Introduction
Lung cancer is a major public health problem and
presents the leading cause of cancer-associated mortality worldwide
since 2007 (1,2). Oncology studies have indicated that lung
cancer is divided into non-small cell lung cancer (NSCLC) and small
cell lung cancer (SCLC), which have been demonstrated to comprise
~85 and ~15% of lung cancer cases respectively (3). A systematic review has indicated that
radiotherapy with curative intent is a favorable treatment option
in patients with early-stage NSCLC (4). At present, a number of clinical
therapeutic methods have been explored and applied for the
treatments of patients with NSCLC, including radiotherapy,
chemotherapy, Chinese medicinal herb treatment, immunotherapy,
genetic and targeted therapies (5,6). However,
the overall 5-year survival has remained poor, at <15% in
patients with NSCLC in developing countries since 2008 (7–9).
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) is a potential anticancer protein that strongly
lyses various human tumors cells by inducing apoptosis (10). Analysis of mechanisms identified that
TRAIL can induce apoptosis of tumor cells by binding with TRAIL
receptors, which further induces caspase- or
mitochondrial-dependent apoptosis (11). Studies have demonstrated that TRAIL
exerts anticancer effects by inhibiting NSCLC cell aggressiveness
and promoting NSCLC cell apoptosis (12,13).
Further study has indicated that TRAIL can lead to augmentation of
paclitaxel-induced apoptosis in human NSCLC cells by upregulation
of Bcl-2-associated death protein (Bad) and Bcl-2-associated X
protein (Bax) expression and downregulation of Bcl-2 and Bcl-w
expression (14). In addition, TRAIL
therapy enhances sensitization to death receptor-mediated apoptosis
by the proteasome inhibitor bortezomib in NSCLC cells, and
preclinical data has indicated that a combination therapy of TRAIL
and bortezomib may be an effective strategy for treatment of NSCLC
(15). Furthermore, combination of
TRAIL and actinomycin D liposomes has been identified to
significantly promote anti-tumor effects in NSCLC cells (16). Additionally, TRAIL downregulates
fibronectin (FN), Vimentin (VN) and E-cadherin (EN) expression,
which further leads to growth inhibition of tumor cells (17). These studies suggest that TRAIL may be
a potential anti-cancer agent for the treatment of human NSCLC.
Iodine-131 (I-131) therapy has been accepted for the
treatment of thyroid cancer and other carcinomas as the decay of
I-131 emits β rays that further destroy tumor cells (18–20). The
efficacy of I-131 has been widely investigated to establish the
side effects for patients with cancer (21,22). A
pivotal study demonstrated that I-131-labeled chimeric tumor
necrosis radioimmunotherapy achieved an objective response rate
(ORR) of 34.6% (complete response, 3.7%; partial response, 30.8%;
no change, 55.1%; and progressive disease, 10.3%) in all patients
and 33% in 97 NSCLC patients (23).
The efficacies of Iodine-131 in theranostic action or ionizing
effect on the cells mimicking the role of radiotherapy have been
investigated in clinical studies (24,25).
Notably, the in vitro and in vivo targeting
properties of iodine-123- or I-131-labeled monoclonal antibody 14C5
provided a novel antibody-based agent for radioimmunodetection and
radioimmunotherapy of patients bearing antigen 14C5-expressing
NSCLC tumors (26). Additionally,
studies have indicated that TRAIL induced apoptosis of tumor cells
through inhibition of activator protein-1 (AP-1) and vascular
endothelial growth factor (VEGF) expression (27,28).
However, the sole use of I-131 is not sufficient for the treatment
of NSCLC.
Based on the efficacy of TRAIL and I-131 for
inhibition of NSCLC cells, we hypothesized that a combination of
TRAIL and I-131 would generate an additive inhibition of NSCLC
cells. In the present study, the efficacy of additive treatment of
TRAIL and I-131 on NSCLC cells was investigated in vitro and
in vivo, the inhibitory effects of additive treatment of
TRAIL and I-131 on growth and aggressiveness in NSCLC cells were
also analyzed. The numbers of apoptotic cells were examined and
caspase-9 activation was also investigated in NSCLC tissue and
cells. The present study presents evidence that the combination of
TRAIL and I-131 significantly inhibits the growth and
aggressiveness of NSCLC cells through upregulation of apoptosis,
and the apoptosis of NSCLC cells may be markedly promoted by
additive management of TRAIL and I-131.
Materials and methods
Ethics statement
The experiments were implemented according to the
Guidelines for the Care and Use of Laboratory Animals of the Animal
Protection Institute of China. This study was also approved by the
Ethics Committee of the Central Hospital of Zibo (Zibo, Shandong,
P.R. China).
Cells and reagents
The NSCLC cell lines A549 and H358 were purchased
from American Type Culture Collection. All tumor cells were
cultured in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) medium (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.). All cells were cultured in a 37°C humidified atmosphere of
5% CO2.
MTT assay
A549 and H358 cells (1×103/well) were
cultured for 12 h at 37°C and then incubated with I-131 (10 mg/ml)
or/and TRAIL (10 mg/ml) in 96-well plates for 24, 48 and 72 h in
triplicate at 37°C. Following incubation, 20 µl of MTT (5 mg/ml) in
PBS solution was added to each well and was further incubated for 4
h at 37°C. The medium was then removed and 100 µl DMSO was added
into the wells to solubilize the crystals. The optical density (OD)
was measured by a microplate reader (BD Biosciences, Franklin
Lakes, NJ, USA) at a wavelength of 570 nm.
Cells invasion and migration
assays
A549 and H358 cells were incubated with I-131 (10
mg/ml) or/and TRAIL (10 mg/ml), with non-treated cells as control.
Cultured A549 or H358 cells were suspended at a density of
1×106 in 100 µl in serum-free DMEM for 24 h using a
Transwell insert (BD Biosciences, Franklin Lakes, NJ, USA) instead
of a Matrigel invasion chamber to assess migration. DMEM medium
with 5% PBS were placed in the lower chamber of the BD BioCoat
Matrigel (BD Biosciences). Cells were then added to the upper
chambers of BD BioCoat Matrigel Migration or invasion Chambers (BD
Biosciences) according to the manufacturer's protocol. A549 and
H358 cell migration and invasion were stained with 1% crystal
violet (Beyotime, Shanghai, China) for 15 min at 37°C. At least
three randomly selected fields from each sample were examined using
light a microscope at ×40, magnification. The extent of migration
and invasion of A549 and H358 cells was mapped and quantified the
results analysis.
