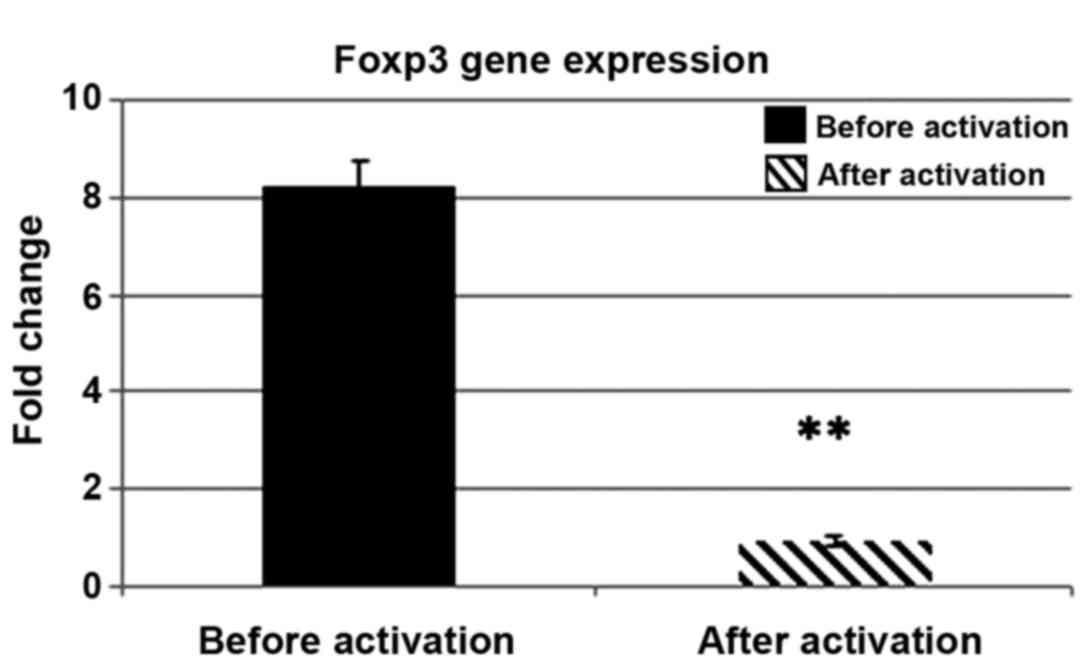
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee J, Park S, Kim S, Kim J, Ryu J, Park
HS, Kim SI and Park BW: Characteristics and survival of breast
cancer patients with multiple synchronous or metachronous primary
cancers. Yonsei Med J. 56:1213–1220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ibrahim AS, Khaled HM, Mikhail NN, Baraka
H and Kamel H: Cancer incidence in Egypt: Results of the national
population-based cancer registry program. J Cancer Epidemiol.
2014:4379712014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caffarel MM, Andradas C, Pérez-Gómez E,
Guzmán M and Sánchez C: Cannabinoids: A new hope for breast cancer
therapy? Cancer Treat Rev. 38:911–918. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang P, Yi S, Li X, Liu R, Jiang H, Huang
Z, Liu Y, Wu J and Huang Y: Preparation of triple-negative breast
cancer vaccine through electrofusion with day-3 dendritic cells.
PLoS One. 9:e1021972014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berneman Z, Van de Velde A, Anguille S,
Willemen Y, Huizing M, Germonpré P, Saevels K, Nijs G, Cools N, Van
Driessche A, et al: Vaccination with Wilms' Tumor Antigen (WT1)
mRNA-electroporated dendritic cells as an adjuvant treatment in 60
cancer patients: Report of clinical effects and increased survival
in acute myeloid leukemia, metastatic breast cancer, glioblastoma
and mesothelioma. Cytotherapy. 18:S13–S14. 2016. View Article : Google Scholar
|
|
7
|
Bigalke I, Honnashagen K, Lundby M, Solum
G, Skoge L, Inderberg EMS, Kasten J, Saboe-Larssen S, Schendel DJ
and Kvalheim G: Abstract 2516: A new generation of dendritic cells
to improve cancer therapy shows prolonged progression-free survival
in patients with solid tumors. Cancer Res. 75 15 Suppl:S25162015.
