Introduction
Hepatocellular carcinoma (HCC) is among the most
prevalent types of malignant neoplasm, and its incidence and
mortality rates have progressively increased worldwide (1,2). There are
>700,000 new cases diagnosed globally each year (1). The majority of HCC cases are associated
with established risk factors, including chronic viral hepatitis
and alcohol abuse (3). Hepatitis
infection is common in China, where HCC is also very commonly
diagnosed and among the leading causes of cancer-associated
mortality (4). Despite improvements
in the diagnosis and treatment of HCC, the overall survival (OS)
time of patients with HCC remains poor (5,6).
Therefore, the discovery of novel tumor biomarkers is required to
aid the devising of optimal treatment strategies and to improve the
prognosis of patients with HCC.
Human matrix metallopeptidase 12 (MMP12), also known
as macrophage metalloelastase, was first identified in human
alveolar macrophages (7). MMP12
belongs to a family of zinc-dependent proteases that are involved
in the degradation of extracellular matrix components (8). The inactive form of human MMP12 is a
54-kDa protein, which is processed to generate a 45-kDa form and,
subsequently, a 22-kDa active form via loss of its N- and
C-terminal residues (7). MMP12 serves
crucial roles in various diseases, including chronic obstructive
pulmonary disease (9), skin diseases
(10), aneurysms (11) and cancer (12). However, the mechanism of MMP12
activity remains controversial in various types of cancer.
Antitumor effects of MMP12 have been demonstrated in gastric
(13) and colorectal cancer (14). In contrast, MMP12 has been
demonstrated to be overexpressed, and associated with tumor
occurrence and progression, in non-small cell lung cancer (15), skin cancer (10), ovarian cancer (16), esophageal squamous cell carcinoma
(12) and pancreatic cancer (17).
Regulatory T cells (Tregs) were first described as
suppressor T cells 1970, and demonstrated to serve important roles
in maintaining immune tolerance and controlling inflammatory
diseases (18,19). Forkhead box P3 (FOXP3) functions as a
master regulator during the development and control of Tregs
(20,21). It is widely viewed as the most
specific and reliable surface marker of Tregs (22–24).
FOXP3-expressing Tregs, which suppress aberrant immune responses
against self-antigens, also suppress the antitumor immune response
(25). In humans, the infiltration of
large numbers of Tregs into tumor tissues is considered a biomarker
and prognostic indicator of malignant tumors (26).
In the present study, the expression of MMP12 and
infiltration of FOXP3-expressing (FOXP3+) Tregs were
investigated in whole-tissue sections obtained from a cohort of 158
patients with HCC. Furthermore, the prognostic significance of
MMP12 protein expression in HCC was investigated.
Materials and methods
Patients and samples
The present study was approved by the Research
Ethics Committee of Sun Yat-Sen University Cancer Center
(Guangzhou, China). All participants provided written informed
consent according to the Declaration of Helsinki (27). Paired tumor and non-tumor tissues were
obtained from 42 patients who underwent HCC resection at Sun
Yat-Sen University Cancer Center (Guangzhou, China) between January
2015 and December 2016. The HCC tissue microarrays consisted of
tissues obtained from 158 patients who were diagnosed with HCC
between October 2005 and December 2011 at Sun Yat-Sen University
Cancer Center (Guangzhou, China). The tissue microarray was
constructed according to a previously described method (28). The inclusion criteria for patient
enrollment were as follows: i) Histologically confirmed diagnosis;
ii) no distant metastasis; iii) no neoadjuvant chemotherapy or
radiotherapy prior to surgery; iv) no serious complications or
other malignant diseases, and v) complete follow-up data available.
The tumor stage was determined according to the 7th Edition
Tumor-Node-Metastasis (TNM) classification system (29). Tumor differentiation was graded
according to the Edmondson grading system (30). The clinicopathological characteristics
of the patients are summarized in Table
I.
 | Table I.Association between MMP12 expression
and clinicopathological characteristics of patients with
hepatocellular carcinoma (n=158). |
Table I.
Association between MMP12 expression
and clinicopathological characteristics of patients with
hepatocellular carcinoma (n=158).