Apoptosis assay
A549 and H358 cells were grown at 37°C with 5%
CO2 until they reached 90% confluence. The apoptosis
rate of tumor cells was assessed by incubating the cells with I-131
(10 mg/ml) or/and TRAIL (10 mg/ml) for 48 h. After incubation, the
tumor cells were trypsinized and collected. The cells were then
washed in cold PBS, adjusted to 1×106 cells/ml with PBS,
labeled with Annexin V-FITC and propidium iodide (Annexin V-FITC
Kit; BD Biosciences), and analyzed with a FACScan flow cytometer
(BD Biosciences). The treatments were performed in triplicate, and
the percentage of labeled cells undergoing apoptosis in each group
was determined and calculated using Expo32-ADC software (version
1.2B; Beckman Coulter Inc., Brea, CA, USA).
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from A549 and H358 cells was extracted
using RNAzol (Sigma-Aldrich; Merck KgaA), and DNase RNase-free was
adopted to digest total RNA at 37°C for 15 min. An RNeasy kit was
used to purify RNA and its concentration was adjusted to 1 µg/µl.
The RNA (2 µg) was used as the template to synthesize cDNA by
reacting with reverse transcriptase at 37°C for 120 min, at 99°C
for 4 min, and at 4°C for 3 min using a High Capacity cDNA Reverse
Transcription Kit (Thermo Fisher Scientific, Inc.). Subsequently,
qPCR was adopted to amplify the gene expression of FN, VN and EN
(Table I) to determine the
transcription level of mRNA, and β-actin was used as the internal
control group. Agarose electrophoresis with 1% ethidium bromide was
adopted to check the PCR amplified products. Relative mRNA
expression changes were calculated by the 2−ΔΔCq method
(29). The results are expressed as
fold expression compared with β-actin.
 | Table I.Sequences of primers used in the
present study. |
Table I.
Sequences of primers used in the
present study.
| Gene name | Sequence |
|---|
| FN | Reverse:
5′-CGTTGCCCTATCTCCGTCTC-3′ |
|
| Forward:
5′-GGGAGCAATCGGGTAATTTTCC-3′ |
| VN | Forward:
5′-ATAACATCAAGCCCAAATCTGC-3′ |
|
| Reverse:
5′-TTCCTTTTTTCTTTCCCAACA-3′ |
| EN | Forward:
5′-GCCAATCCTGATGAAATTGGAA-3′ |
|
| Reverse:
5′-CAGAACCACTGCCCTCGTAATC-3′ |
| β-actin | Reverse:
5′-CGGAGTCAACGGATTTGGTC-3′ |
|
| Forward:
5′-AGCCTTCTCCATGGTCGTGA-3′ |
Western blotting
A549 and H358 cells were then incubated with I-131
(10 mg/ml) or/and TRAIL (10 mg/ml) for 48 h. Cells were collected
and lysed in RIPA buffer (M-PER reagent for the cells and T-PER
reagent for the tissues; Thermo Fisher Scientific Inc.) followed
homogenized at 4°C for 10 min. Protein concentration was measured
using a bicinchoninic acid protein assay kit (Thermo Fisher
Scientific, Inc.). A total of 20 µg protein extracts was
electrophoresed on 12.5% polyacrylamide gradient gels and then
transferred to nitrocellulose membranes. The membranes were
incubated in blocking buffer comprising 5% bovine serum albumin
(BSA; Sigma-Aldrich; Merck KGaA) for 2 h at 37°C prior to
incubation with primary antibodies at 4°C overnight. The primary
rabbit anti-mouse antibodies used in the immunoblotting assays
were: Bcl-2 (dilution 1:200, ab3214), Bcl-w (dilution 1:1,000,
ab32370), Bax (dilution 1:500; ab32503), Bad (dilution 1:500;
ab32445), FN (dilution 1:500; ab2413), VN (dilution 1:500; ab8978),
EN (dilution 1:1,000; ab76055), VEGF (dilution 1:500; ab72278),
AP-1 (dilution 1:500; ab207196) and β-actin (dilution 1:500;
ab8226) (all from Abcam, Cambridge, UK). Horseradish
peroxidase-conjugated anti-rabbit IgG (cat no. 5204-2504; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) were used at a 1:5,000
dilution and detected using a western blotting Luminol Reagent (cat
no. 32106; Thermo Fisher Scientific, Inc.). Experiments were
repeated at least three times. Densitometric quantification of the
immunoblot data was performed by using Quantity-One software
(version 1.5; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Animal study
A total of 80 specific pathogen-free male Balb/c
mice (8-weeks old; body weight, 32–38 g) were purchased from Slack
Life Co., Ltd (Shanghai, China). All mice were housed in a
temperature-controlled facility at 23±1°C and relative humidity of
50±5% with a 12 h light/dark cycle and free access to food and
water. Experimental mice were injected with A549 or H358 tumor
cells (1×106 cells) into subcutaneous tissue and were
divided into four groups: PBS, I-131, TRAIL and combination
(I-131+TRAIL) (n=10 in each group). Mice were treated with of I-131
(10 mg/kg) or/and TRAIL (10 mg/kg) once a day, a total of 10 times.
Treatments were initiated on day 3 after tumor implantation
(diameter, 5–6 mm). The tumor volumes in each group were recorded
from three randomly selected experimental mice. The tumor volumes
were calculated according to a previous study (30). Mice were sacrificed when tumor
diameter reached 16 mm.
Immunohistochemistry
NSCLC tumors from xenograft mice were fixed in
formaldehyde (10%) for 12 h at 4°C followed with embedding in
paraffin. Tumor tissues were fabricated to 4 µm-thick sections.
Tissues were deparaffinized in 100% xylene for 30 min at 37°C and
rehydrated in a graded alcohol series (70, 80, 90, 95 and 100%).
Antigen retrieval was performed using Lab Vision™ Tris-HCl buffer
for heat-induced epitope retrieval (cat no. AP-9005-050; Thermo
Fisher Scientific, Inc.) and tumor sections were blocked with 5%
BSA for 2 h at 37°C, then incubated with primary antibodies
targeting caspase-9 (1: 1,200, ab32539; Abcam) for 12 h at 4°C.
Subsequently, horseradish peroxidase-conjugated anti-rabbit IgG
(1:2,000; cat. no., sc-362260; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) were applied for specimens for 12 h at 4°C. A
Ventana Benchmark automated staining system was used for
observation of caspase-9 expression. The slides were examined with
a Keyence Biozero BZ8100E fluorescence microscope (Keyence, Osaka,
Japan) at a magnification of ×40.
