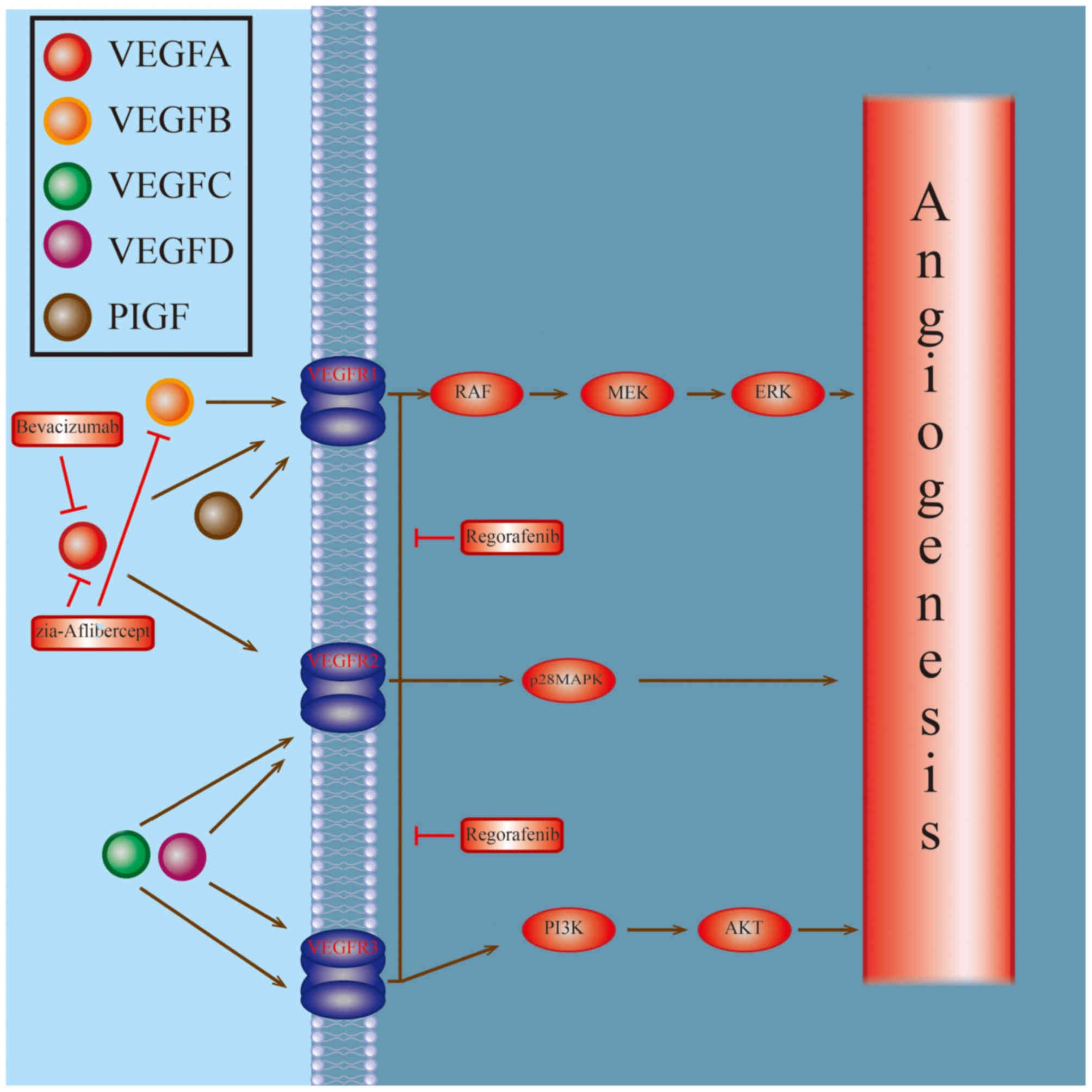
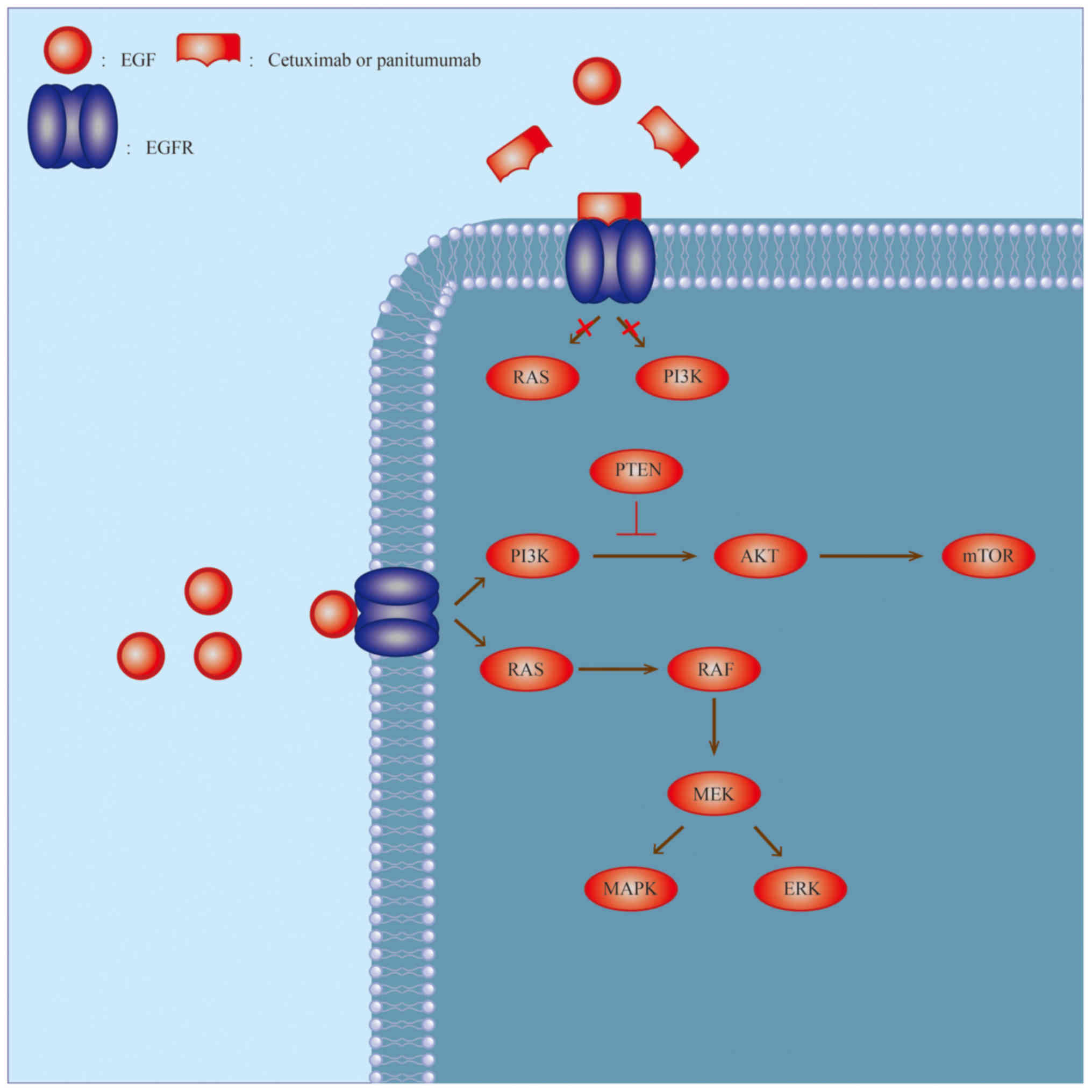
|
1
|
Kumar B, Bhat ZI, Bansal S, Saini S,
Naseem A, Wahabi K, Burman A, Kumar GT, Saluja SS and Rizvi MMA:
Association of mitochondrial copy number variation and T16189C
polymorphism with colorectal cancer in North Indian population.
