Introduction
Approximately 75% of patients with ovarian cancer
are diagnosed with advanced disease, namely, International
Federation of Gynecology and Obstetrics (FIGO) stage III–IV disease
(1). Ovarian cancer metastasizes
mainly through direct extension and abdominal implantation,
followed by use of lymphatic channels, particularly the
intra-peritoneal route, so that most advanced ovarian cancer is
confined in the abdominal and pelvic cavity (2,3). Distant
metastasis occurs in patients with stage IV disease, mainly through
the lymphatic channels and via hematogenous dissemination, and
these metastatic sites can be the lungs, liver, brain and distant
lymph node. Distant metastatic lymph nodes are rare in the primary
presence of ovarian cancer (4). Only
a few cases of supra-clavicular, axillary, mediastinal and inguinal
lymph node metastasis have been reported (5–8).
The standard treatment for ovarian cancer is
surgical staging and maximal cytoreduction, with adjuvant platinum
and taxane combination chemotherapy. For patients with stage III to
IV disease whose tumor is too large to be treated surgically,
neoadjuvant chemotherapy (NACT) may be considered as the primary
treatment. No more than four cycles of NACT and interval debulking
surgery can be a good option for these patients. A new radiological
study should be performed following every two cycles of NACT. A
complete pathological response (pCR) is uncommon in patients who
have received chemotherapy, so is uncommon in ovarian cancer. Only
a small percentage of patients (6.5%) achieve a pathologic complete
response (pCR) following NACT (9,10).
Furthermore, pCR in patients with advanced ovarian cancer receiving
NACT is associated with longer progression-free survival and
overall survival times compared with those in women with no pCR
(10).
The present study reports the case of a patient who
initially presented with metastatic left supra-clavicular lymph
nodes from ovarian cancer and achieved a pCR following two cycles
of NACT.
Case report
A 43-year-old woman was admitted to the Department
of Radiotherapy and Chemotherapy (Gynecological Oncology) in
Zhongnan Hospital of Wuhan University (Wuhan, China) in January
2014, with complaints of several enlarged left supra-clavicular
lymph nodes that had been apparent for 1 week. A number of the
