Introduction
Lung cancer is one of the most common types of tumor
and the leading cause of cancer-associated mortality in the United
States in 2015 (1). Non-small cell
lung cancer (NSCLC) accounts for ~80% of all incidents lung cancer
cases (2). Despite various improved
treatments, including surgery, radiotherapy and chemotherapy, the
5-year overall survival rate of patients with NSCLC is ~15%
(3). Therefore, investigating
potential target genes for NSCLC treatment is vital.
Circular RNAs (circRNAs) are a novel type of
universal and endogenous RNA. Unlike linear RNAs, circRNAs form a
covalently closed continuous loop without 5′ or 3′ ends (4,5).
Accumulating evidence indicates that the aberrant expression of
circRNAs is involved in tumor development and progression. The
circular RNA ciRS-7 serves as a risk factor for hepatic
microvascular invasion in hepatocellular carcinoma (6); hsa_circ_0005075 may participate in cell
adhesion during hepatocellular carcinoma (HCC) development and
function as a potential HCC biomarker (7); hsa_circ_0001649 expression is
significantly downregulated in HCC tissues and is correlated with
tumor size and the occurrence of tumor embolus in HCC (8); and circ_001569 serves as a miRNA sponge
to suppress miR-145 expression, subsequently increasing
transcription factor E2F5, BAG family molecular chaperone regulator
4 and formin-like protein 2 expression levels to promote the
proliferation and invasion of colorectal cancer (9). To the best of our knowledge, the role of
circ_001569 in NSCLC progression and its underlying mechanism have
not been investigated.
In the present study, it was identified that the
levels of circ_001569 were significantly increased in NSCLC tissues
compared with in adjacent normal tissues. Knockdown of circ_001569
inhibited cell proliferation in NSCLC. In addition, downregulation
of circ_001569 suppressed the Wnt/β-catenin pathway in NSCLC cells.
Therefore, these results indicated circ_001569 may be a potential
target of NSCLC treatment.
Materials and methods
Clinical tissue specimens
A total of 56 pairs of NSCLC and adjacent normal
tissues were obtained from patients (41 male cases and 15 female
cases; mean age, 51.22 years; range, 38–70 years) who had undergone
surgery at the Nantong Tumor Hospital (Nantong, China) between
April 2011 and December 2015. The tissues were immediately
snap-frozen in liquid nitrogen and stored at −80°C. No patient had
received radiotherapy or chemotherapy prior to surgery. The Ethics
Board of Nantong Tumor Hospital approved the study. Written
informed consent was obtained from all patients. All patients with
NSCLC were staged according to the 7th edition of the American
Joint Committee on Cancer Tumor-Node-Metastasis (TNM) staging
system for lung cancer (10).
Cell culture
A total of 4 human NSCLC H460, H1299, SPC-A1 and
A549 cell lines and the non-tumorigenic bronchial epithelium
BEAS-2B cell line were purchased from the Type Culture Collection
of the Chinese Academy of Sciences (Shanghai, China). Cells were
cultured in RPMI-1640 medium (Life Technologies; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Life Technologies; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin in a 5%
CO2 humidified atmosphere at 37°C.
Cell transfection with small
interfering (si)RNAs
The hsa-circ_001569 siRNA (si-circ_001569:
5′-GCATCGTGCAGGACTGGAA-3′) and negative control (NC) were purchased
from Guangzhou RiboBio Co., Ltd (Guangzhou, China), and used
according to the manufacturer's protocol. When cells reached 80–90%
confluency, they were transfected with 20 nM hsa-circ_001569 siRNA
and negative control. Cells were transfected with
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), in accordance with the manufacturer's protocol.
At 48 h post transfection, the cells were harvested and used in
subsequent analysis.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from tissues and cells using
TRIzol® reagent (Life Technologies; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Reverse transcription was performed using 10 ng total RNA and a
PrimeScript™ RT Reagent kit (Takara Biotechnology Co., Ltd.,
Dalian, China). RT-qPCR was performed using an Applied Biosystems
PRISM 7900HT Fast Real-Time PCR System (Thermo Fisher Scientific,
Inc.) with SYBR® Premix Ex Taq™II (Takara Bio, Inc.,
Otsu, Japan) with the following conditions: 50°C for 2 min; 95°C
for 10 min; and 45 cycles of 95°C for 15 sec and 60°C for 60 sec.
