Introduction
Gastric cancer (GC) is the fourth most common cancer
and the second leading cause of cancer-associated mortality
worldwide, with >950,000 newly diagnosed cases every year and an
estimated 720,000 cases of GC-associated mortality in 2012
(1–3).
Despite the development of surgical techniques and chemotherapy,
the 5-year survival rate remains low, as the majority of GCs are
diagnosed at advanced or metastatic stages (4,5).
Therefore, it is of great clinical importance to establish specific
and sensitive biomarkers for the early diagnosis of GC and to
identify effective therapeutic targets to prevent the metastasis of
GC.
Several studies have demonstrated that elevated
lipogenesis is associated with a poor prognosis in a number of
cancer types including ovarian cancer, breast cancer and prostate
cancer (6–8), and that it is involved in signal
transduction of several types of tumors, including non-small cell
lung cancer, prostate cancer and hepatocellular cancer (9–11). Fatty
acid synthase (FASN), the main enzyme involved in de novo
lipogenesis, is overexpressed in several types of tumor tissues
including breast cancer, colorectal cancer and gastric cancer, and
its overexpression is significantly associated with tumor cell
proliferation, metastasis, epithelial-mesenchymal transition (EMT)
and a poor prognosis (12–15). However, there are only a few studies
focusing on FASN in GC (15–17), and these indicate that FASN is
overexpressed in the GC tissues and blood serum of patients with
GC, and that its overexpression is connected with poor survival
rate. These data suggest that FASN serves a critical role in the
development and progression of GC, but the precise functions and
internal mechanisms of FASN in GC cell proliferation and metastasis
remain elusive.
Available data indicate that the EMT serves a
crucial role in tumor cell metastasis and invasion, which is
accompanied by upregulation of mesenchymal-associated genes,
including Vimentin, and downregulation of epithelial-associated
markers, including E-cadherin (18,19).
Several types of signaling pathways and molecules are involved in
the regulation of EMT (18,20), and one of the most important signaling
pathways is the Hedgehog (Hh) pathway. Furthermore, there is strong
evidence that the Hh pathway and its effector zinc finger protein
GLI1 (Gli1), one of the glioma-associated oncogenes, serve crucial
roles in the development and progression of GC (21–24).
The present study investigated the role of FASN in
GC development and determined its effect on the regulation of the
Hh signaling pathway effector Gli1, which is strongly associated
with cell proliferation, metastasis and EMT in GC (21–24). To
the best of our knowledge, for the first time, the present in
vitro studies identified that FASN functions as a novel
regulator for Gli1 expression to mediate GC cell proliferation and
metastasis, with potential implications for novel approaches to GC
therapy.
Materials and methods
Human GC tissues and cell lines
A total of 18 paired human GC tissues and adjacent
normal tissues were collected immediately from patients with GC (10
male and 8 female) with a median age of 64 years (range, 31–75
years), who underwent surgical resection between January 2014 to
December 2015 at the First Affiliated Hospital of Soochow
University (Suzhou, Jiangsu, China). Written informed consent was
obtained from all patients in this study, which was approved by the
Biomedical Research Ethics Committee of the First Affiliated
Hospital of Soochow University. The experiments performed on human
tissues were in compliance with the Helsinki Declaration. The human
GC SGC-7901 and MGC-803 cell lines were purchased from the Cell
Bank of the Chinese Academy of Sciences (Shanghai, China) and were
cultured in Roswell Park Memorial Institute (RPMI) 1640 medium
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) containing
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 100 U/ml penicillin G sodium and 100 µg/ml
streptomycin sulfate (Gibco; Thermo Fisher Scientific, Inc.). All
cells were maintained at 37°C in a humidified atmosphere containing
5% CO2.
Transfection of small interfering RNA
(siRNA)
The siRNA against FASN (specific target sequence,
5′-TACGACTACGGCCCTCATT-3′) and a negative control siRNA (target
sequence, 5′-TTCTCCGAACGTGTCACGTTT-3′) were synthesized by
GenePharma (Shanghai, China). GC SGC-7901 and MGC-803 cell lines
were transfected with control or FASN siRNA at a final
concentration of 20 nM using Lipofectamine™ RNAiMax (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. The cells were harvested for further experiments at
48–72 h post-transfection.
Protein extraction and western blot
analysis
Whole protein extracts of tissues or cell lines
(SGC7901 and MGC803) were lysed in ice-cold RIPA lysis buffer
containing cocktails of protease and phosphatase inhibitors
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) according to the
manufacturer's protocols. Protein concentrations were determined
using a BCA protein assay kit (Pierce; Thermo Fisher Scientific,
Inc.) Total proteins (10 µg) from each lysate were separated by 8%
SDS-PAGE and transferred onto polyvinylidene difluoride membranes
(Bio-Rad Laboratories, Inc., Hercules, CA, USA), and then blocked
with 5% skimmed milk in TBS/0.1% Tween for 1 h at room temperature.
The membranes were then probed with the indicated primary
antibodies diluted using PBS at 4°C with gentle agitation
overnight, and then incubated with horseradish
peroxidase-conjugated secondary antibodies diluted using PBS
(dilution, 1:5,000; OriGene Technologies, Inc., Rockville, MD, USA)
for 1 h at room temperature. Next, the proteins were visualized
using chemiluminescence kit (EMD Millipore, Billerica, MA, USA) and
signals were quantified by ImageJ software (version 1.46; National
Institutes of Health, Bethesda, MD, USA). Antibodies used in this
study are listed in Table I.
 | Table I.Antibody information. |
Table I.
Antibody information.
| Name | Company | Catalog no. | Concentration |
|---|
| FASN | Cell Signaling
Technology, Inc. | 3180 | 1:1,000 |
| E-cadherin | Cell Signaling
Technology, Inc. | 3195 | 1:500 |
| Vimentin | Cell Signaling
Technology, Inc. | 5741 | 1:500 |
| Phospho-AMPKα
(Thr172) | Cell Signaling
Technology, Inc. | 4535 | 1:1,000 |
| Phospho-Akt
(Ser473) | Cell Signaling
Technology | 4058 | 1:1,000 |
| Phospho-mTOR
(S2448) | Cell Signaling
Technology, Inc. | 9205 | 1:1,000 |
| Gli1 | Abcam | Ab15179 | 1:2,000 |
| GAPDH | Beyotime | AG019 | 1:2,000 |
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The mRNA expression of FASN, E-cadherin and Vimentin
in SGC-7901 and MGC-803 cells transfected with negative control
siRNA or siRNA against FASN was quantified by RT-qPCR. Total RNA
was extracted from the cells using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) and cDNA was synthesized from 2 µg
RNA using the First Strand cDNA Synthesis kit (Fermentas; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocols.
