Introduction
With >600,000 new cases diagnosed yearly, head
and neck cancer is the sixth most common cancer worldwide (1). It is associated with impairments like
pain, severe disfigurement and difficulties in swallowing,
breathing and speech. Most tumors of this group are head and neck
squamous cell carcinomas (HNSCC). Despite partial advances in
therapy of patients suffering from head and neck cancer, prognosis
still remains poor with minimal improvement in survival over the
past several decades (2). Thus, new
treatment modalities are needed for a better outcome of patients
with this aggressive disease.
In the past, the increase of reactive oxygen species
(ROS) has been seen as a disadvantageous circumstance associated
with carcinogenesis and cancer progression. In contrast, there are
agents known to kill cancer cells in vitro by promoting
cellular ROS accumulation (3–5). Additionally, since some cancer cells are
more sensitive to ROS than normal cells, (6) selective generation of ROS may be a
promising strategy for killing cancer cells without significantly
harming benign tissue (7–9).
Shin et al (10) synthesized the novel polyphenol
conjugate
(E)-3-(3′',5′'-dimethoxyphenyl)-1-(2′-methoxyphenyl)prop-2-en-1-one
(DPP-23). They assessed its effects on cell lines of human colon
cancer, glioblastoma, breast cancer and pancreatic cancer in
vitro as well as in vivo on the human colon cancer cell
line HCT116 in athymic nude mice. Their data suggests that DPP-23
may be a promising therapeutic agent with antitumor effects by the
selective generation of ROS and targeting the unfolded protein
response in the endoplasmatic reticulum, resulting in a growth
inhibition of cancer cells via caspase-dependent apoptosis.
Furthermore, DPP-23 induced autophagy in various cancer cell types.
Notably, there was no indication of toxicity in normal tissues
in vivo. (10).
In a recent study of Kim et al (11), the selective killing of HNSCC cells
and increased cisplatin antitumor activity in resistant HNSCC cells
by interfering with Nrf2 antioxidant systems, via activation of p53
expression and accumulation of cellular ROS could be shown in
vitro and in vivo. For their investigations, they used
oral keratinocytes, fibroblasts and HN3, HN4 and HN9 cells
(11).
Due to their high phenotypic and cellular
plasticity, human mesenchymal stem cells like human bone marrow
stem cells (hBMSC) are a suitable model for in vitro
toxicological assessment of various biological and chemical agents
(12). Furthermore, since
systematically applied substances come into contact with peripheral
blood lymphocytes, the latter is relevant for toxicological
evaluations as well.
Hence, the present study was performed to further
elucidate the role of DPP-23 as a possible agent in head and neck
oncology, and to investigate its cytotoxic effects on
well-established HNSCC cell lines like HLaC 78 and FaDu, as well as
primary hBMSC and human peripheral blood lymphocytes in
vitro.
Materials and methods
Chemical synthesis and analysis of
DPP-23
Reagents and devices
All reagents used were of commercial quality.
Organic solvents were dried and distilled prior to use.
2′-Methoxyacetophenone and 3,5-dimethoxybenzaldehyde were purchased
from Sigma-Aldrich (Steinheim, Germany). Reactions were monitored
by thin-layer chromatography (TLC) on aluminum plates coated with
silica gel 60 F254 (Merck, Darmstadt, Germany). Column
chromatography was performed on Merck silica gel (0.063–0.200
mm).
General methods
Melting points (uncorrected) were determined using a
Reichert-Jung Thermovar hot-stage apparatus. Infrared spectra (IR)
were obtained with a Jasco FT/IR-410 spectrophotometer (Jasco,
Inc., Easton, MD, USA), and are reported in wave numbers
(cm−1). Proton and carbon-13 spectra were recorded on a
Bruker Avance spectrometer (Bruker, Billerica, MA, USA) at 400 MHz
(for 1H NMR), and at 100 MHz (for 13C NMR) at
ambient temperature. Chemical shifts (δ) are reported in parts per
million (ppm) with the proton or carbon-13 signals of chloroform
(1H, δ=7.26 ppm; 13C, δ=77.16 ppm) in the
deuterated solvent used as internal reference. Coupling constants
(J) are given in Hertz (Hz). The following abbreviations are used:
s, singulet; d, doublet; dd, double doublet; t, triplet. Electron
ionization mass spectra (70 eV) were obtained using a Finnigan MAT
8200 mass spectrometer in the positive mode (70 eV), the relative
intensities are given in brackets. Matrix-assisted mass spectra
(MALDI) were measured in the positive mode via a Bruker Autoflex II
mass spectrometer using DCTB as the matrix. High-resolution
electrospray ionization mass spectra (HRESIMS) were determined with
a Bruker microOTOF spectrometer.