GAPDH was used as a normalization control. The 2−ΔΔCq
method was used to analyze relative mRNA expression (11). The primer sequences were as follows:
circ_001569 forward, 5′-TCCCCTGAACATTCTCCCCAT-3′; circ_001569
reverse, 5′-GAAAGCACTTGGTGAAGTCGG-3′; GAPDH forward,
5′-CACCGTAGCCTTCCGAGTA-3′; and GAPDH reverse,
5′-GCCCTTGATGAGCTGTTGA-3′.
Cell proliferation assay
For this assay, 5×103 cells/well,
transfected with si-NC or si-circ_001569 for 24 h at 37°C, were
seeded into 96-well plates. Cell proliferation was examined at the
indicated time points, 0, 24, 48 and 72 h, using a CCK-8 assay
(Dojindo Molecular Technologies, Kumamoto, Japan), according to the
manufacturer's protocol. The absorbance was read at a wavelength of
450 nm using a microplate reader (Molecular Devices, LLC,
Sunnyvale, CA, USA).
Cell colony formation assay
A cell colony assay was performed using 6-well
plates. Briefly, 200 cells/well in RPMI-1640 containing 10% FBS
were seeded into 6-well plates. Following culture in a 5%
CO2 humidified atmosphere at 37°C for 14 days, cells
were fixed in 100% methanol at room temperature, stained with 0.5%
crystal violet for 20 min at room temperature, and then the number
of colony-forming cells were calculated using an AID iSpot Reader
(Autoimmun Diagnostika GmbH, Strassberg, Germany). Three
independent experiments were performed.
Western blot analysis
Total protein was extracted from cells using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology Co., Ltd., Haimen, China). Protein concentrations
were determined using a Bicinchoninic Acid Protein Assay kit
(Beyotime Institute of Biotechnology, Beijing, China). Equal
amounts of protein (40 ng) were separated by 10% SDS-PAGE and
transferred onto the nitrocellulose membranes (EMD Millipore,
Billerica, MA, USA). The membranes were blocked with 5% skim milk
for 2 h at room temperature, and then incubated with primary
antibodies against proto-oncogene Wnt1 (WNT1; cat. no. sc-514531;
1:1,000), β-catenin (cat. no. sc-376841; 1:2,000), transcription
factor 4 (TCF4; cat. no. sc-271287; 1:500) (all from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and GAPDH (1:1,000, cat. no.
2118; Cell Signaling Technology, Inc., Danvers, MA, USA) overnight
at 4°C. The blots were then incubated with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibodies
(dilution, 1:2,000; cat. no. A21020; Abbkine, Wuhan, China). for 2
h at room temperature and visualized using an Enhanced
Chemiluminescence Plus kit (GE Healthcare Bio-Sciences, Pittsburgh,
PA, USA). The intensity of the bands was quantified using Image
Lab™ Software (version 4.6.9; Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS v.20.0
software (IBM Corp., Armonk, NY, USA). All data are expressed as
the mean ± standard deviation. The experiments were repeated ≥3
times. Differences between two groups were assessed using the
Student's t-test (two-tailed) and differences among multiple groups
were analyzed using one-way analysis of variance (ANOVA). The
Student-Newman-Keuls test was used as a post-hoc test following
ANOVA. The associations between clinical variables and circ_001569
expression were analyzed using the Pearson χ2 test.
Survival curves were plotted using the Kaplan-Meier method and
assessed with a log-rank test to identify significant differences.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Circ_001569 expression is higher in
NSCLC tissues and cells
To investigate the expression pattern of circ_001569
in 56 pairs of NSCLC and adjacent normal tissues, RT-qPCR assays
were performed. As demonstrated in Fig.