View Article : Google Scholar
|
|
8
|
Song QK, Ren J, Zhou XN, Wang XL, Song GH,
Di LJ, Yu J, Hobeika A, Morse MA, Yuan YH, et al: The prognostic
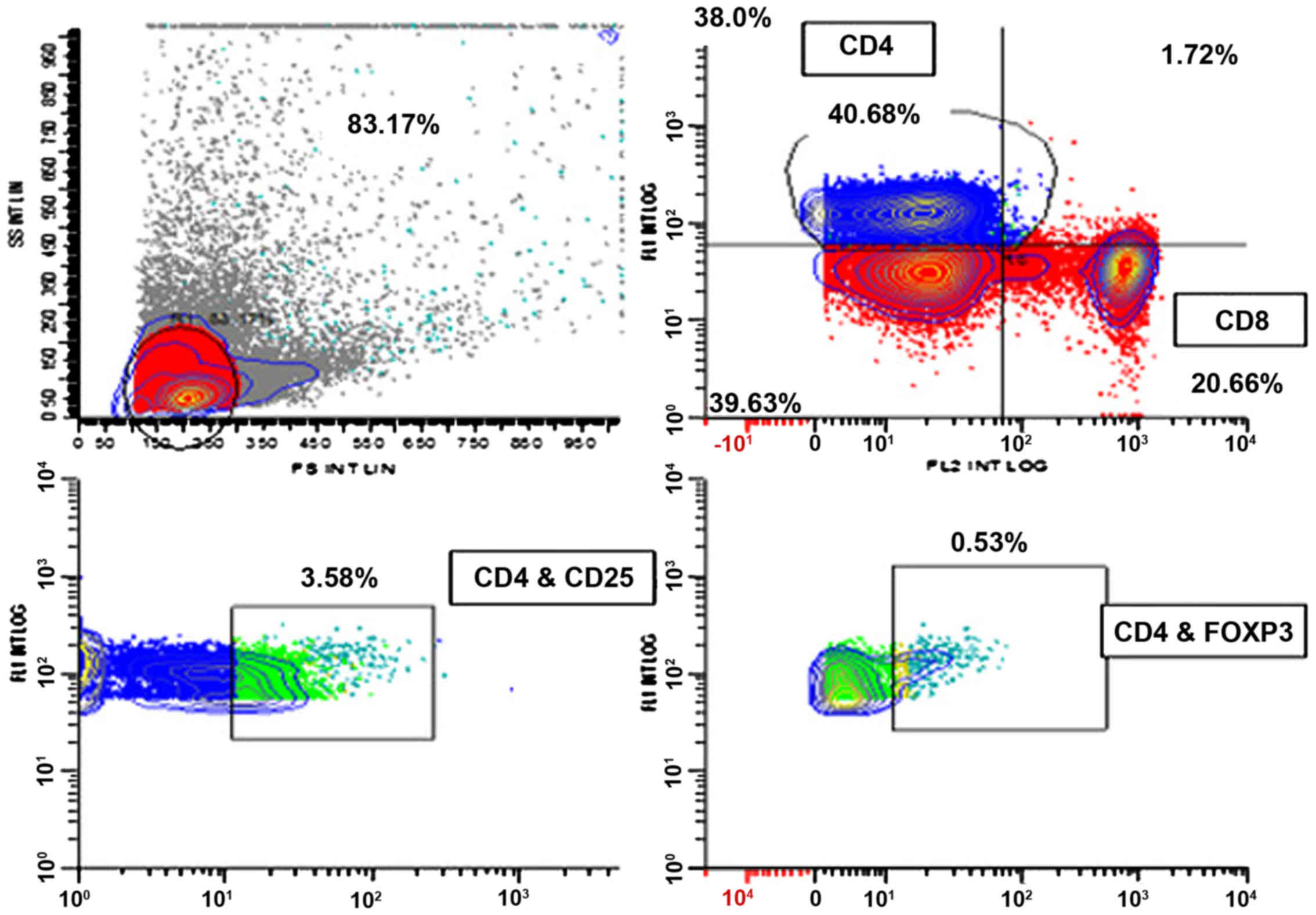
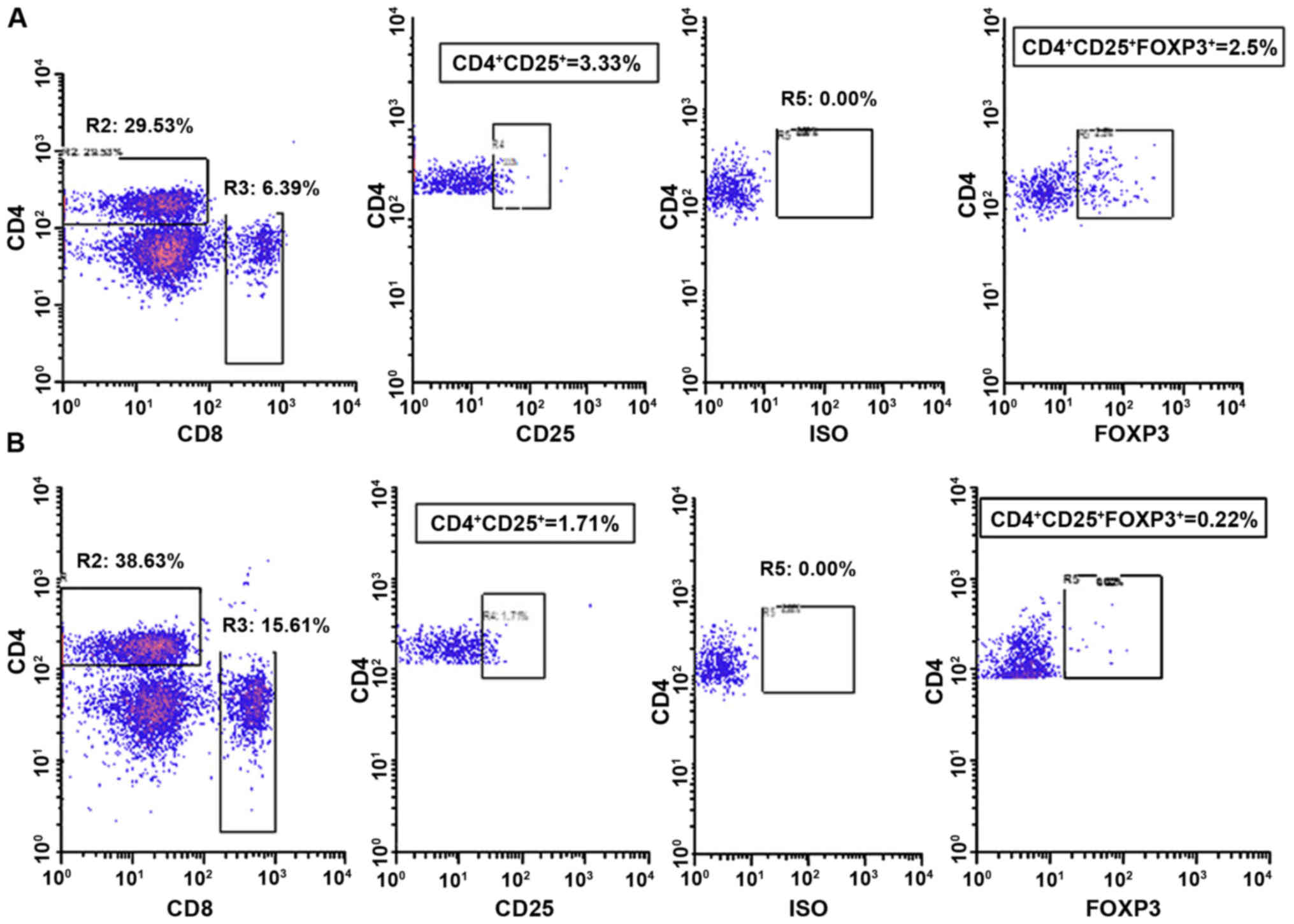
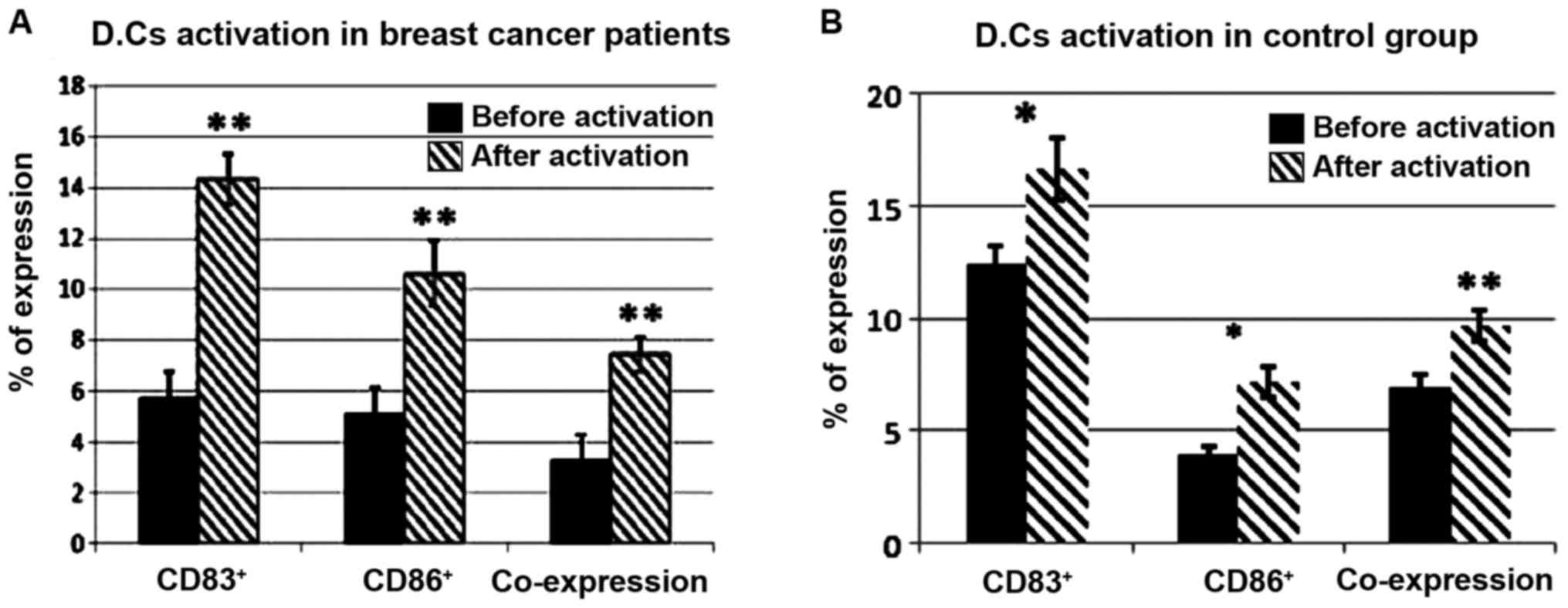
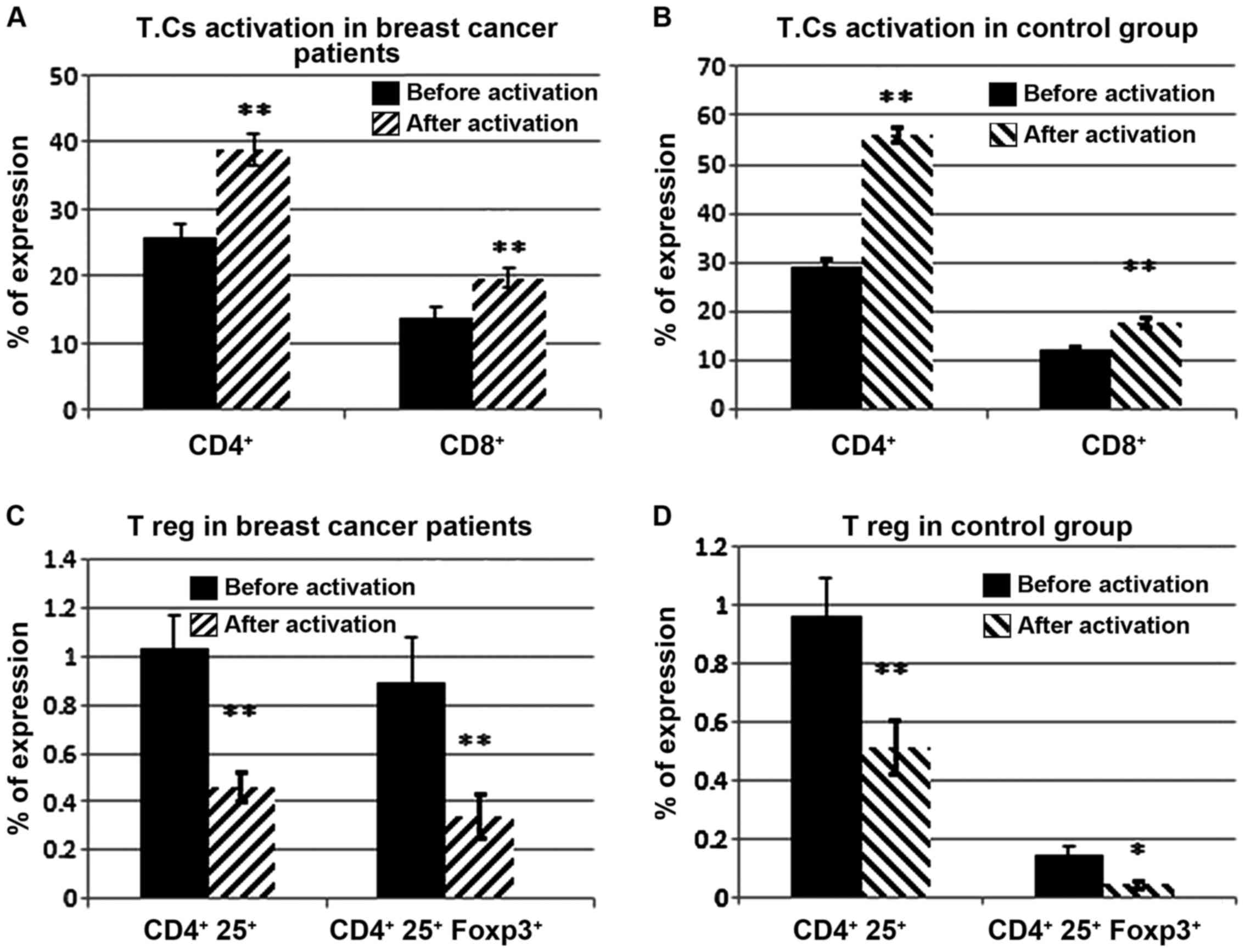
value of peripheral CD4+CD25+ T lymphocytes
among early stage and triple negative breast cancer patients
receiving dendritic cells-cytokine induced killer cells infusion.
Oncotarget. 6:41350–41359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iranpour S, Nejati V, Delirezh N, Biparva
P and Shirian S: Enhanced stimulation of anti-breast cancer T cells