enlarged lymph nodes were tender. Chest computed tomography (CT)
showed no abnormality in the bilateral lungs, but a magnetic
resonance imaging scan of the pelvis showed gross masses.
Fine-needle aspiration cytology of the lymphadenopathy was
obtained, which showed poorly differentiated carcinoma, and the
metastatic supra-clavicular lymph nodes were revealed to be ovarian
cancer according to the following immunohistochemical staining
results: Activin receptor-like kinase 1-negative, carbonic
anhydrase 9-negative, cluster of differentiation 30-negative,
homeobox protein CDX-2-negative, chromogranin A-negative,
cytokeratin (CK)20-negative, CK7-postive, Epstein-Barr encoding
region (in situ hybridization)-negative, Ki-67-positive
(90%), paired box protein Pax-2 (PAX2)-positive, PAX8-positive,
synaptophysin-negative and thyroid transcription factor 1-negative.
For Immunohistochemical staining, the fixative was 4%
paraformaldehyde, at room temperature, for 24 h. The resin was
paraffin and the thickness of sections was 4 µm. The blocking
reagent was 10% goat serum (OriGene Technologies, Inc., Beijing
China), for 30 min at room temperature. The details of primary and
secondary antibody are presented in Table
I. All the sections were analyzed under a phase-contrast
positive microscope (Eclipse 80i; Nikon Corporation, Tokyo, Japan).
Further immunostaining for Wilms tumor 1, tumor protein p53, p16,
cancer antigen 125 (CA125), creatine kinase, estrogen receptor,
postmeiotic segregation increased 2 protein, mutS homolog 2
protein, mutL homolog 1 protein and mutS homolog 6 protein was
positive, while immunostaining for aspartic proteinases A and
progestogen receptor was negative. These results showed that the
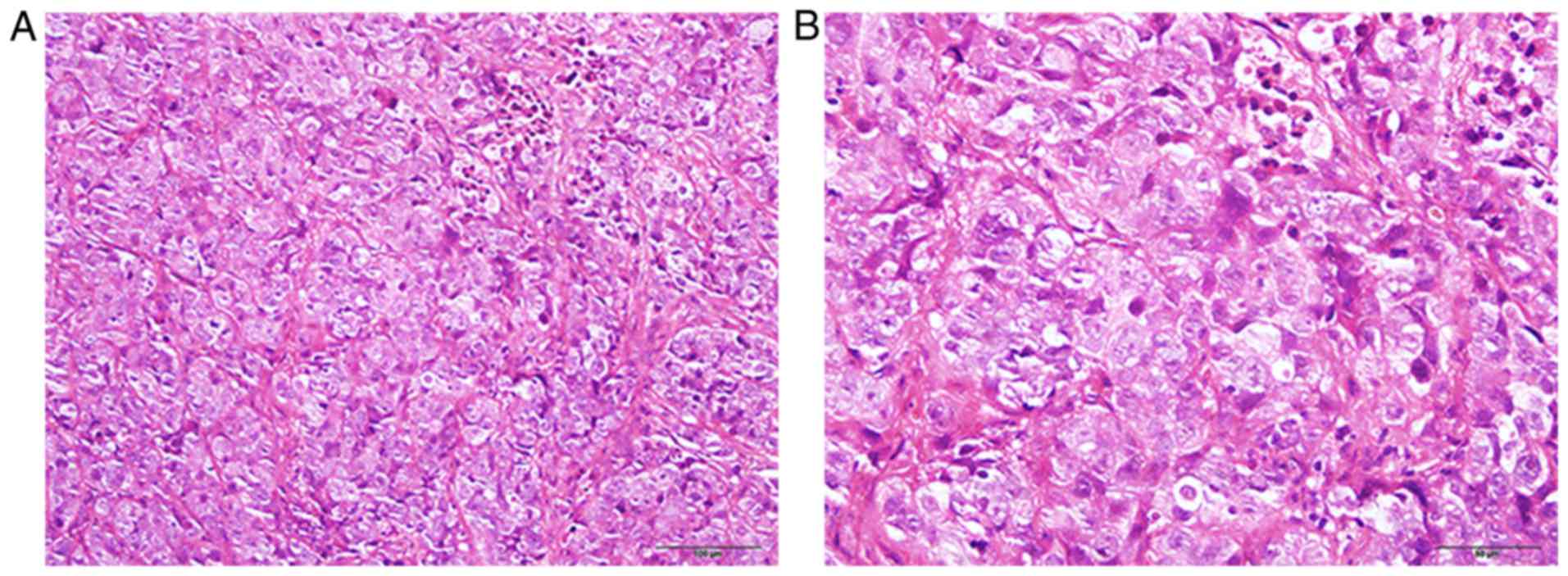
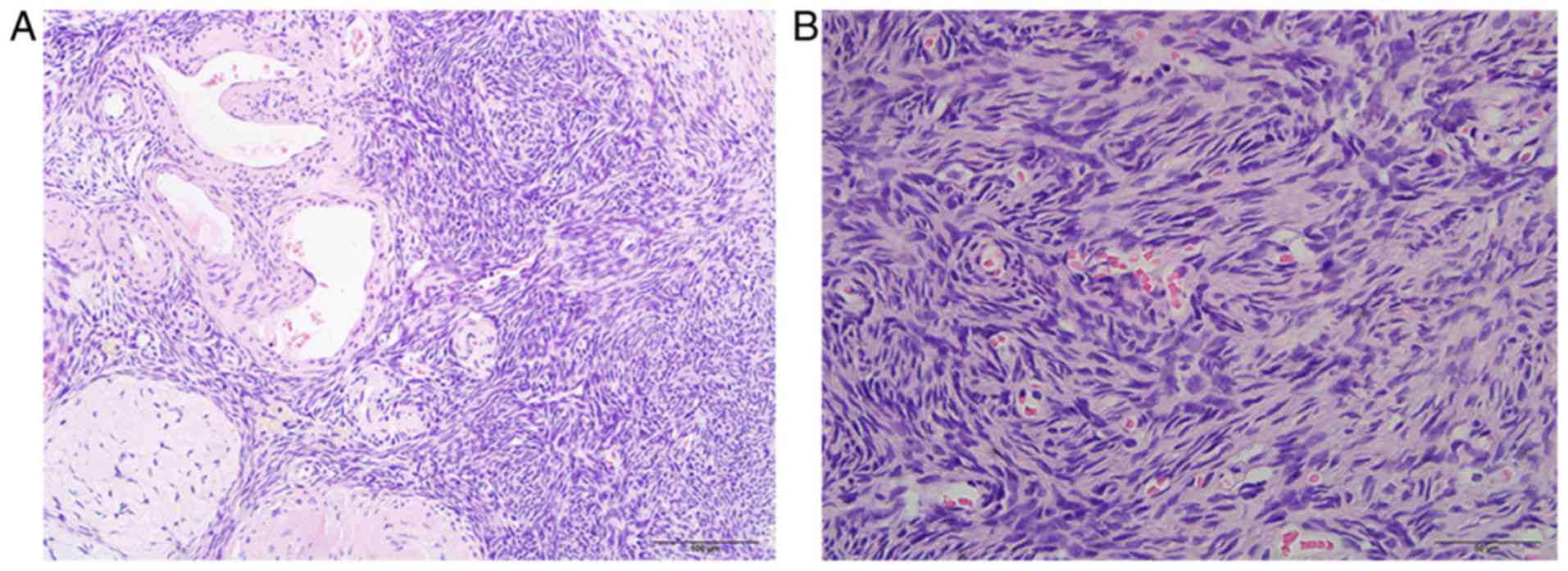
metastatic supra-clavicular lymph nodes were poorly differentiated
serous carcinoma (Fig. 1). At the
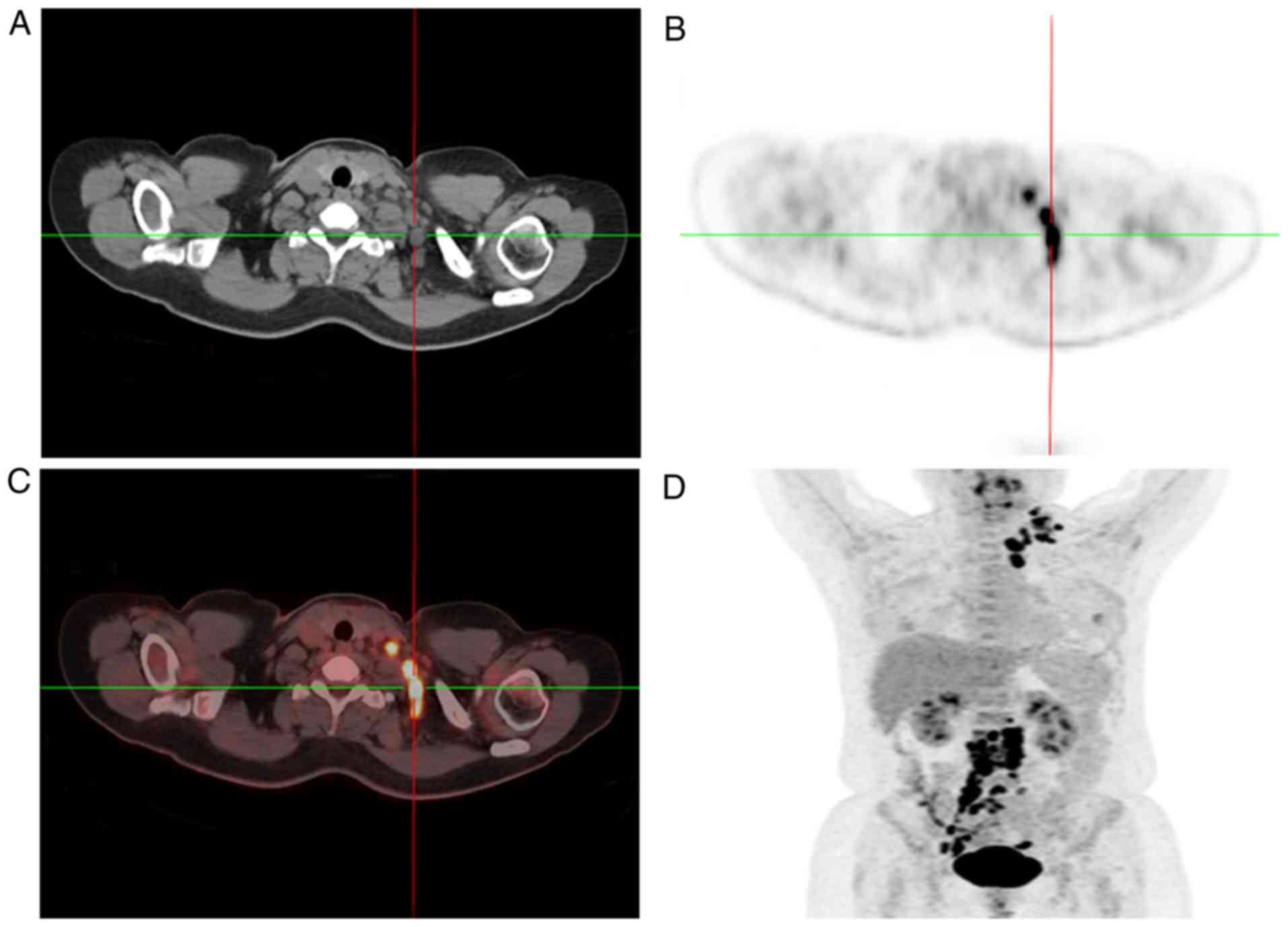
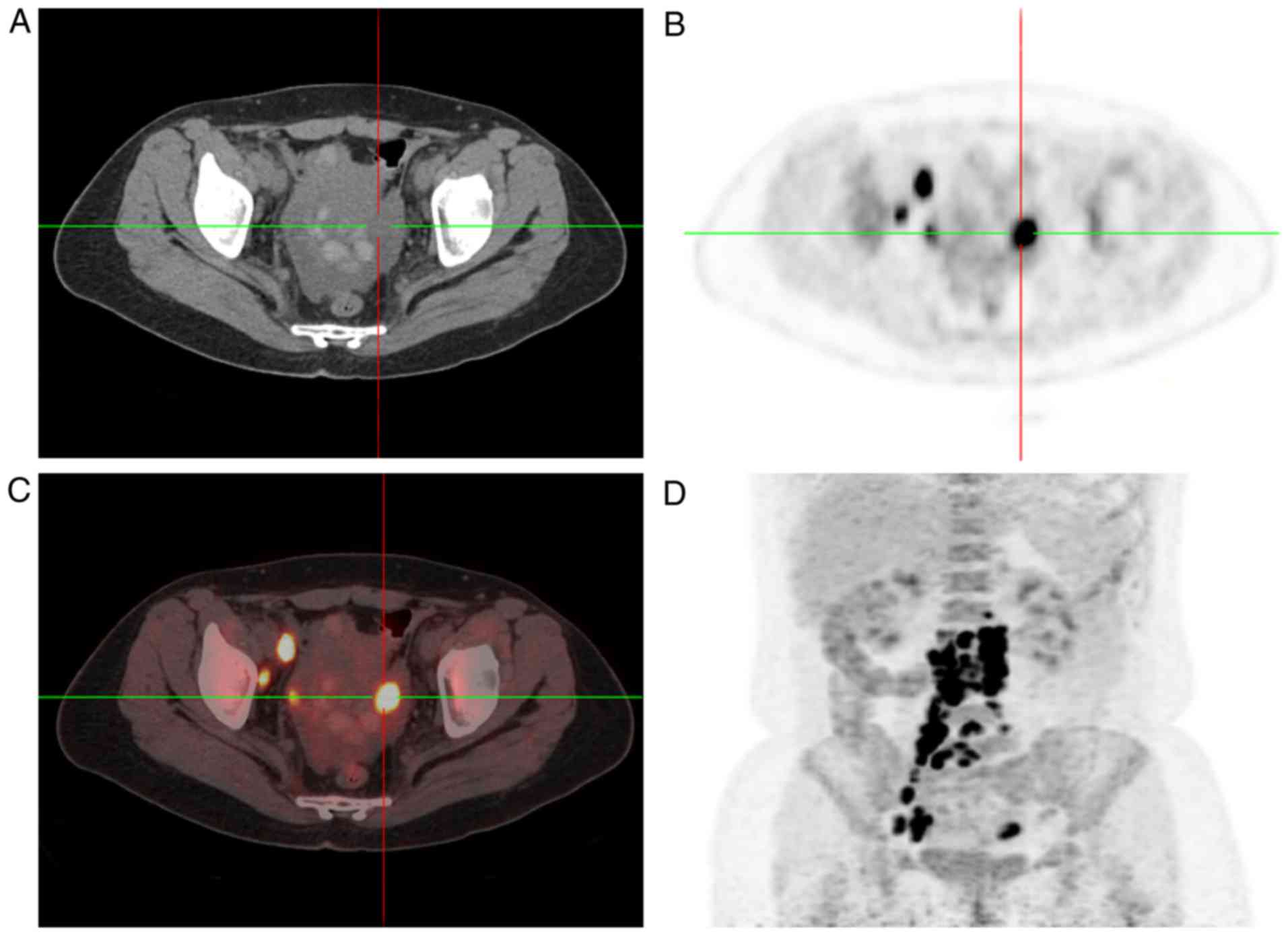
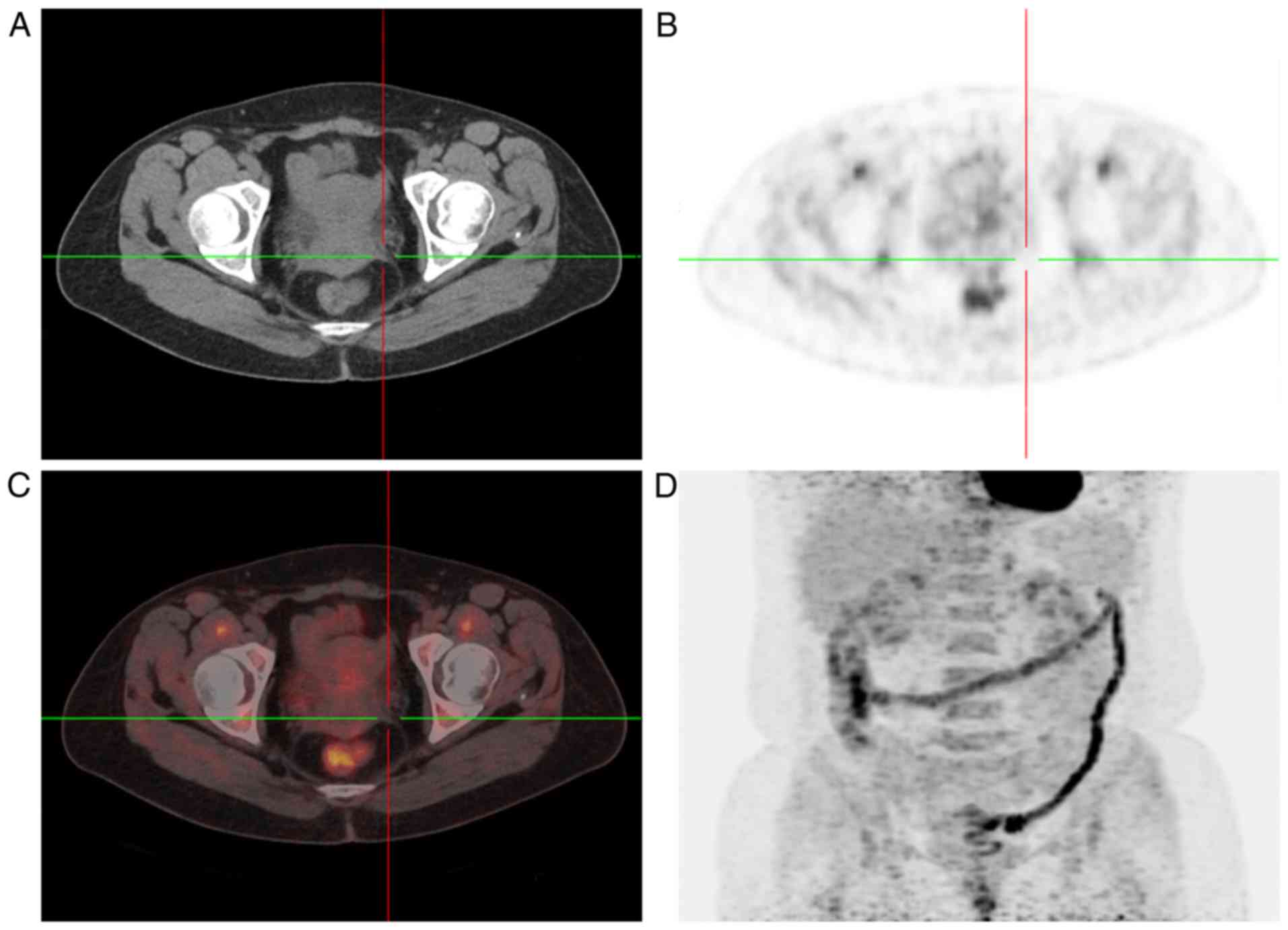
same time, 18F-fluorodeoxyglucose-positron emission tomography/CT
(FDG-PET/CT) scan revealed increased FDG uptake in the bilateral
adnexal areas and in multiple lymph nodes (left supra-clavicular,
mediastinal, retroperitoneal and pelvic) (Figs. 2 and 3).
