Introduction
Breast cancer is a major cause of mortality in women
worldwide (1). The multistep process
of breast cancer progression results from acquisition of genetic
and epigenetic alterations in oncogenes and tumor suppressor genes,
which confer growth and/or survival advantages to mammary cells.
Subsequent molecular alterations may convert premalignant cells
into malignant cells with invasive and metastatic abilities
(2). These acquired capabilities,
namely cell proliferation, tissue invasion and adhesion, are
essential for malignant growth and progression (2).
Proprotein convertase subtilisin/kexin type 6
(PCSK6) is a neuroendocrine-specific mammalian
subtilisin-associated endoprotease and a calcium-dependent serine
proteinase (3). PCSK6 is able to
cleave precursor proteins at basic residues within the following
motif, (K/R)-(X)n-(K/R)↓, where n=0, 2, 4 or 6 and X refers to any
amino acid except Cys (3). A total of
8 isoforms of PCSK6 have been reported: PCSK6A-I, A-II, B, C, CS,
D, E-I and E-II (4). These isoforms
are distributed throughout various human tissues and function in
the secretory pathway (5). PCSK6
serves a pivotal role in the activation of proteins in the
extracellular matrix (ECM) (4). The
ECM contains numerous active proteins, including growth factors,
cell adhesion molecules and oncogene products (5), which are important in the occurrence and
progression of cancer. Previous studies have indicated that PCSK6
serves an important role in enhancing the progression of prostate
cancer (6) and ovarian cancer
(7). Furthermore, PCSK6 inhibition
decreases skin cancer cell proliferation, tumorigenesis and
metastasis (8).
The role of PCSK6 in cancer progression renders it
an attractive target for cancer treatment. A previous study
indicated that the proprotein convertase inhibitor, α1-PDX, and the
PCSK6 pro-segment, ppPCSK6, may increase breast cancer cell
motility, migration and invasion (9).
In addition, our previous study demonstrated that short interfering
RNA inhibition of PCSK6 reduces proliferation, invasion and
migration abilities in MDA-MB-231 cells arrested in
G0/G1 phase (10). Therefore, the present study aimed to
further investigate the direct effects and mechanisms of PCSK6 on
breast cancer cells.
Materials and methods
Cell culture
The human breast cancer MDA-MB-231 cell line was
obtained from the Shanghai Institutes for Biological Sciences
(Chinese Academy of Sciences, Shanghai, China) and cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco, Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (FBS; Hyclone; GE Healthcare, Chicago, IL, USA), 100 U/ml
penicillin and 100 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in 5% CO2.
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China)
with protease inhibitors (PMSF; Beyotime Institute of
Biotechnology) on ice for 30 min. The cell lysates were centrifuged
at 12,000 × g for 30 min at 4°C. Protein concentration was
determined using a Bio-Rad protein assay system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), according to the
manufacturer's protocol. A total of 25 µg protein was separated by
10% SDS-PAGE, transferred into polyvinylidene difluoride membranes
(GE Healthcare, Chicago, IL, USA), and blocked at room temperature
for 30 min in 10 mM Tris-buffered saline containing 0.1% (v/v)
Tween-20 (TBST) and 5% (w/v) skimmed milk.
The membranes were incubated overnight at 4°C with
the following primary antibodies at a dilution of 1:1,000: signal
transducer and activator of transcription 3 (STAT3; cat. no.
30835), phosphorylated STAT3 (p-STAT3; cat. no. 9134), Wnt family
member 3A (WNT3A; cat. no. 2721), extracellular signal-regulated
kinase (ERK; p44; cat. no. 4695) and p-ERK (cat. no. 4370; Cell
Signaling Technology, Inc., Danvers, MA, USA), as well as GAPDH
(cat. no. sc47724; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). Subsequent to washing with TBST, the membranes were incubated
with mouse anti-rabbit IgG (no conjugate; cat. no. 3677; Cell
Signaling Technology, Inc.) as a secondary antibody at a dilution
of 1:2,000 for 1 h at 37°C. The proteins were visualized using an
enhanced chemiluminescence kit (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The densitometric
analyses were performed using ImageJ software (version k1.45;
National Institutes of Health, Bethesda, MD, USA). All experiments
were repeated ≥3 times.