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
Apoptotic cells in tumor specimens were analyzed
using a TUNEL assay (DeadEnd™ Colorimetric Tunel System; Promega
Corporation, Madison, WI, USA) according to the manufacturer's
protocol. Tumor sections were fixed with 4% PFA for 1 h at room
temperature and then incubated with the reaction mixture (terminal
deoxynucleotidyl transferase, equilibration buffer and biotinylated
nucleotide mix) for 1 h at 37°C. Subsequently, Streptavidin- and
DAB-bound biotin were applied and the specimens were counterstained
with hemalaun (Merck Millipore, Darmstadt, Germany) and mounted in
aquatex (Merck Millipore) for 30 min at 37°C. DNA fragmentation was
detected by selecting three random fields in the tumor sections
(×40 magnification). The tissue sections were captured using a
Keyence Biozero BZ8100E fluorescence microscope at a magnification
of ×40.
Statistical analysis
All data are expressed as the mean and standard
deviation of triplicate dependent experiments and analyzed using
one-way ANOVA (with Tukey HSD test). All data were analyzed using
SPSS Statistics 19.0 (IBM Corp., Armonk, NY, USA) and Graphpad
Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
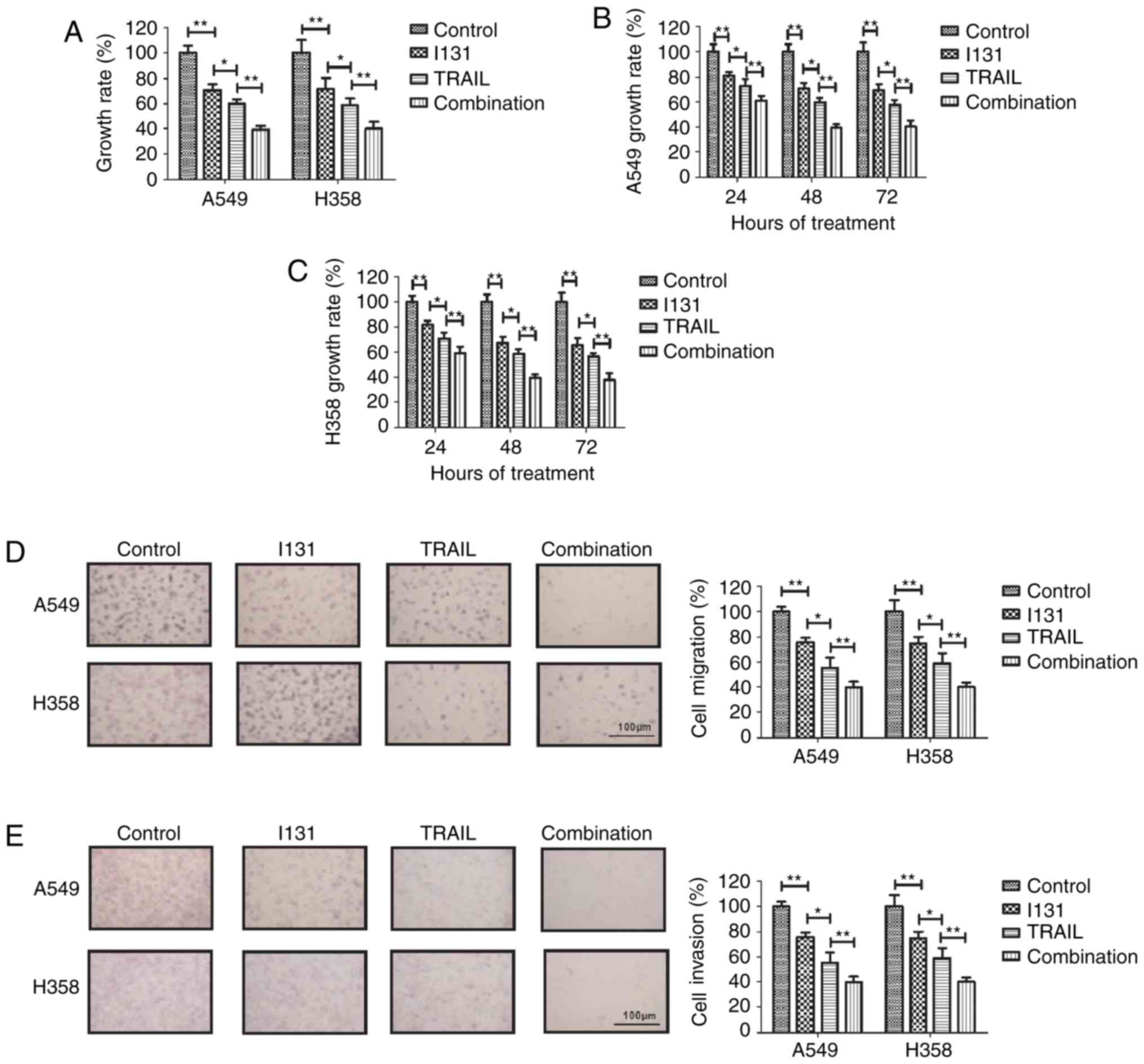
Additive treatment of TRAIL and I-131
inhibits growth and aggressiveness of NSCLC cells
To evaluate the inhibitory effects of TRAIL-I-131 on
NSCLC cell growth and aggressiveness, NSCLC cells were investigated
following treatment with TRAIL or I-131. As shown in Fig. 1A, TRAIL-I-131 treatment significantly
inhibited the growth of NSCLC cells compared with TRAIL or I-131
treatment. Fig. 1B and C revealed
that TRAIL-I-131 treatment could achieve the maximum inhibitory
effect against A549 and H358 cells after 48 h incubation. Migration
and invasion assays demonstrated that TRAIL-I-131 treatment
suppressed the migration and invasion of A549 and H358 cells in
vitro (Fig. 1D and E). These
assays confirmed that the TRAIL was more efficient in inhibiting
NSCLC cell growth and aggressiveness compared with I-131. Taken
together, these results suggest that TRAIL-I-131 additive treatment
can significantly inhibit the growth and aggressiveness of NSCLC
cells.
Additive treatment of TRAIL and I-131
(TRAIL-I-131) promotes apoptosis of NSCLC cells
In order to investigate the role of TRAIL-I-131 in
NSCLC cells, the efficacy of TRAIL-I-131 treatment in inducing
apoptosis of NSCLC cells was analyzed. The results demonstrated
that TRAIL-I-131 treatment markedly promoted apoptosis of A549 and
H358 cells compared with either TRAIL or I-131 treatment (Fig. 2A). Western blot analysis demonstrated
that TRAIL-I-131 treatment significantly decreased the levels of
the anti-apoptotic proteins Bcl-2 and Bcl-w in A549 and H358 cells,
while pro-apoptosis Bad and Bax protein levels were increased by
TRAIL-I-131 treatment compared with either TRAIL or I-131 treatment
(Fig. 2B). TRAIL-I-131 treatment was
also demonstrated to increase caspase-8 and caspase-9 activation in
A549 and H358 cells compared with the TRAIL, I-131 and control
groups (Fig. 2C and D). The results
also demonstrated TRAIL-I-131 treatment inhibited VEGF and AP-1
expression in A549 and H358 cells (Fig.
