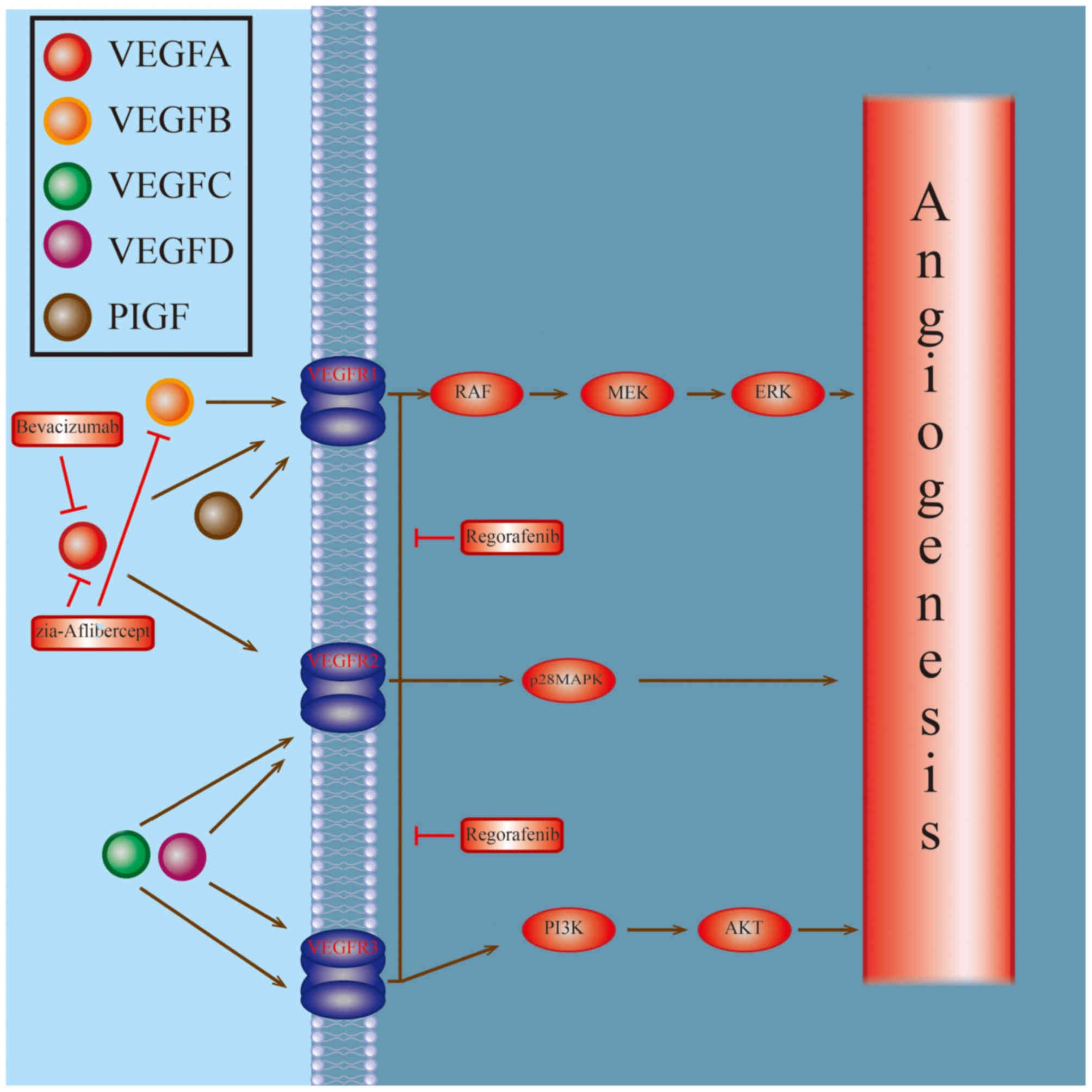
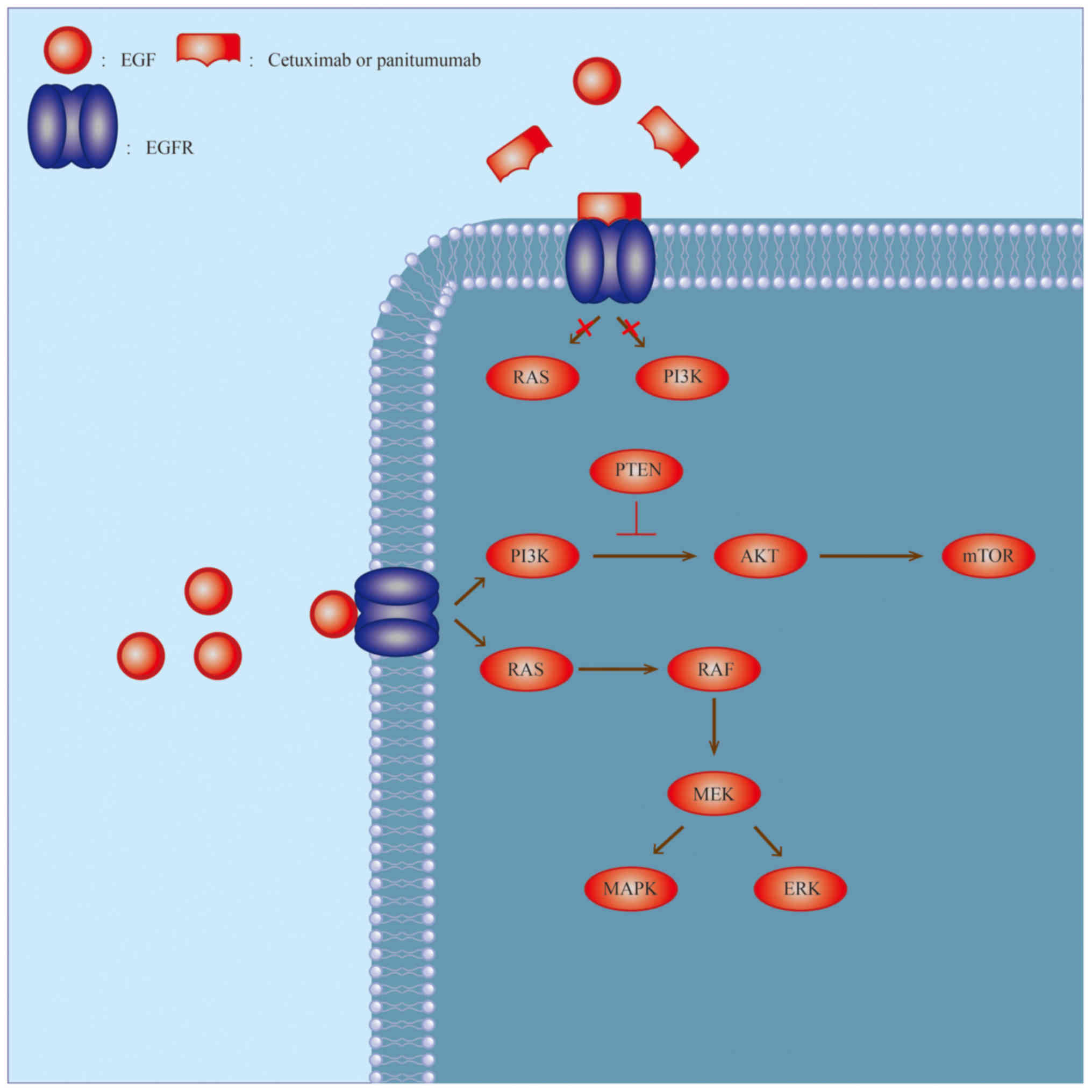
Approximately 20% of all cases with colorectal
cancer are stage IV (tumor, node and metastasis staging system;
www.nccn.org/patients) at the first
diagnosis, and the 5-year survival rate of these cases is only 13%
(4,6–8).
Chemotherapy is the main treatment for metastatic and local
late-stage colorectal cancer; however, the toxicity and adverse
side effects maybe intolerable for patients with a poor prognosis
(9). In comparison, targeted therapy
is associated with improved compliance, decreased toxicity and
fewer side effects in addition to improved prognosis (10,11).
However, increasingly, adverse events associated
with targeted therapy have been reported in previous years
(12–15). At present, the approved targeted drugs
for metastatic colorectal cancer include monoclonal antibodies
targeting vascular endothelial growth factor (VEGF) and those
targeting the epidermal growth factor receptor (EGFR). The
representatives of the first category include bevacizumab,
ziv-aflibercept and regorafenib; and those of the second category
include cetuximab and panitumumab. The present review assesses the
adverse events and the corresponding treatments following the use
of these two categories of targeted drugs.
The common adverse events arising from the use of
VEGF inhibitors are outlined below.
VEGF induces the synthesis of nitric oxide synthase
via endothelial cells and results in the release of nitric oxide, a
notable vasodilator (21). The
blocking of VEGF-associated signaling pathways may decrease the
secretion of nitric oxide synthase and result in hypertension
(22,23). A previous study suggested that VEGF
inhibitors induce hypertension by altering the
rennin-angiotensin-aldosterone system (24). Another study hypothesized that VEGF
inhibitors decrease the microvessel density of the internal organs
in patients with hypertension, thus decreasing the blood flow rate
and resulting in hypertension (25).