Synthesis, purification, and
characterization of DPP-23
DPP-23: The title compound was synthesized according
to a published procedure (13), which
was slightly modified: To a solution of 1.0 eq. of
2′-methoxyacetophenone (1.25 g, 8.22 mmol) and 1.0 eq. of
3,5-dimethoxybenzaldehyde (1.37 g, 8.22 mmol) in 30 ml of anhydrous
ethanol, 2.0 eq. of KOH (923 mg, 16.5 mmol) was added. The reaction
was allowed to proceed at room temperature (RT) for 45 min with
stirring, and monitored by TLC (eluent:n-Hexan/Et2O,
1:1). The reaction mixture was then treated with water (20 ml), and
extracted with ethyl acetate (2×20 ml). The combined organic layers
were dried over anhydrous Na2SO4, filtered,
and concentrated under reduced pressure. The crude residue was
purified by column chromatography on silica gel
(eluent:n-Hexan/Et2O, 3:1). The remaining yellowish oil
was then crystallized from methanol at 4°C by storage of the
solution overnight in a refrigerator, providing pure DPP-23 (2.20
g, 7.36 mmol, 90% yield) as pale yellow crystals. The spectroscopic
data (1H NMR, 13C NMR) were in agreement with
those reported in the literature (14) and were found to be as follows:
1H NMR (400 MHz, CDCl3):
δ=3.82 (s, 6H), 3.89 (s,3H), 6.50 (t, J=2.3, 2.3 Hz, 1H),
6.72 (d, J=2.3 Hz, 1H), 6.73 (d, J=2.3 Hz, 1H), 7.00 (dd,
J=8.4, 1.0 Hz, 1H), 7.04 (td, J=7.5, 1.0 Hz, 1H),
7.31 (d, J=15.9 Hz, 1H), 7.47 (ddd, J=8.3, 7.4, 1.9
Hz, 1H), 7.52 (d, J=15.9 Hz, 1H), 7.61 (dd, J=7.6,
1.8 Hz, 1H). 13C NMR (101 MHz, CDCl3):
δ=55.58, 55.90, 102.57, 106.43, 111.75, 120.87, 127.72, 129.33,
130.45, 133.02, 137.16, 143.41, 158.21, 161.10, 193.19. M.p.
53–55°C (MeOH). IR (ATR) v/cm−1: 2939 (w), 2837
(w), 1652 (w), 1587 (s), 1483 (m), 1456 (m), 1425 (m), 1347 (w),
1288 (s), 1240 (m), 1202 (s), 1151 (s), 1113 (m), 1059 (m), 1018
(m), 977 (m), 925 (w), 834 (m), 752 (s), 671 (m), 637 (m). EI-MS
(70 eV) m/z (%): 77 (36), 135 (54), 152 (28), 239 (49), 267 (49),
298 (100), 299 (20). MALDI
calculated for C18H19O4 [M +
H]+: 299.128; found: 299.074. ESI-MS (positive) exact
mass calculated for C18H18O4Na [M
+ Na]+: 321.10973; found: 321.10920.
Cell lines and culture
HNSCC cell lines FaDu, HLaC 78 and Cal 27 were
cultured in RPMI 1640 medium supplemented with 10% fetal calf serum
(FCS), 100 U/ml penicillin, 100 lg/ml streptomycin, 1% 100 mM
sodium pyruvate and 1% of a 100-fold concentration of non-essential
amino acids (Biochrom, Berlin, Germany). The cells were maintained
in a tissue culture incubator equilibrated with 95% air and 5%
CO2 at 37°C in 150 cm2 flasks.
Human bone marrow cells were harvested from six
voluntary donors undergoing surgery in the Department of
Orthopedics, and written informed consent was obtained from all of
the individuals included. The study was approved by the Ethics
Committee of the Medical Faculty, University of Wuerzburg
(Wuerzburg, Germany; 12/06). As previously described, (15) hBMSC were isolated from fresh bone
marrow aspirates by several washing and centrifugation steps. The
cells were incubated in Dulbecco's modified Eagle's medium (DMEM)
(Gibco; Invitrogen, Karlsruhe, Germany) expansion medium (DMEM-EM)
supplemented with 10% FCS and 1% penicillin/streptomycin
(Sigma-Aldrich) at 37°C and 5% CO2. At a confluence of
>70%, cells were trypsinized, resuspended and subcultured at a
concentration of 2,000 cells/cm2.
Human lymphocytes were obtained by venous puncture
from healthy volunteers. Lymphocytes were separated by
density-gradient centrifugation (10 min, 1,000 × g) at RT on equal
amounts of Ficoll (Biochrom), using a membrane containing 50 ml
cell tube (Greiner Bio-One, Frickenhausen, Germany). After washing
twice in PBS, lymphocytes were resuspended in RPMI (Biochrom)
containing the supplement of bovine serum albumin (Linaris,
Wertheim-Bettingen, Germany), 1% sodium-pyruvate, 1% non-essential
amino acids, and 1% penicillin-streptomycin (all Biochrom). The
study was approved by the Ethics Commission of the Medical Faculty,
Julius-Maximilian-University Wuerzburg, (Wuerzburg, Germany) and
all participants gave written informed consent.