Cell proliferation assay
MDA-MB-231 cells were seeded onto 96-well culture
plates and cultured to 80% confluence. Cultured cells were
stimulated with recombinant human PCSK6 (rHuPCSK6) at 1, 10, 50,
100 or 150 ng/ml. Cells with only PBS were used as a control.
Following incubation for 24, 48 and 72 h, 20 µl 5 mg/ml MTT was
added to each well, and the cultures were incubated for 4 h at
37°C. The MTT solution was removed and 150 µl dimethyl sulfoxide
(DMSO) was added to dissolve the formazan products at room
temperature for 10 min. Absorbance was measured in triplicate at
490 nm using a spectrophotometer. Cell proliferation was plotted
over 72 h relative to each starting value at 24 h. Stimulation was
apparent at 150 ng/ml rHuPCSK6 with no cytotoxicity; therefore,
this concentration was used in subsequent experiments. All
experiments were repeated ≥3 times.
Cell invasion assay
Cell invasion ability was analyzed using a Transwell
apparatus (BD Biosciences, Franklin Lakes, NJ, USA). MDA-MB-231
cells were plated at a density of 3×104 cells/well and
incubated at 37°C with 150 ng/ml rHuPCSK6 in the upper chamber of
the Transwell apparatus for 8 h. The upper and lower chambers were
incubated with DMEM without FBS for 12 h, then DMEM containing 20%
FBS was placed in the lower chamber for 48 h. Cells remaining in
the upper chamber were removed with cotton swabs, and the cells
which had invaded the lower chamber were stained with 10% Giemsa
for 10 min at room temperature (cat no. G1015; Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China). The number of
invasive cells were counted in 5 random fields using a light
microscope (Nikon Corporation, Tokyo, Japan; magnification,
×100).
Wound-healing assay
To assess the migratory ability of MDA-MB-231 cells,
they were plated onto 24-well plates and cultured at 37°C to 80%
confluence. The cell layers were scratched linearly using a cell
scraper, followed by treatment with 150 ng/ml rHuPCSK6. After 24 h,
the number of cells that migrated over the scratched line was
calculated under a light microscope (magnification, ×100; Nikon
Corporation). The mean of random 5 fields was calculated. The
experiment was repeated ≥3 times.
Cell cycle analysis
Cells were plated onto Costar 6-well culture plates
(Corning Incorporated, Corning, NY, USA) at 1.0×106
cells/well and treated with 150 ng/ml rHuPCSK6. After 24 h, the
cells were harvested by trypsinization, washed twice with
phosphate-buffered saline (PBS), and fixed overnight with 70%
ethanol at 4°C. The fixed cells were rinsed and resuspended in PBS,
and stained for 30 min at 37°C with 1 ml 0.05 mg/ml propidium
iodide solution containing 10 µg/ml RNase (Beijing Dingguo
Biotechnology, Beijing, China). Cells were detected with a Coulter
Epics XL flow cytometer, and DNA content was analyzed using Beckman
Coulter EXPO32 ADC software (version 1.2B; both Beckman Coulter,
Inc., Brea, CA, USA). The experiments were repeated 3 times in
triplicate.
Apoptosis assay
MDA-MB-231 cells were cultured in 2% FBS in the
presence or absence of 150 ng/ml rHuPCSK6 for 24 h and stained
using an Annexin V-fluorescein isothiocyanate apoptosis detection
kit (BD Biosciences), according to the manufacturer's protocol.
Cells were detected using a Coulter Epics XL flow cytometer, and
DNA content was analyzed with Beckman Coulter EXPO32 ADC software
(version 1.2B; both Beckman Coulter, Inc.). The experiments were
repeated 3 times in triplicate.