The CA125 level was 290.4 U/ml (normal range, 0–35 U/ml) prior to
treatment. In addition, although the patient had undergone
in-vitro fertilization twice, no success had been obtained,
and the patient's menstrual cycle was 28/3 days, which means the
first day of the period follows the first day of the preceding
period by 28 days, and the duration of flow is 3 days.
 | Table I.Details of primary and secondary
antibody. |
Table I.
Details of primary and secondary
antibody.
| The primary
antibody | Catalog no. | Dilution |
|---|
| Activin receptor-like
kinase 1 | ZM-0248 | 1:100 |
| Carbonic anhydrase
9 | TA336805 | 1:200 |
| Cluster of
differentiation 30 | ZA-0591 | 1:100 |
| Homeobox protein
CDX-2 | ZA-0520 | 1:100 |
| Chromogranin A | ZM-0076 | 1:100 |
| CK20 | ZA-0574 | 1:100 |
| CK7 | ZM-0071 | 1:100 |
| Epstein-Barr encoding
region (in situ hybridization) | ZM-0105 | 1:100 |
| Ki-67 | ZM-0167 | 1:25 |
| PAX2 | ZA-0467 | 1:20 |
| PAX8 | ZM-0468 | 1:50 |
| Synaptophysin | ZM-0246 | 1:100 |
| Thyroid transcription
factor 1 | ZM-0270 | 1:100 |
| Wilms tumor 1 | ZM-0269 | 1:50 |
| Tumor protein
p53 | ZA-0501 | 1:100 |
| Tumor protein
p16 | ZM-0205 | 1:100 |
| CA125 | ZM-0019 | 1:50 |
| Creatine kinase | ZM-0069 | 1:100 |
| Estrogen
receptor | ZA-0102 | 1:50 |
| Postmeiotic
segregation increased 2 protein | ZA-0542 | 1:20 |
| MutS homolog 2
protein | ZA-0622 | 1:100 |
| MutL homolog 1
protein | ZM-0154 | 1:10 |
| MutS homolog 6
protein | ZA-0541 | 1:100 |
| Aspartic proteinases
A | ZM-0473 | 1:100 |
| Progestogen
receptor | ZA-0255 | 1:100 |
The patient was stage IV by FIGO staging system
(1) and a complete resection was
difficult, so NACT was planned as the primary treatment followed by
interval debulking surgery. The NACT regimen was a platinum and
taxane combination (260 mg paclitaxel and 850 mg carboplatin, every
3 weeks). The CA125 level following the first cycle was 41.0 and
the second cycle was 14.6 U/ml. The CA125 level decreased to normal
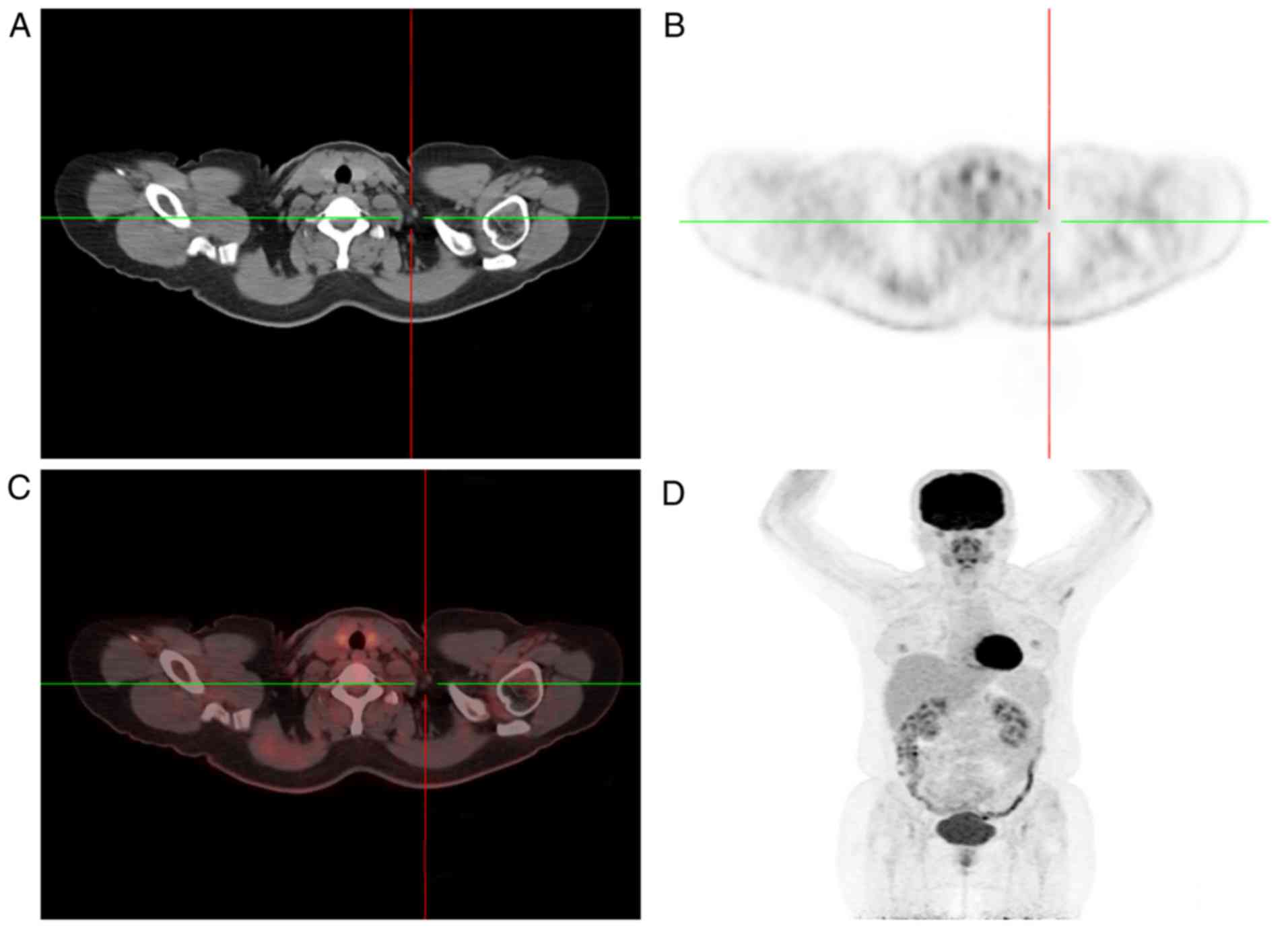
following the second cycle of NACT. PET/CT was used for follow-up
imaging subsequent to two cycles of NACT, and it showed no markedly
increased FDG uptake in the areas that were abnormal on the first
PET/CT scan (Figs. 4 and 5). The patient achieved a complete response
(CR) following two cycles of NACT. Next, the patient was managed
with surgery plus hyperthermic intraoperative interperitoneal
chemotherapy (HIPEC). The surgical therapy included a total
laparoscopic hysterectomy, a bilateral salpingo-oophorectomy, an
abdominal wall lesionectomy, an omentectomy, and a pelvic and
para-aortic lymphadenectomy. Intraoperative views concluded that
the uterus and bilateral adnexa were normal, that a small amount of
ascites was present in the pelvic cavity, that there was no evident
abnormality in the bowel, the large omentum, on the surface of the
liver and spleen, and that only four small lesions were in the
right lower abdominal wall. All specimens were sent for
histopathological examination. Intraoperative rapid frozen section
(frozen at −20°C, thickness was 5 µm; observed under a
phase-contrast positive microscope at room temperature) indicated a
lack of malignant cells. Next, lobaplatin (50 mg) was administered,
at a temperature ranging from 42–43°C, for 60 min. The second HIPEC
with same dose and temperature as the first HIPEC was completed 3
days later. Final histological findings detected no malignant cells
in the bilateral adnexa or the omentum, and the pelvic and
para-aortic lymph nodes were confirmed to exhibit only chronic
inflammation (Fig. 6). pCR was
confirmed according to the postoperative histological findings. The
patient was administered another four cycles of chemotherapy (270
mg paclitaxel and 120 mg nedaplatin, every 3 weeks). No recurrence