MTT assay
For evaluation of the cytotoxic effects of DPP-23 on
tumor cells and hBMSC the
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide]
(MTT; Sigma-Aldrich) colorimetric staining method according to
Mosmann (18) was used. Cells were
seeded onto 24-well plates at a concentration of 1×105
cells/ml and treated with DPP-23 at various concentrations for 24
h. Respective concentrations of dimethyl sulfoxide were added to
controls. After subsequent incubation with 100 µl of MTT solution
(1 mg/ml), MTT was removed and 100 µl of isopropanol was added for
30 min. Then, the absorption values of the blue formazan dye were
determined with a multi-plate reader at a wavelength of 570 nm
(Titertek Multiskan PLUS MK II; Labsystems, Helsinki, Finland). All
measurements were performed in triplicate.
Annexin V-propidium iodide test
Apoptosis and necrosis were evaluated by flow
cytometry using an Annexin V-propidium iodide kit (BD Bioscience,
Heidelberg, Germany) according to the manufacturer's manual. Cells
were washed twice with cold PBS and resuspended in an Annexin V
binding buffer containing 10 mM Hepes/NaOH (pH 7.4), 140 mM NaCl
and 2.5 mM CaCl2 at a concentration of 1×106
cells/ml. 100 µl aliquots of this cell suspension (1×105
cells) were then incubated with 5 µl of Annexin V-APC and 5 µl of
propidium iodide (PI for 15 min in the dark at RT). After
resuspension with 400 µl 1:10 binding buffer, viable (Annexin
V−/PI-), apoptotic cells (Annexin
V+/PI−) and necrotic cells (Annexin
V+/PI+) were measured using a flow
cytometer.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism version 6.0c for Mac (GraphPad Software, La Jolla, CA, USA;
www.graphpad.com). For determination of the half
maximal inhibitory concentration (IC50), dose response
curves were generated by nonlinear regression analysis.
For detection of significant differences between
treated and untreated samples, the paired Student's t-test was
applied. P<0.05 was considered to indicate a statistically
significant difference. All results are expressed as the mean ±
SD.
Results
Chemical characterization of
DPP-23
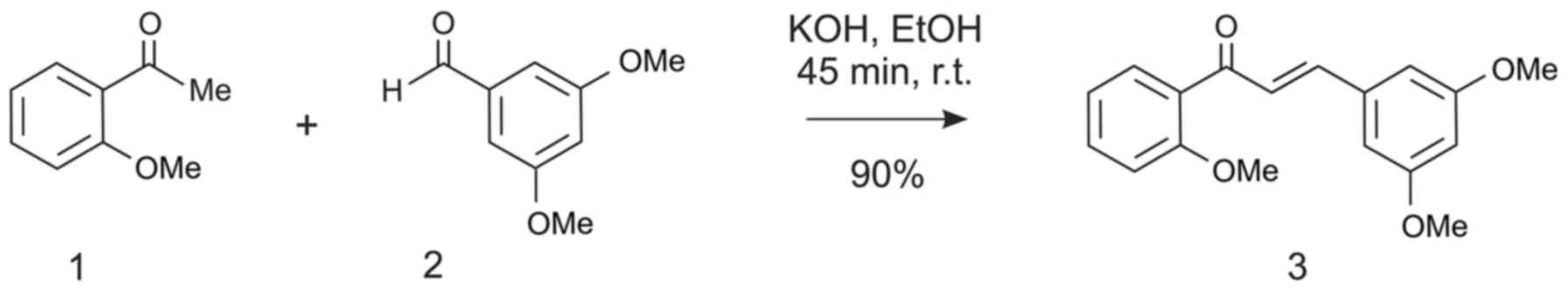
The chalcone DPP-23 was synthesized via a ‘cold’
procedure of the Claisen-Schmidt condensation (13), by running the reaction of
2-methoxyacetophenone with 3,5-dimethoxybenzaldehyde in ethanol
under basic conditions at RT (Fig.