1A, the results indicated that circ_001569 expression was
significantly upregulated compared with in the adjacent normal
tissues (P<0.001). Additionally, circ_001569 expression was
significantly upregulated in the 4 human NSCLC H460, H1299, SPCA-1
cell lines and in the A549 cells, when compared with in the
non-tumorigenic bronchial epithelium BEAS-2B cell line (Fig. 1B). The mean expression of circ_001569
in tumor tissues was used for cut-off levels to divide patients
into the higher and lower expression groups. As determined by
χ2 test analysis, and as summarized in Table I, circ_001569 expression levels were
demonstrated to be closely associated with tumor differentiation
(P=0.035), lymph node metastasis (P=0.012) and TNM classification
(P=0.010) in NSCLC. Patients who exhibited higher circ_001569
expression exhibited a poor survival outcome, compared with lower
circ_001569 expression (log rank=10.641; P<0.001; Fig. 1C).
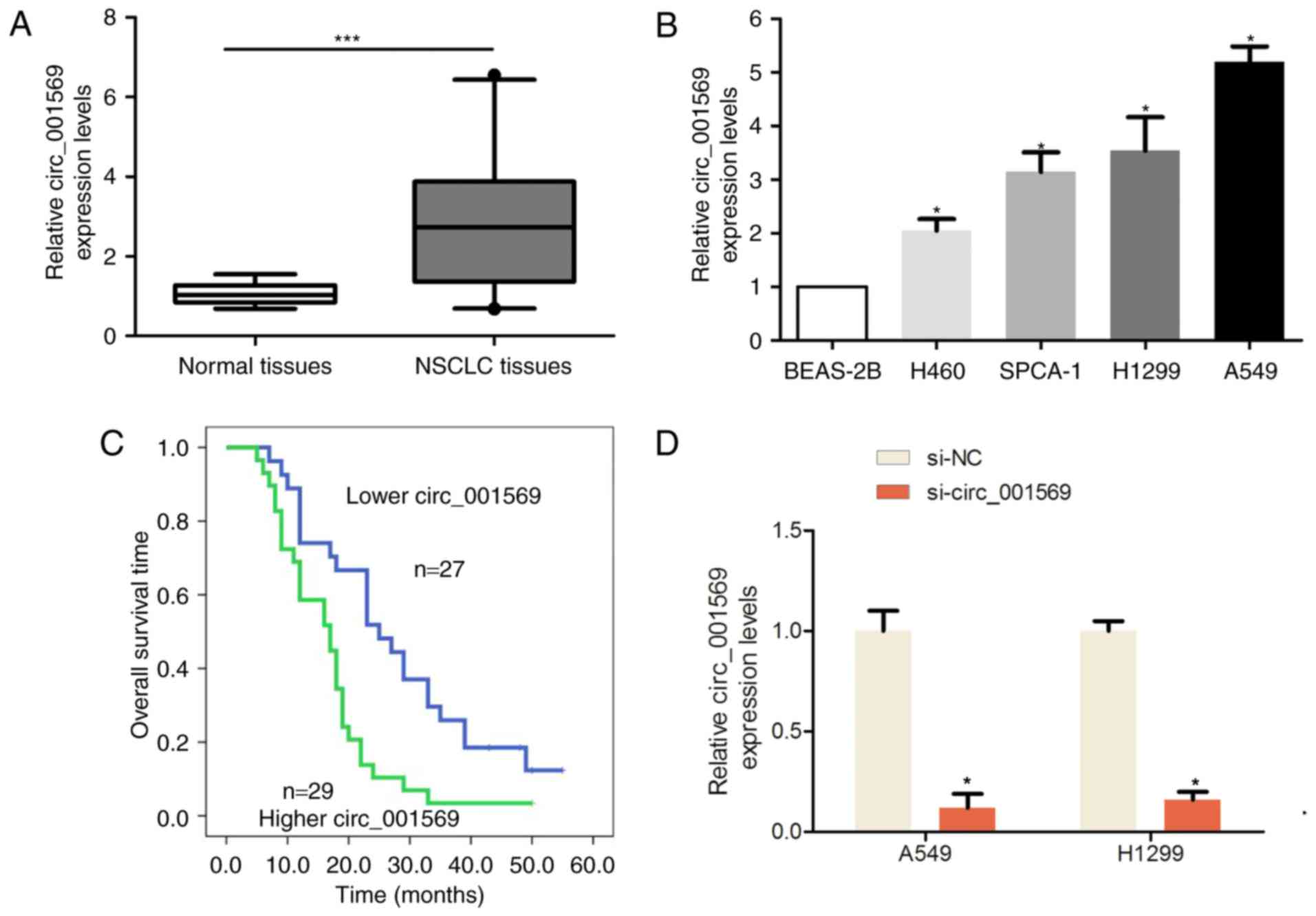 | Figure 1.Expression of circ_001569 was
significantly upregulated in NSCLC tissues and cells. (A)
Expression of circ_001569 was upregulated in NSCLC tissues compared
with in adjacent normal tissues (n=56), ***P<0.001. (B)
Expression of circ_001569 was upregulated in human NSCLC H460,
H1299, SPCA-1 and A549 cell lines when compared with that in the
non-tumorigenic bronchial epithelium cell line BEAS-2B, *P<0.05.