Statistical analysis
All results were confirmed in ≥3 independent
experiments. Multiple comparisons were conducted using one-way
analysis of variance followed by Bonferroni's correction. The data
are expressed as mean ± standard error of the mean. All statistical
analyses were performed using GraphPad Prism 5 (GraphPad Software,
Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
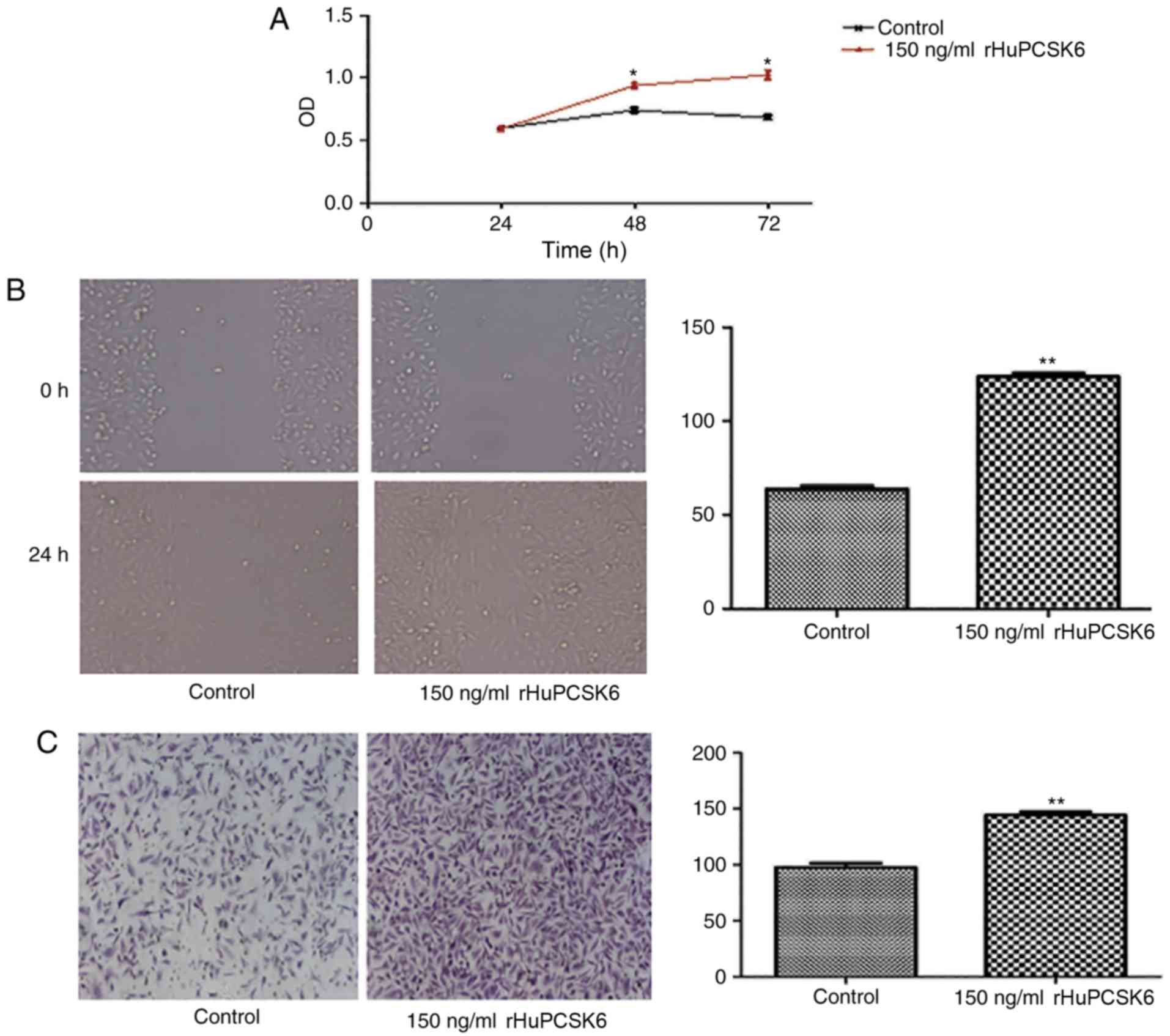
rHuPCSK6 promotes the proliferation,
migration and invasion of MDA-MB-231 cells
In our previous study, PCSK6 knockdown by RNA
interference was demonstrated to reduce the proliferation,
migration and invasion of MDA-MB-231 cells. Due to the fact that
PCSK6 is a secreted protein, extracellular PCSK6 may also have an
effect. MDA-MB-231 cells stimulated with 150 ng/ml rHuPCSK6
exhibited significantly increased proliferation compared with
control cells (P<0.05; Fig. 1A).
In addition, wound-healing assays revealed that cells stimulated
with rHuPCSK6 exhibited significantly increased migration
capability compared with control cells (P=0.01; Fig. 1B). Transwell assays indicated that
cells stimulated with rHuPCSK6 had enhanced invasion capability
compared with control cells (P=0.01; Fig.