RT-qPCR was performed using Power SYBR® Green PCR Master
mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) on the
7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The 18S rRNA was used as a loading control for
each specific gene. The sequences for the sense (S) and antisense
(AS) primers were as follows: Human-FASN-S,
5′-CTTCCGAGATTCCATCCTACGC-3′ and human-FASN-AS,
5′-TGGCAGTCAGGCTCACAAACG-3′; human-E-cadherin-S,
5′-CGGGAATGCAGTTGAGGATC-3′ and human-E-cadherin-AS,
5′-AGGATGGTGTAAGCGATGGC-3′; human-Vimentin-S,
5′-GAGAACTTTGCCGTTGAAGC-3′ and human-Vimentin-AS,
5′-GCTTCCTGTAGGTGGCAATC-3′; and human-18S rRNA-S,
5′-GTAACCCGTTGAACCCCATT-3′ and human-18S rRNA-AS,
5′-CCATCCAATCGGTAGTAGCG-3′. The PCR conditions consisted of 5 min
at 95°C for 1 cycle, followed by 30 sec at 95°C, 30 sec at 55°C, 30
sec at 72°C and 7 min at 72°C for 40 cycles. The relative
fold-changes in mRNA expression were calculated using the
2−∆∆Cq method (25), where
the mean of the ∆Cq values for the amplicon of interest was
normalized to that of 18S rRNA and compared with the control
specimens.
MTT assay of cell proliferation
Cell proliferation was determined using an MTT assay
kit (Amresco LLC, Solon, OH, USA). Following transfection, 4,000
cells were seeded in 96-well plates for 24, 48 and 72 h, and then
incubated with MTT solution-containing culture medium at 37°C for 4
h. The supernatants were then removed and formazan crystals were
dissolved in 150 µl dimethyl sulfoxide. Following gentle agitation
for 10 min, the absorbance at 490 nm was measured using a
microplate reader.
Colony formation assay
In total, 1,000 cells were placed in 6-well plates
for 10 days, and then fixed with 4% paraformaldehyde (Beyotime
Institute of Biotechnology; Haimen, China) for 10 min at room
temperature and stained with 0.1% crystal violet (Beyotime
Institute of Biotechnology) for 15 min at room temperature. The
number of foci containing >100 cells was determined at ×40
magnification using an optical microscope (Nikon Corporation;
Tokyo, Japan), and the images were captured by a digital camera
(Nikon Corporation).
Cell migration assay
GC cell migration was assessed using Transwell
chambers (pore size, 8.0 µm; Corning Inc., Corning, New York, USA).
The cells were re-suspended in serum-free RPMI medium, then cell
suspensions (200 µl containing 50,000 cells) were seeded onto the
filters in 24-well chambers; 750 µl medium containing 10% FBS was
placed in the lower chambers as a chemoattractant. The cells were
allowed to migrate for 24 h at 37°C. Cells remaining on the upper
surface of the membrane were then removed using a cotton swab. The
filters were fixed with 4% paraformaldehyde (Beyotime Institute of
Biotechnology) for 10 min at room temperature, and the cells were
stained with 0.1% crystal violet solution (Beyotime Institute of
Biotechnology) for 15 min at room temperature. The cells that had
migrated from the upper to the lower side of the filter were
counted in 5 randomly selected fields per sample using a light
microscope (magnification, ×200; Nikon Corporation).
Statistical analysis
Data of MTT assay are presented as the standard
deviation and the other are presented as mean ± standard error.
Statistical significance was analyzed using Student's t-test
(unpaired, two-tailed) or one-way analysis of variance followed by
the Student-Newman-Keuls test. P<0.05 was considered to indicate
a statistically significant difference. All statistical analyses
were performed with SPSS17.0 software (SPSS, Inc., Chicago, IL,
USA).
Results
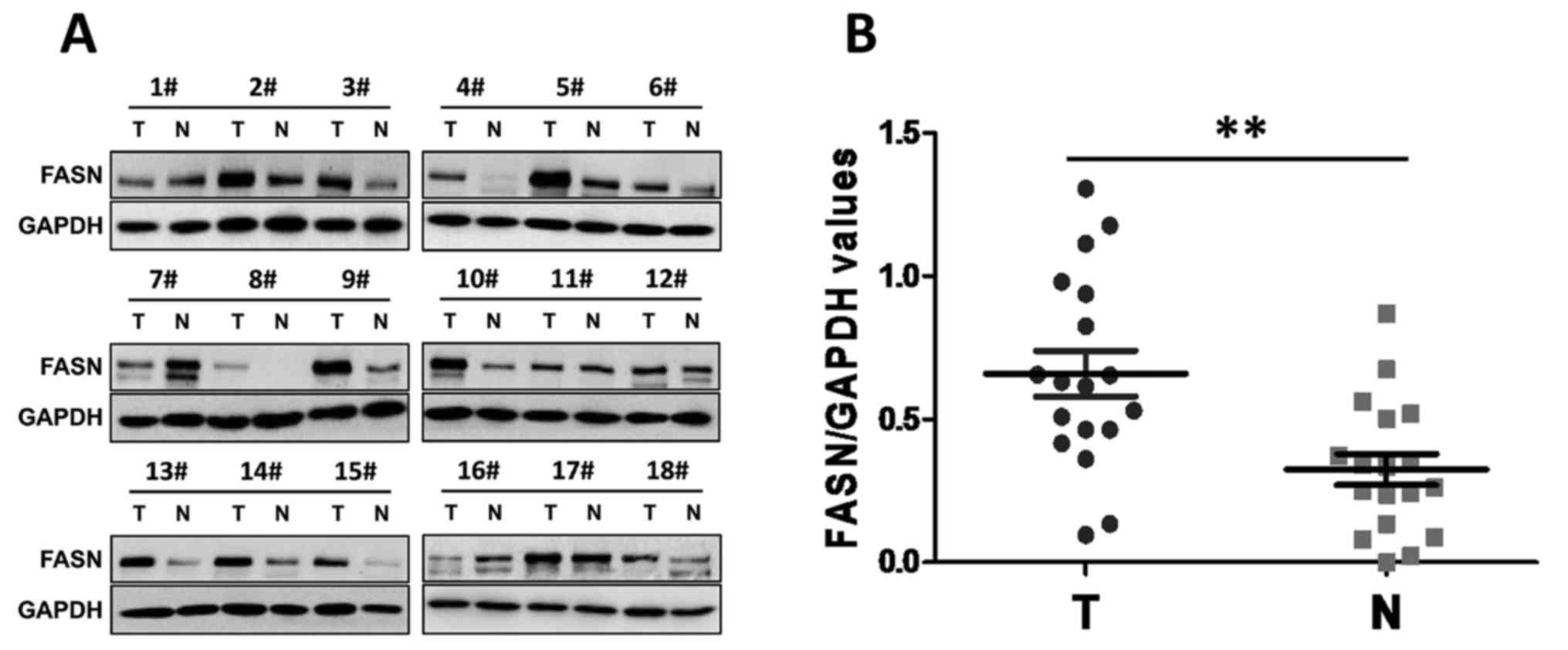
FASN is overexpressed in human GC
Previous studies (15–17) have
provided evidence that FASN is overexpressed in GC tissues and that
its overexpression is closely associated with GC metastasis and
patient survival. To further investigate the potential role of FASN
in the development of GC, western blotting was used in the present
study to examine FASN expression in human GC tissues and paired
adjacent normal tissues. Notably, the results showed that abundant
FASN expression was present in the primary GC tumors. By contrast,
the expression of FASN was significantly lower in the matched
paraneoplastic tissues (Fig. 1A and
B). These data suggested that increased FASN expression may be
associated with the development of GC.