(C) Patients who exhibited higher circ_001569 expression also
exhibited a poorer survival outcome, compared with patients with
lower circ_001569 expression, as determined via Kaplan-Meier
analysis and the log rank test. (D) Expression of circ_001569 was
measured by reverse transcription quantitative polymerase chain
reaction analysis following the introduction of si-NC or
si-circ_001569 into A549 and H1299 cells compared to si-NC groups,
*P<0.05. NSCLC, non-small cell lung cancer; si, small
interfering; NC, negative control. |
 | Table I.Association between circ_001569
expression and the clinicopathological factors of patients with
non-small cell lung cancer. |
Table I.
Association between circ_001569
expression and the clinicopathological factors of patients with
non-small cell lung cancer.
|
|
| Circ_001569
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
factors | Patients (n) | Lower (n=27) | Higher (n=29) | P-value |
|---|
| Age |
|
|
| 0.877 |
| ≤60 | 42 | 20 | 22 |
|
|
>60 | 14 | 7 | 7 |
|
| Sex |
|
|
| 0.643 |
| Male | 41 | 19 | 22 |
|
|
Female | 15 | 8 | 7 |
|
| Smoking |
|
|
| 0.106 |
| Yes | 29 | 17 | 12 |
|
| No | 27 | 10 | 17 |
|
| Differentiation |
|
|
| 0.035a |
| High and
moderate | 38 | 22 | 16 |
|
|
Lower | 18 | 5 | 13 |
|
| Tumor size, cm |
|
|
| 0.188 |
|
<3 | 20 | 12 | 8 |
|
|
>3 | 36 | 15 | 21 |