1C).
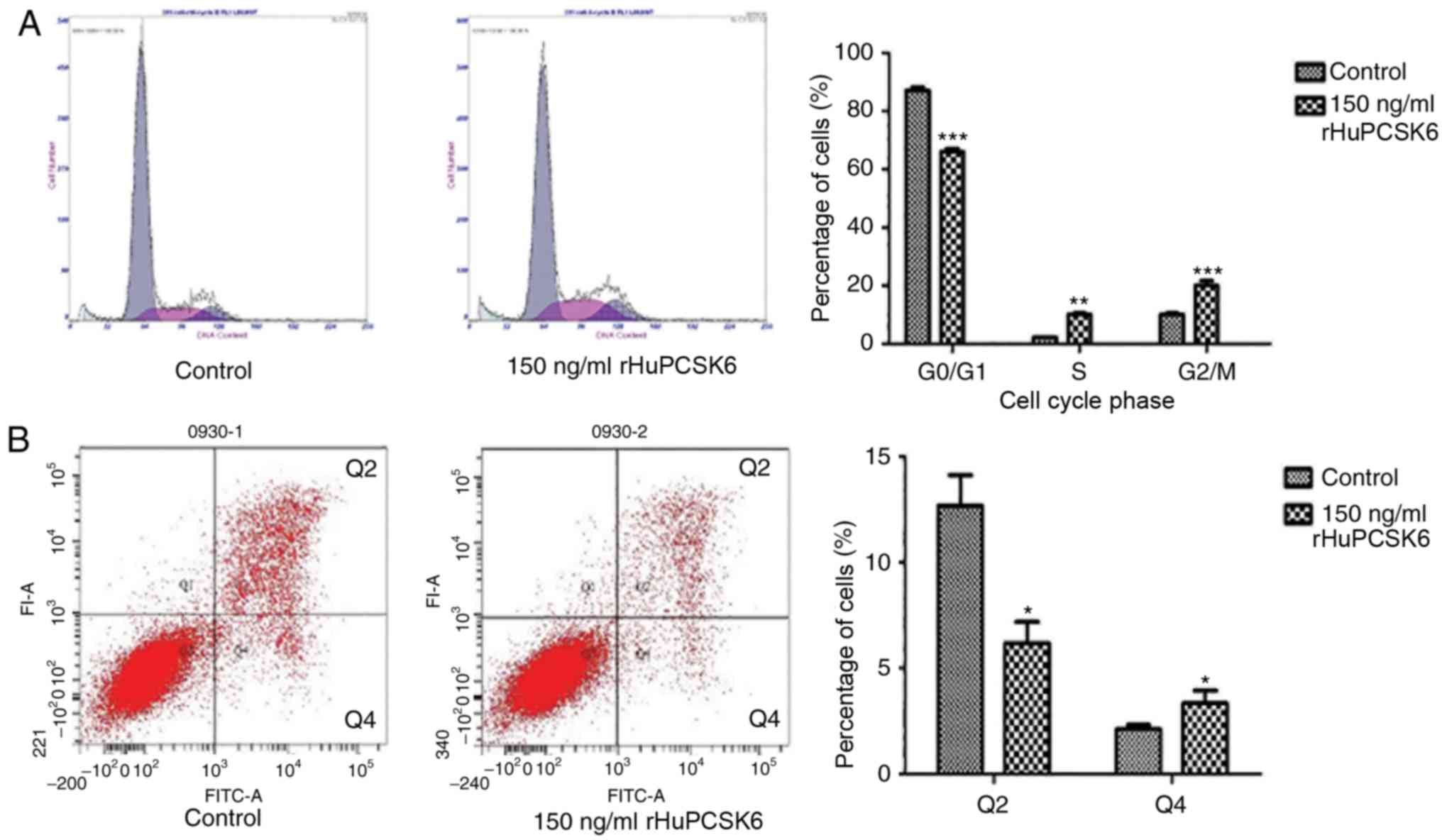
rHuPCSK6 reduces cell cycle arrest and
prevents apoptosis of MDA-MB-231 cells
Flow cytometric analysis demonstrated that
MDA-MB-231 cells stimulated with 150 ng/ml rHuPCSK6 had a
significantly lower ratio of G0/G1 phase
cells (P<0.001) and a significantly higher ratio of S phase
(P<0.01) and G2/M phase (P<0.001) cells compared with control
cells (Fig. 2A). Furthermore,
rHuPCSK6 treatment significantly reduced Annexin V-stained late
apoptotic cells compared with control cells (P=0.02; Fig. 2B).