FASN deficiency inhibits GC cell
proliferation and metastasis in vitro
Since FASN expression is upregulated in GC tissues,
the present study assessed whether FASN has a causal role in
regulating GC cell phenotypes. First, FASN expression was knocked
down using FASN-specific siRNA in the GC SGC-7901 cell line.
Furthermore, the knockdown efficiency was confirmed by RT-qPCR
(Fig. 2A). In order to determine the
effect of FASN on the proliferation of SGC-7901 cells in
vitro, the proliferation curves were detected by MTT assays at
24, 48 and 72 h following 48 h of transfection (Fig. 2B). It was found that the SGC-7901
cells transfected with siRNA against FASN experienced significant
inhibition of cell proliferation compared with the SGC-7901 cells
transfected with control siRNA or the wild-type untreated SGC-7901
cells. In agreement with this result, colony formation assays also
revealed that the ability of SGC-7901 cells with downregulated FASN
to form foci displayed an apparent reduction compared with that of
the control-siRNA and untreated cells, respectively (Fig. 2C). In a Transwell migration assay, the
migration ability of FASN-depleted SGC-7901 cells was greatly
inhibited compared with that of the control-siRNA and untreated
cells (Fig. 2D). These results
indicated that the knockdown of FASN by siRNA inhibits GC cell
proliferation and metastasis in vitro.
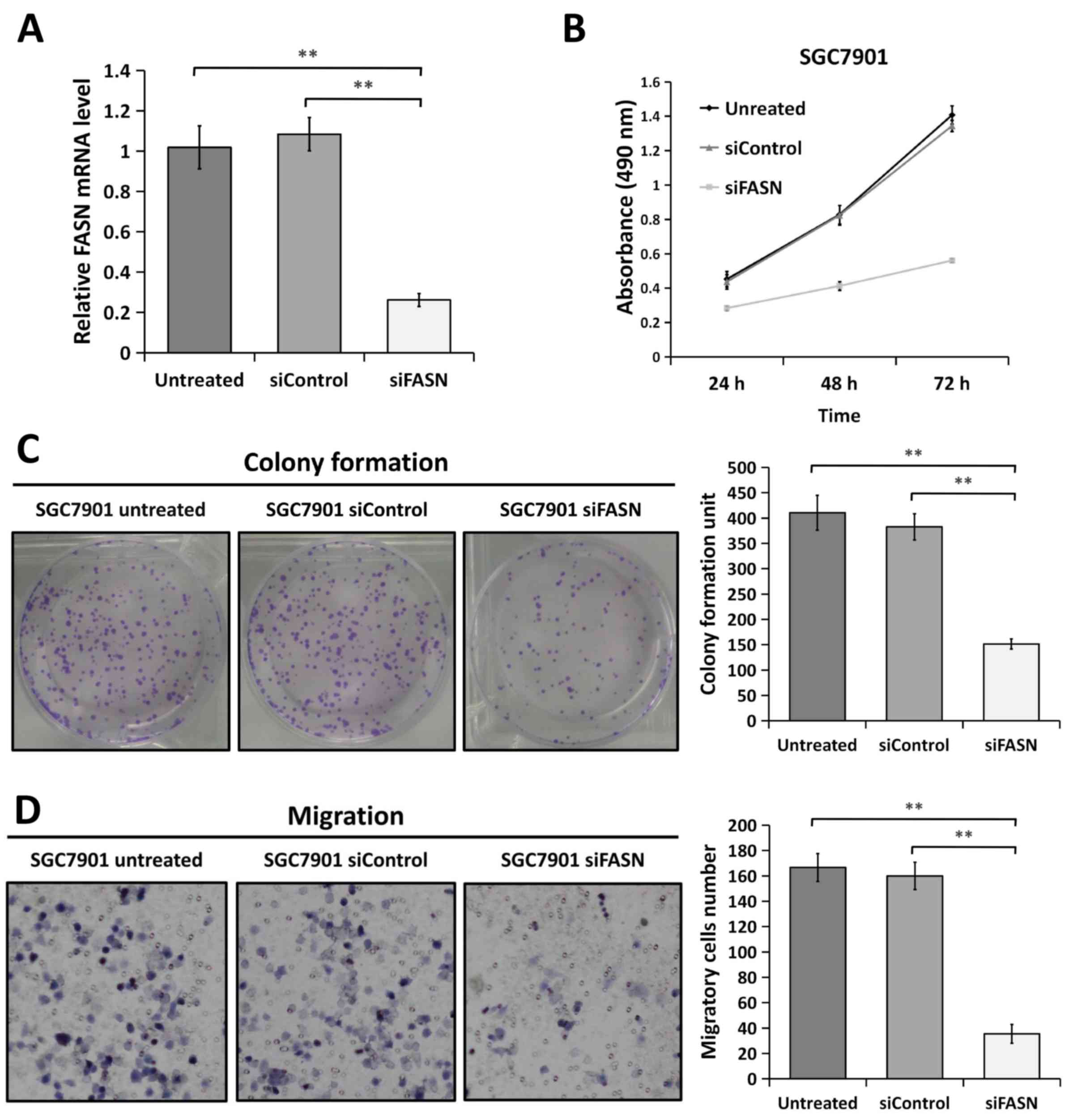 | Figure 2.Knockdown of FASN by siRNA inhibits
gastric cancer cell proliferation and metastasis in vitro.
(A) Reverse transcription-quantitative polymerase chain reaction
analysis indicated that FASN expression was strongly decreased in
FASN-depleted SGC-7901 cells at the mRNA level. 18S rRNA was used
as a loading control. (B) Downregulation of FASN expression
inhibited the proliferation of SGC-7901 cells compared with that in
the untreated cells and control siRNA-transfected cells, as
determined by MTT assay. Figures are curves of SGC-7901 cell
proliferation following transfection for 24, 48 and 72 h for MTT
assays. Data are presented as the mean ± standard deviation (n=3).