|
|
| MMP12
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
variable | n | Negative, n | Positive, n | P-value |
|---|
| Age, years |
|
|
|
|
|
≤50 | 78 | 38 | 40 | 0.429 |
|
>50 | 80 | 44 | 36 |
|
| Sex |
|
|
|
|
|
Male | 143 | 75 | 68 | 0.670 |
|
Female | 15 | 7 | 8 |
|
| Hepatitis B surface
antigen |
|
|
|
|
|
Negative | 18 | 8 | 10 | 0.501 |
|
Positive | 140 | 74 | 66 |
|
| Serum
α-fetoprotein, ng/ml |
|
|
|
|
|
≤400 | 93 | 57 | 36 | 0.005 |
|
>400 | 65 | 25 | 40 |
|
| Liver
cirrhosis |
|
|
|
|
| No | 100 | 51 | 49 | 0.143 |
|
Yes | 58 | 33 | 22 |
|
| Tumor size, cm |
|
|
|
|
| ≤5 | 63 | 40 | 23 | 0.018 |
|
>5 | 95 | 42 | 53 |
|
| Tumor number |
|
|
|
|
|
Solitary | 115 | 59 | 56 | 0.807 |
|
Multiple | 43 | 23 | 20 |
|
| Microvascular
invasion |
|
|
|
|
| No | 140 | 75 | 65 | 0.241 |
|
Yes | 18 | 7 | 11 |
|
| Differentiation
grade |
|
|
|
|
|
I+II | 82 | 42 | 40 | 0.325 |
|
III+IV | 76 | 42 | 34 |
|
| TNM stage |
|
|
|
|
| I | 101 | 54 | 47 | 0.600 |
|
II+III | 57 | 28 | 29 |
|
Immunohistochemistry (IHC)
IHC was performed as described in our previous study
(26). Briefly, the sections were
deparaffinized in dimethyl benzene, then progressively rehydrated
in 100, 95, 90, 80 and 70% ethanol solutions. Endogenous peroxidase
activity was blocked by incubating the sections in 0.3% hydrogen
peroxide at room temperature for 10 min. Subsequently, the slides
were boiled (100°C, 5 min; 95°C, 25 min) in citrate-hydrochloric
acid (pH 6.0) for heat-induced epitope retrieval. The tissue
sections were then incubated with an MMP12 primary antibody
(dilution, 1:200 cat. no. ab52879) and a FOXP3 primary antibody
(dilution, 1:100; cat. no. ab20034; both Abcam, Cambridge, UK)
overnight at 4°C. A horseradish peroxidase-conjugated
anti-rabbit/mouse Dako REAL™ EnVision™ detection system (cat. no.
K5007; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) was
then used according to the manufacturer's protocol. Finally, the
sections were counterstained with hematoxylin at room temperature
for 2 min. A negative control was provided by replacing the primary
antibody with normal rabbit IgG (cat. no. 12-370; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). To assess the expression level of
MMP12/FOXP3, a Vectra-inForm image analysis system (PerkinElmer,
Inc., Waltham, MA, USA) was used as described in previous studies
(31,32).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from tumor and non-tumor
liver tissues using TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocol.
RNA concentrations were calculated using a NanoDrop 2000
Spectrophotometer (Thermo Fisher Scientific, Inc.). RNA with an
absorbance ratio from 1.8–2.0 at 260 and 280 nm (260/280) was
regarded as pure. cDNA was synthesized from 2 µg pure total RNA
using a Revert Aid First-Strand cDNA Synthesis kit (Toyobo Life
Science, Osaka, Japan). The resulting cDNA was then subjected to
RT-qPCR to determine relative MMP12 mRNA expression levels. The
reference gene, GAPDH, served as an internal control. RT-qPCR
assays (ReverTra Ace® qPCR RT Master Mix; cata. no.
FSQ-201; Toyobo Life Science) were performed in triplicate at a
final volume of 10 µl. The reactions consisted of 5 µl 2X SYBR
Green master mix (Toyobo Life Science), 0.4 µl 20 mmol/l forward
primer, 0.4 µl reverse primer, 0.75 µl sample cDNA and 3.45 µl
RNase-free water. The thermocycling conditions used were as
follows: An initial step in which the mixture was preheated to 95°C
for 10 min, followed by 45 cycles at 95°C for 30 sec and 60°C for
60 sec. The following specific primers were used: MMP12 forward,
5′-CCCTGGTTATCCCAAACTGA-3′ and reverse,
5′-CCAAACCAGCTATTGCTTTTC-3′; and GAPDH forward,
5′-CTCCTCCTGTTCGACAGTCAGC-3′ and reverse,
5′-CCCAATACGACCAAATCCGTT-3′ (Beijing Ruibiotech Co., Ltd., Beijing,
China). The data were analyzed using the comparative threshold
cycle (2−ΔΔCq) method (33) and Roche LightCycler 480 software
(version 1.5; Roche Diagnostics, Basel, Switzerland), and the
results were averaged, normalized and expressed in relative
expression units.
Statistical analysis
SPSS 20.0 software (IBM Corp., Armonk, NY, USA) and
GraphPad Prism V6.0 (GraphPad Software, Inc., La Jolla, CA, USA)
were used for statistical analysis. The differences in MMP12 mRNA
levels between HCC tissues and matched non-tumor liver tissues were
analyzed using paired t-tests. The association between MMP12
expression status and clinicopathological features was analyzed
using χ2 or Fisher's exact tests, as appropriate.