2E). TRAIL-treated A549 cells exhibited lower Bcl-2 and Bcl-w
expression than I-131-treated A549 cells, and TRAIL and I-131 had
similar effects on Bad and Bax expression in A549 and H358 cells.
These results suggest that TRAIL-I-131 additive treatment can
markedly promote apoptosis of NSCLC cells by regulating
apoptosis-associated gene expression.
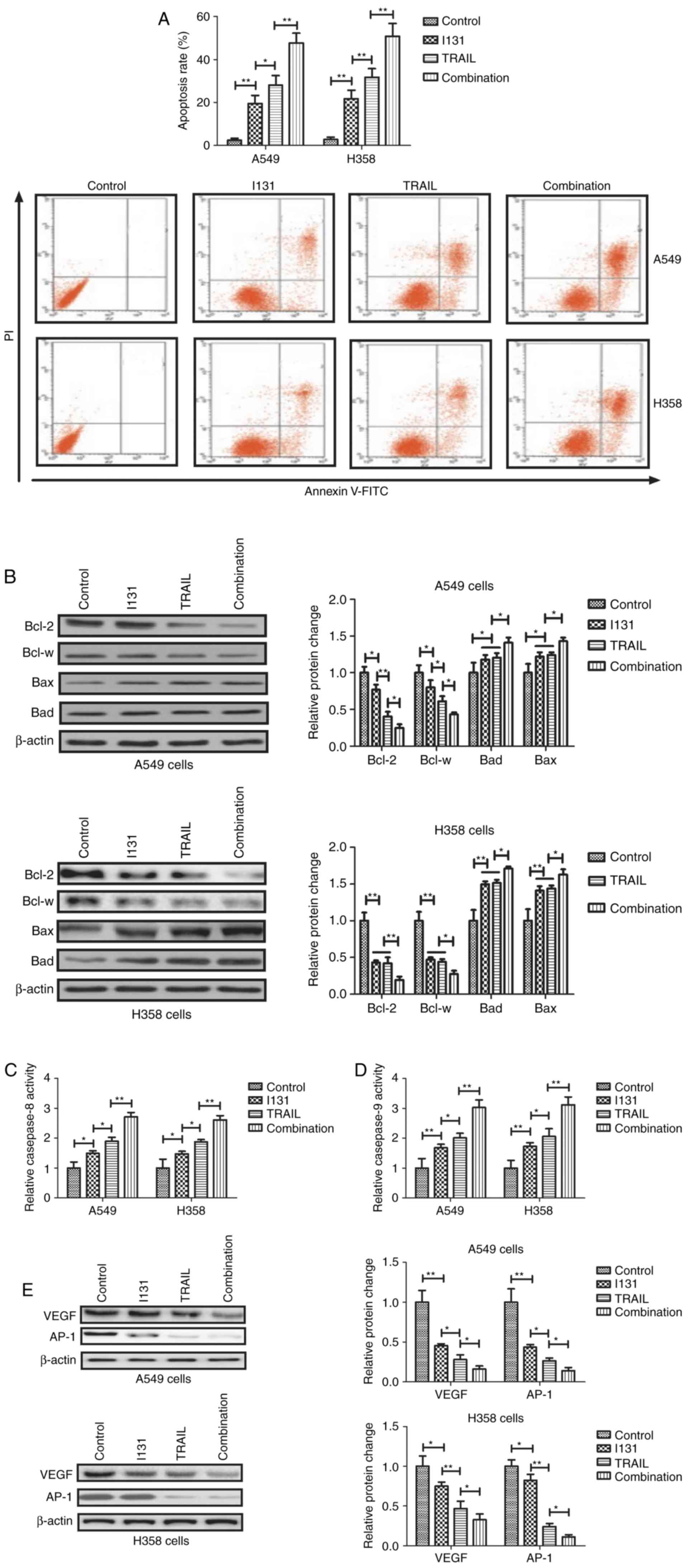 | Figure 2.Additive treatment of TRAIL and I-131
promotes apoptosis of NSCLC cells. (A) TRAIL-I-131 treatment
promoted the apoptosis of A549 and H358 cells compared with either
TRAIL, I-131 or control treatment. (B) TRAIL-I-131 treatment
increased proapoptosic Bad and Bax protein levels, and decreased
antiapoptotic Bcl-2 and Bcl-w protein levels in A549 and H358
cells. (C and D) TRAIL-I-131 treatment increased (C) caspase-8 and
(D) caspase-9 activation in A549 and H358 cells. (E) TRAIL-I-131
treatment downregulated VEGF and AP-1 expression levels in A549 and
H358 cells. All data are expressed as the mean and standard
deviation, and were analyzed using one-way analysis of variance
with Tukey HSD test. *P<0.05, TRAIL vs. I-131; **P<0.01,
I-131 vs. control, combination vs. TRAIL. TRAIL, tumor necrosis
factor related apoptosis-inducing ligand; I-131, Iodine-131; PI,
propidium iodide; FITC, fluorescein isothiocyanate; VEGF, vascular
endothelial growth factor; AP-1, activator protein-1. |
Additive treatment of TRAIL and I-131
downregulates metastasis-associated gene expression levels
Metastasis-associated gene and protein expression
levels were subsequently analyzed to understand the inhibitory
effects of TRAIL-I-131 on NSCLC cells. RT-qPCR demonstrated that
FN, VN and EN expression levels were downregulated by TRAIL-I-131
treatment in A549 and H358 cells (Fig. 3A
and B). No statistically significantly differences in miRNA
levels were observed between the TRAIL and I-131 groups in A549 and
H358 cells. Western blot analysis also demonstrated that
TRAIL-I-131 treatment significantly decreased FN, VN and EN
expression levels in A549 and H358 cells (Fig. 3C and D). TRAIL treatment significantly
downregulated FN, VN and EN expression levels compared with the
I-131 group in A549 and H358 cells, suggesting that TRAIL-I-131
additive treatment can decrease NSCLC metastasis-related FN, VN and
EN expression levels in A549 and H358 cells.