(C) SGC-7901 cells transfected with control siRNA or siRNA against
FASN were maintained in culture medium for 10 days and then fixed
and stained with 0.1% crystal violet, and the colonies containing
>100 cells were counted manually. The representative images are
presented (left; magnification, ×1), and the relative number of
colonies was counted (right). The bands were quantified and
presented as the mean ± SEM of three independent experiments. (D)
Migration assays were performed in wild-type SGC-7901 cells and in
cells transfected with negative control siRNA or siRNA against
FASN. Representative images are presented (left; magnification,
×200) and the relative numbers of migratory cells (right) were
counted. The bands were quantified and presented as the mean ± SEM
of three independent experiments. Statistical significance was
determined by one-way analysis of variance. **P<0.01;
***P<0.001. FASN, fatty acid synthase; siRNA, small interfering
RNA; SEM, standard error of the mean; siControl, control siRNA;
siFASN, siRNA against FASN; untreated, wild-type cells. |
Silencing FASN expression by siRNA
regulates EMT in GC cells
Since FASN is involved in GC metastasis, it is
possible that FASN may regulate EMT, which is an early event in the
metastasis of cancer (18,19). To test this, the expression of the
epithelial marker E-cadherin and the mesenchymal marker Vimentin
was analyzed using western blotting and RT-qPCR assays. The western
blotting analysis revealed that cells transfected with siRNA
against FASN exhibited a significant decrease in Vimentin protein
expression compared with cells transfected with control siRNA and
the wild-type untreated cells, as well as a significant increase in
E-cadherin protein expression in the SGC-7901 (Fig. 3A and B) and MGC-803 (Fig. 3D and E) GC cell lines. Consistent with
the western blot analysis, the downregulation of FASN expression
significantly enhanced E-cadherin gene expression and attenuated
Vimentin gene expression at the mRNA level in the SGC-7901
(Fig. 3C) and MGC-803 (Fig. 3F) GC cell lines. All these data
indicated that FASN exerts a crucial role in modulating EMT in GC
cells.
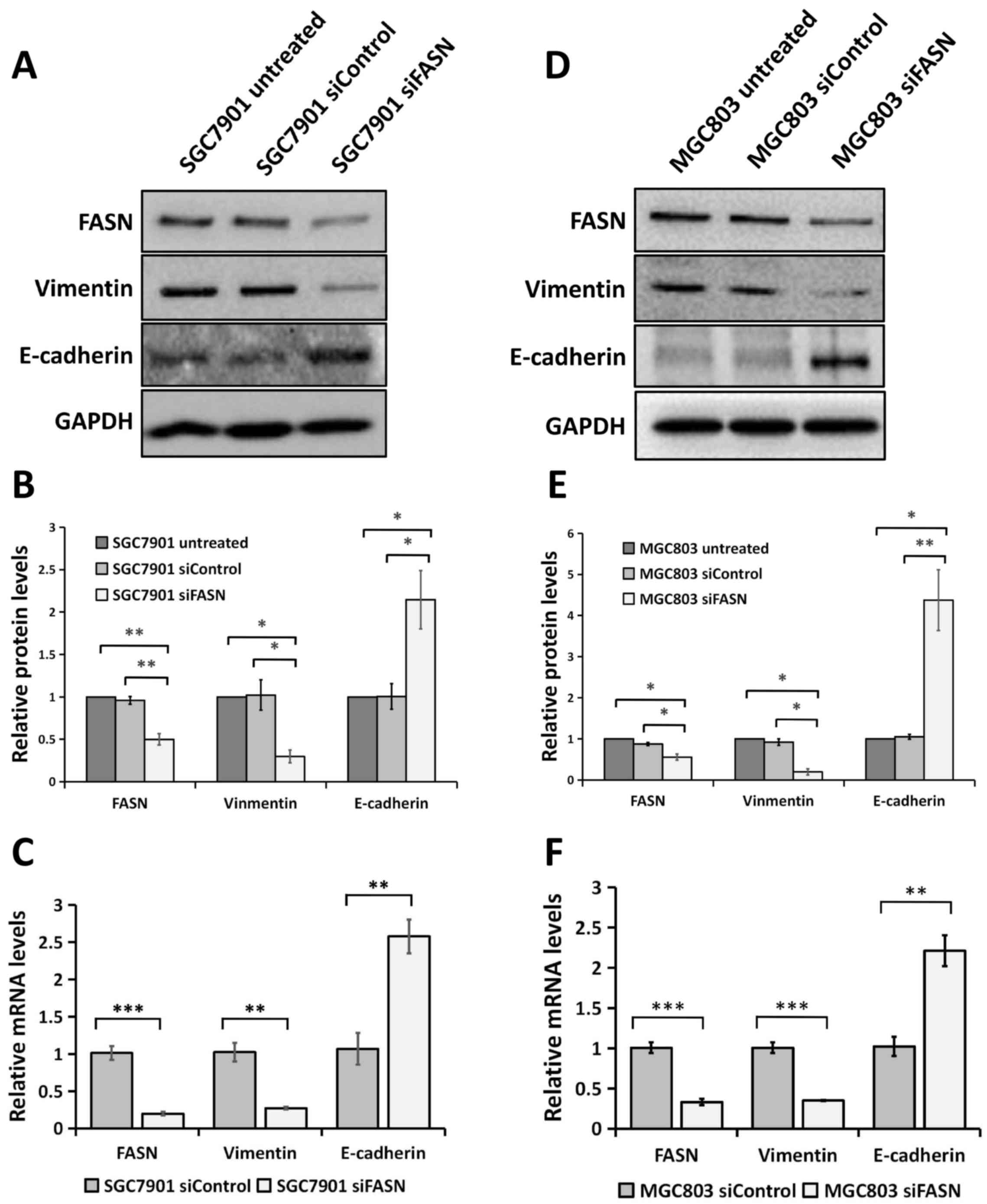 | Figure 3.Knockdown of FASN expression by siRNA
reversed EMT at the protein and mRNA levels in gastric cancer
cells. (A) Western blot analysis of the Vimentin and E-cadherin
expression in wild-type SGC-7901 cells and in cells transfected
with negative control siRNA or siRNA against FASN. GAPDH was used
as a loading control. (B) The bands were quantified and presented
as the mean ± SEM of three independent experiments. (C) RT-qPCR
analysis of Vimentin and E-cadherin expression in SGC-7901 cells
transfected with negative control siRNA or siRNA against FASN. The
bands were presented as the mean ± SEM (n=4). 18S rRNA was used as
a loading control. (D) Western blotting analysis of the Vimentin
and E-cadherin expression in wild-type MGC-803 cells and in cells
transfected with negative control siRNA or siRNA against FASN.
GAPDH was used as a loading control. (E) The bands were quantified
and presented as the mean ± SEM of three independent experiments.
(F) RT-qPCR analysis of Vimentin and E-cadherin expression in
MGC-803 cells transfected with negative control siRNA or siRNA
against FASN. The bands were presented as the mean ± SEM (n=4). 18S
rRNA was used as a loading control. Statistical significance was
determined by Student's t-test (unpaired, two-tailed) or one-way
analysis of variance. *P<0.05; **P<0.01; ***P<0.001.