The following principles should be followed in order
to prevent and treat hypertension induced by VEGF inhibitors
(32,33): i) Blood pressure monitoring should be
performed for patients treated using VEGF inhibitors at least once
every 2–3 weeks, and frequency of monitoring should be increased
during treatment. ii) VEGF inhibitors should not be administered
unless blood pressure is properly controlled. iii) If hypertension
was once induced or aggravated by VEGF inhibitors for the patient,
blood pressure monitoring should be continued even subsequent to
the cessation of VEGF inhibitor treatment. iv) Any antihypertensive
drugs may be used, however, the angiotensin converting enzyme
inhibitor is considered to be the superior drug, as it may prevent
or treat other side effects arising from treatment with VEGF
inhibitors, namely, proteinuria.
Proteinuria is another side effect resulting from
the use of VEGF inhibitors. If the protein content in the urine is
>300 mg/dl, this usually indicates proteinuria (34–39).
Proteinuria caused by the use of VEGF inhibitors is asymptomatic
(34) without obvious pathological
changes of the kidney (35). As to
the mechanism of occurrence of proteinuria, a previous study
(36) proposed the intervention of a
podocyte-derived VEGF signal axis. However, the glomerular
podocytes may constitutively express VEGF and activate VEGFR2 on
glomerular vascular endothelial cells, thus establishing and
maintaining basic liver functions (36,37).
The incidence of proteinuria appears to be dependent
on the dose of VEGF inhibitors and the severity of hypertension
(38,39). Generally speaking, VEGF inhibitors are
more likely to induce hypertension compared with proteinuria. As
demonstrated by a previous meta-analysis (40,41), the
relative risk (RR) caused by VEGF inhibitors was 3.46 and that of
proteinuria was 2.51 compared with the control group. Another
meta-analysis included 6,882 cases from a total of 33 clinical
trials, and the results revealed that the incidence of proteinuria
was 18.7% among patients receiving VEGF inhibitor treatment and the
incidence of advanced proteinuria (grade 3 or above) was 2.4%
(42).
Prior to the use of VEGF inhibitors, screening for
proteinuria should be performed. For patients that are negative for
proteinuria, only screening is required prior to each treatment;
for patients that are positive for proteinuria, evaluation by
physicians in nephrology is required if the treatment with VEGF
inhibitors is to be administered and the treatment should be highly
individualized (43,44).
However, no standards have been established so far
for the treatment of proteinuria caused by VEGF inhibitors.
According to US Food and Drug Administration guidelines (44), anti-angiogenic drugs should be disused
if protein content in the urine >2 g/24 h. Furthermore, if
hypertension is induced by VEGF inhibitors and complicated by
proteinuria, ACEI and angiotensin receptor blockers are often used
for the effect of decreasing the level of protein in the urine and
protecting the blood vessels (34).
The EGFR signaling pathway is one of the first
pathways discovered to be involved in the targeted therapy of
tumors (Fig. 2). This pathway affects
the proliferation, differentiation, migration and apoptosis of
cells (51,52) and usually demonstrates abnormal
expression and activation in various solid tumor types (52–54). The
common adverse events associated with EGFR tyrosine kinase
inhibitors (TKIs) are outlined below.
Hypomagnesemia is negatively associated with age,
potentially due to the easier loss of Mg2+ (61,62).
Furthermore, severe hypomagnesemia may lead to changes in muscle
strength (including cramps, muscle weakness and ataxia), heart
lesions (including coronary spasms, arrhythmia and long Q-T
syndrome) and psychotic symptoms (including epilepsy, insanity,
depression and anxiety) (63). These
symptoms are easily confused with paraneoplastic syndrome (64). Thus, an electrolyte test is
recommended prior to treatment, particularly for elderly patients,
together with reexamination once every 2–4 weeks.
For grade 1 hypomagnesemia, which is usually
asymptomatic, no interventions are recommended clinically (65). For grade 2 hypomagnesemia or above,
intravenous infusion of Mg2+ is usually required as oral
administration, which may induce diarrhea (61).
At present, the underlying mechanism of
hypopotassemia occurrence caused by EGFR TKI is not well
understood. It is a generally held opinion that EGFR TKIs may cause
nephrotoxicity (66). A previous
study (67) revealed the function of
inhibited Mg2+ channel TRPM-6 in this process. When
hypomagnesemia occurs, increased K+ is required for the
repair of Na-K-adenosine triphosphatase. This may result in
decreased renal potassium conservation and hence
hypopotassemia.