was determined during 3 years of follow-up.
Discussion
Ovarian cancer is caused by a variety of factors,
including genetic, environment and reproductive factors. One of the
known risks of ovarian cancer is being childless; women who give
birth multiple times have an 8% reduction in risk for each
additional pregnancy compared with nulliparous women (11). The patient in the present study was
not able to become pregnant despite undergoing IVF twice.
Ovarian cancer is confined to the intraperitoneal
route of dissemination by the direct exfoliation of malignant
cells. The cancer can also metastasize through the lymphatic
channels and the hematogenous route to the retroperitoneal and
extra-peritoneal lymph nodes and other distant sites, including the
lungs and bones (4,12). Distant metastatic lymph nodes can
occur at the time of diagnosis of ovarian cancer or during its
evolution.
Ovarian cancer with metastatic lymph nodes is stage
IV disease and the prognosis of these patients is naturally poor.
Data from the literature concerning distant metastases are scarce,
and much less is known about the effect of treatment and the
prognosis of these patients compared with stage IV patients without
distant metastases. A CR has rarely been reported in cases of
ovarian cancer with metastatic lymph nodes. A review of the
literature on ovarian cancer with distant metastatic lymph nodes,
including supra-clavicular (5,13,14), axillary (6,15–22), and inguinal (8,23) lymph
node metastasis, is shown in Table
II. These reports all emphasized how rare these diseases were,
and how to correctly diagnose and provide them with appropriate
systemic treatment. The effects of the treatments in these reports
were not distinctive from the ordinary measures. Cormio et
al (12) analyzed 50 patients
with distant disease from 162 patients with epithelial ovarian
carcinoma. Only 13 patients presented with distant metastatic
disease at the time of diagnosis and not more than 5 patients had
metastatic extra-abdominal lymph nodes. The study finally concluded
that the duration between the ovarian cancer diagnosis and the
documentation of the distant metastasis was the most important
prognostic factor associated with survival, and that the survival
time was longer when this duration was longer. Cheng et al
(7) reported a retrospective study of
20 cases of epithelial ovarian carcinoma with extra-abdominal
metastases and 645 cases without extra-abdominal metastases. Only 2
patients presented initially with extra-abdominal metastases at the
time of diagnosis, and not more than 3 patients had metastatic
extra-abdominal lymph nodes. In conclusion, this study indicated
that the Karnofsky performance status (KPS) score, sensitivity of
primary chemotherapy, metastatic site and systemic therapy
following the diagnosis of extra-abdominal metastases were the
factors that were significantly associated with survival. Zang
et al (24) reviewed 25
patients with epithelial ovarian cancer who were diagnosed with
initial extra-abdominal metastases. The study demonstrated that the
prognosis of patients with supraclavicular lymphadenopathy or
malignant pleural effusion was improved compared with that for
other stage IV patients with epithelial ovarian cancer. The present
report may indicate improved treatment effects and a longer
survival time compared with other patients with stage IV disease
from the aforementioned study (7,24), with a
KPS score of 1, left supra-clavicular metastatic lymph nodes and
active chemotherapy. In fact, subsequent to two cycles of
chemotherapy, the patient achieved CR. Intraoperative examination
and histopathological examination were negative for disease, as was
the histopathological examination following the surgery.