|
| Histology style |
|
|
| 0.301 |
| Squamous
carcinoma | 20 | 7 | 13 |
|
|
Adenocarcinoma | 24 | 14 | 10 |
|
| Other
type | 12 | 6 | 6 |
|
| Lymph node
metastasis |
|
|
| 0.012a |
| No | 34 | 21 | 13 |
|
| Yes | 22 | 6 | 16 |
|
| TNM
classification |
|
|
| 0.010a |
| I–II | 36 | 22 | 14 |
|
| III | 20 | 5 | 15 |
|
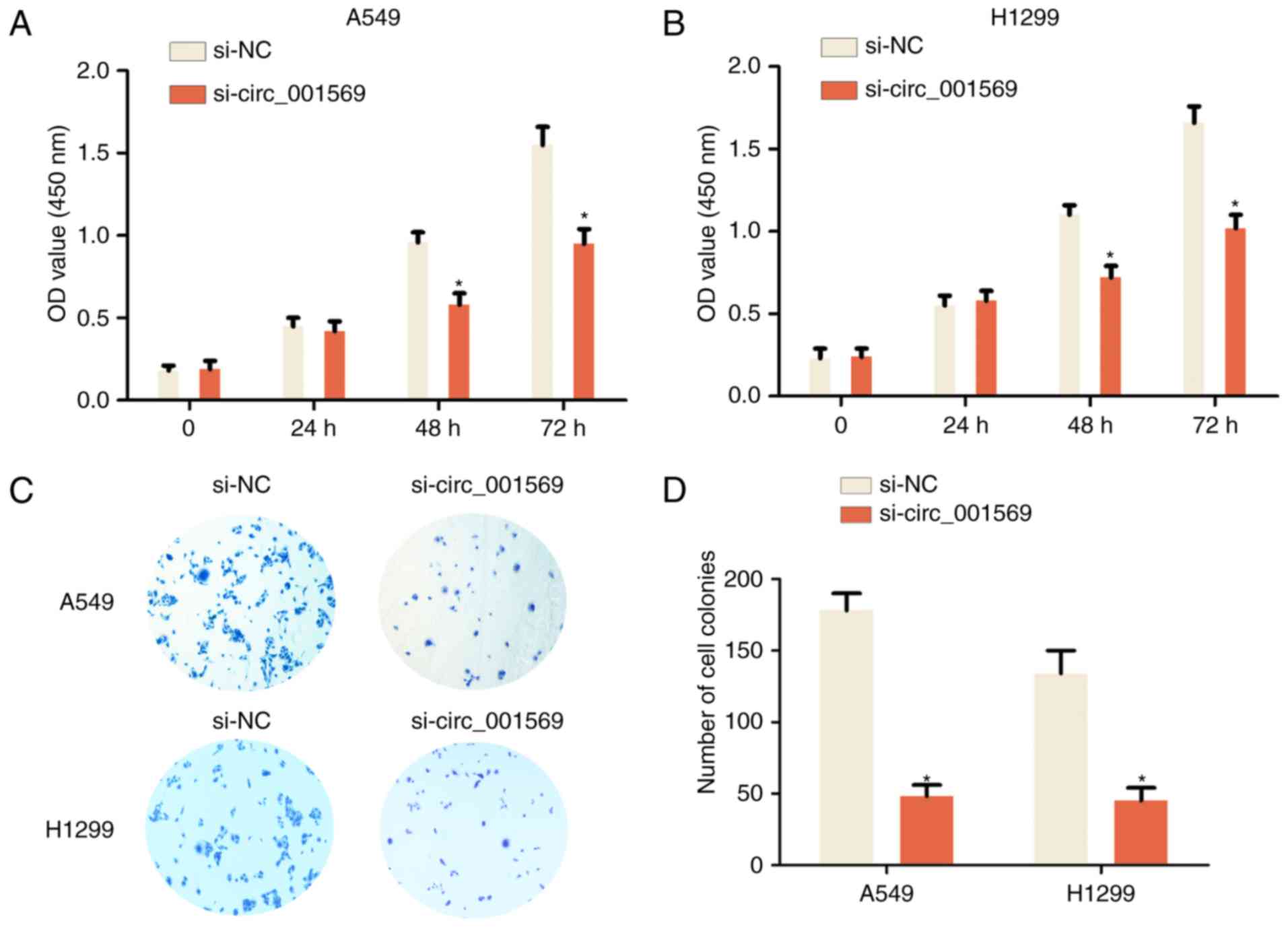
Downregulation of circ_001569 inhibits
cell proliferation of NSCLC
To additionally explore the function of circ_001569
in the development of NSCLC, loss-of-function assays were
performed. siRNA was used to silence circ_001569 expression in A549
and H1299 cells, as these two cell lines exhibited higher
circ_001569 expression levels when compared with H460 and SPC-A1
cells (Fig. 1D). Following
transfection of the A549 and H1299 cells with si-circ_001569 at 48
and 72 h, the CCK-8 cell proliferation assay results revealed that
the proliferation rate was significantly reduced compared with that
of the si-NC group (Fig. 2A and B).
Following transfection of the A549 and H1299 cells with
si-circ_001569 at 14 days, the cell colony formation assay results
observed that the number of cell colonies was significantly reduced
compared with the si-NC group (Fig. 2C
and D). Therefore, the results indicated that the
downregulation of circ_001569 inhibited NSCLC cell
proliferation.