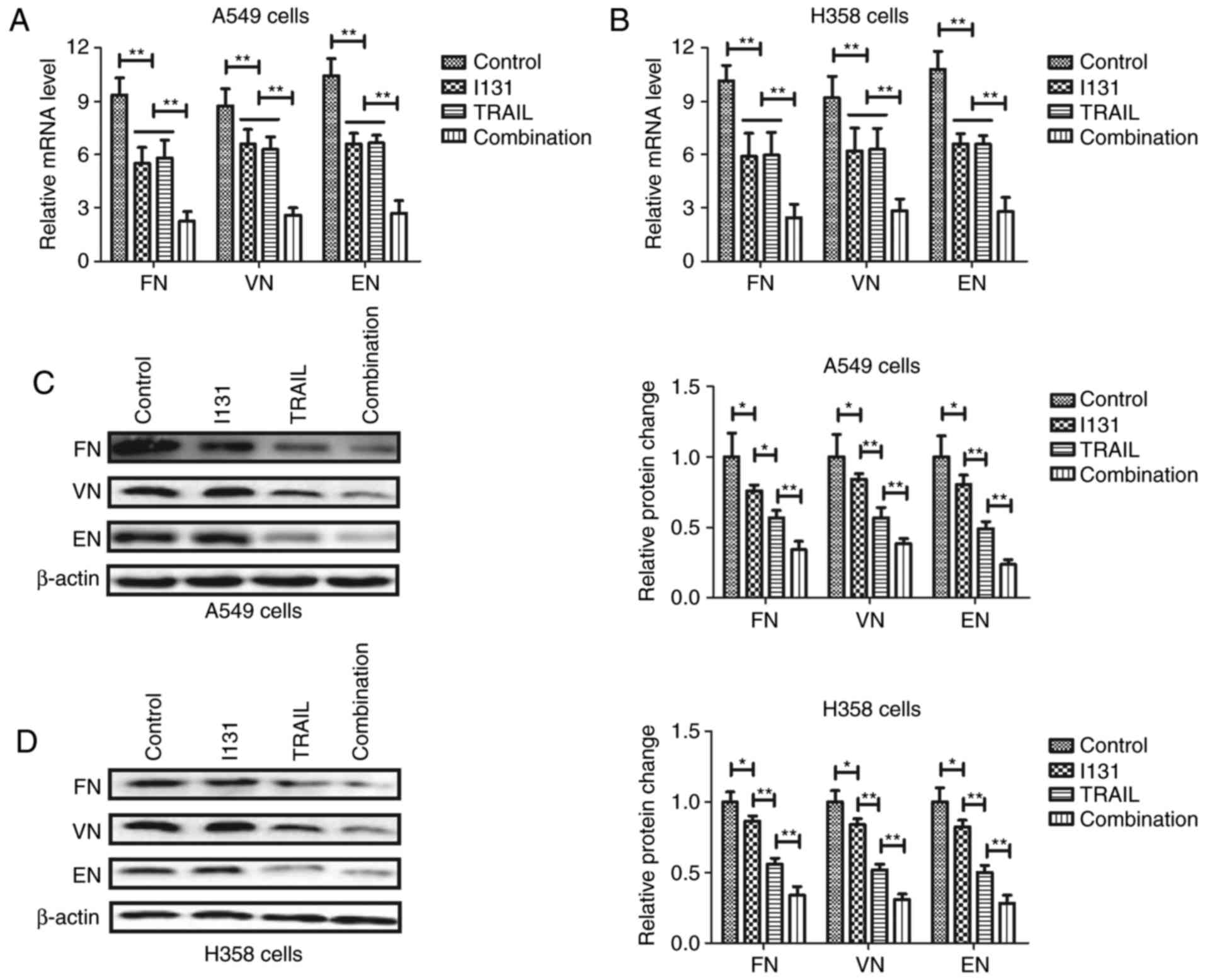 | Figure 3.Additive treatment of TRAIL and I-131
downregulates metastasis-associated gene expression levels. (A and
B) TRAIL-I-131 treatment downregulated FN, VN and EN mRNA
expression levels in (A) A549 and (B) H358 cells. TRAIL-I-131
treatment downregulated FN, VN and EN protein expression levels in
(C) A549 and (D) H358 cells. All data are expressed as the mean and
standard deviation, and were analyzed using one-way analysis of
variance with Tukey HSD test. *P<0.05; **P<0.01; I-131 vs.
control, TRAIL vs. I-131, combination vs. I-131 or TRAIL. TRAIL,
tumor necrosis factor-related apoptosis-inducing ligand; I-131,
Iodine-131; FN, fibronectin; VN, vimentin; EN, E-cadherin. |
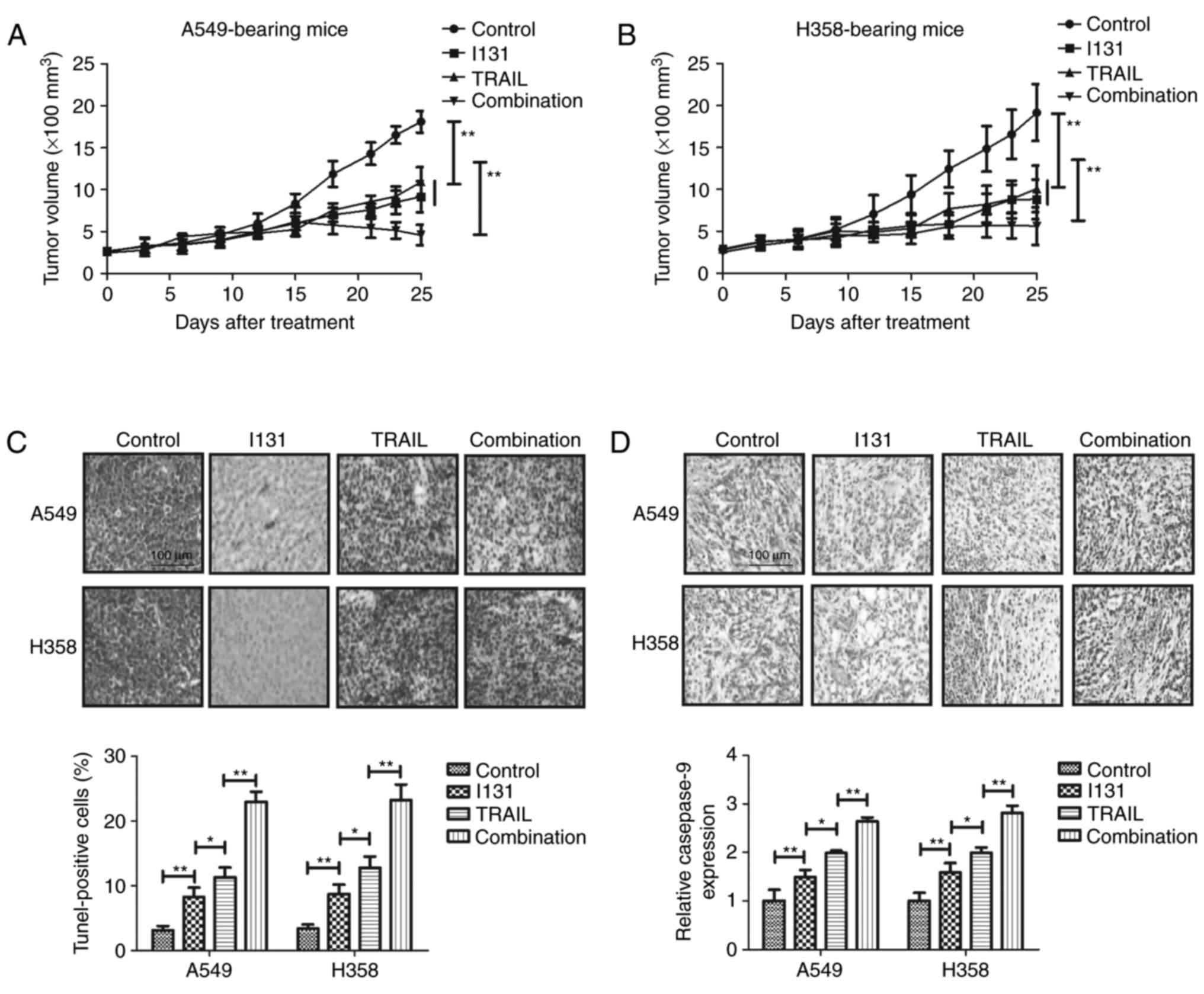
Additive treatment of TRAIL and I-131
inhibits tumor growth and promotes apoptosis in tumor tissues
To further investigate the role of TRAIL-I-131,
in vivo anti-cancer effects of TRAIL-I-131 were analyzed in
NSCLC-bearing mice. As demonstrated in Fig. 4A and B, TRAIL-I-131 treatment was
observed to inhibit tumor growth in A549- and H358-bearing mice
compared with the TRAIL, I-131 and PBS groups. No significant
difference was identified between the TRAIL and I-131 groups. A
TUNEL assay demonstrated that TRAIL-I-131 treatment increased the
number of apoptotic cells present in the tumor tissues examined
(Fig. 4C). Immunohistochemistry
demonstrated that caspase-9 expression was upregulated in tumor
tissue in TRAIL-I-131-treated tumors compared with the TRAIL, I-131
and PBS groups (Fig. 4D). These
results demonstrate that TRAIL-treated tumors present a higher
apoptosis rate and relative caspase-9 expression compared with the
I-131 group in A549- and H358-bearing mice. Taken together, these
results suggest that additive treatment of TRAIL and I-131 could
inhibit tumor growth through increasing apoptosis of NSCLC
cells.
Discussion
Systematic review and meta-analysis have indicated
that drug-induced apoptosis contributes to the inhibition of tumor
cell growth and aggressiveness (31).
A previous study also identified the advantages of using TRAIL for
human tumor cells by selectively inducing apoptosis by binding with
death receptors in NSCLC cells (32).
In addition, I131 radioimmunotherapy has presented more advantages
in the treatment of human thyroid cancer due to its easy excretion
and high accuracy (33). In the
present study, the additive therapeutic effects of TRAIL and I-131
were investigated for NSCLC cells and an NSCLC-bearing mouse model,
where TRAIL was demonstrated to possess increased efficiency
compared with I-131 in the inhibition of NSCLC cell growth.
However, the similar effects of TRAIL and I-131 on Bcl-2, Bcl-w,
Bad, Bax, FN, VN and EN expression levels have been observed in