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; FASN, fatty acid synthase; siRNA, small interfering RNA;
SEM, standard error of the mean; siControl, control siRNA; siFASN,
siRNA against FASN; untreated, wild-type cells; EMT,
epithelial-mesenchymal transition; GAPDH, glyceraldehyde
3-phosphate dehydrogenase. |
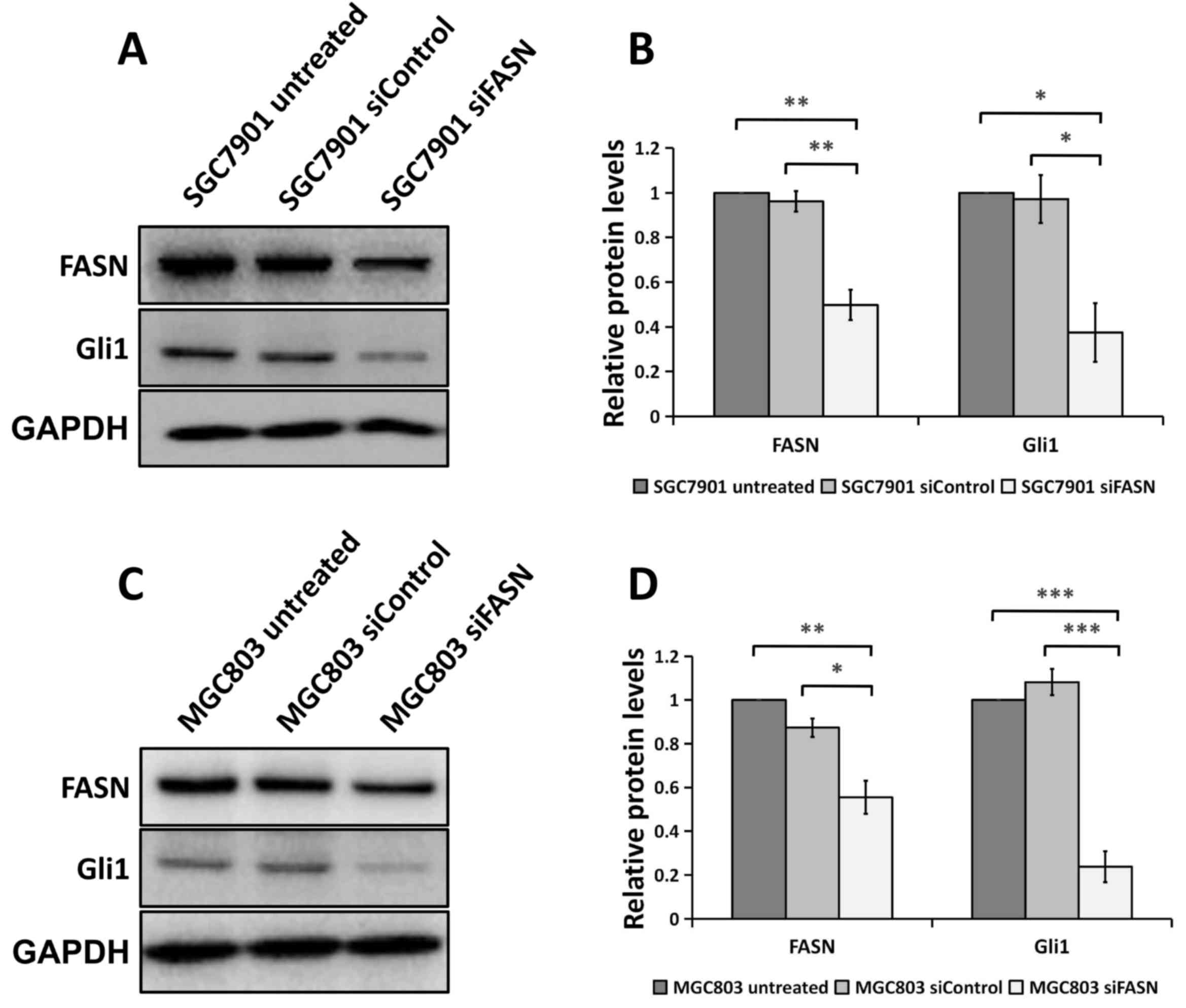
FASN loss decreases Gli1 level in GC
cells
Since aberrant Gli1 expression in the Hh pathway
underlies the development and metastasis of cancer (18,20,21), it
was assessed whether FASN serves any role in modulating Gli1
expression with regard to GC development and progression.
Unexpectedly, as shown in Fig. 4A and
B, cells transfected with siRNA against FASN exhibited a
significant decrease in Gli1 protein expression compared with cells
transfected with control siRNA or the wild-type untreated cells,
indicating that FASN may regulate Gli1 expression in SGC-7901 and
MGC-803 GC cells.
FASN modulates Gli1 expression through
regulating the mTOR signaling pathway in GC cells
As aforementioned, the blockade of FASN attenuated
Gli1 protein expression in GC cells. Next, the present study
determined how FASN regulates Gli1 expression in GC. A range of
recent findings have shown that FASN can stimulate mTOR signaling
and that by contrast, silencing FASN impairs mTOR signaling in
ovarian cancer (26–28). Furthermore, it has been reported that
activated mTOR can promote Gli1 transcriptional activity and
oncogenic function through ribosomal protein S6 kinase β1
(S6K1)-mediated Gli1 phosphorylation in esophageal adenocarcinoma
(23). The present study investigated
whether FASN modulates Gli1 expression via regulating mTOR
signaling in GC. As expected, the blockade of FASN by siRNA in the
SGC-7901 and MGC-803 cells greatly decreased phosphorylation of
mTOR while mTOR expression itself has not changed, which indicated
that knockdown of FASN suppressed the activation of mTOR (Fig. 5A and B), indicating that FASN
modulates Gli1 expression probably via its regulation of the mTOR
signaling pathway in GC cells. These data suggested that FASN
modulates the proliferation and metastasis of GC potentially via
regulation of the mTOR/Gli1 signaling pathway.