Tumour Biol. 39:10104283177402962017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang J, Wang TY and Niu XC: Increased
plasma levels of pentraxin 3 are associated with poor prognosis of
colorectal carcinoma patients. Tohoku J Exp Med. 240:39–46. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Edge SB and Compton CC: The American Joint
Committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kumar SK, Callander NS, Alsina M,
Atanackovic D, Biermann JS, Chandler JC, Costello C, Faiman M, Fung
HC, Gasparetto C, et al: Multiple myeloma, version 3.2017, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
15:230–269. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Benson AB III, Venook AP, Cederquist L,
Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC,
Fichera A, et al: Colon cancer, version 1.2017, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
15:370–398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gibson RJ, Keefe DM, Lalla RV, Bateman E,
Blijlevens N, Fijlstra M, King EE, Stringer AM, van der Velden WJ,
Yazbeck R, et al: Systematic review of agents for the management of
gastrointestinal mucositis in cancer patients. Support Care Cancer.
21:313–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chien Chang CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Isobe T, Uchino K, Makiyama C, Ariyama H,
Arita S, Tamura S, Komoda M, Kusaba H, Shirakawa T, Esaki T, et al:
Analysis of adverse events of bevacizumab-containing systemic
chemotherapy for metastatic colorectal cancer in Japan. Anticancer
Res. 34:2035–2040. 2014.PubMed/NCBI
|
|
13
|
Folprecht G, Pericay C, Saunders MP,
Thomas A, Lopez Lopez R, Roh JK, Chistyakov V, Höhler T, Kim JS,
Hofheinz RD, et al: Oxaliplatin and 5-FU/folinic acid (modified
FOLFOX6) with or without aflibercept in first-line treatment of
patients with metastatic colorectal cancer: The AFFIRM study. Ann
Oncol. 27:1273–1279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calcagno F, Lenoble S, Lakkis Z, Nguyen T,
Limat S, Borg C, Jary M, Kim S and Nerich V: Efficacy, safety and
cost of regorafenib in patients with metastatic colorectal cancer
in French clinical practice. Clin Med Insights Oncol. 10:59–66.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang D, Ye J, Xu T and Xiong B: Treatment
related severe and fatal adverse events with cetuximab in
colorectal cancer patients: A meta-analysis. J Chemother.
25:170–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferrara N: Vascular endothelial growth
factor as a target for anticancer therapy. Oncologist. 9 Suppl
1:S2–S10. 2004. View Article : Google Scholar
|
|
17
|
Correale P, Botta C, Ciliberto D, Pastina
P, Ingargiola R, Zappavigna S, Tassone P, Pirtoli L, Caraglia M and
Tagliaferri P: Immunotherapy of colorectal cancer: New perspectives
after a long path. Immunotherapy. 8:1281–1292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klein A and Loewenstein A: Therapeutic
monoclonal antibodies and fragments: Bevacizumab. Dev Ophthalmol.
55:232–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Syed YY and McKeage K: Aflibercept: A
review in metastatic colorectal cancer. Drugs. 75:1435–1445. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun W: Angiogenesis in metastatic
colorectal cancer and the benefits of targeted therapy. J Hematol
Oncol. 5:632012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shiojima I, Sato K, Izumiya Y, Schiekofer
S, Ito M, Liao R, Colucci WS and Walsh K: Disruption of coordinated
cardiac hypertrophy and angiogenesis contributes to the transition
to heart failure. J Clin Invest. 115:2108–2118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Al-Husseini A, Kraskauskas D, Mezzaroma E,
Nordio A, Farkas D, Drake JI, Abbate A, Felty Q and Voelkel NF:
Vascular endothelial growth factor receptor 3 signaling contributes
to angioobliterative pulmonary hypertension. Pulm Circ. 5:101–116.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Voelkel NF and Gomez-Arroyo J: The role of
vascular endothelial growth factor in pulmonary arterial
hypertension. The angiogenesis paradox. Am J Respir Cell Mol Biol.
51:474–484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robinson ES, Khankin EV, Choueiri TK,
Dhawan MS, Rogers MJ, Karumanchi SA and Humphreys BD: Suppression
of the nitric oxide pathway in metastatic renal cell carcinoma
patients receiving vascular endothelial growth factor-signaling
inhibitors. Hypertension. 56:1131–1136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gurevich F and Perazella MA: Renal effects
of anti-angiogenesis therapy: Update for the internist. Am J Med.