 | Table II.Ovarian cancer with distant metastatic
lymph nodes reported in the literature. |
Table II.
Ovarian cancer with distant metastatic
lymph nodes reported in the literature.
|
|
| Distant lymph
node |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| First author
(year) | Age, years | Site | Side | Time | Site of primary
lesion | Pathology | (Refs.) |
|---|
| Cebesoy et al
(2008) | – | Supra-clavicular | Left | The time of
diagnosis | – | Serous | (13) |
| Rahman et al
(2012) | 49 | Supra-clavicular | Left | The time of
diagnosis | – | Serous | (5) |
| Fanti et al
(2006) | 51 | Supra-clavicular | Left | After initial
surgery | Left | Poorly
differentiated | (14) |
|
| 65 | Supra-clavicular | Left | The time of
diagnosis | – | Poorly differentiated
serous-papillary |
| Ceccarelli et
al (2011) | 48 | Axillary | Right | The time of
diagnosis | Right | Poorly differentiated
serous-papillary | (6) |
| Hockstein et
al (1997) | 78 | Axillary | Right | The time of
diagnosis | Bilateral | Adenocarcinoma | (15) |
| Saxena et al
(2014) | 45 | Axillary | Right | The time of
diagnosis | Right | Serous | (16) |
| Sibio et al
(2014) | 49 | Axillary | – | The time of
diagnosis | – | Serous
papillary | (17) |
| Singer et al
(2001) | 46 | Axillary | Right | 15 years after
OC | Bilateral | Serous
papillary | (18) |
|
| 63 | Axillary | Left | Several years after
OC | Bilateral | Serous
papillary |
|
|
| 68 | Axillary | – | Several years after
OC | – | Serous
papillary |
|
| Aydin et al
(2009) | 47 | Axillary | – | Two years after
surgery | – | Intermediate
differentiated serous | (19) |
| Ozmen et al
(2007) | 74 | Axillary | Right | 4 years after
OC | – | Serous
papillary | (20) |
|
| 38 | Axillary | Right | 2 years after
OC | – | Papillary |
|
| Skagias et
al (2008) | 63 | Axillary | Right | Several years after
OC | – | Poorly
differentiated | (21) |
| Orris et al
(1999) | 63 | Axillary | Bilateral | Several years after
OC | – | Adenocarcinoma | (22) |
| Ang et al
(2007) | 59 | Inguinal | Right | The time of
diagnosis | Left | Moderately
differentiated papillary serous | (23) |
| Yang et al
(2014) | 54 | Inguinal | Right | The time of
diagnosis | Right | Low-grade
differentiated serous papillary | (8) |
When a patient presents with metastatic lymph nodes,
it is crucial to locate the primary tumor and begin active
treatment. Due to the use of PET/CT, it is easy to locate the exact
origin of metastatic diseases. CR has rarely been achieved
following chemotherapy for ovarian cancer, but it is possible. In
the present case, the patient presented with distant metastatic
lymph nodes and was nulliparous. CR was achieved following two
cycles of NACT. Thus, the authors speculate whether patients who
initially present with distant metastatic lymph nodes and are
nulliparous, as in the present case, experience an improved
prognosis and also avoid overtreatment compared with patients
presenting differently. Further studies are required to confirm
this and to explain the reason why such patients have a better
prognosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this case are
included in this published article.
Authors' contributions
HQ, ZM and HZ analyzed and interpreted the patient
data. SL and HC performed the histological and immunohistochemical
of all the operative specimens. LH was a major contributor in
writing the manuscript, analyzing and interpreting the patient
data, gave final approval of the version to be published, and
agreed to be accountable for all aspects of the article in ensuring
that questions related to the accuracy. HQ performed critical
revisions of the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Ethics approval and consent to participate were
authorized by the ethical committee of Zhongnan Hospital of Wuhan
University (approval no. 2017060). Informed consent was obtained
from all individual participants included in the study.
Consent for publication
Written consent for publication was obtained from
the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Prat J: FIGO Committee on Gynecologic
Oncology: Staging classification for cancer of the ovary, fallopian
tube, and peritoneum. Int J Gynaecol Obstet. 124:1–5. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goff BA, Mandel LS, Melancon CH and Muntz
HG: Frequency of symptoms of ovarian cancer in women presenting to
primary care clinics. JAMA. 291:2705–2712. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kamura T and Jeon JD: Lymph node
metastasis in a gynecologic malignancy. Yonsei Med J. 43:783–791.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rahman M, Nakayama K, Rahman MT, Katagiri
H, Ishibashi T and Miyazaki K: Enlarged Virchow's node as an
initial complaint of serous ovarian adenocarcinoma. Eur J Gynaecol
Oncol. 33:546–548. 2012.PubMed/NCBI
|
|
6
|
Ceccarelli F, Barberi S, Pontesilli A,
Zancla S and Ranieri E: Ovarian carcinoma presenting with axillary
lymph node metastasis: A case report. Eur J Gynaecol Oncol.