NSCLC cells (14). These results may
be associated with the different mechanisms of action of TRAIL and
I-131 (34,35). The present study demonstrated that
additive therapy of TRAIL and I-131 significantly inhibited NSCLC
cell growth in vitro and in vivo through inducing
apoptosis.
The therapeutic efficiency of I-131 in theranostic
action has previously been identified in tumor therapy (36,37). In
the present study, the inhibitory effects of I-131 on NSCLC cells
in vitro and in vivo were analyzed, with results
demonstrating that I-131 treatment inhibited the growth of NSCLC
cells. The ionizing effects of I-131 target radiotherapy on cells,
improving anticancer efficacy of vascular disruption treatment have
been identified in rabbit VX2 tumor models (38). The results of the present study have
indicated that I-131 decreased aggressiveness and induced apoptosis
of NSCLC cells. However, the efficacy of single I-131 treatment was
limited with unsatisfactory effects on tumor inhibition. In the
present study, the results obtained indicated that additive
treatment of TRAIL and I-131 enhanced the inhibitory effects of
TRAIL or I-131 for NSCLC cells. Kaewput and Pusuwan(39) previously
reported that I-131 therapy may inhibit tumor growth and metastasis
in a patient with papillary thyroid cancer and the results of the
present study identified that I-131 promoted TRAIL-induced
apoptosis and growth inhibition for NSCLC cells.
A previous study indicated that Bcl-2 mRNA
expression may be regarded as a biomarker for patients with
curatively resected NSCLC (40). The
present study demonstrated that TRAIL-I-131 treatment decreased
anti-apoptotic Bcl-2 and Bcl-w expression levels and increased
pro-apoptotic Bad and Bax expression levels in A549 and H358 cells.
The study of Checinska et al (41) suggested that caspase-9 may initiate a
scaffold function and may serve as caspase substrate during NSCLC
apoptosis. Additionally, therapy that targets VEGF has been
demonstrated to be beneficial for the treatment of NSCLC (42,43).
Furthermore, TRAIL-I-131 treatment markedly inhibited VEGF and AP-1
expression in A549 and H358 cells, which potentially contributed to
the inhibitory efficacy of TRAIL-I-131 treatment for tumor growth
in the NSCLC-bearing mouse model. Furthermore, the sensitizing
apoptotic effect of TRAIL could enhance the inhibitory effects for
human lung cancer PC9 cells (44).
Additionally, the study of Nazim et al (45) ascertained that peroxisome
proliferator-activated receptor-γ activation by troglitazone
enhanced human lung cancer cell apoptosis induced by TRAIL. In the
present study, an in vivo assay demonstrated that
TRAIL-I-131 treatment increased the apoptotic rate and caspase-9
expression in tumor tissues compared with either TRAIL or
I-131-treated tumors.