1). The resulting chalcone was obtained as a pure compound in
high chemical yields (ca. 90%) after purification of the crude
reaction mixture by chromatography on silica gel, followed by
subsequent crystallization of the resulting pale yellow oil from
methanol. As expected, the substance was found to possess a
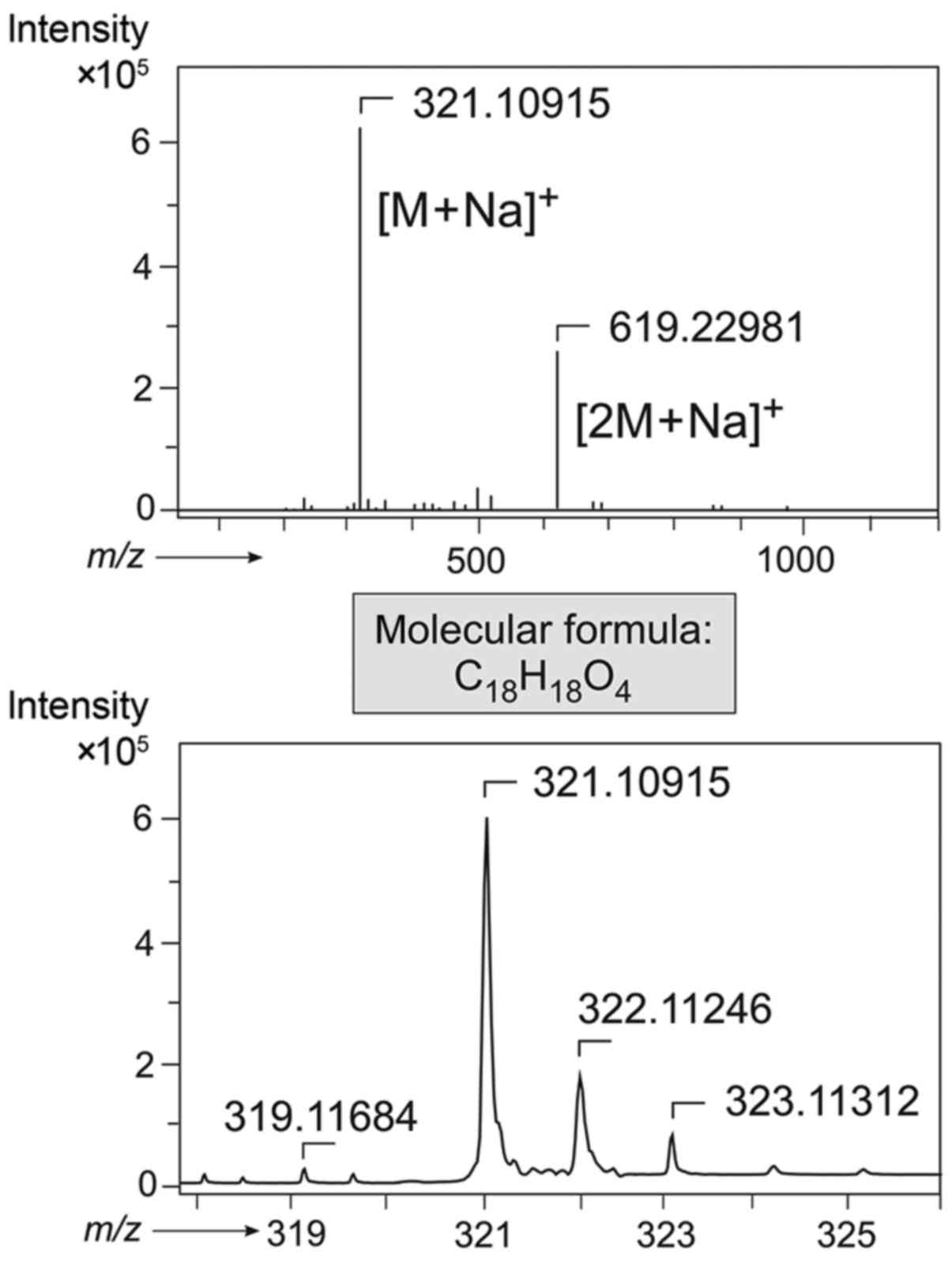
molecular formula of C18H18O4 due
to the most abundant molecular mass peaks of m/z 321.1092 [M +
Na]+ and m/z 619.2299 [2 M + Na]+, as
evidenced from high-resolution electrospray ionization mass
spectrometry (HRESIMS) (Fig. 2). The
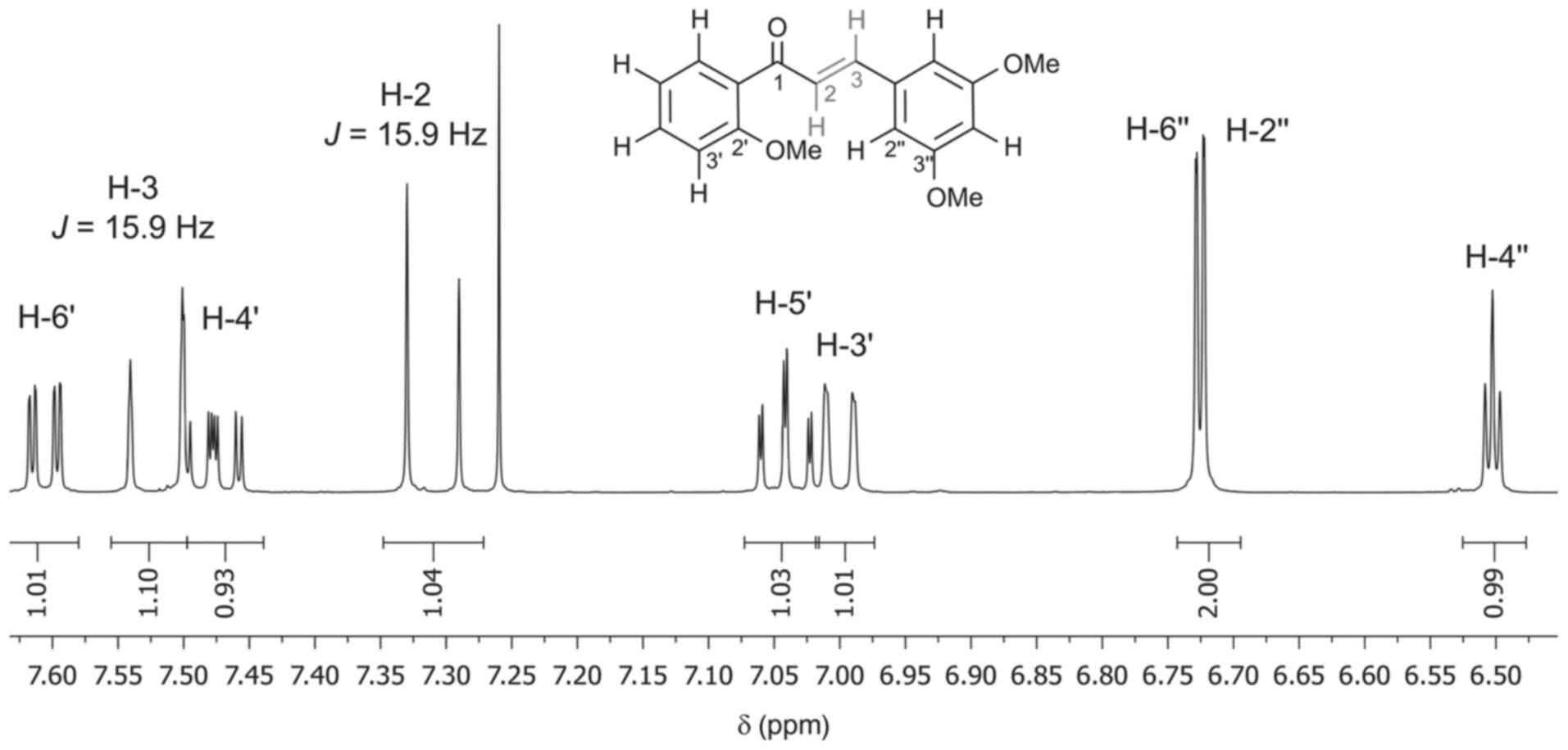
selective, and thus exclusive formation of DPP-23 with an
(E)-configuration at the double bond, as outlined in Scheme 1, was
unequivocally confirmed by the large coupling constant of 15.9 Hz
between H-2 and H-3, as determined by NMR measurements (Fig. 3).
Cytotoxicity
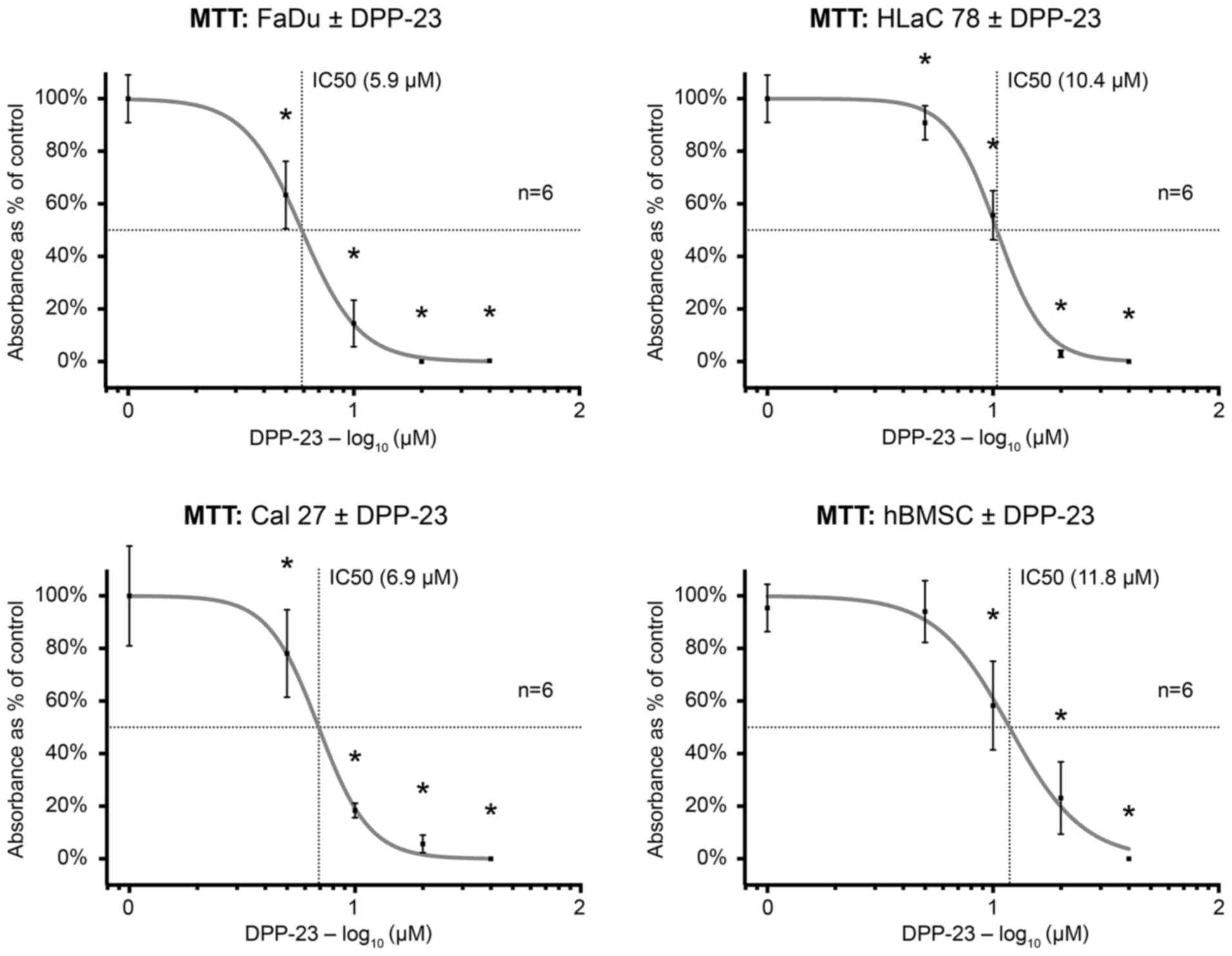
The MTT test indicated a reduction in cell viability
on FaDu, HLaC 78 and Cal 27 cells, as well as hBMSC after treatment