32:237–239. 2011.PubMed/NCBI
|
|
7
|
Cheng B, Lu W, Xiaoyun W, YaXia C and Xie
X: Extra-abdominal metastases from epithelial ovarian carcinoma: An
analysis of 20 cases. Int J Gynecol Cancer. 19:611–614. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang XJ, Zheng FY, Xu YS and Ou RY:
Ovarian cancer initially presenting with isolated ipsilateral
superficial inguinal lymph node metastasis: A case study and review
of the literature. J Ovarian Res. 7:202014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fagotti A and Scambia G: Counterpoint:
Primary Debulking surgery vs neoadjuvant chemotherapy for newly
diagnosed advanced ovarian cancer. Oncology (Williston Park).
31(453): 458. 460–461. 2017.
|
|
10
|
Petrillo M, Zannoni GF, Tortorella L,
Anchora Pedone L, Salutari V, Ercoli A, Margariti PA, Scambia G and
Fagotti A: Prognostic role and predictors of complete pathologic
response to neoadjuvant chemotherapy in primary unresectable
ovarian cancer. Am J Obstet Gynecol. 211:632.e1–e8. 2014.
View Article : Google Scholar
|
|
11
|
Tsilidis KK, Allen NE, Key TJ, Dossus L,
Lukanova A, Bakken K, Lund E, Fournier A, Overvad K, Hansen L, et
al: Oral contraceptive use and reproductive factors and risk of
ovarian cancer in the European prospective investigation into
cancer and nutrition. Br J Cancer. 105:1436–1442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cormio G, Rossi C, Cazzolla A, Resta L,
Loverro G, Greco P and Selvaggi L: Distant metastases in ovarian
carcinoma. Int J Gynecol Cancer. 13:125–129. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cebesoy FB, Balatt O and Aydin A:
Virchow's node as a first manifestation of ovarian serous
carcinoma: Case report. Eur J Gynaecol Oncol. 29:182–183.
2008.PubMed/NCBI
|
|
14
|
Fanti S, Nanni C, Castellucci P, Farsad M,
Rampin L, Gross MD, Mariani G and Rubello D: Supra-clavicular lymph
node metastatic spread in patients with ovarian cancer disclosed at
18F-FDG-PET/CT: An unusual finding. Cancer Imaging. 6:20–23. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hockstein S, Keh P, Lurain JR and Fishman
DA: Ovarian carcinoma initially presenting as metastatic axillary
lymphadenopathy. Gynecol Oncol. 65:543–547. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saxena AK, Goyal N, Singhal J and Kumar P:
Primary ovarian serous adenocarcinoma with ipsilateral axillary
lymph node metastasis: A case report. Indian J Surg Oncol.
5:224–226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sibio S, Sammartino P, Accarpio F, dei
Malatesta Framarino ML, Biacchi D, Sollazzo BM and Di Giorgio A:
Axillary lymph node metastasis as first presentation of peritoneal
carcinomatosis from serous papillary ovarian cancer: Case report
and review of the literature. Eur J Gynaecol Oncol. 35:170–173.
2014.PubMed/NCBI
|
|
18
|
Singer C, Blankstein E, Koenigsberg T,
Mercado C, Pile-Spellman E and Smith SJ: Mammographic appearance of
axillary lymph node calcification in patients with metastatic
ovarian carcinoma. AJR Am J Roentgenol. 176:1437–1440. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aydin C, Unalp HR, Baloğlu A, Inci AG,
Yiğit S and Yavuzcan A: Axillary lymph node metastasis from serous
ovarian cancer: A case report and review of the literature. Arch
Gynecol Obstet. 279:203–207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ozmen V, Asoglu O, Karanlik H, Cabioglu N,
Kecer M and Bakkaloglu H: Primary ovarian cancer presenting with
axillary lymph node metastases: A report of two cases. Acta Chir
Belg. 107:75–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Skagias L, Ntinis A, Vasou O, Kondi-Pafiti
A and Politi E: Ovarian carcinoma presenting with axillary lymph
node metastasis: A case diagnosed by fine-needle aspiration and
brief review of the literature. Diagn Cytopathol. 36:891–893. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Orris BG, Geisler JP and Geisler HE:
Ovarian carcinoma metastatic to bilateral axillary lymph nodes. A
case report. Eur J Gynaecol Oncol. 20:189–190. 1999.PubMed/NCBI
|
|
23
|
Ang D, Ng KY, Tan HK, Chung AY, Yew BS and
Lee VK: Ovarian carcinoma presenting with isolated contralateral
inguinal lymph node metastasis: A case report. Ann Acad Med
Singapore. 36:427–430. 2007.PubMed/NCBI
|
|
24
|
Zang RY, Zhang ZY, Cai SM, Tang MQ, Chen J
and Li ZT: Epithelial ovarian cancer presenting initially with
extraabdominal or intrahepatic metastases: A preliminary report of
25 cases and literature review. Am J Clin Oncol. 23:416–419. 2000.
View Article : Google Scholar : PubMed/NCBI
|