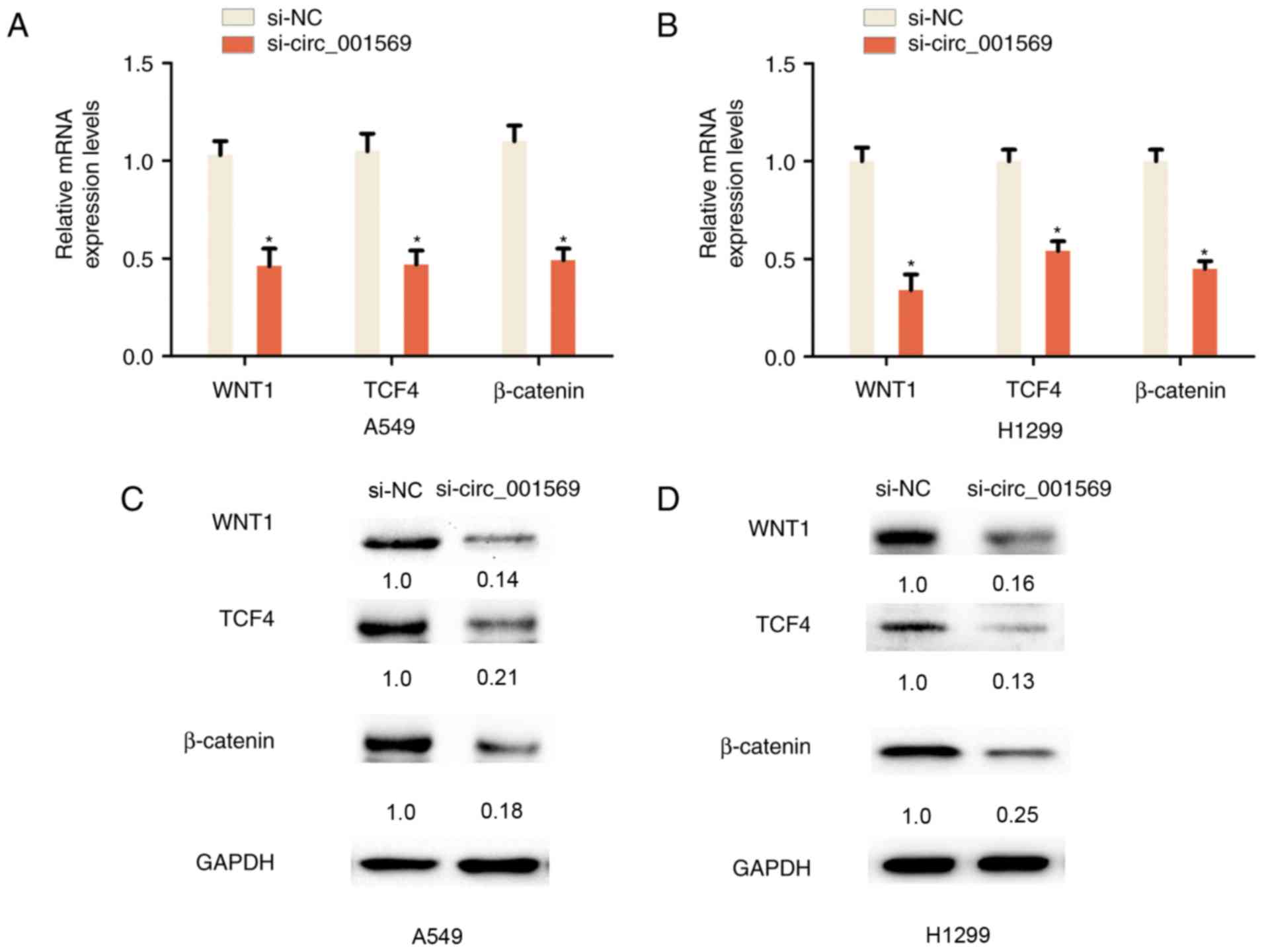
Reduced circ_001569 expression
inhibits the Wnt/β-catenin pathway in NSCLC cells
The Wnt/β-catenin pathway is associated with cell
proliferation and invasion in NSCLC; miRNA-148a serves as a
prognostic factor, and suppresses cell migration and invasion
through WNT1 in NSCLC (12). The
association between circ_001569 expression and the Wnt/β-catenin
pathway in NSCLC was additionally examined. RT-qPCR analysis
indicated that the knockdown of circ_001569 reduced the mRNA
expression levels of WNT1, β-catenin and TCF4 in A549 and H1299
cells, as compared with in the control group (Fig. 3A and B). Consistently, it was also
demonstrated that the knockdown of circ_001569 reduced the protein
expression levels of WNT1, β-catenin and TCF4 in A549 and H1299
cells, as compared with in the control group (Fig. 3C and D). Therefore, these results
indicated that the knockdown of circ_001569 inhibits the
Wnt/β-catenin pathway in NSCLC cells.