122:322–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kabbinavar FF, Schulz J, McCleod M, Patel
T, Hamm JT, Hecht JR, Mass R, Perrou B, Nelson B and Novotny WF:
Addition of bevacizumab to bolus fluorouracil and leucovorin in
first-line metastatic colorectal cancer: Results of a randomized
phase II trial. J Clin Oncol. 23:3697–3705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoshida M, Muro K, Tsuji A, Hamamoto Y,
Yoshino T, Yoshida K, Shirao K, Miyata Y, Takahari D, Takahashi T
and Ohtsu A: Combination chemotherapy with bevacizumab and S-1 for
elderly patients with metastatic colorectal cancer (BASIC trial).
Eur J Cancer. 51:935–941. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qi WX, Shen Z, Tang LN and Yao Y: Risk of
hypertension in cancer patients treated with aflibercept: A
systematic review and meta-analysis. Clin Drug Investig.
34:231–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen J, Lu Y and Zheng Y: Incidence and
risk of hypertension with bevacizumab in non-small-cell lung cancer
patients: A meta-analysis of randomized controlled trials. Drug Des
Devel Ther. 9:4751–4760. 2015.PubMed/NCBI
|
|
30
|
Sclafani F and Cunningham D: Bevacizumab
in elderly patients with metastatic colorectal cancer. J Geriatr
Oncol. 5:78–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hamnvik OP, Choueiri TK, Turchin A, McKay
RR, Goyal L, Davis M, Kaymakcalan MD and Williams JS: Clinical risk
factors for the development of hypertension in patients treated
with inhibitors of the VEGF signaling pathway. Cancer. 121:311–319.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hurwitz H and Saini S: Bevacizumab in the
treatment of metastatic colorectal cancer: Safety profile and
management of adverse events. Semin Oncol. 33 5 Suppl 10:S26–S34.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pande A, Lombardo J, Spangenthal E and
Javle M: Hypertension secondary to anti-angiogenic therapy:
Experience with bevacizumab. Anticancer Res. 27:3465–3470.
2007.PubMed/NCBI
|
|
34
|
Izzedine H, Massard C, Spano JP,
Goldwasser F, Khayat D and Soria JC: VEGF signalling
inhibition-induced proteinuria: Mechanisms, significance and
management. Eur J Cancer. 46:439–448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang JC, Haworth L, Sherry RM, Hwu P,
Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX and
Rosenberg SA: A randomized trial of bevacizumab, an anti-vascular
endothelial growth factor antibody, for metastatic renal cancer. N
Engl J Med. 349:427–434. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Eremina V, Sood M, Haigh J, Nagy A, Lajoie
G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH and Quaggin SE:
Glomerular-specific alterations of VEGF-A expression lead to
distinct congenital and acquired renal diseases. J Clin Invest.
111:707–716. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dimke H, Sparks MA, Thomson BR, Frische S,
Coffman TM and Quaggin SE: Tubulovascular cross-talk by vascular
endothelial growth factor a maintains peritubular microvasculature
in kidney. J Am Soc Nephrol. 26:1027–1038. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu X, Wu S, Dahut WL and Parikh CR: Risks
of proteinuria and hypertension with bevacizumab, an antibody
against vascular endothelial growth factor: Systematic review and
meta-analysis. Am J Kidney Dis. 49:186–193. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang ZF, Wang T, Liu LH and Guo HQ: Risks
of proteinuria associated with vascular endothelial growth factor
receptor tyrosine kinase inhibitors in cancer patients: A
systematic review and meta-analysis. PLoS One. 9:e901352014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Izzedine H, Rixe O, Billemont B, Baumelou
A and Deray G: Angiogenesis inhibitor therapies: Focus on kidney
toxicity and hypertension. Am J Kidney Dis. 50:203–218. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Faruque LI, Lin M, Battistella M, Wiebe N,
Reiman T, Hemmelgarn B, Thomas C and Tonelli M: Systematic review
of the risk of adverse outcomes associated with vascular
endothelial growth factor inhibitors for the treatment of cancer.