Briefly, if n<5, Fisher's exact tests were used; otherwise,
χ2 tests were used. Pearson's correlation coefficient
was used to assess the correlation between MMP12 and FOXP3
expression, as analyzed by IHC. OS curves were generated using the
Kaplan-Meier method and analyzed using the log-rank test.
Parameters demonstrated to be significant in univariate analysis
were further assessed in a multivariate Cox proportional hazards
model. P<0.05 was considered to indicate a statistically
significant difference.
Results
MMP12 expression in hepatocellular
carcinoma tissues
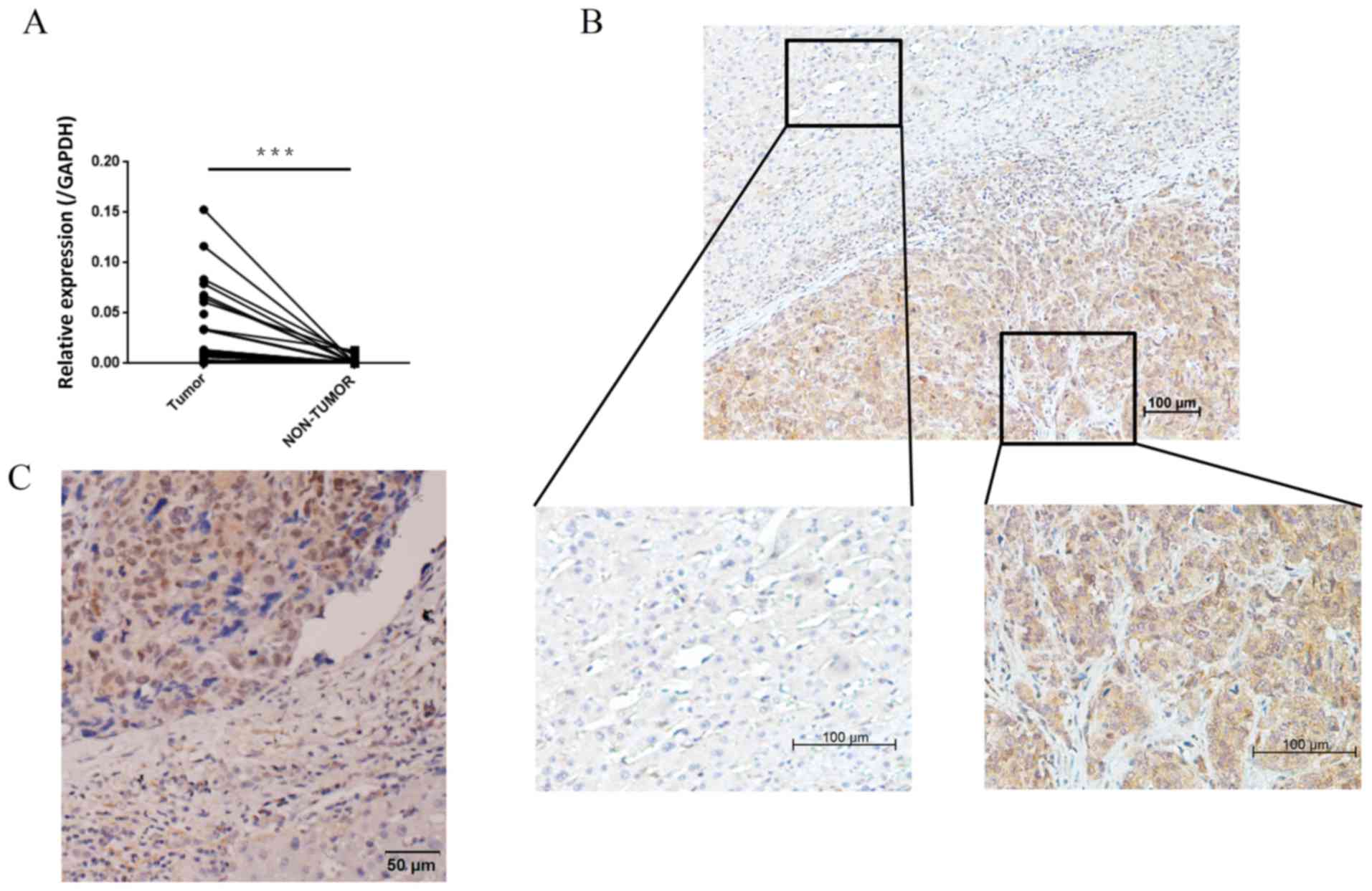
To compare the expression of MMP12 in paired HCC
tumor and adjacent non-tumor tissues, the MMP12 mRNA expression
level was analyzed in 42 pairs of tissue using RT-qPCR. It was
demonstrated that the expression level of MMP12 mRNA was
significantly higher in HCC tumor tissues compared with the matched
adjacent non-tumor tissues (Fig. 1A).