Discussion
The majority of the circRNAs have been demonstrated
to be exon-containing circular RNAs, and the crucial roles and
underlying functions of circRNAs are not well-understood at present
(13,14). Certain circRNAs have exhibited
significant roles in cancer; for example: hsa_circ_0003159
expression was significantly downregulated in gastric cancer
tissues and significantly negatively associated with sex, distal
metastasis and TNM stage (15).
CircMTO1 suppresses HCC progression by acting as a sponge for
oncogenic miR-9 in order to promote cyclin-dependent kinase
inhibitor 1 expression, suggesting that circMTO1 is a potential
target for HCC treatment (16);
hsa_circ_0001895 expression levels were significantly downregulated
and correlated with cell differentiation, Borrmann type and tissue
carcinoembryonic antigen expression in gastric cancer (17); in NSCLC development and progression, a
previous study identified that circRNA_100876 expression levels
were demonstrated to be higher in NSCLC tissues and cells and to
exhibit a close correlation with lymph node metastasis and TNM
stage in NSCLC, In addition, patients with high circRNA_100876
expression exhibited significantly shorter survival rates compared
with lower expression groups of patients with NSCLC (18). In the present study, the results
indicated that circ_001569 expression was significantly upregulated
in NSCLC tissues and cells. Circ_001569 expression was closely
associated with differentiation, lymph node metastasis and TNM
classification in NSCLC. Patients who exhibited higher circ_001569
expression had a poorer survival outcome when compared with
patients with lower circ_001569 expression.
Furthermore, to explore the function of circ_001569
in the development of NSCLC, the present study identified that,
following the transfection of A549 and H1299 cells with
si-circ_001569, the proliferation rate and cell colony number were
significantly reduced, compared with the control group. The Wnt
signaling pathway is associated with NSCLC progression, and
serine-arginine protein kinase 1 promotes a cancer stem cell-like
phenotype through the activation of Wnt/β-catenin signaling in
NSCLC (19). DEP Domain Containing
protein 1B enhances cell migration and invasion rate of NSCLC via
activation of the Wnt/β-catenin signaling pathway (20). Armadillo repeat-containing protein 8 α
promotes the proliferation and invasion of NSCLC cells by
activating the canonical Wnt signaling pathway (21). Knockdown of Homeobox B5 inhibits
proliferation, migration and invasion in non-small cell lung cancer
cells through inactivation of the Wnt/β-catenin pathway (22). The present study demonstrated that the
knockdown of circ_001569 inhibited Wnt signaling by reducing the
mRNA and protein expression levels of the Wnt signaling
pathway-associated genes WNT1, β-catenin and TCF4 in NSCLC cells.
In conclusion, the present study demonstrated that circ_001569
expression is higher in NSCLC tissues, and that the knockdown of
circ_001569 inhibits proliferation in NSCLC. Furthermore, reduced
circ_001569 expression may inhibit the Wnt/β-catenin signaling
pathway. These results indicate that circ_001569 may present a
potential target for NSCLC treatment.
Acknowledgements
Not applicable.
Funding
Funding information is not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DLC and ZXD made substantial contributions to the
conception and design, or acquisition of data, or analysis and
interpretation of data. ZXD, YWD, GJ and LJG performed the
experiments. ZXD, YWD and GJ drafted the manuscript.