PLoS One. 9:e1011452014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qi WX, Sun YJ, Tang LN, Shen Z and Yao Y:
Risk of gastrointestinal perforation in cancer patients treated
with vascular endothelial growth factor receptor tyrosine kinase
inhibitors: A systematic review and meta-analysis. Crit Rev Oncol
Hematol. 89:394–403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cartwright TH: Adverse events associated
with antiangiogenic agents in combination with cytotoxic
chemotherapy in metastatic colorectal cancer and their management.
Clin Colorectal Cancer. 12:86–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Grenon NN: Managing toxicities associated
with antiangiogenic biologic agents in combination with
chemotherapy for metastatic colorectal cancer. Clin J Oncol Nurs.
17:425–433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tabernero J, Takayuki Y and Cohn AL:
Correction to Lancet Oncol 2015; 16: 499–508. Ramucirumab versus
placebo in combination with second-line FOLFIRI in patients with
metastatic colorectal carcinoma that progressed during or after
first-line therapy with bevacizumab, oxaliplatin, and a
fluoropyrimidine (RAISE): A randomised, double-blind, multicentre,
phase 3 study. Lancet Oncol. 16:e2622015.PubMed/NCBI
|
|
46
|
Liu S and Kurzrock R: Toxicity of targeted
therapy: Implications for response and impact of genetic
polymorphisms. Cancer Treat Rev. 40:883–891. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Economopoulou P, Kotsakis A, Kapiris I and
Kentepozidis N: Cancer therapy and cardiovascular risk: Focus on
bevacizumab. Cancer Manag Res. 7:133–143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Van Cutsem E, Rivera F, Berry S,
Kretzschmar A, Michael M, DiBartolomeo M, Mazier MA, Canon JL,
Georgoulias V, Peeters M, et al: Safety and efficacy of first-line
bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in
metastatic colorectal cancer: The BEAT study. Ann Oncol.
20:1842–1847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
McLellan B, Ciardiello F, Lacouture ME,
Segaert S and Van Cutsem E: Regorafenib-associated hand-foot skin
reaction: Practical advice on diagnosis, prevention, and
management. Ann Oncol. 26:2017–2026. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Elmalik HH, ElAzzazy S, Salem KS and
Bujassoum S: A grave outcome of posterior reversible encephalopathy
syndrome in a patient receiving avastin (Bevacizumab) for
metastatic High-grade serous ovarian cancer. Case Rep Oncol.
8:290–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ayuso-Sacido A, Moliterno JA, Kratovac S,
Kapoor GS, O'Rourke DM, Holland EC, García-Verdugo JM, Roy NS and
Boockvar JA: Activated EGFR signaling increases proliferation,
survival, and migration and blocks neuronal differentiation in
post-natal neural stem cells. J Neurooncol. 97:323–337. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Takahashi N, Yamada Y, Furuta K, Honma Y,
Iwasa S, Takashima A, Kato K, Hamaguchi T and Shimada Y: Serum
levels of hepatocyte growth factor and epiregulin are associated
with the prognosis on anti-EGFR antibody treatment in KRAS
wild-type metastatic colorectal cancer. Br J Cancer. 110:2716–2727.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hasegawa Y, Ando M, Maemondo M, Yamamoto
S, Isa S, Saka H, Kubo A, Kawaguchi T, Takada M, Rosell R, et al:
The role of smoking status on the progression-free survival of
non-small cell lung cancer patients harboring activating epidermal
growth factor receptor (EGFR) mutations receiving first-line EGFR
tyrosine kinase inhibitor versus platinum doublet chemotherapy: A
meta-analysis of prospective randomized trials. Oncologist.