The treatment of hypopotassemia is not difficult
clinically. Regular potassium tests are necessary during
medication, and potassium may be infused if necessary. If
hypopotassemia is complicated by hypomagnesemia, Mg2+
infusion is necessary (71).
The most common side effect of EGFR TKIs is a skin
rash, which was one of the first identified side effects (72–76). A
number of studies and literature reviews are focused on the topic
of the skin rash caused by EGFR TKIs (77–79).
Targeted therapy has unique advantages in treating
colorectal cancer, and the progression of this therapy is fueled by
an enhanced understanding of the tumor types it aims to target at a
genetic level (8,85–87).
However, as targeting remains imprecise, certain adverse events are
consistently reported (26–30,39,41,45–51).
This is particularly true when the targeted therapy is combined
with chemotherapy (27,28) Therefore, gaining a deeper
understanding of the underlying mechanisms of the side effects
occurring as a result of targeted therapy and identifying methods
to treat them are highly prioritized. According to a previous
study, the optimal method for coping with the side effects
associated with targeted therapy is not to decrease the dosage, but
through symptomatic treatment, which is capable of avoiding
toxicity and adverse side effects (33). For example, VEGFR TKIs may cause
hypertension, which may be prevented by proper preventive measures.
The active control of blood pressure during targeted therapy can
avoid damage of relevant target organs caused by hypertension and
prevent progression of hypertension, as a medical consensus,
ordinary patients with hypertension also require active control of
blood pressure.
In conclusion, proficient understanding of the
underlying molecular mechanisms of targeted drugs and the potential
adverse events in addition to the proper treatments for these
adverse effects is crucial for improving the prognosis of patients
with cancer.
The present study was supported by the National
Natural Science Foundation of China (grant no. 81502147) and the
Youth Scientific Innovation Foundation of Zhejiang Cancer Hospital
(grant no. QN201402).
The authors declare that they have no competing
interests.
|
1
|
Kumar B, Bhat ZI, Bansal S, Saini S,
Naseem A, Wahabi K, Burman A, Kumar GT, Saluja SS and Rizvi MMA:
Association of mitochondrial copy number variation and T16189C
polymorphism with colorectal cancer in North Indian population.
Tumour Biol. 39:10104283177402962017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang J, Wang TY and Niu XC: Increased
plasma levels of pentraxin 3 are associated with poor prognosis of
colorectal carcinoma patients. Tohoku J Exp Med. 240:39–46. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Edge SB and Compton CC: The American Joint
Committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kumar SK, Callander NS, Alsina M,
Atanackovic D, Biermann JS, Chandler JC, Costello C, Faiman M, Fung
HC, Gasparetto C, et al: Multiple myeloma, version 3.2017, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
15:230–269. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Benson AB III, Venook AP, Cederquist L,
Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC,
Fichera A, et al: Colon cancer, version 1.2017, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
15:370–398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gibson RJ, Keefe DM, Lalla RV, Bateman E,
Blijlevens N, Fijlstra M, King EE, Stringer AM, van der Velden WJ,
Yazbeck R, et al: Systematic review of agents for the management of
gastrointestinal mucositis in cancer patients. Support Care Cancer.
21:313–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chien Chang CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Isobe T, Uchino K, Makiyama C, Ariyama H,
Arita S, Tamura S, Komoda M, Kusaba H, Shirakawa T, Esaki T, et al:
Analysis of adverse events of bevacizumab-containing systemic
chemotherapy for metastatic colorectal cancer in Japan. Anticancer
Res. 34:2035–2040. 2014.PubMed/NCBI
|
|
13
|
Folprecht G, Pericay C, Saunders MP,
Thomas A, Lopez Lopez R, Roh JK, Chistyakov V, Höhler T, Kim JS,
Hofheinz RD, et al: Oxaliplatin and 5-FU/folinic acid (modified
FOLFOX6) with or without aflibercept in first-line treatment of
patients with metastatic colorectal cancer: The AFFIRM study. Ann
Oncol. 27:1273–1279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calcagno F, Lenoble S, Lakkis Z, Nguyen T,
Limat S, Borg C, Jary M, Kim S and Nerich V: Efficacy, safety and
cost of regorafenib in patients with metastatic colorectal cancer
in French clinical practice. Clin Med Insights Oncol. 10:59–66.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang D, Ye J, Xu T and Xiong B: Treatment
related severe and fatal adverse events with cetuximab in
colorectal cancer patients: A meta-analysis. J Chemother.