In conclusion, the results of the present study
demonstrated that additive TRAIL and I-131 treatment effectively
inhibited NSCLC cell growth and aggressiveness. These findings
suggested that I-131 enhanced TRAIL-inhibited growth of NSCLC cells
through the induction of apoptosis. Importantly, additive TRAIL and
I-131 treatment demonstrated efficient tumor-suppressing effects on
NSCLC tumor cells in vitro and in vivo, which
suggests that additive TRAIL and I-131 treatment may be a promising
anticancer therapeutic strategy for the treatment of NSCLC.
However, this requires corroboration with further studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NY designed the present study; SY and DL performed
all experiments and analyzed all data in the present study.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Central Hospital of Zibo (Zibo, Shandong,
China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baker S, Dahele M, Lagerwaard FJ and Senan
S: A critical review of recent developments in radiotherapy for
non-small cell lung cancer. Radiat Oncol. 11:1152016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang T, Zhai C, Su C, Ren S and Zhou C:
The diagnostic value of circulating cell free DNA quantification in
non-small cell lung cancer: A systematic review with meta-analysis.
Lung Cancer. 100:63–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhukovsky M, Varaksin A and Pakholkina O:
Statistical analysis of observational study of the influence of
radon and other risk factors on lung cancer incidence. Radiat Prot
Dosimetry. 160:108–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Falkson CB, Vella ET, Yu E, El-Mallah M,
Mackenzie R, Ellis PM and Ung YC: Radiotherapy with curative intent
in patients with Early-stage, medically inoperable, non-small-cell
lung cancer: A systematic review. Clin Lung Cancer. 18:105–121.e5.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Onesti CE, Iacono D, Angelini S, Lauro S,
Mazzotta M, Occhipinti MA, Giusti R and Marchetti P: Unexpected
long survival of brain oligometastatic non-small cell lung cancer
(NSCLC) treated with multimodal treatment: A single-center
experience and review of the literature. Transl Lung Cancer Res.
5:712–719. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cadranel J, Park K, Arrieta O, Pless M,
Bendaly E, Patel D, Sasane M, Nosal A, Swallow E, Galebach P, et
al: Characteristics, treatment patterns, and survival among ALK+
non-small cell lung cancer (NSCLC) patients treated with
crizotinib: A chart review study. Lung Cancer. 98:9–14. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McKay C, Knight KA and Wright C: Beyond
cancer treatment-a review of total lymphoid irradiation for heart
and lung transplant recipients. J Med Radiat Sci. 61:202–209. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han RX, Liu X, Pan P, Jia YJ and Yu JC:
Effectiveness and safety of chemotherapy combined with dendritic
cells co-cultured with cytokine-induced killer cells in the
treatment of advanced non-small-cell lung cancer: A systematic
review and meta-analysis. PLoS One. 9:e1089582014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuykendall A and Chiappori A: Advanced
EGFR mutation-positive non-small-cell lung cancer: Case report,
literature review, and treatment recommendations. Cancer Control.
21:67–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grosse-Wilde A, Voloshanenko O, Bailey SL,
Longton GM, Schaefer U, Csernok AI, Schütz G, Greiner EF, Kemp CJ
and Walczak H: TRAIL-R deficiency in mice enhances lymph node
metastasis without affecting primary tumor development. J Clin
Invest. 118:100–110. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dai X, Zhang J, Arfuso F, Chinnathambi A,
Zayed ME, Alharbi SA, Kumar AP, Ahn KS and Sethi G: Targeting
TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural
products as a potential therapeutic approach for cancer therapy.
Exp Biol Med (Maywood). 240:760–773. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang F, Shi P, Xi X, Yi S, Li H, Sun Q and
Sun M: Recombinant adenoviruses expressing TRAIL demonstrate
antitumor effects on non-small cell lung cancer (NSCLC). Med Oncol.
23:191–204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang X, Cheung RM, Komaki R, Fang B and
Chang JY: Radiotherapy sensitization by tumor-specific TRAIL gene
targeting improves survival of mice bearing human non-small cell
lung cancer. Clin Cancer Res. 11:6657–6668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Odoux C, Albers A, Amoscato AA, Lotze MT
and Wong MK: TRAIL, FasL and a blocking anti-DR5 antibody augment
paclitaxel-induced apoptosis in human non-small-cell lung cancer.
Int J Cancer. 97:458–465. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Voortman J, Resende TP, El Hassan Abou MA,
Giaccone G and Kruyt FA: TRAIL therapy in non-small cell lung
cancer cells: Sensitization to death receptor-mediated apoptosis by
proteasome inhibitor bortezomib. Mol Cancer Ther. 6:2103–2112.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo L, Fan L, Ren J, Pang Z, Ren Y, Li J,
Wen Z, Qian Y, Zhang L, Ma H and Jiang X: Combination of TRAIL and
actinomycin D liposomes enhances antitumor effect in non-small cell
lung cancer. Int J Nanomedicine. 7:1449–1460. 2012.PubMed/NCBI
|
|
17
|
Cho C, Horzempa C, Jones D and
McKeown-Longo PJ: The fibronectin III-1 domain activates a
PI3-Kinase/Akt signaling pathway leading to αvβ5 integrin
activation and TRAIL resistance in human lung cancer cells. BMC
Cancer. 16:5742016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kao PF, Chang HY, Tsai MF, Lin KJ, Tzen KY
and Chang CN: Breast uptake of iodine-131 mimicking lung metastases
in a thyroid cancer patient with a pituitary tumour. Br J Radiol.
74:378–381. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu S, Feng F, Wang K, Men C, Lin C, Liu Q,
Yang D and Gao Z: The therapeutic efficacy of I131-PSCA-mAb in
orthotopic mouse models of prostate cancer. Eur J Med Res.
18:562013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schachter P, Shimonov M and Lorberboim M:
Combined radioiodine (I-131) treatment and radio-guided surgery for
recurrent cervical well-differentiated thyroid cancer. Harefuah.