20:307–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Philip PA and Lutz MP: Targeting epidermal
growth factor receptor-related signaling pathways in pancreatic
cancer. Pancreas. 44:1046–1052. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Groenestege WM, Thébault S, van der Wijst
J, van den Berg D, Janssen R, Tejpar S, van den Heuvel LP, van
Cutsem E, Hoenderop JG, Knoers NV and Bindels RJ: Impaired
basolateral sorting of pro-EGF causes isolated recessive renal
hypomagnesemia. J Clin Invest. 117:2260–2267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Petrelli F, Borgonovo K, Cabiddu M,
Ghilardi M and Barni S: Risk of anti-EGFR monoclonal
antibody-related hypomagnesemia: Systematic review and pooled
analysis of randomized studies. Expert Opin Drug Saf. 11 Suppl
1:S9–S19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Maliakal P and Ledford A: Electrolyte and
protein imbalance following anti-EGFR therapy in cancer patients: A
comparative study. Exp Ther Med. 1:307–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Fakih MG, Wilding G and Lombardo J:
Cetuximab-induced hypomagnesemia in patients with colorectal
cancer. Clin Colorectal Cancer. 6:152–156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen P, Wang L, Li H, Liu B and Zou Z:
Incidence and risk of hypomagnesemia in advanced cancer patients
treated with cetuximab: A meta-analysis. Oncol Lett. 5:1915–1920.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cao Y, Liao C, Tan A, Liu L and Gao F:
Meta-analysis of incidence and risk of hypomagnesemia with
cetuximab for advanced cancer. Chemotherapy. 56:459–465. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tejpar S, Piessevaux H, Claes K, Piront P,
Hoenderop JG, Verslype C and Van Cutsem E: Magnesium wasting
associated with epidermal-growth-factor receptor-targeting
antibodies in colorectal cancer: A prospective study. Lancet Oncol.
8:387–394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Streb J, Püsküllüoğlu M, Glanowska I,
Ochenduszko S, Konopka K, Łupkowski R, Michalowska-Kaczmarczyk A,
Bochenek-Cibor J, Majka M and Krzemieniecki K: Assessment of
frequency and severity of hypomagnesemia in patients with
metastatic colorectal cancer treated with cetuximab, with a review
of the literature. Oncol Lett. 10:3749–3755. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
de Baaij JH, Hoenderop JG and Bindels RJ:
Magnesium in man: Implications for health and disease. Physiol Rev.
95:1–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cosmai L, Gallieni M and Porta C: Renal
toxicity of anticancer agents targeting HER2 and EGFR. J Nephrol.
28:647–657. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Arora N, Gupta A and Singh PP: Biological
agents in gastrointestinal cancers: Adverse effects and their
management. J Gastrointest Oncol. 8:485–498. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cao Y, Liu L, Liao C, Tan A and Gao F:
Meta-analysis of incidence and risk of hypokalemia with
cetuximab-based therapy for advanced cancer. Cancer Chemother
Pharmacol. 66:37–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dimke H, Monnens L, Hoenderop JG and
Bindels RJ: Evaluation of hypomagnesemia: Lessons from disorders of
tubular transport. Am J Kidney Dis. 62:377–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang Q, Qi Y, Zhang D, Gong C, Yao A, Xiao
Y, Yang J, Zhou F and Zhou Y: Electrolyte disorders assessment in
solid tumor patients treated with anti-EGFR monoclonal antibodies:
A pooled analysis of 25 randomized clinical trials. Tumour Biol.
36:3471–3482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Geyikoglu F, Emir M, Colak S, Koc K,
Turkez H, Bakir M, Hosseinigouzdagani M, Cerig S, Keles ON and Ozek
NS: Effect of oleuropein against chemotherapy drug-induced
histological changes, oxidative stress, and DNA damages in rat
kidney injury. J Food Drug Anal. 25:447–459. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Oh CJ, Ha CM, Choi YK, Park S, Choe MS,
Jeoung NH, Huh YH, Kim HJ, Kweon HS, Lee JM, et al: Pyruvate
dehydrogenase kinase 4 deficiency attenuates cisplatin-induced
acute kidney injury. Kidney Int. 91:880–895. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Abbas A, Mirza MM, Ganti AK and Tendulkar
K: Renal toxicities of targeted therapies. Target Oncol.