IHC staining of 16 specimens was performed, revealing that MMP12
was primarily localized in tumor cell cytoplasm. 1C). The adjacent
non-tumor cells were negative for MMP12 expression (Fig. 1B). MMP12 protein was localized in the
tumor nuclei of some specimens (Fig.
1). Furthermore, in HCC tissue specimens, it was observed that
non-tumor cells also expressed MMP12 protein. Based on previous
research (7), it was hypothesized
that these cells may be tumor associated-macrophages.
Associations between MMP12 expression
and clinicopathological characteristics
Patients were divided into the following groups
based on MMP12 expression in the tumor cell cytoplasm, determined
using the receiver operating characteristic (ROC) curve:
MMP12-(negative expression in tumor cells) and MMP12+ (positive
expression in tumor cells). These groups included 51.9 and 48.1% of
all included samples, respectively. Patients in the MMP12+ group
exhibited a significantly larger average tumor size (P=0.018) and
significantly higher serum α-fetoprotein (AFP) levels (P=0.005)
compared with the MMP12-group (Table
I). However, there was no significant difference in MMP12
protein expression status in HCC tissues according to age, sex,
tumor number, presence of invasive microvasculature,
differentiation grade or TNM stage (Table
I).
Prognostic significance of MMP12
protein expression in HCC patients
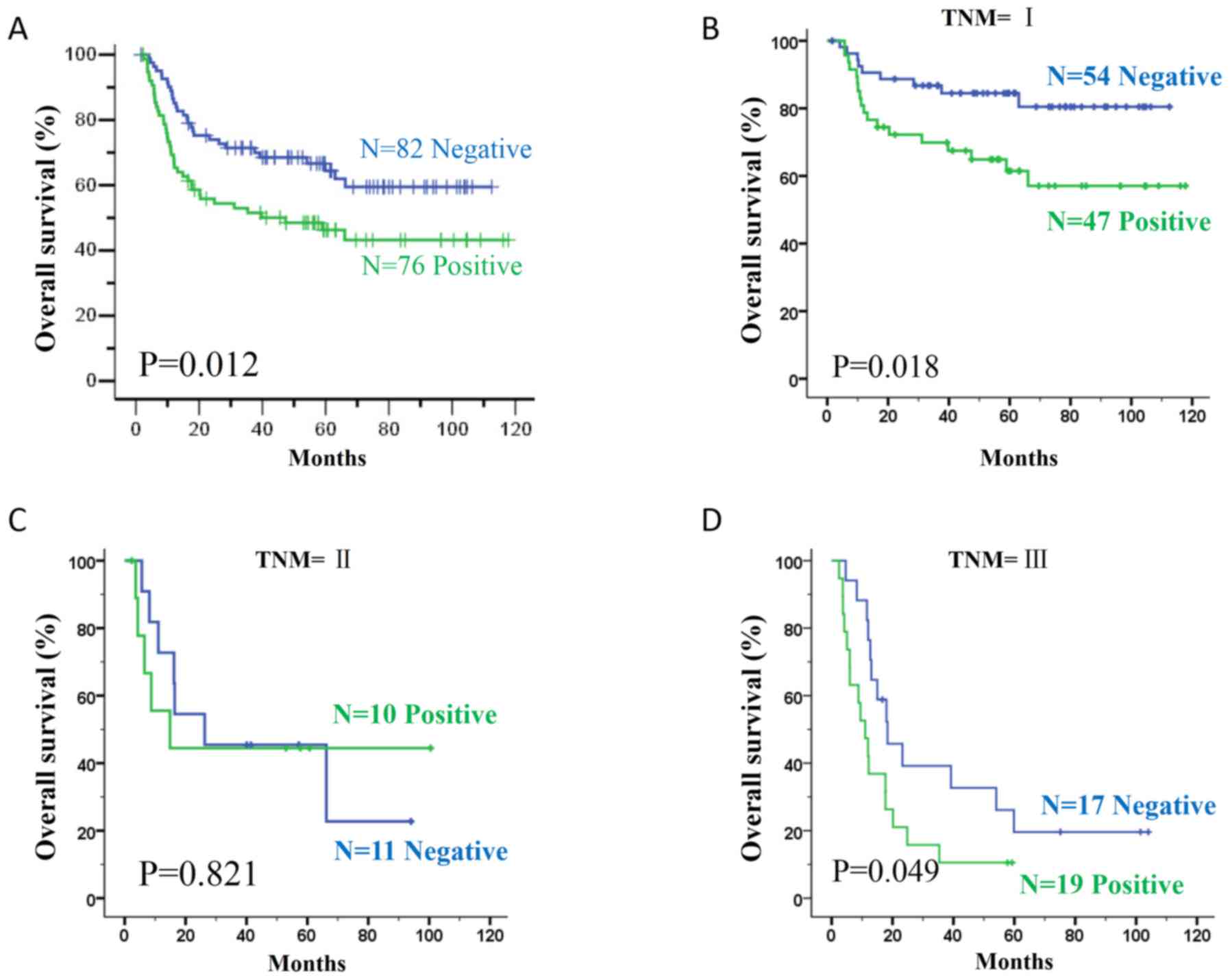
Kaplan-Meier survival analysis revealed that
patients in the MMP12+ group had a shorter OS time than patients in
the MMP12-group (P=0.012; Fig. 2A).