25:170–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferrara N: Vascular endothelial growth
factor as a target for anticancer therapy. Oncologist. 9 Suppl
1:S2–S10. 2004. View Article : Google Scholar
|
|
17
|
Correale P, Botta C, Ciliberto D, Pastina
P, Ingargiola R, Zappavigna S, Tassone P, Pirtoli L, Caraglia M and
Tagliaferri P: Immunotherapy of colorectal cancer: New perspectives
after a long path. Immunotherapy. 8:1281–1292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klein A and Loewenstein A: Therapeutic
monoclonal antibodies and fragments: Bevacizumab. Dev Ophthalmol.
55:232–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Syed YY and McKeage K: Aflibercept: A
review in metastatic colorectal cancer. Drugs. 75:1435–1445. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun W: Angiogenesis in metastatic
colorectal cancer and the benefits of targeted therapy. J Hematol
Oncol. 5:632012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shiojima I, Sato K, Izumiya Y, Schiekofer
S, Ito M, Liao R, Colucci WS and Walsh K: Disruption of coordinated
cardiac hypertrophy and angiogenesis contributes to the transition
to heart failure. J Clin Invest. 115:2108–2118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Al-Husseini A, Kraskauskas D, Mezzaroma E,
Nordio A, Farkas D, Drake JI, Abbate A, Felty Q and Voelkel NF:
Vascular endothelial growth factor receptor 3 signaling contributes
to angioobliterative pulmonary hypertension. Pulm Circ. 5:101–116.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Voelkel NF and Gomez-Arroyo J: The role of
vascular endothelial growth factor in pulmonary arterial
hypertension. The angiogenesis paradox. Am J Respir Cell Mol Biol.
51:474–484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robinson ES, Khankin EV, Choueiri TK,
Dhawan MS, Rogers MJ, Karumanchi SA and Humphreys BD: Suppression
of the nitric oxide pathway in metastatic renal cell carcinoma
patients receiving vascular endothelial growth factor-signaling
inhibitors. Hypertension. 56:1131–1136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gurevich F and Perazella MA: Renal effects
of anti-angiogenesis therapy: Update for the internist. Am J Med.
122:322–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kabbinavar FF, Schulz J, McCleod M, Patel
T, Hamm JT, Hecht JR, Mass R, Perrou B, Nelson B and Novotny WF:
Addition of bevacizumab to bolus fluorouracil and leucovorin in
first-line metastatic colorectal cancer: Results of a randomized
phase II trial. J Clin Oncol. 23:3697–3705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoshida M, Muro K, Tsuji A, Hamamoto Y,
Yoshino T, Yoshida K, Shirao K, Miyata Y, Takahari D, Takahashi T
and Ohtsu A: Combination chemotherapy with bevacizumab and S-1 for
elderly patients with metastatic colorectal cancer (BASIC trial).
Eur J Cancer. 51:935–941. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qi WX, Shen Z, Tang LN and Yao Y: Risk of
hypertension in cancer patients treated with aflibercept: A
systematic review and meta-analysis. Clin Drug Investig.
34:231–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen J, Lu Y and Zheng Y: Incidence and
risk of hypertension with bevacizumab in non-small-cell lung cancer
patients: A meta-analysis of randomized controlled trials. Drug Des
Devel Ther. 9:4751–4760. 2015.PubMed/NCBI
|
|
30
|
Sclafani F and Cunningham D: Bevacizumab
in elderly patients with metastatic colorectal cancer. J Geriatr
Oncol. 5:78–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hamnvik OP, Choueiri TK, Turchin A, McKay
RR, Goyal L, Davis M, Kaymakcalan MD and Williams JS: Clinical risk
factors for the development of hypertension in patients treated
with inhibitors of the VEGF signaling pathway. Cancer. 121:311–319.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hurwitz H and Saini S: Bevacizumab in the
treatment of metastatic colorectal cancer: Safety profile and
management of adverse events. Semin Oncol. 33 5 Suppl 10:S26–S34.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pande A, Lombardo J, Spangenthal E and
Javle M: Hypertension secondary to anti-angiogenic therapy:
Experience with bevacizumab. Anticancer Res. 27:3465–3470.