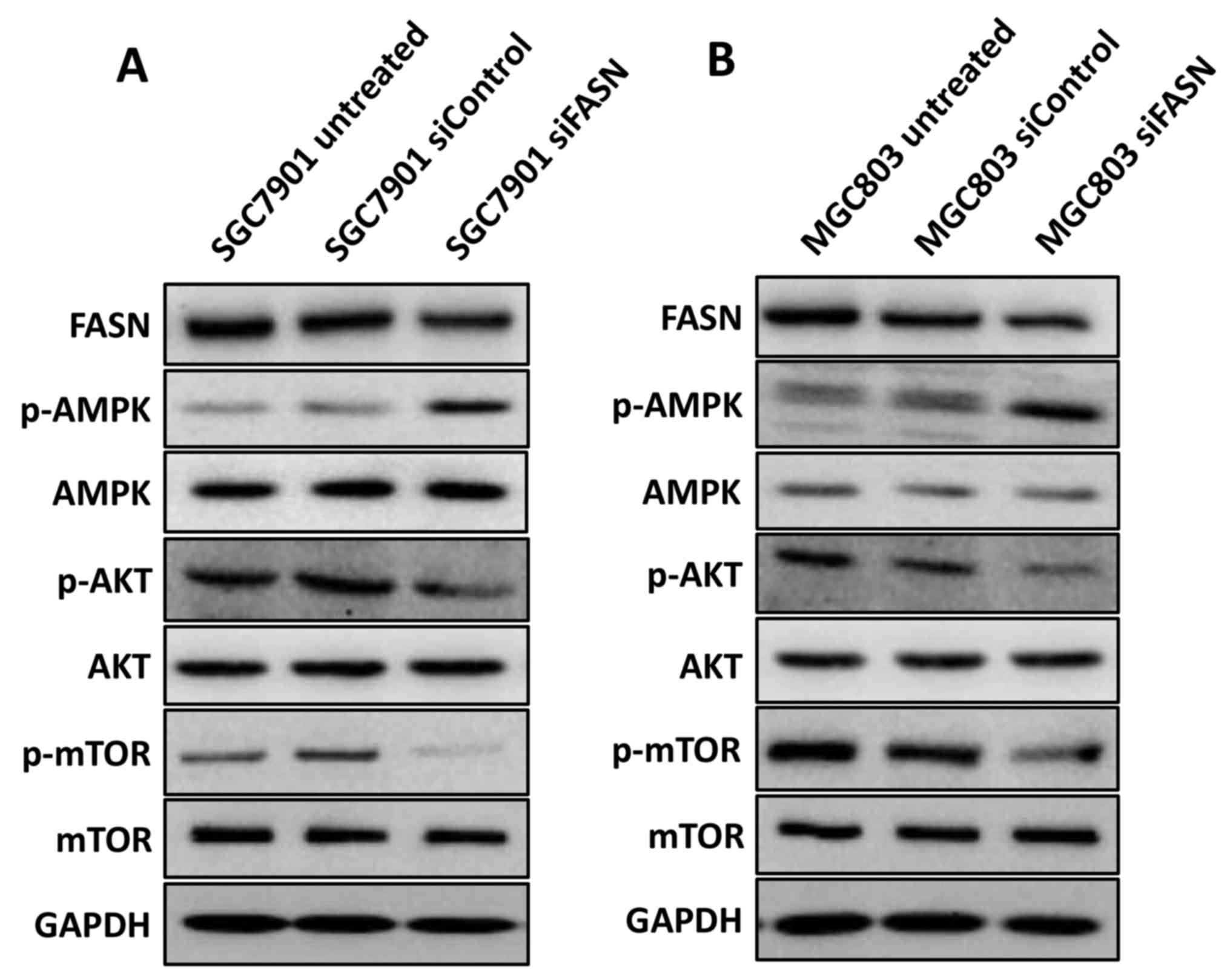 | Figure 5.FASN modulates the activation of
AMPK, AKT and mTOR in gastric cancer cells. (A) Western blotting
analysis of the p-AMPK, p-AKT and p-mTOR expression in wild-type
SGC-7901 cells and in cells transfected with negative control siRNA
or siRNA against FASN. GAPDH was used as a loading control. (B)
Western blotting analysis of the p-AMPK, p-AKT and p-mTOR
expression in wild-type MGC-803 cells and in cells transfected with
negative control siRNA or siRNA against FASN. GAPDH was used as a
loading control. FASN, fatty acid synthase; siRNA, small
interfering RNA; siControl, control siRNA; siFASN, siRNA against
FASN; untreated, wild-type cells; AMPK, AMP-activated protein
kinase; AKT, protein kinase B; mTOR, mechanistic target of
rapamycin; p-, phosphorylated; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
FASN regulates AMP-activated protein
kinase (AMPK) and protein kinase B (AKT) in GC cells
Available data suggest that blockade of FASN
activates the mTOR repressor AMPKα causing the mTOR signaling
inhibition in ovarian cancer (28),
and FASN inhibition can also inactivate the activity of AKT in
various types of cancer (29–31). Since the activation of AMPK and AKT
serves a vital role in regulating mTOR activity in cancer (32), the present study assessed whether AMPK
and AKT are involved in the regulation of FASN-mediated mTOR/Gli1
activation in GC. Unexpectedly, as shown in Fig. 5A and B, silencing FASN markedly
increased phosphorylation of AMPK and attenuated phosphorylation of
AKT while total AMPK or AKT expression has not changed in the
SGC-7901 and MGC-803 cell lines, indicating that FASN modulates
mTOR activation, probably via its regulation of AMPK and AKT in GC
cells.
Discussion
Fatty acid metabolism serves a crucial role in
carcinogenesis and is strongly involved in the signal transduction
of several types of tumor cells (9–11). FASN is
a key enzyme of de novo fatty acid synthesis, which supplies
lipids for membrane production. In cancer cells, FASN is commonly
overexpressed, providing cancer cells with an extra source of
cellular fatty acids, and is significantly associated with tumor
cell proliferation, metastasis and a poor prognosis (12–15).
Inhibition of tumor FASN activity attenuates tumor cell
proliferation and metastasis, and induces apoptosis in vitro
and in vivo (11,14,27,28,30,31),
suggesting that FASN is an attractive target for cancer
therapy.
However, studies focusing upon FASN in GC are
limited. Previous studies (15–17) have
provided evidence that FASN is overexpressed in GC tissues, and
that its overexpression is closely associated with GC metastasis
and survival, indicating that FASN contributes to the development
and progression of GC. However, the functional role of FASN
expression in GC cells remains unclear, and the concrete molecular
mechanisms of the regulation of cell proliferation and metastasis
by FASN are not well understood.
The present study demonstrated that the expression
of FASN in GC tissues was increased compared with that in adjacent
normal tissues, as indicated by other previous studies (15,16). To
the best of our knowledge, the present study also provided the
first evidence that the knockdown of FASN via siRNA inhibited the
proliferation and migration of GC cells. In view of the important
role of EMT in tumor metastasis during tumor progression (18,19), the
role of FASN on the EMT of GC cells was also investigated for the
first time in the present study, and it was revealed that FASN can
modulate the expression of EMT markers E-cadherin and Vimentin in
GC SGC-7901 and MGC-803 cells.
Emerging literature suggests that the Hh pathway and
its effector Gli1 are highly involved in the proliferation,
metastasis and EMT of numerous types of malignant tumors, including
GC (18,20–24). The
Hh pathway includes the canonical and non-canonical signaling
pathways (33). The Hh ligands binds
and inactivates the Hh receptor, protein patched homolog 1 (PTCH1),
leading to the release of the G-coupled receptor-like signal
transducer Smoothened homolog (Smo), which then activates Gli by
blocking their inhibitory partner, suppressor of fused homolog
(SuFu); this is the canonical Hh signaling pathway. Besides being
activated by the Hh ligand-PTCH1-Smo axis, Gli proteins, mainly
Gli1, can also be activated by other signaling molecules, including
mTOR/S6K1 and mitogen-activated protein kinase/extracellular
signal-regulated kinase (33,34), which is termed non-canonical Hh
signaling.