144(168–172): 231–232. 2005.
|
|
21
|
van Ginkel RJ, Limburg PC, Piers DA, Koops
HS and Hoekstra HJ: Value of continuous leakage monitoring with
radioactive iodine-131-labeled human serum albumin during
hyperthermic isolated limb perfusion with tumor necrosis
factor-alpha and melphalan. Ann Surg Oncol. 9:355–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dewaraja YK, Ljungberg M and Koral KF:
Monte Carlo evaluation of object shape effects in iodine-131 SPET
tumor activity quantification. Eur J Nucl Med. 28:900–906. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen S, Yu L, Jiang C, Zhao Y, Sun D, Li
S, Liao G, Chen Y, Fu Q, Tao Q, et al: Pivotal study of
iodine-131-labeled chimeric tumor necrosis treatment
radioimmunotherapy in patients with advanced lung cancer. J Clin
Oncol. 23:1538–1547. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song JJ, Lin YS, Zhu L and Li F: Efficacy
of iodine-131 in treating hyperthyroid heart disease. Zhongguo Yi
Xue Ke Xue Yuan Xue Bao. 35:166–170. 2013.(In Chinese). PubMed/NCBI
|
|
25
|
Gultekin SS and Sahmaran T: The efficacy
of patient-dependent practices on exposure rate in patients
undergoing iodine-131 ablation. Health Phys. 104:454–458. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burvenich I, Schoonooghe S, Cornelissen B,
Blanckaert P, Coene E, Cuvelier C, Mertens N and Slegers G: In
vitro and in vivo targeting properties of iodine-123- or
iodine-131-labeled monoclonal antibody 14C5 in a non-small cell
lung cancer and colon carcinoma model. Clin Cancer Res.
11:7288–7296. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin CY, Park C, Hong SH, Han MH, Jeong JW,
Xu H, Liu H, Kim GY, Kim WJ, Yoo YH and Choi YH: Synergistic
induction of TRAIL-mediated apoptosis by anisomycin in human
hepatoma cells via the BH3-only protein Bid and c-Jun/AP-1
signaling pathway. Biomed Pharmacother. 67:321–328. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Na HJ, Hwang JY, Lee KS, Choi YK, Choe J,
Kim JY, Moon HE, Kim KW, Koh GY, Lee H, et al: TRAIL negatively
regulates VEGF-induced angiogenesis via caspase-8-mediated
enzymatic and non-enzymatic functions. Angiogenesis. 17:179–194.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bai FL, Yu YH, Tian H, Ren GP, Wang H,
Zhou B, Han XH, Yu QZ and Li DS: Genetically engineered Newcastle
disease virus expressing interleukin-2 and TNF-related
apoptosis-inducing ligand for cancer therapy. Cancer Biol Ther.
15:1226–1238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shivapurkar N, Reddy J, Chaudhary PM and
Gazdar AF: Apoptosis and lung cancer: A review. J Cell Biochem.
88:885–898. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ouyang W, Yang C, Liu Y, Xiong J, Zhang J,
Zhong Y, Zhang G, Zhou F, Zhou Y and Xie C: Redistribution of DR4
and DR5 in lipid rafts accounts for the sensitivity to TRAIL in
NSCLC cells. Int J Oncol. 39:1577–1586. 2011.PubMed/NCBI
|
|
33
|
Howard DM, Kearfott KJ, Wilderman SJ and
Dewaraja YK: Comparison of I-131 radioimmunotherapy tumor
dosimetry: Unit density sphere model versus patient-specific Monte
Carlo calculations. Cancer Biother Radiopharm. 26:615–621. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
De Miguel D, Gallego-Lleyda A, Ayuso JM,
Erviti-Ardanaz S, Pazo-Cid R, del Agua C, Fernández LJ, Ochoa I,
Anel A and Martinez-Lostao L: TRAIL-coated lipid-nanoparticles
overcome resistance to soluble recombinant TRAIL in non-small cell
lung cancer cells. Nanotechnology. 27:1851012016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zidan J, Hefer E, Iosilevski G, Drumea K,
Stein ME, Kuten A and Israel O: Efficacy of I131 ablation therapy
using different doses as determined by postoperative thyroid scan
uptake in patients with differentiated thyroid cancer. Int J Radiat
Oncol Biol Phys. 59:1330–1336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Castellani MR, Aktolun C, Buzzoni R,
Seregni E, Chiesa C, Maccauro M, Aliberti GL, Vellani C, Lorenzoni
A and Bombardieri E: Iodine-131 metaiodobenzylguanidine (I-131
MIBG) diagnosis and therapy of pheochromocytoma and paraganglioma:
Current problems, critical issues and presentation of a sample
case. Q J Nucl Med Mol Imaging. 57:146–152. 2013.PubMed/NCBI
|
|
37
|
Harisankar CN, Mittal BR, Bhattacharya A,
Kashyap R and Bhansali A: Iodine-131 meta-iodobezylguanidine single
photon emission computed tomography/computerized tomography in
diagnosis of neuro-endocrine tumors. Indian J Nucl Med. 27:55–58.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shao H, Zhang J, Sun Z, Chen F, Dai X, Li
Y, Ni Y and Xu K: Necrosis targeted radiotherapy with
iodine-131-labeled hypericin to improve anticancer efficacy of
vascular disrupting treatment in rabbit VX2 tumor models.
Oncotarget. 6:14247–14259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kaewput C and Pusuwan P: Severe
hyponatremia: A comorbidity with I131 therapy in a patient with
papillary thyroid cancer. J Med Assoc Thai. 97:886–890.
2014.PubMed/NCBI
|
|
40
|
Grimminger PP, Schneider PM, Metzger R,
Vallböhmer D, Danenberg KD, Danenberg PV, Hölscher AH and Brabender
J: The prognostic role of Bcl-2 mRNA expression in curatively
resected non-small cell lung cancer (NSCLC). Lung Cancer. 70:82–87.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Checinska A, Giaccone G, Rodriguez JA,
Kruyt FA and Jimenez CR: Comparative proteomics analysis of
caspase-9-protein complexes in untreated and cytochrome c/dATP
stimulated lysates of NSCLC cells. J Proteomics. 72:575–585. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ballas MS and Chachoua A: Rationale for
targeting VEGF, FGF, and PDGF for the treatment of NSCLC. Onco
Targets Ther. 4:43–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rades D, Setter C, Dunst J, Dahl O, Schild
SE and Noack F: Prognostic impact of VEGF and VEGF receptor 1
(FLT1) expression in patients irradiated for stage II/III non-small
cell lung cancer (NSCLC). Strahlenther Onkol. 186:307–314. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li J, Wang Y, Liu L, Yuan Y and Bao Y:
Thioridazine sensitizes apoptotic effect of TRAIL in human lung
cancer PC9 cells through ER stress mediated upregulation of DR5.
Zhongguo Fei Ai Za Zhi. 20:80–87. 2017.(In Chinese). PubMed/NCBI
|
|
45
|
Nazim UM, Moon JH, Lee YJ, Seol JW and
Park SY: PPARγ activation by troglitazone enhances human lung
cancer cells to TRAIL-induced apoptosis via autophagy flux.
Oncotarget. 8:26819–26831. 2017. View Article : Google Scholar : PubMed/NCBI
|