responses by dendritic cells loaded with poly lactic-co-glycolic
acid (PLGA) nanoparticle encapsulated tumor antigens. J Exp Clin
Cancer Res. 35:1682016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ciccocioppo R, Ricci G, Rovati B, Pesce I,
Mazzocchi S, Piancatelli D, Cagnoni A, Millimaggi D, Danova M and
Corazza GR: Reduced number and function of peripheral dendritic
cells in coeliac disease. Clin Exp Immunol. 149:487–496. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bøyum A: Isolation of lymphocytes,
granulocytes and macrophages. Scand J Immunol. Suppl 5:S9–S15.
1976. View Article : Google Scholar
|
|
12
|
Kwok S: Procedures to minimize PCR-product
carry-over. PCR protocols: A Guide Met Appl. 142–145. 1990.
|
|
13
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei FQ, Sun W, Wong TS, Gao W, Wen YH, Wei
JW, Wei Y and Wen WP: Eliciting cytotoxic T lymphocytes against
human laryngeal cancer-derived antigens: Evaluation of dendritic
cells pulsed with a heat-treated tumor lysate and other
antigen-loading strategies for dendritic-cell-based vaccination. J
Exp Clin Cancer Res. 35:182016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fields R, Shimizu K and Mulé JJ: Murine
dendritic cells pulsed with whole tumor lysates mediate potent
antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci
USA. 95:9482–9487. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nesrua FO, Atuaolcl S, Gilliet M, Sun Y,
Grabbe S, Dummer R, Burg G and Schadendorf D: Vaccination of
melanoma patients with peptide- or tumor lysate-pulsed dendritic
cells. Nat Med. 4:328–332. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jouanneau E, Poujol D, Gulia S, Le Mercier
I, Blay J, Belin MF and Puisieux I: Dendritic cells are essential
for priming but inefficient for boosting antitumour immune response
in an orthotopic murine glioma model. Cancer Immunol Immunother.