In addition, all patients were stratified according to the TNM
staging system. There were 101 patients at stage I, 21 patients at
stage II and 36 patients at stage III. In patients with stage I or
III tumors, the surgical prognosis more positive in the
MMP12-compared with the MMP12+ group (stage I; P=0.018; Fig. 2B, and stage III P=0.049; Fig. 2D). However, this association was not
demonstrated in stage II patients (P=0.821; Fig. 2C). To determine whether positive MMP12
expression in tumor cells is an independent prognostic factor for
HCC, multivariate survival analysis was performed. As demonstrated
in Table II, positive MMP12
expression was an independent prognostic factor for OS in patients
with HCC (HR=2.013; 95% CI=1.211–3.347; P=0.007).
 | Table II.Univariate and multivariate analyses
of prognostic factors in hepatocellular carcinoma (n=158). |
Table II.
Univariate and multivariate analyses
of prognostic factors in hepatocellular carcinoma (n=158).
|
| Overall
survival |
|
|---|
|
|
|
|
|---|
| Variable | Univariate analysis
P-value | Multivariate
analysis hazard ratio (95% CI) | P-value |
|---|
| Age, years |
|
|
|
|
≤50 |
0.284 |
|
|
|
>50 |
|
|
|
| Sex |
|
|
|
|
Male |
0.955 |
|
|
|
Female |
|
|
|
| Serum
α-fetoprotein, ng/ml |
|
|
|
|
≤400 |
0.192 |
|
|
|
>400 |
|
|
|
| Liver
cirrhosis |
|
|
|
|
Negative |
0.001 | 1 |
|
|
Positive |
| 1.907
(1.154–3.153) |
0.012 |
| Tumor size, cm |
|
|
|
| ≤5 | <0.001 | 1 |
|
|
>5 |
| 2.224
(1.234–4.009) |
0.008 |
| Tumor number |
|
|
|
|
Solitary | <0.001 | 1 | <0.001 |
|
Multiple |
| 4.844
(2.927–8.015) |
|
| Microvascular
invasion |
|
|
|
| No | <0.001 | 1 |
0.001 |
|
Yes |
| 2.961
(1.545–5.675) |
|
| Differentiation
grade |
|
|
|
|
I+II |
0.139 |
|
|
|
III+IV |
|
|
|
| MMP12
expression |
|
|
|
|
Negative |
0.012 | 1 |
0.007 |
|
Positive |
| 2.013
(1.211–3.347) |
|
Correlation between MMP12 expression
and FOXP3+ T cell number in human HCC tissues
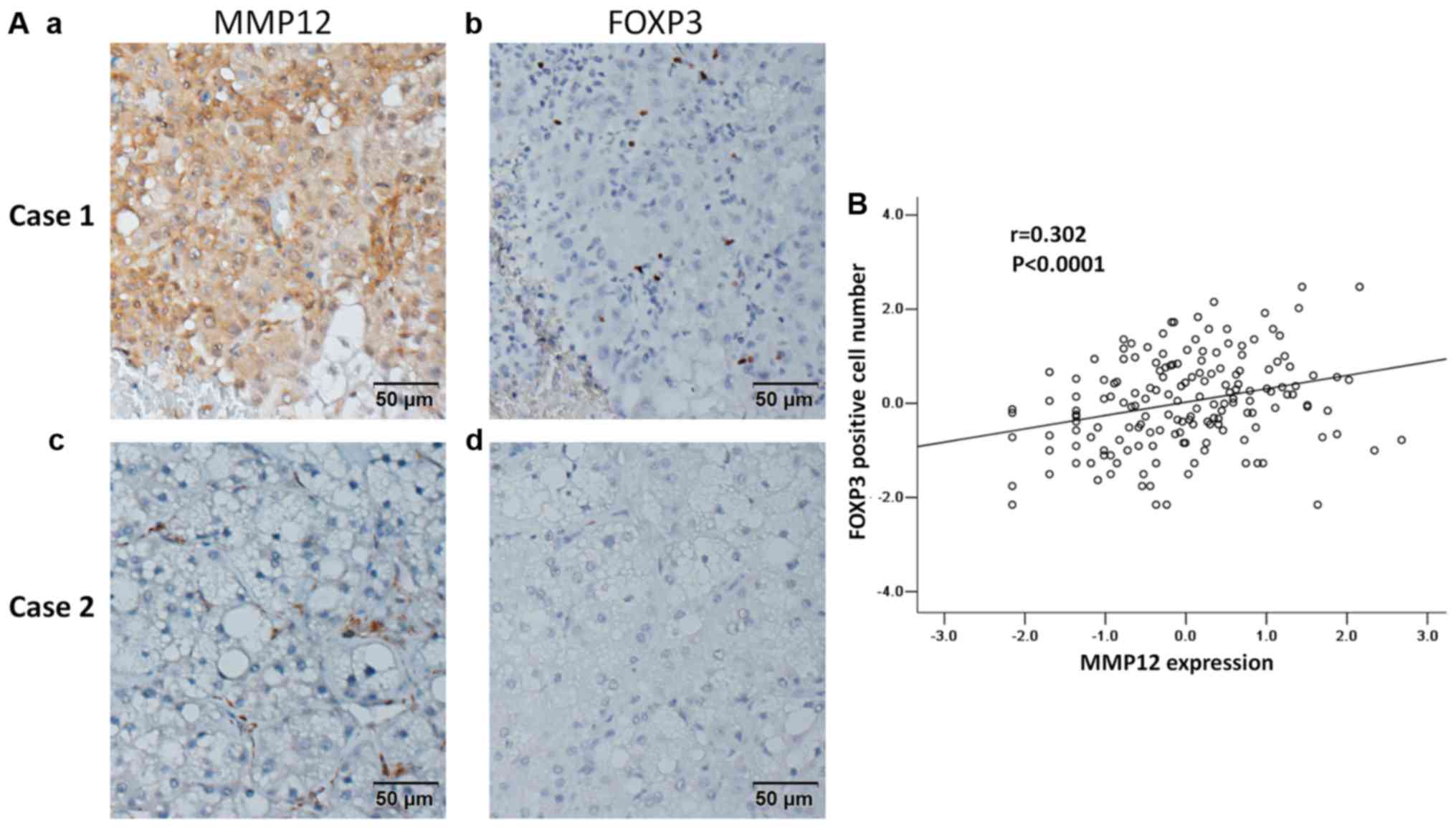
To investigate the association of MMP12 expression
and FOXP3 expression in human HCC tissues, IHC staining for MMP12
and FOXP3 was performed in human HCC tissue sections. Tissues
positively expressing MMP12 also expressed high levels of FOXP3
(Fig. 3Aa and Ab). Conversely,