2007.PubMed/NCBI
|
|
34
|
Izzedine H, Massard C, Spano JP,
Goldwasser F, Khayat D and Soria JC: VEGF signalling
inhibition-induced proteinuria: Mechanisms, significance and
management. Eur J Cancer. 46:439–448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang JC, Haworth L, Sherry RM, Hwu P,
Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX and
Rosenberg SA: A randomized trial of bevacizumab, an anti-vascular
endothelial growth factor antibody, for metastatic renal cancer. N
Engl J Med. 349:427–434. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eremina V, Sood M, Haigh J, Nagy A, Lajoie
G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH and Quaggin SE:
Glomerular-specific alterations of VEGF-A expression lead to
distinct congenital and acquired renal diseases. J Clin Invest.
111:707–716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dimke H, Sparks MA, Thomson BR, Frische S,
Coffman TM and Quaggin SE: Tubulovascular cross-talk by vascular
endothelial growth factor a maintains peritubular microvasculature
in kidney. J Am Soc Nephrol. 26:1027–1038. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu X, Wu S, Dahut WL and Parikh CR: Risks
of proteinuria and hypertension with bevacizumab, an antibody
against vascular endothelial growth factor: Systematic review and
meta-analysis. Am J Kidney Dis. 49:186–193. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang ZF, Wang T, Liu LH and Guo HQ: Risks
of proteinuria associated with vascular endothelial growth factor
receptor tyrosine kinase inhibitors in cancer patients: A
systematic review and meta-analysis. PLoS One. 9:e901352014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Izzedine H, Rixe O, Billemont B, Baumelou
A and Deray G: Angiogenesis inhibitor therapies: Focus on kidney
toxicity and hypertension. Am J Kidney Dis. 50:203–218. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Faruque LI, Lin M, Battistella M, Wiebe N,
Reiman T, Hemmelgarn B, Thomas C and Tonelli M: Systematic review
of the risk of adverse outcomes associated with vascular
endothelial growth factor inhibitors for the treatment of cancer.
PLoS One. 9:e1011452014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qi WX, Sun YJ, Tang LN, Shen Z and Yao Y:
Risk of gastrointestinal perforation in cancer patients treated
with vascular endothelial growth factor receptor tyrosine kinase
inhibitors: A systematic review and meta-analysis. Crit Rev Oncol
Hematol. 89:394–403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cartwright TH: Adverse events associated
with antiangiogenic agents in combination with cytotoxic
chemotherapy in metastatic colorectal cancer and their management.
Clin Colorectal Cancer. 12:86–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Grenon NN: Managing toxicities associated
with antiangiogenic biologic agents in combination with
chemotherapy for metastatic colorectal cancer. Clin J Oncol Nurs.
17:425–433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tabernero J, Takayuki Y and Cohn AL:
Correction to Lancet Oncol 2015; 16: 499–508. Ramucirumab versus
placebo in combination with second-line FOLFIRI in patients with
metastatic colorectal carcinoma that progressed during or after
first-line therapy with bevacizumab, oxaliplatin, and a
fluoropyrimidine (RAISE): A randomised, double-blind, multicentre,
phase 3 study. Lancet Oncol. 16:e2622015.PubMed/NCBI
|
|
46
|
Liu S and Kurzrock R: Toxicity of targeted
therapy: Implications for response and impact of genetic
polymorphisms. Cancer Treat Rev. 40:883–891. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Economopoulou P, Kotsakis A, Kapiris I and
Kentepozidis N: Cancer therapy and cardiovascular risk: Focus on
bevacizumab. Cancer Manag Res. 7:133–143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Van Cutsem E, Rivera F, Berry S,
Kretzschmar A, Michael M, DiBartolomeo M, Mazier MA, Canon JL,
Georgoulias V, Peeters M, et al: Safety and efficacy of first-line
bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in
metastatic colorectal cancer: The BEAT study. Ann Oncol.
20:1842–1847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
McLellan B, Ciardiello F, Lacouture ME,
Segaert S and Van Cutsem E: Regorafenib-associated hand-foot skin
reaction: Practical advice on diagnosis, prevention, and
management. Ann Oncol. 26:2017–2026. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Elmalik HH, ElAzzazy S, Salem KS and
Bujassoum S: A grave outcome of posterior reversible encephalopathy
syndrome in a patient receiving avastin (Bevacizumab) for
metastatic High-grade serous ovarian cancer. Case Rep Oncol.