10:487–499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Qi WX, Sun YJ, Shen Z and Yao Y: Risk of
anti-EGFR monoclonal antibody-related skin rash: An up-to-date
meta-analysis of 25 randomized controlled trials. J Chemother.
26:359–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Vaubel J, Livingstone E, Schadendorf D and
Zimmer L: Retarded low-dose doxycycline for EGFR or MEK
inhibitor-induced papulopustular rash. J Eur Acad Dermatol
Venereol. 28:1685–1689. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Singh N, Vishwanath G, Aggarwal AN and
Behera D: Clinical experience on use of oral EGFR-TKIs as
first-line treatment of advanced NSCLC from a tertiary care centre
in North India and implications of skin rash. Indian J Chest Dis
Allied Sci. 56:149–152. 2014.PubMed/NCBI
|
|
75
|
Liu HB, Wu Y, Lv TF, Yao YW, Xiao YY, Yuan
DM and Song Y: Skin rash could predict the response to EGFR
tyrosine kinase inhibitor and the prognosis for patients with
non-small cell lung cancer: A systematic review and meta-analysis.
PLoS One. 8:e551282013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jaka A, Gutiérrez-Rivera A, Ormaechea N,
Blanco J, La Casta A, Sarasqueta C, Izeta A and Tuneu A:
Association between EGFR gene polymorphisms, skin rash and response
to anti-EGFR therapy in metastatic colorectal cancer patients. Exp
Dermatol. 23:751–753. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ocvirk J, Heeger S, McCloud P and Hofheinz
RD: A review of the treatment options for skin rash induced by
EGFR-targeted therapies: Evidence from randomized clinical trials
and a meta-analysis. Radiol Oncol. 47:166–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Gerber PA, Meller S, Eames T, Buhren BA,
Schrumpf H, Hetzer S, Ehmann LM, Budach W, Bölke E, Matuschek C, et
al: Management of EGFR-inhibitor associated rash: A retrospective
study in 49 patients. Eur J Med Res. 17:42012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Eaby-Sandy B and Lynch K: Side effects of
targeted therapies: Rash. Semin Oncol Nurs. 30:147–154. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Stremitzer S, Sebio A, Stintzing S and
Lenz HJ: Panitumumab safety for treating colorectal cancer. Expert
Opin Drug Saf. 13:843–851. 2014.PubMed/NCBI
|
|
81
|
Lv ZC, Ning JY and Chen HB: Efficacy and
toxicity of adding cetuximab to chemotherapy in the treatment of
metastatic colorectal cancer: A meta-analysis from 12 randomized
controlled trials. Tumour Biol. 35:11741–11750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
André T and Dumont SN: Regorafenib
approved in metastatic colorectal cancer. Bull Cancer.
100:1027–1029. 2013.(In French). PubMed/NCBI
|
|
83
|
Saif MW, Syrigos KI, Hotchkiss S, Shanley
J, Grasso J, Ferencz TM, Syrigos K and Shah MM: Successful
desensitization with cetuximab after an infusion reaction to
panitumumab in patients with metastatic colorectal cancer. Cancer
Chemother Pharmacol. 65:107–112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Garcia-Foncillas J and Diaz-Rubio E:
Progress in metastatic colorectal cancer: Growing role of cetuximab
to optimize clinical outcome. Clin Transl Oncol. 12:533–542. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Emmanouilides C, Sfakiotaki G, Androulakis
N, Kalbakis K, Christophylakis C, Kalykaki A, Vamvakas L, Kotsakis
A, Agelaki S, Diamandidou E, et al: Front-line bevacizumab in
combination with oxaliplatin, leucovorin and 5-fluorouracil
(FOLFOX) in patients with metastatic colorectal cancer: A
multicenter phase II study. BMC Cancer. 7:912007. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Douillard JY, Siena S, Cassidy J,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Randomized, phase III trial of panitumumab with
infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4)
versus FOLFOX4 alone as first-line treatment in patients with
previously untreated metastatic colorectal cancer: The PRIME study.
J Clin Oncol. 28:4697–4705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Saltz LB, Clarke S, Diaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|