with DPP-23 in a dose-dependent manner (Fig. 4). There was a cytotoxic effect at a
concentration of 10 µM of DPP-23, respectively. The calculated
IC50 was 5.9 µM in FaDu, 10.4 µM in HLaC 78, 6.9 µM in
Cal 27 and 12.1 µM in hBMSC.
The results of the MTT test were confirmed by the
Annexin V-propidium iodide test. The latter showed a dose-dependent
enhancement of cellular apoptosis by DPP-23 in FaDu and
lymphocytes. In detail, FaDu cells were subdivided into 92.3%
viable, 2.0% apoptotic and 5.1% necrotic cells in the control
group, whereas in the treatment group (40 µM DPP-23) there were
79.8% viable, 4.6% apoptotic and 13.1% necrotic cells. Flow
cytometry of lymphocytes revealed 83.8% viable, 8.0% apoptotic and
8.1% necrotic cells in the control group, whereas there were 52.3%
viable, 44.2% apoptotic and 3.4% necrotic cells in the treatment
group (Fig. 5).
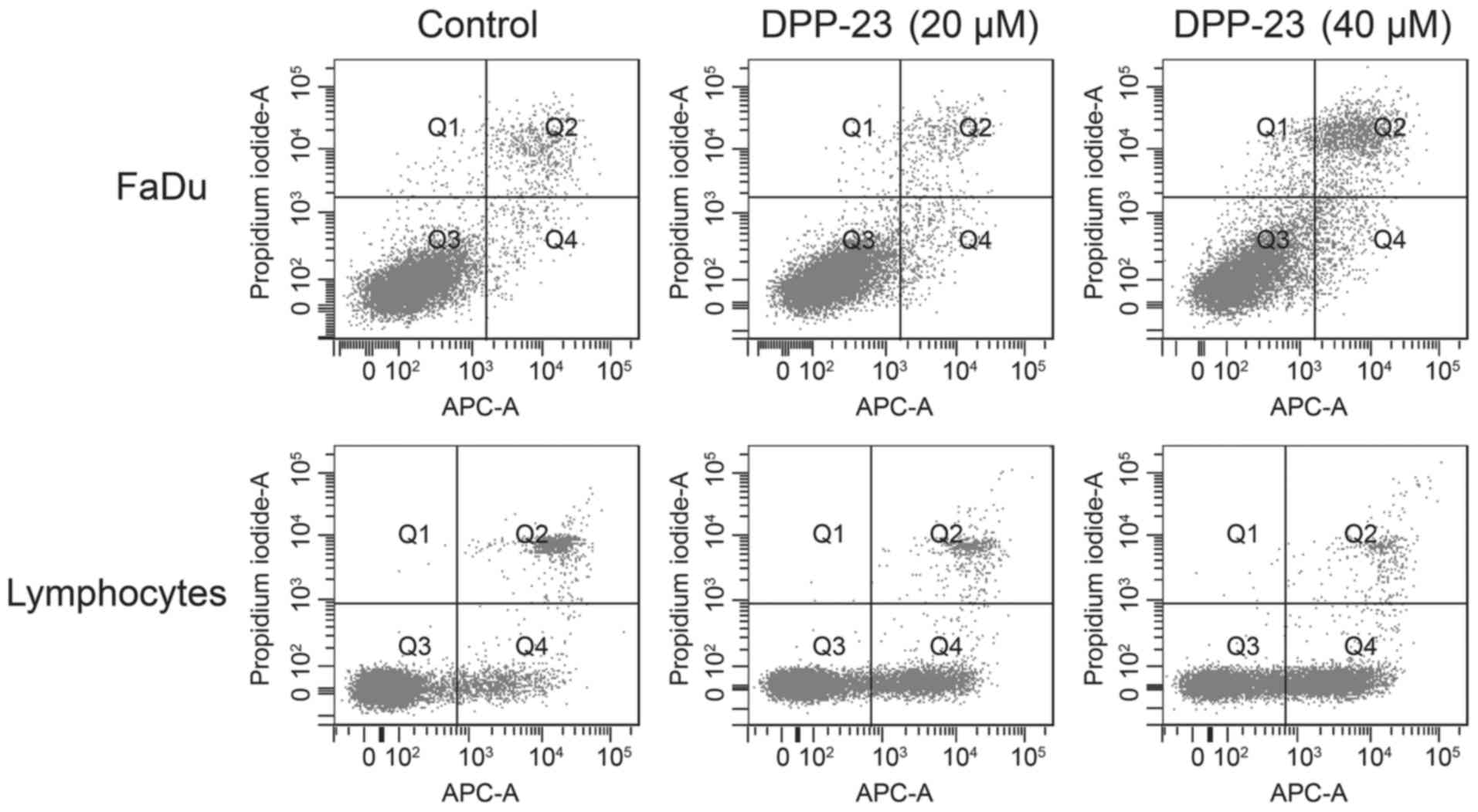 | Figure 5.Dot plots of Annexin V-propidium
iodide flow cytometry revealed a dose-dependent induction of
apoptosis and necrosis in FaDu cells as well as human lymphocytes.