tissues that did not express MMP12 also expressed low levels of
FOXP3 (Fig. 3Ac and Ad). Statistical
analysis indicated a significantly positive correlation between
MMP12 expression and FOXP3 expression (r=0.302, P<0.001;
Fig. 3B).
Discussion
In the present study, it was demonstrated that MMP12
protein was usually in tumor cell cytoplasm, but was sometimes
localized in the nucleus. According to RT-qPCR analysis, MMP12 was
expressed at significantly higher levels in HCC tissues than in
non-tumor tissues, and MMP12 overexpression was also associated
with malignant clinicopathological characteristics, including
larger tumor size and high serum AFP levels. However, there was no
association between MMP12 expression in HCC tissues and patient
age, sex, tumor number, microvascular invasion or TNM stage. In the
present study, MMP12 expression was positively correlated with
tumor size but not with tumor number or microvascular invasion.
Therefore, MMP12 expression was not significantly correlated with
TNM stage. These results are in agreement with a previous study
(34). It was also demonstrated that
this pattern of MMP12 protein expression in tumor cells was
associated with a relatively poor prognosis. Furthermore, MMP12
protein expression level and FOXP3+ Treg infiltration
were positively correlated. Taken together, these conclusions
suggest that MMP12 may not serve important roles in metastasis, but
may affect prognosis by influencing the immune system in HCC
patients.
In a previous study, the overexpression of MMP12
mRNA was demonstrated to be associated with the presence of venous
infiltration, high serum AFP levels, early tumor recurrence and
poor overall survival time in patients with HCC (34). However, the results of a different
study indicated that MMP12 mRNA overexpression was significantly
associated with an improved overall survival time in patients with
HCC who underwent curative resection (35). This study also demonstrated that the
expression of MMP12 was positively correlated with angiostatin
production and hypovascular tumors. However, while these studies
determined MMP12 expression levels using a quantitative method,
neither detected MMP12 protein expression levels in tumor tissues.