Ethics approval and consent to
participate
The Ethics Board of Nantong Tumor Hospital approved
the study. Written informed consent was obtained from all
patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Cos Sanchez J, Gonzalez Sojo MA,
Montero MV, Calvo Pérez MC, Vicente MJ and Valle MH: Non-small cell
lung cancer and silent brain metastasis. Survival and prognostic
factors. Lung Cancer. 63:140–145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P
and Wu M: CircRNA: Functions and properties of a novel potential
biomarker for cancer. Mol Cancer. 16:942017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He J, Xie Q, Xu H, Li J and Li Y: Circular
RNAs and cancer. Cancer Lett. 396:138–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu L, Zhang M, Zheng X, Yi P, Lan C and Xu
M: The circular RNA ciRS-7 (Cdr1as) acts as a risk factor of
hepatic microvascular invasion in hepatocellular carcinoma. J
Cancer Res Clin Oncol. 143:17–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shang X, Li G, Liu H, Li T, Liu J, Zhao Q
and Wang C: Comprehensive circular RNA profiling reveals that
hsa_circ_0005075, a new circular RNA biomarker, is involved in
hepatocellular crcinoma development. Medicine (Baltimore).
95:e38112016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qin M, Liu G, Huo X, Tao X, Sun X, Ge Z,
Yang J, Fan J, Liu L and Qin W: Hsa_circ_0001649: A circular RNA
and potential novel biomarker for hepatocellular carcinoma. Cancer
Biomark. 16:161–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie H, Ren X, Xin S, Lan X, Lu G, Lin Y,
Yang S, Zeng Z, Liao W, Ding YQ and Liang L: Emerging roles of
circRNA_001569 targeting miR-145 in the proliferation and invasion
of colorectal cancer. Oncotarget. 7:26680–26691. 2016.PubMed/NCBI
|
|
10
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Min L, Ren C, Xu X, Yang J, Sun X,
Wang T, Wang F, Sun C and Zhang X: miRNA-148a serves as a
prognostic factor and suppresses migration and invasion through
Wnt1 in non-small cell lung cancer. PLoS One. 12:e01717512017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qu S, Yang X, Li X, Wang J, Gao Y, Shang
R, Sun W, Dou K and Li H: Circular RNA: A new star of noncoding
RNAs. Cancer Lett. 365:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hansen TB, Kjems J and Damgaard CK:
Circular RNA and miR-7 in cancer. Cancer Res. 73:5609–5612. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tian M, Chen R, Li T and Xiao B: Reduced
expression of circRNA hsa_circ_0003159 in gastric cancer and its
clinical significance. J Clin Lab Anal. 32:2018. View Article : Google Scholar
|
|
16
|
Han D, Li J, Wang H, Su X, Hou J, Gu Y,
Qian C, Lin Y, Liu X, Huang M, et al: Circular RNA circMTO1 acts as
the sponge of microRNA-9 to suppress hepatocellular carcinoma
progression. Hepatology. 66:1151–1164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shao Y, Chen L, Lu R, Zhang X, Xiao B, Ye
G and Guo J: Decreased expression of hsa_circ_0001895 in human
gastric cancer and its clinical significances. Tumour Biol.
39:10104283176991252017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao JT, Zhao SH, Liu QP, Lv MQ, Zhou DX,
Liao ZJ and Nan KJ: Over-expression of CircRNA_100876 in non-small
cell lung cancer and its prognostic value. Pathol Res Pract.
213:453–456. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong L, Song J, Lin X, Wei F, Zhang C,
Wang Z, Zhu J, Wu S, Chen Y, Liang J, et al: Serine-arginine
protein kinase 1 promotes a cancer stem cell-like phenotype through
activation of Wnt/β-catenin signalling in NSCLC. J Pathol.
240:184–196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Y, Liu L, Cai J, Wu J, Guan H, Zhu X,
Yuan J and Li M: DEPDC1B enhances migration and invasion of
non-small cell lung cancer cells via activating Wnt/β-catenin
signaling. Biochem Biophys Res Commun. 450:899–905. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie C, Jiang G, Fan C, Zhang X, Zhang Y,
Miao Y, Lin X, Wu J, Wang L, Liu Y, et al: ARMC8α promotes
proliferation and invasion of non-small cell lung cancer cells by
activating the canonical Wnt signaling pathway. Tumour Biol.
35:8903–8911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang B, Li N and Zhang H: Knockdown of
homeobox B5 (HOXB5) inhibits cell proliferation, migration, and
invasion in non-small cell lung cancer cells through inactivation
of the Wnt/β-catenin pathway. Oncol Res. 26:37–44. 2018. View Article : Google Scholar : PubMed/NCBI
|