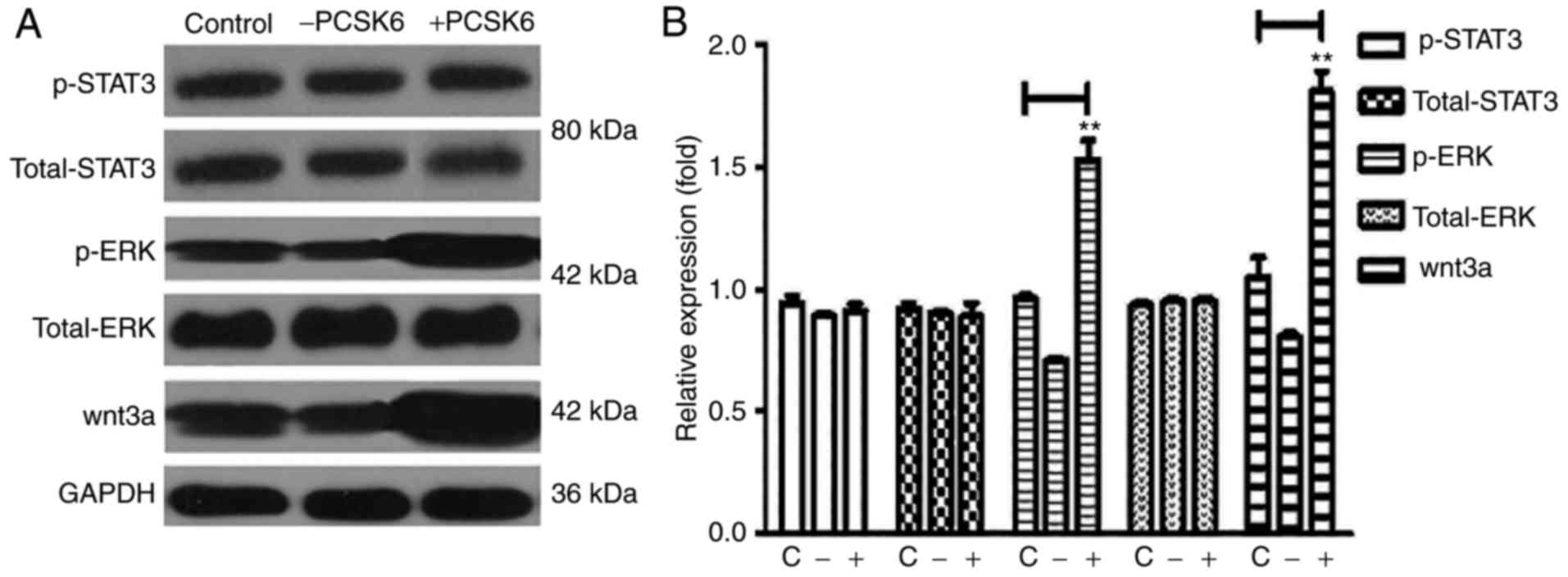
rHuPCSK6 activates ERK1/2 and WNT3A
pathways in MDA-MB-231 cells
ERK1/2, are catalytically activated by
phosphorylation and are associated with cancer cell proliferation
(11). To investigate the pathway by
which PCSK6 directly or indirectly regulates cell proliferation,
ERK1/2 activity was analyzed in MDA-MB-231 cells following
stimulation with 150 ng/ml rHuPCSK6 for 30 min by western blotting.
The western blots demonstrated that rHuPCSK6 treatment increased
ERK1/2 phosphorylation in MDA-MB-231 cells (Fig. 3).
The WNT signaling pathway mediates biological
processes, including cell adhesion, migration, proliferation,
differentiation and survival (12).
WNT3A is overexpressed in breast cancer (13–15);
therefore, the present study examined whether its expression may be
influenced by PCSK6. Stimulation of MDA-MB-231 cells with rHuPCSK6
increased the expression of WNT3A (Fig.