FaDu: control/40 µM DPP-23-viable cells, 92.3/79.8%; apoptosis,
2.0/4.6%; necrosis, 5.1/13.1%; other, 0.6/2.5%. Lymphocytes:
control/40 µM DPP-23-viable cells, 83.8/52.3%; apoptosis,
8.0/44.2%; necrosis, 8.1/3.4%; other, 0.0/0.1%. DPP-23,
(E)-3-(3′,5′-dimethoxyphenyl)-1-(2′-methoxyphenyl) prop-2-en-1-one;
APC-A, allophycocyanin A. |
Discussion
Novel therapeutic approaches with lower adverse
effects than with currently performed treatments are needed for
HNSCC (16). In the present study,
the chalcone DPP-23 was synthesized according to the structural
formula published by Shin et al (10), and using this substance to test a
novel idea, we assessed its effects on well-established HNSCC tumor
cell lines and primary non-malignant cells. In particular, the
hBMSC test system used in this study is considered to be very
suitable for the prediction of toxicological behavior since it
provides a more accurate simulation of in vivo conditions
than traditional in vitro systems with transformed or
immortalized cell lines. Furthermore, hBMSC are highly
proliferative and can be cultivated over several passages. DNA
stability was demonstrated over up to 10 passages (17). In contrast to other primary cells,
they are suitable for long-term toxicological evaluations,
especially for the determination of DNA fragmentation and repair
capacity. In addition, functional properties like cytokine
secretion, migration or differentiation can be assessed (12). Therefore, hBMSC are an optimal cell
entity for further investigations of the effects of DPP-23 on
non-malignant cells.
The data from the MTT assay and the Annexin
V-propidium iodide test suggest a dose-dependent cytotoxicity in
the tested HNSCC tumor cell lines, as well as in hBMSC and
lymphocytes. The applied concentrations of DPP-23 are similar to
the doses reported by Shin et al (10), whereas Kim et al (11) partially administered lower doses in
combination with longer exposure times (up to 72 h). As a
limitation of the MTT assay, it should be mentioned that its
results do not directly reflect the cell viability, but the
activity of the mitochondria (18).