The results of the present study suggest that the MMP12 protein is
usually localized in the cytoplasm of tumor cells. Furthermore,
multivariate analysis revealed that MMP12 expression was an
independent and significant risk factor affecting overall survival
time of patients with HCC who underwent curative resection. Matrix
metallopeptidases (MMPs) are secreted proteases that degrade the
extracellular matrix during various cellular processes. Several
studies have demonstrated that MMPs are located in the nucleus of
human cells (36,37). In one study, MMP12 was demonstrated to
be trafficked into the nucleus, where it bound specific DNA
sequences to regulate the expression of specific genes (37). In the present study, it was observed
that the MMP12 protein was localized in tumor cell nuclei in HCC
specimens. The transcriptional functions of MMP12 in HCC should be
investigated, in order to have confidence that the intracellular
localization of MMP12 is not an artifact.
MMPs can activate, deactivate or modify the
activities of signaling cytokines, chemokines and receptors acting
as modulators of inflammation and innate immunity (38,39). In a
bi-transgenic mouse model, MMP12 overexpression in the myeloid
lineage cells contributed to modulation of myelopoiesis, immune
suppression and lung tumorigenesis (40). In the same study, the number of Tregs
was increased in bi-transgenic mice overexpressing MMP12. In the
present study, the association between MMP12 expression and FOXP3
expression (FOXP3 is a marker of Tregs) was analyzed in HCC
tissues. A positive correlation between MMP12 expression and FOXP3
expression was identified. The upregulated expression level of
MMP12 in HCC patients indicated they were in a state of immune
suppression.
In summary, the results of the present study suggest
that MMP12 mRNA and protein expression levels are higher in HCC
patients. In HCC tumor tissues, MMP12 protein overexpression was
associated with poor overall survival time in patients with HCC who
had undergone hepatectomy. However, the precise mechanism
underlying the effect of MMP12 expression on the occurrence and
progression of HCC remains to be investigated.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81625017 and
81572385) and the Fundamental Research Funds for the Central
Universities of China (grant no. 16ykjc36).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors have read and approved the manuscript.
MKH designed the study, performed the experiment and statistical
analyses, and wrote the manuscript. YL participated in the design
of the study, performed part of the statistical analyses and helped
draft the manuscript. YFZ, HYO, PEJ, ZSY and LJW participated in
clinical data collection. MS supervised the whole study, was
involved in the study design and supervised the draft of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Sun Yat-Sen University Cancer Center. All
participants provided written informed consent according to the
Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hassan MM, Hwang LY, Hatten CJ, Swaim M,
Li D, Abbruzzese JL, Beasley P and Patt YZ: Risk factors for
hepatocellular carcinoma: Synergism of alcohol with viral hepatitis
and diabetes mellitus. Hepatology. 36:1206–1213. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Villanueva A, Hoshida Y, Battiston C,
Tovar V, Sia D, Alsinet C, Cornella H, Liberzon A, Kobayashi M,
Kumada H, et al: Combining clinical, pathology, and gene expression
data to predict recurrence of hepatocellular carcinoma.
Gastroenterology. 140:1501–1512.e2. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bruix J, Boix L, Sala M and Llovet JM:
Focus on hepatocellular carcinoma. Cancer Cell. 5:215–219. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shapiro SD, Kobayashi DK and Ley TJ:
Cloning and characterization of a unique elastolytic
metalloproteinase produced by human alveolar macrophages. J Biol
Chem. 268:23824–23829. 1993.PubMed/NCBI
|
|
8
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hunninghake GM, Cho MH, Tesfaigzi Y,
Soto-Quiros ME, Avila L, Lasky-Su J, Stidley C, Melén E, Söderhäll
C, Hallberg J, et al: MMP12, lung function, and COPD in high-risk
populations. N Engl J Med. 361:2599–2608. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kerkelä E, Ala-Aho R, Jeskanen L, Rechardt
O, Grénman R, Shapiro SD, Kähäri VM and Saarialho-Kere U:
Expression of human macrophage metalloelastase (MMP-12) by tumor
cells in skin cancer. J Invest Dermatol. 114:1113–1119. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Curci JA, Liao S, Huffman MD, Shapiro SD
and Thompson RW: Expression and localization of macrophage elastase
(matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin
Invest. 102:1900–1910. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kerkelä E, Ala-aho R, Klemi P, Grénman S,
Shapiro SD, Kähäri VM and Saarialho-Kere U: Metalloelastase
(MMP-12) expression by tumour cells in squamous cell carcinoma of
the vulva correlates with invasiveness, while that by macrophages
predicts better outcome. J Pathol. 198:258–269. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng P, Jiang FH, Zhao LM, Dai Q, Yang
WY, Zhu LM, Wang BJ, Xu C, Bao YJ and Zhang YJ: Human macrophage
metalloelastase correlates with angiogenesis and prognosis of
gastric carcinoma. Dig Dis Sci. 55:3138–3146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang W, Arii S, Gorrin-Rivas MJ, Mori A,
Onodera H and Imamura M: Human macrophage metalloelastase gene
expression in colorectal carcinoma and its clinicopathologic
significance. Cancer. 91:1277–1283. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho NH, Hong KP, Hong SH, Kang S, Chung KY
and Cho SH: MMP expression profiling in recurred stage IB lung
cancer. Oncogene. 23:845–851. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Jia JH, Kang S, Zhang XJ, Zhao J,
Wang N, Zhou RM, Sun DL, Duan YN and Wang DJ: The functional
polymorphisms on promoter region of matrix metalloproteinase-12,
−13 genes may alter the risk of epithelial ovarian carcinoma in
Chinese. Int J Gynecol Cancer. 19:129–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Balaz P, Friess H, Kondo Y, Zhu Z,
Zimmermann A and Buchler MW: Human macrophage metalloelastase
worsens the prognosis of pancreatic cancer. Ann Surg. 235:519–527.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gershon RK and Kondo K: Cell interactions
in the induction of tolerance: The role of thymic lymphocytes.