55:254–267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Yoneyama H, Wang Y, Ishikawa S,
Hashimoto S, Gao JL, Murphy P and Matsushima K: Mobilization of
dendritic cell precursors into the circulation by administration of
MIP-1alpha in mice. J Natl Cancer Inst. 96:201–209. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hersey P, Menzies SW, Halliday GM, Nguyen
T, Farrelly ML, DeSilva C and Lett M: Phase I/II study of treatment
with dendritic cell vaccines in patients with disseminated
melanoma. Cancer Immunol Immunother. 53:125–134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Höltl L, Zelle-Rieser C, Gander H, Papesh
C, Ramoner R, Bartsch G, Rogatsch H, Barsoum AL, Coggin JH Jr and
Thurnher M: Immunotherapy of metastatic renal cell carcinoma with
tumor lysate-pulsed autologous dendritic cells. Clin Cancer Res.
8:3369–3376. 2002.PubMed/NCBI
|
|
22
|
Ascierto ML, Idowu MO, Zhao Y, Khalak H,
Payne KK, Wang XY, Dumur CI, Bedognetti D, Tomei S, Ascierto PA, et
al: Molecular signatures mostly associated with NK cells are
predictive of relapse free survival in breast cancer patients. J
Transl Med. 11:1452013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Idoyaga J, Moreno J and Bonifaz L: Tumor
cells prevent mouse dendritic cell maturation induced by TLR
ligands. Cancer Immunol Immunother. 56:1237–1250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vieira PL, de Jong EC, Wierenga EA,
Kapsenberg ML and Kaliński P: Development of Th1-inducing capacity
in myeloid dendritic cells requires environmental instruction. J
Immunol. 164:4507–4512. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Benchetrit F, Gazagne A, Adotevi O,
Haicheur N, Godard B, Badoual C, Fridman WH and Tartour E:
Cytotoxic T lymphocytes: Role in immunosurveillance and in
immunotherapy. Bull Cancer. 90:677–685. 2003.PubMed/NCBI
|
|
26
|
Bailur Kini J, Gueckel B and Pawelec G:
Prognostic impact of high levels of circulating plasmacytoid
dendritic cells in breast cancer. J Transl Med. 14:1512016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morse MA, Secord AA, Blackwell K, Hobeika
AC, Sinnathamby G, Osada T, Hafner J, Philip M, Clay TM, Lyerly HK
and Philip R: MHC class I-presented tumor antigens identified in
ovarian cancer by immunoproteomic analysis are targets for T-cell
responses against breast and ovarian cancer. Clin Cancer Res.
17:3408–3419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Woo EY, Yeh H, Chu CS, Schlienger K,
Carroll RG, Riley JL, Kaiser LR and June CH: Cutting edge:
Regulatory T cells from lung cancer patients directly inhibit
autologous T cell proliferation. J Immunol. 168:4272–4276. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang ZK, Yang B, Liu H, Hu Y, Yang JL, Wu
LL, Zhou ZH and Jiao SC: Regulatory T cells increase in breast
cancer and in stage IV breast cancer. Cancer Immunol Immunother.
61:911–916. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jaberipour M, Habibagahi M, Hosseini A,
Habibabad SR, Talei A and Ghaderi A: Increased CTLA-4 and FOXP3
transcripts in peripheral blood mononuclear cells of patients with
breast cancer. Pathol Oncol Res. 16:547–551. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suzuki S, Ishida T, Yoshikawa K and Ueda
R: Progress in clinical use of CC chemokine receptor 4 antibody for
regulatory T cell suppression. Inflam Immun Cancer: Springer.
207–227. 2015.
|
|
32
|
Liu F, Lang R, Zhao J, Zhang X, Pringle
GA, Fan Y, Yin D, Gu F, Yao Z and Fu L: CD8+ cytotoxic T
cell and FOXP3+ regulatory T cell infiltration in
relation to breast cancer survival and molecular subtypes. Breast
Cancer Res Treat. 130:645–655. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hamidinia M, Boroujerdnia Ghafourian M,
Talaiezadeh A, Solgi G, Roshani R, Iranprast S and Khodadadi A:
Increased P-35, EBI3 transcripts and other treg markers in
peripheral blood mononuclear cells of breast cancer patients with
different clinical stages. Adv Pharm Bull. 5:261–267. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Faloppi L, Bianconi M, Memeo R, Gardini
Casadei A, Giampieri R, Bittoni A, Andrikou K, Del Prete M, Cascinu
S and Scartozzi M: Lactate dehydrogenase in hepatocellular
carcinoma: Something old, something new. Biomed Res Int.
2016:71962802016. View Article : Google Scholar : PubMed/NCBI
|