Nevertheless, the Annexin V-propidium iodide test allows a precise
estimation of apoptotic incidence (19). In contrast to the findings of Shin
et al (10) and Kim et
al (11), our preliminary results
suggest surprisingly higher cytotoxic effects of DPP-23 in benign
cells than suspected. This may indicate a limitation in the
feasibility, or at least the systemic application of this
substance. One reason for the discrepancy between the findings of
Shin et al (10) and Kim et
al (11) vs. the results of the
present study could be on the one hand due to different cell types
and in vitro test systems, and on the other hand due to
different surrounding conditions of in vivo and in
vitro settings. Compensatory mechanisms like maintaining the
redox balance via the cells' antioxidant systems should be
discussed as a possible explanation for the differences. However, a
substance with less cytotoxic effects in mesenchymal stem cells may
be a more favorable candidate for a chemotherapeutic agent with
presumably low adverse effects.
Future investigations should address the
cytotoxicity of DPP-23 using additional in vitro test
systems like spheroids. These three-dimensional cell culture models
can be constructed for stem cells and tumor cells. Due to the
possibility of intercellular communication, they are a very
distinct test system for evaluating xenobiotics in human cells.
Furthermore, the comparison between multilayer cellular test
systems for human squamous cell carcinomas and multilayer systems
for the human oropharyngeal mucosa will provide more detailed
information about the underlying mechanisms of DPP-23-induced
toxicity (20).
Taken together, our results show that prior to the
application of DPP-23 in clinical studies, further molecular
evaluations are warranted to understand the effects and mechanisms
of DPP-23 in malignant as well as benign cell populations.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pulte D and Brenner H: Changes in survival
in head and neck cancers in the late 20th and early 21st century: A
period analysis. Oncologist. 15:994–1001. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang P, Feng L, Oldham EA, Keating MJ and
Plunkett W: Superoxide dismutase as a target for the selective
killing of cancer cells. Nature. 407:390–395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pelicano H, Feng L, Zhou Y, Carew JS,
Hileman EO, Plunkett W, Keating MJ and Huang P: Inhibition of
mitochondrial respiration: A novel strategy to enhance drug-induced
apoptosis in human leukemia cells by a reactive oxygen
species-mediated mechanism. J Biol Chem. 278:37832–37839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park SW, Kim JE, Oh SM, Cha WJ, Hah JH and
Sung MW: Anticancer effects of anandamide on head and neck squamous
cell carcinoma cells via the production of receptor-independent
reactive oxygen species. Head Neck. 37:1187–1192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schumacker PT: Reactive oxygen species in
cancer cells: Live by the sword, die by the sword. Cancer Cell.
10:175–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trachootham D, Zhou Y, Zhang H, Demizu Y,
Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J and
Huang P: Selective killing of oncogenically transformed cells
through a ROS-mediated mechanism by beta-phenylethyl
isothiocyanate. Cancer Cell. 10:241–252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Raj L, Ide T, Gurkar AU, Foley M, Schenone
M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, et al:
Selective killing of cancer cells by a small molecule targeting the
stress response to ROS. Nature. 475:231–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shin SY, Lee JM, Lee MS, Koh D, Jung H,
Lim Y and Lee YH: Targeting cancer cells via the reactive oxygen
species-mediated unfolded protein response with a novel synthetic
polyphenol conjugate. Clin Cancer Res. 20:4302–4313. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim EH, Jang H and Roh JL: A novel
polyphenol conjugate sensitizes cisplatin-resistant head and neck
cancer cells to cisplatin via Nrf2 inhibition. Mol Cancer Ther.
15:2620–2629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scanu M, Mancuso L and Cao G: Evaluation
of the use of human mesenchymal stem cells for acute toxicity
tests. Toxicol In Vitro. 25:1989–1995. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lahtchev KL, Batovska DI, Parushev SP,
Ubiyvovk VM and Sibirny AA: Antifungal activity of chalcones: A
mechanistic study using various yeast strains. Eur J Med Chem.
43:2220–2228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hwang D, Hyun J, Jo G, Koh D and Lim Y:
Synthesis and complete assignment of NMR data of 20 chalcones. Magn
Reson Chem. 49:41–45. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh
K, Bae YC and Jung JS: Characterization and expression analysis of
mesenchymal stem cells from human bone marrow and adipose tissue.
Cell Physiol Biochem. 14:311–324. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Froelich K, Mickler J, Steusloff G,
Technau A, Tirado Ramos M, Scherzed A, Hackenberg S, Radeloff A,
Hagen R and Kleinsasser N: Chromosomal aberrations and
deoxyribonucleic acid single-strand breaks in adipose-derived stem
cells during long-term expansion in vitro. Cytotherapy. 15:767–781.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Engeland M, Nieland LJ, Ramaekers FC,
Schutte B and Reutelingsperger CP: Annexin V-affinity assay: A
review on an apoptosis detection system based on phosphatidylserine
exposure. Cytometry. 31:1–9. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schweinlin M, Rossi A, Lodes N, Lotz C,
Hackenberg S, Steinke M, Walles H and Groeber F: Human barrier
models for the in vitro assessment of drug delivery. Drug Deliv
Transl Res. 7:217–227. 2017. View Article : Google Scholar : PubMed/NCBI
|