Immunology. 18:723–737. 1970.PubMed/NCBI
|
|
19
|
Sakaguchi S, Yamaguchi T, Nomura T and Ono
M: Regulatory T cells and immune tolerance. Cell. 133:775–787.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yagi H, Nomura T, Nakamura K, Yamazaki S,
Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S and
Sakaguchi S: Crucial role of FOXP3 in the development and function
of human CD25+CD4+ regulatory T cells. Int Immunol. 16:1643–1656.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Campbell DJ and Ziegler SF: FOXP3 modifies
the phenotypic and functional properties of regulatory T cells. Nat
Rev Immunol. 7:305–310. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fontenot JD, Gavin MA and Rudensky AY:
Foxp3 programs the development and function of CD4+CD25+ regulatory
T cells. Nat Immunol. 4:330–336. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khattri R, Cox T, Yasayko SA and Ramsdell
F: Pillars article: An essential role for Scurfin in CD4+CD25+ T
regulatory cells. Nat. Immunol. 4: 337–342. J Immunol. 198:993–998.
2017.PubMed/NCBI
|
|
25
|
Tanaka A and Sakaguchi S: Regulatory T
cells in cancer immunotherapy. Cell Res. 27:109–118. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schreiber TH: The use of FoxP3 as a
biomarker and prognostic factor for malignant human tumors. Cancer
Epidemiol Biomarkers Prev. 16:1931–1934. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
World medical association declaration of
helsinki: Ethical principles for medical research involving human
subjects. JAMA. 284:3043–3045. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu J, Ding T, He Q, Yu XJ, Wu WC, Jia WH,
Yun JP, Zhang Y, Shi M, Shao CK, et al: An in situ molecular
signature to predict early recurrence in hepatitis B virus-related
hepatocellular carcinoma. J Hepatol. 57:313–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: A study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang W, Hennrick K and Drew S: A colorful
future of quantitative pathology: Validation of Vectra technology
using chromogenic multiplexed immunohistochemistry and prostate
tissue microarrays. Hum Pathol. 44:29–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li L, Xu L, Yan J, Zhen ZJ, Ji Y, Liu CQ,
Lau WY, Zheng L and Xu J: CXCR2-CXCL1 axis is correlated with
neutrophil infiltration and predicts a poor prognosis in
hepatocellular carcinoma. J Exp Clin Cancer Res. 34:1292015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ng KT, Qi X, Kong KL, Cheung BY, Lo CM,
Poon RT, Fan ST and Man K: Overexpression of matrix
metalloproteinase-12 (MMP-12) correlates with poor prognosis of
hepatocellular carcinoma. Eur J Cancer. 47:2299–2305. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gorrin-Rivas MJ, Arii S, Mori A, Takeda Y,
Mizumoto M, Furutani M and Imamura M: Implications of human
macrophage metalloelastase and vascular endothelial growth factor
gene expression in angiogenesis of hepatocellular carcinoma. Ann
Surg. 231:67–73. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shimizu-Hirota R, Xiong W, Baxter BT,
Kunkel SL, Maillard I, Chen XW, Sabeh F, Liu R, Li XY and Weiss SJ:
MT1-MMP regulates the PI3Kδ Mi-2/NuRD-dependent control of
macrophage immune function. Genes Dev. 26:395–413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Marchant DJ, Bellac CL, Moraes TJ,
Wadsworth SJ, Dufour A, Butler GS, Bilawchuk LM, Hendry RG,
Robertson AG, Cheung CT, et al: A new transcriptional role for
matrix metalloproteinase-12 in antiviral immunity. Nat Med.
20:493–502. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Page-McCaw A, Ewald AJ and Werb Z: Matrix
metalloproteinases and the regulation of tissue remodelling. Nat
Rev Mol Cell Biol. 8:221–233. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qu P, Yan C and Du H: Matrix
metalloproteinase 12 overexpression in myeloid lineage cells plays
a key role in modulating myelopoiesis, immune suppression and lung
tumorigenesis. Cancer Res. 117:4476–4489. 2011.
|