3). Oncogenic STAT3 hyperactivation has been observed in breast
cancer (16). However, activation of
STAT3 by rHuPCSK6 treatment was not demonstrated in MDA-MB-231
cells in the present study.
Discussion
The critical role of proprotein convertases in
proteolytic maturation of multiple precursor substrates implicated
in neoplasia makes them attractive targets for the development of
potent and selective inhibitors of tumorigenesis (17,18). In
recent years, the association between proprotein convertases and
cancer has been extensively studied. PCSK6 is often overexpressed
in breast cancer, and Lapierre et al (9) demonstrated that overexpression of the
ppPCSK6 prosegment in MDA-MB-231 breast cancer cells significantly
enhanced cell motility, migration and invasion abilities in
vitro. However, the mechanistic role of PCSK6 in these
processes remains unclear.
In our previous study (19), it was demonstrated that knockdown of
PCSK6 expression in breast cancer MDA-MB-231 cells inhibited
proliferation, invasion and migration abilities. As PCSK6 is a
neuroendocrine-specific mammalian subtilisin-related endoprotease
(20) that exhibits a C-terminal
cysteine-rich region (10) and
functions in the secretory pathway, it may serve important roles in
the ECM. Therefore, in the present study, the effect of exogenous
PCSK6 on MDA-MB-231 cells was investigated. The results of the
present study were in line with those of previous studies using
siRNA knockdown (19).
In breast cancer, increased cell proliferation
and/or decreased apoptosis contribute to tumor cell hyperplasia,
which contributes to breast cancer pathogenesis (21). In the present study, rHuPCSK6
stimulation of MDA-MB-231 cells was demonstrated to significantly
promote cell proliferation, migration and invasion abilities,
suggesting that PCSK6 may serve an important role in breast cancer
hyperplasia. Due to the fact that rHuPCSK6 was isolated from wheat
germ, there was no risk of lipopolysaccharide contamination. In our
previous study (19), it was
demonstrated that knockdown of PACE4 (PCSK6) is able to decrease
the migration, invasion and proliferation abilities of MDA-MB-231
cells, which is consistent with the report by Lapierre et al
(9). In the present study, it was
indicated that stimulation with recombinant PCSK6 significantly
increased the proliferation, invasion and migration abilities of
breast cancer cells compared with control cells. PCSK6 was also
indicated to reduce cell cycle arrest and prevent apoptosis of
MDA-MB-231 cells, and increase the expression of the phosphorylated
forms of ERK1/2 and WNT3A, compared with control cells. These
results are consistent with our study on rheumatoid arthritis
synovial fibroblasts and in prostate cancer (10), and a previous study by Lapierre et
al (9), in which α1-antitrypsin
Portland (α1-PDX) was used as an inhibitor of proprotein
convertases, including PCSK6, and reduced the mRNA expression level
of furin but increased that of PCSK6. In the cell migration assay,
α1-PDX was demonstrated to increase the migratory ability of
MDA-MB-231 cells, suggesting that PCSK6 may induce the migration of
MDA-MB-231 cells.
Overall, the results of the present study suggested
that PCSK6 may promote the proliferation of MDA-MB-231 cells by
disturbing cell cycle arrest through the mitogen-activated protein
kinase pathway. The present study also demonstrates that
rHuPCSK6-mediated activation of MDA-MB-231 cells likely occurs via
the ERK1/2 and WNT3A pathways, as the level of phosphorylation of
these proteins was increased following rHuPCSK6 treatment. ERK1/2
isoforms serve a major role in cell proliferation signaling
(10). The main limitation of the
present study was that only MDA-MB-231 cells were used. Further
research should analyze the role of PCSK6 in other breast cancer
cell lines. In summary, exogenous PCSK6 may exacerbate the
progression of breast cancer through its effects on tumor cells.
Furthermore, the ERK1/2 and WNT3A signaling pathways may mediate
the stimulatory role of PCSK6 in breast cancer. Therefore, PCSK6
may be a potential therapeutic target of breast cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81671624), the
Natural Science Foundation of Shandong Province (grant no.
ZR2015LY029) and the Innovation Program of Shandong Academy of
Medical Sciences (2016).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PW and FW performed the experiments. LW analyzed the
data. JP designed the study.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
La Vecchia C, Bosetti C, Lucchini F,
Bertuccio P, Negri E, Boyle P and Levi F: Cancer mortality in
Europe, 2000–2004, and an overview of trends since 1975. Ann Oncol.