The present study identified siRNA-mediated
knockdown of FASN decreased Gli1 level in GC cells, suggesting that
FASN may serve as a regulator of the Hh signaling pathway effector
Gli1 in GC tumorigenesis and metastasis. Considering that FASN can
stimulate mTOR signaling and FASN inhibition impairs this mTOR
signaling (26–28,31) and as
activated mTOR can induce Gli1 transcriptional activity and
oncogenic function (34), the present
study assessed whether FASN modulates Gli1 expression via
regulating the mTOR signaling in GC. In accordance with other
studies (26–28,31), the
blockade of FASN suppressed the phosphorylation of mTOR in GC cells
(Fig. 5A and B), suggesting that FASN
modulates the proliferation and metastasis of GC potentially via
the regulation of the mTOR/Gli1 signaling pathway.
Recent studies suggest that the inhibition of FASN
activates the mTOR repressor AMPKα (28) and suppresses the activity of AKT
(29–31). Since the activation of AMPK and AKT
serves a vital role in regulating mTOR activity in cancer (32), the present study next investigated
whether AMPK and AKT are involved in the regulation of
FASN-mediated mTOR/Gli1 activation in GC. Unexpectedly, the study
showed that silencing FASN activated AMPK and attenuated
phosphorylation of AKT in the GC SGC-7901 and MGC-803 cell lines,
indicating that FASN modulates mTOR activation, at least in part,
by the regulation of AMPK and AKT in GC cells.
Available data suggest that mTOR signaling
upregulates FASN by the induction of sterol regulatory element
binding protein-1 (35), and FASN in
turn can stimulate mTOR signaling, with its blockade impairing mTOR
signaling (26–28,31),
implying the presence of positive feedback loops between FASN and
mTOR signaling in cancer. The impact of mTOR signaling on FASN is
already well characterized, but reverse actions from FASN towards
mTOR remain elusive. A recent study revealed that AMPK links
FASN-blockade to mTOR suppression and growth inhibition (28). In the present study, in GC cells, it
was confirmed that FASN modulates mTOR activation via regulation of
AMPK and AKT.
Extensive studies have demonstrated that Hh
signaling triggers FASN expression in a Smo-dependent manner to
regulate de novo lipid synthesis in progenitor cells and
medulloblastoma (36,37), but whether FASN serves any role in
regulating the Hh pathway remains unclear. To the best of our
knowledge, the present study provided the first evidence that
siRNA-mediated FASN-knockdown decreased the expression of the Hh
pathway effector Gli1, at least in part via regulation of AMPK/mTOR
and AKT/mTOR signaling in GC cells, but whether FASN regulates Gli1
expression in a Smo-dependent manner was not elucidated and
warrants future investigation.
There were certain limitations in the present study.
Firstly, only western blot analysis was used to examine FASN
expression in human GC tissues and paired adjacent normal tissues.
The level of FASN could be analyzed by immunohistochemical staining
and the association with clinicopathological parameters in GC
patients could be assessed. Secondly, AMPK/mTOR activity or Gli1
expression was not detected in the GC and paired normal tissues,
nor was the effect of the depletion of FASN on GC tumorigenesis and
metastasis measured in vivo. Thirdly, multi-level
suppression of FASN or FASN overexpression in GC cells was not
performed and siRNA was only used against FASN to inhibit FASN
expression. The mechanism of FASN regulation of AMPK and AKT in GC
will be investigated in a future study. Further research should be
undertaken to further investigate the function of FASN in GC. Taken
together, the results of the current study indicate that the
knockdown of FASN by siRNA suppresses GC proliferation and
metastasis through targeting mTOR/Gli1 signaling, indicating that
it may serve as a potential target for the treatment of GC.
In summary, the present in vitro experiments
not only indicated that FASN functions as a novel regulator of GC
cell proliferation and metastasis, but also emphasized the
potential role of FASN in regulating Gli1 expression. Moreover, a
critical mechanism was revealed for FASN in the regulation of GC
tumorigenesis and metastasis through its participation in the
non-classical Hh signaling pathway. This may highlight a novel
entry point for treating GC by targeting the FASN/mTOR/Gli1
signaling axis.
Acknowledgements
Not applicable.
Funding
This study was supported by the Project of Nature
Science Foundation of China (grant no. 81672348), the National
Science Foundation of Jiangsu Province, China (grant no.
BK2016255), the Six Major Talent Peak Project of Jiangsu Province,
China (grant no. 2015-WSW-014), the Six One Project for Advanced
Medical Talent of Jiangsu Province, China (grant no. LGY2016031)
and the Jiangsu Provincial Medical Youth Talent (grant no.
QNRC2016735).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS and SH conducted the research, analyzed the data
and wrote the manuscript. YY, GP, SZ, WS, TL, JY, KT, LJ and SS
contributed to data collection and analysis. LS, XZ and SH designed
the study and supervised the manuscript.
Ethics approval and consent to
participate
The study was approved by the Biomedical Research
Ethics Committee of the First Affiliated Hospital of Soochow
University. The experiments performed on human tissues were in
compliance with the Helsinki Declaration.
Consent for publication
Written informed consent for publication was
obtained from all patients in this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gores GJ and Lieberman D: Good news-bad
news: Current status of GI cancers. Gastroenterology. 151:13–16.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shridhar R, Almhanna K, Hoffe SE, Fulp W,
Weber J, Chuong MD and Meredith KL: Increased survival associated
with surgery and radiation therapy in metastatic gastric cancer: A
surveillance, epidemiology, and end results database analysis.