8:290–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ayuso-Sacido A, Moliterno JA, Kratovac S,
Kapoor GS, O'Rourke DM, Holland EC, García-Verdugo JM, Roy NS and
Boockvar JA: Activated EGFR signaling increases proliferation,
survival, and migration and blocks neuronal differentiation in
post-natal neural stem cells. J Neurooncol. 97:323–337. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Takahashi N, Yamada Y, Furuta K, Honma Y,
Iwasa S, Takashima A, Kato K, Hamaguchi T and Shimada Y: Serum
levels of hepatocyte growth factor and epiregulin are associated
with the prognosis on anti-EGFR antibody treatment in KRAS
wild-type metastatic colorectal cancer. Br J Cancer. 110:2716–2727.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hasegawa Y, Ando M, Maemondo M, Yamamoto
S, Isa S, Saka H, Kubo A, Kawaguchi T, Takada M, Rosell R, et al:
The role of smoking status on the progression-free survival of
non-small cell lung cancer patients harboring activating epidermal
growth factor receptor (EGFR) mutations receiving first-line EGFR
tyrosine kinase inhibitor versus platinum doublet chemotherapy: A
meta-analysis of prospective randomized trials. Oncologist.
20:307–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Philip PA and Lutz MP: Targeting epidermal
growth factor receptor-related signaling pathways in pancreatic
cancer. Pancreas. 44:1046–1052. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Groenestege WM, Thébault S, van der Wijst
J, van den Berg D, Janssen R, Tejpar S, van den Heuvel LP, van
Cutsem E, Hoenderop JG, Knoers NV and Bindels RJ: Impaired
basolateral sorting of pro-EGF causes isolated recessive renal
hypomagnesemia. J Clin Invest. 117:2260–2267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Petrelli F, Borgonovo K, Cabiddu M,
Ghilardi M and Barni S: Risk of anti-EGFR monoclonal
antibody-related hypomagnesemia: Systematic review and pooled
analysis of randomized studies. Expert Opin Drug Saf. 11 Suppl
1:S9–S19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Maliakal P and Ledford A: Electrolyte and
protein imbalance following anti-EGFR therapy in cancer patients: A
comparative study. Exp Ther Med. 1:307–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Fakih MG, Wilding G and Lombardo J:
Cetuximab-induced hypomagnesemia in patients with colorectal
cancer. Clin Colorectal Cancer. 6:152–156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen P, Wang L, Li H, Liu B and Zou Z:
Incidence and risk of hypomagnesemia in advanced cancer patients
treated with cetuximab: A meta-analysis. Oncol Lett. 5:1915–1920.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cao Y, Liao C, Tan A, Liu L and Gao F:
Meta-analysis of incidence and risk of hypomagnesemia with
cetuximab for advanced cancer. Chemotherapy. 56:459–465. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tejpar S, Piessevaux H, Claes K, Piront P,
Hoenderop JG, Verslype C and Van Cutsem E: Magnesium wasting
associated with epidermal-growth-factor receptor-targeting
antibodies in colorectal cancer: A prospective study. Lancet Oncol.
8:387–394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Streb J, Püsküllüoğlu M, Glanowska I,
Ochenduszko S, Konopka K, Łupkowski R, Michalowska-Kaczmarczyk A,
Bochenek-Cibor J, Majka M and Krzemieniecki K: Assessment of
frequency and severity of hypomagnesemia in patients with
metastatic colorectal cancer treated with cetuximab, with a review
of the literature. Oncol Lett. 10:3749–3755. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
de Baaij JH, Hoenderop JG and Bindels RJ:
Magnesium in man: Implications for health and disease. Physiol Rev.
95:1–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cosmai L, Gallieni M and Porta C: Renal
toxicity of anticancer agents targeting HER2 and EGFR. J Nephrol.
28:647–657. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Arora N, Gupta A and Singh PP: Biological
agents in gastrointestinal cancers: Adverse effects and their
management. J Gastrointest Oncol. 8:485–498. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cao Y, Liu L, Liao C, Tan A and Gao F:
Meta-analysis of incidence and risk of hypokalemia with
cetuximab-based therapy for advanced cancer. Cancer Chemother
Pharmacol. 66:37–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dimke H, Monnens L, Hoenderop JG and
Bindels RJ: Evaluation of hypomagnesemia: Lessons from disorders of
tubular transport. Am J Kidney Dis. 62:377–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang Q, Qi Y, Zhang D, Gong C, Yao A, Xiao
Y, Yang J, Zhou F and Zhou Y: Electrolyte disorders assessment in
solid tumor patients treated with anti-EGFR monoclonal antibodies:
A pooled analysis of 25 randomized clinical trials. Tumour Biol.