21:1323–1360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Byler S, Goldgar S, Heerboth S, Leary M,
Housman G, Moulton K and Sarkar S: Genetic and epigenetic aspects
of breast cancer progression and therapy. Anticancer Res.
34:1071–1077. 2014.PubMed/NCBI
|
|
3
|
Seidah NG and Chrétien M: Proprotein and
prohormone convertases: A family of subtilases generating diverse
bioactive polypeptides. Brain Res. 848:45–62. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagahama M, Taniguchi T, Hashimoto E,
Imamaki A, Mori K, Tsuji A and Matsuda Y: Biosynthetic processing
and quaternary interactions of proprotein convertase SPC4 (PACE4).
FEBS Lett. 434:155–159. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mains RE, Berard CA, Denault JB, Zhou A,
Johnson RC and Leduc R: PACE4: A subtilisin-like endoprotease with
unique properties. Biochem J. 321:587–593. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Couture F, D'Anjou F, Desjardins R,
Boudreau F and Day R: Role of proprotein convertases in prostate
cancer progression. Neoplasia. 14:1032–1042. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Longuespée R, Couture F, Levesque C,
Kwiatkowska A, Desjardins R, Gagnon S, Vergara D, Maffia M,
Fournier I, Salzet M and Day R: Implications of proprotein
convertases in ovarian cancer cell proliferation and tumor
progression: Insights for PACE4 as a therapeutic target. Transl
Oncol. S1936–S5233. 2014.
|
|
8
|
Bassi DE, Zhang J, Cenna J, Litwin S,
Cukierman E and Klein-Szanto AJ: Proprotein convertase inhibition
results in decreased skin cell proliferation, tumorigenesis, and
metastasis. Neoplasia. 12:516–526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lapierre M, Siegfried G, Scamuffa N,
Bontemps Y, Calvo F, Seidah NG and Khatib AM: Opposing function of
the proprotein convertases furin and PACE4 on breast cancer cells'
malignant phenotypes: Role of tissue inhibitors of
metalloproteinase-1. Cancer Res. 67:9030–9034. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang F, Wang L, Jiang H, Chang X and Pan
J: Inhibition of PCSK6 may play a protective role in the
development of rheumatoid arthritis. J Rheumatol. 42:161–169. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sansone P and Bromberg J: Targeting the
interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol.
30:1005–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akiyama T and Kawasaki Y: Wnt signalling
and the actin cytoskeleton. Oncogene. 25:7538–7544. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Larue L and Delmas V: The WNT/Beta-catenin
pathway in melanoma. Front Biosci. 11:733–742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai SL, Chien AJ and Moon RT: Wnt/Fz
signaling and the cytoskeleton: Potential roles in tumorigenesis.
Cell Res. 19:532–545. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Teo JL and Kahn M: The Wnt signaling
pathway in cellular proliferation and differentiation: A tale of
two coactivators. Adv Drug Deliv Rev. 62:1149–1155. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bromberg JF, Wrzeszczynska MH, Devgan G,
Zhao Y, Pestell RG, Albanese C and Darnell JE Jr: Stat3 as an
oncogene. Cell. 98:295–303. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bontemps Y, Scamuffa N, Calvo F and Khatib
AM: Potential opportunity in the development of new therapeutic
agents based on endogenous and exogenous inhibitors of the
proprotein convertases. Med Res Rev. 27:631–648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Basak A: Inhibitors of proprotein
convertases. J Mol Med. 83:844–855. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang F, Wang L and Pan J: PACE4 regulates
proliferation, migration and invasion in human breast cancer
MDA-MB-231 cells. Mol Med Rep. 11:698–704. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fu J, Bassi DE, Zhang J, Li T, Cai KQ,
Testa CL, Nicolas E and Klein-Szanto AJ: Enhanced UV-induced skin
carcinogenesis in transgenic mice overexpressing proprotein
convertases. Neoplasia. 15:169–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li M, Lewis B, Capuco AV, Laucirica R and
Furth PA: WAP-TAg transgenic mice and the study of dysregulated
cell survival, proliferation, and mutation during breast
carcinogenesis. Oncogene. 19:1010–1019. 2000. View Article : Google Scholar : PubMed/NCBI
|