Cancer. 119:1636–1642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Califano D, Pignata S, Losito NS, Ottaiano
A, Greggi S, De Simone V, Cecere S, Aiello C, Esposito F, Fusco A
and Chiappetta G: High HMGA2 expression and high body mass index
negatively affect the prognosis of patients with ovarian cancer. J
Cell Physiol. 229:53–59. 2014.PubMed/NCBI
|
|
7
|
Bi X, Rexer B, Arteaga CL, Guo M and
Mahadevan-Jansen A: Evaluating HER2 amplification status and
acquired drug resistance in breast cancer cells using Raman
spectroscopy. J Biomed Opt. 19:0250012014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koochekpour S, Majumdar S, Azabdaftari G,
Attwood K, Scioneaux R, Subramani D, Manhardt C, Lorusso GD,
Willard SS, Thompson H, et al: Serum glutamate levels correlate
with gleason score and glutamate blockade decreases proliferation,
migration, and invasion and induces apoptosis in prostate cancer
cells. Clin Cancer Res. 18:5888–5901. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang L, Xiao L, Sugiura H, Huang X, Ali
A, Kuro-o M, Deberardinis RJ and Boothman DA: Metabolic
reprogramming during TGFβ1-induced epithelial-to-mesenchymal
transition. Oncogene. 34:3908–3916. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zadra G, Photopoulos C, Tyekucheva S,
Heidari P, Weng QP, Fedele G, Liu H, Scaglia N, Priolo C, Sicinska
E, et al: A novel direct activator of AMPK inhibits prostate cancer
growth by blocking lipogenesis. EMBO Mol Med. 6:519–538. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li L, Pilo GM, Li X, Cigliano A, Latte G,
Che L, Joseph C, Mela M, Wang C, Jiang L, et al: Inactivation of
fatty acid synthase impairs hepatocarcinogenesis driven by AKT in
mice and humans. J Hepatol. 64:333–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Dong L, Wei D, Wang X, Zhang S and
Li H: Fatty acid synthase mediates the epithelial-mesenchymal
transition of breast cancer cells. Int J Biol Sci. 10:171–180.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grube S, Dünisch P, Frietag D, Klausnitzer
M, Sakr Y, Walter J, Kalff R and Ewald C: Overexpression of fatty
acid synthase in human gliomas correlates with the WHO tumor grade
and inhibition with Orlistat reduced cell viability and triggers
apoptosis. J Neurooncol. 118:277–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zaytseva YY, Rychahou PG, Gulhati P,
Elliott VA, Mustain WC, O'Connor K, Morris AJ, Sunkara M, Weiss HL,
Lee EY and Evers BM: Inhibition of fatty acid synthase attenuates
CD44-associated signaling and reduces metastasis in colorectal
cancer. Cancer Res. 72:1504–1517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duan J, Sun L and Liao W, Wu Z, Wang L and
Liao W: Overexpression of fatty acid synthase predicts a poor
prognosis for human gastric cancer. Mol Med Rep. 13:3027–3035.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hou W, Fei M and Qin CY, Zhu X, Greshock
J, Liu P, Zhou Y, Wang H, Ye BC and Qin CY: High overexpression of
fatty acid synthase is associated with poor survival in Chinese
patients with gastric carcinoma. Exp Ther Med. 4:999–1004. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ito T, Sato K, Maekawa H, Sakurada M,
Orita H, Shimada K, Daida H, Wada R, Abe M, Hino O and Kajiyama Y:
Elevated levels of serum fatty acid synthase in patients with
gastric carcinoma. Oncol Lett. 7:616–620. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tania M, Khan MA and Fu J: Epithelial to
mesenchymal transition inducing transcription factors and
metastatic cancer. Tumour Biol. 35:7335–7342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taipale J and Beachy PA: The Hedgehog and
Wntsignalling pathways in cancer. Nature. 411:349–354. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukaya M, Isohata N, Nakanishi Y, Aoyagi
K, Ochiya T, Saeki N, Yanagihara K, Nakanishi Y, Taniguchi H,
Sakamoto H, et al: Hedgehog signal activation in gastric pit cell
and in diffuse-type gastric cancer. Gastroenterology. 131:14–29.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chong Y, Tang D, Xiong Q, Jiang X, Xu C,
Huang Y, Wang J, Zhou H, Shi Y, Wu X and Wang D: Galectin-1 from
cancer-associated fibroblasts induces epithelial-mesenchymal
transition through β1 integrin-mediated upregulation of Gli1 in
gastric cancer. J Exp Clin Cancer Res. 35:1752016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang ZS, Shen Y, Li X, Zhou CZ, Wen YG,
Jin YB and Li JK: Significance and prognostic value of Gli-1 and
Snail/E-cadherin expression in progressive gastric cancer. Tumour
Biol. 35:1357–1363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoo YA, Kang MH, Lee HJ, Kim BH, Park JK,
Kim HK, Kim JS and Oh SC: Sonic hedgehog pathway promotes
metastasis and lymphangiogenesis via activation of Akt, EMT, and
MMP-9 pathway in gastric cancer. Cancer Res. 71:7061–7070. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grunt TW, Wagner R, Grusch M, Berger W,
Singer CF, Marian B, Zielinski CC and Lupu R: Interaction between
fatty acid synthase and ErbB systems in ovarian cancer cells.
Biochem Biophys Res Commun. 385:454–459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tomek K, Wagner R, Varga F, Singer CF,
Karlic H and Grunt TW: Blockade of fatty acid synthase induces
ubiquitination and degradation of phosphatidylinositol-3-kinase
signaling proteins in ovarian cancer. Mol Cancer Res. 9:1767–1779.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wagner R, Stübiger G, Veigel D, Wuczkowski
M, Lanzerstorfer P, Weghuber J, Karteris E, Nowikovsky K,
Wilfinger-Lutz N, Singer CF, et al: Multi-level suppression of
receptor-PI3K-mTORC1 by fatty acid synthase inhibitors is crucial
for their efficacy against ovarian cancer cells. Oncotarget.
8:11600–11613. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Calvisi DF, Wang C, Ho C, Ladu S, Lee SA,
Mattu S, Destefanis G, Delogu S, Zimmermann A, Ericsson J, et al:
Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling,
promotes development of human hepatocellular carcinoma.
Gastroenterology. 140:1071–1083. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng SS, Gao JG, Liu ZJ, Zhang XH, Wu S,
Weng BW, Wang YL, Hou SC and Jiang B: Downregulation of fatty acid
synthase complex suppressed cell migration by targeting
phosphor-AKT in bladder cancer. Mol Med Rep. 13:1845–1850. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang L, Wu P, Senthilkumar R, Tian X, Liu
H, Shen X, Tao Z and Huang P: Loss of fatty acid synthase
suppresses the malignant phenotype of colorectal cancer cells by
down-regulating energy metabolism and mTOR signaling pathway. J
Cancer Res Clin Oncol. 142:59–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shimobayashi M and Hall MN: Making new
contacts: The mTOR network in metabolism and signalling crosstalk.
Nat Rev Mol Cell Biol. 15:155–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Teperino R, Aberger F, Esterbauer H, Riobo
N and Pospisilik JA: Canonical and non-canonical Hedgehog
signalling and the control of metabolism. Semin Cell Dev Biol.
33:81–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Ding Q, Yen CJ, Xia W, Izzo JG,
Lang JY, Li CW, Hsu JL, Miller SA, Wang X, et al: The crosstalk of
mTOR/S6K1 and Hedgehog pathways. Cancer Cell. 21:374–387. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Furuta E, Pai SK, Zhan R, Bandyopadhyay S,
Watabe M, Mo YY, Hirota S, Hosobe S, Tsukada T, Miura K, et al:
Fatty acid synthase gene is upregulated by hypoxia via activation
of Akt and sterol regulatory element binding protein-1. Cancer Res.
68:1003–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bhatia B, Hsieh M, Kenney AM and Nahlé Z:
Mitogenic sonic hedgehog signaling drives E2F1-dependent
lipogenesis in progenitor cells and Medulloblastoma. Oncogene.
30:410–422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bhatia B, Potts CR, Guldal C, Choi S,
Korshunov A, Pfister S, Kenney AM and Nahlé ZA: Hedgehog-mediated
regulation of PPARγ controls metabolic patterns in neural
precursors and shh-driven medulloblastoma. Acta Neuropath.
123:587–600. 2012. View Article : Google Scholar : PubMed/NCBI
|