36:3471–3482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Geyikoglu F, Emir M, Colak S, Koc K,
Turkez H, Bakir M, Hosseinigouzdagani M, Cerig S, Keles ON and Ozek
NS: Effect of oleuropein against chemotherapy drug-induced
histological changes, oxidative stress, and DNA damages in rat
kidney injury. J Food Drug Anal. 25:447–459. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Oh CJ, Ha CM, Choi YK, Park S, Choe MS,
Jeoung NH, Huh YH, Kim HJ, Kweon HS, Lee JM, et al: Pyruvate
dehydrogenase kinase 4 deficiency attenuates cisplatin-induced
acute kidney injury. Kidney Int. 91:880–895. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Abbas A, Mirza MM, Ganti AK and Tendulkar
K: Renal toxicities of targeted therapies. Target Oncol.
10:487–499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Qi WX, Sun YJ, Shen Z and Yao Y: Risk of
anti-EGFR monoclonal antibody-related skin rash: An up-to-date
meta-analysis of 25 randomized controlled trials. J Chemother.
26:359–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Vaubel J, Livingstone E, Schadendorf D and
Zimmer L: Retarded low-dose doxycycline for EGFR or MEK
inhibitor-induced papulopustular rash. J Eur Acad Dermatol
Venereol. 28:1685–1689. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Singh N, Vishwanath G, Aggarwal AN and
Behera D: Clinical experience on use of oral EGFR-TKIs as
first-line treatment of advanced NSCLC from a tertiary care centre
in North India and implications of skin rash. Indian J Chest Dis
Allied Sci. 56:149–152. 2014.PubMed/NCBI
|
|
75
|
Liu HB, Wu Y, Lv TF, Yao YW, Xiao YY, Yuan
DM and Song Y: Skin rash could predict the response to EGFR
tyrosine kinase inhibitor and the prognosis for patients with
non-small cell lung cancer: A systematic review and meta-analysis.
PLoS One. 8:e551282013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jaka A, Gutiérrez-Rivera A, Ormaechea N,
Blanco J, La Casta A, Sarasqueta C, Izeta A and Tuneu A:
Association between EGFR gene polymorphisms, skin rash and response
to anti-EGFR therapy in metastatic colorectal cancer patients. Exp
Dermatol. 23:751–753. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ocvirk J, Heeger S, McCloud P and Hofheinz
RD: A review of the treatment options for skin rash induced by
EGFR-targeted therapies: Evidence from randomized clinical trials
and a meta-analysis. Radiol Oncol. 47:166–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Gerber PA, Meller S, Eames T, Buhren BA,
Schrumpf H, Hetzer S, Ehmann LM, Budach W, Bölke E, Matuschek C, et
al: Management of EGFR-inhibitor associated rash: A retrospective
study in 49 patients. Eur J Med Res. 17:42012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Eaby-Sandy B and Lynch K: Side effects of
targeted therapies: Rash. Semin Oncol Nurs. 30:147–154. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Stremitzer S, Sebio A, Stintzing S and
Lenz HJ: Panitumumab safety for treating colorectal cancer. Expert
Opin Drug Saf. 13:843–851. 2014.PubMed/NCBI
|
|
81
|
Lv ZC, Ning JY and Chen HB: Efficacy and
toxicity of adding cetuximab to chemotherapy in the treatment of
metastatic colorectal cancer: A meta-analysis from 12 randomized
controlled trials. Tumour Biol. 35:11741–11750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
André T and Dumont SN: Regorafenib
approved in metastatic colorectal cancer. Bull Cancer.
100:1027–1029. 2013.(In French). PubMed/NCBI
|
|
83
|
Saif MW, Syrigos KI, Hotchkiss S, Shanley
J, Grasso J, Ferencz TM, Syrigos K and Shah MM: Successful
desensitization with cetuximab after an infusion reaction to
panitumumab in patients with metastatic colorectal cancer. Cancer
Chemother Pharmacol. 65:107–112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Garcia-Foncillas J and Diaz-Rubio E:
Progress in metastatic colorectal cancer: Growing role of cetuximab
to optimize clinical outcome. Clin Transl Oncol. 12:533–542. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Emmanouilides C, Sfakiotaki G, Androulakis
N, Kalbakis K, Christophylakis C, Kalykaki A, Vamvakas L, Kotsakis
A, Agelaki S, Diamandidou E, et al: Front-line bevacizumab in
combination with oxaliplatin, leucovorin and 5-fluorouracil
(FOLFOX) in patients with metastatic colorectal cancer: A
multicenter phase II study. BMC Cancer. 7:912007. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Douillard JY, Siena S, Cassidy J,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Randomized, phase III trial of panitumumab with
infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4)
versus FOLFOX4 alone as first-line treatment in patients with
previously untreated metastatic colorectal cancer: The PRIME study.
J Clin Oncol. 28:4697–4705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Saltz LB, Clarke S, Diaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|