Introduction
Osteosarcoma (OS) is a common malignant bone tumor
occurring in children or teenagers younger than 20 years (1). More specifically, OS usually occurs in
children with bone malignancy, accounting for almost 5% of all
pediatric tumors (2). Moreover,
incidence of OS in primary malignant tumor ranks first, showing a
very high tumor malignant degree and poor prognosis of OS patients
(3). In addition, OS can spread to
the lungs within a few months, resulting in a 3- to 5-year survival
rate of only 5–20% after amputation (4). Thus, finding effective biomarkers for
the diagnosis and therapy of OS is required.
MicroRNAs (miRNAs) have been reported to be
unusually upregulated or downregulated in various human cancers.
For instance, miR-21 was upregulated in colorectal cancer (5), while miR-126 was downregulated in lung
cancer (6). Moreover, the
dysregulated expression of miRNAs was also closely-associated with
the pathogenesis and development of cancers (7). In OS, downregulation of miR-375,
miR-302b and miR-216a was detected in previous studies (8–10).
Furthermore, the upregulation of miR-19, miR-92b and miR-146a was
found in OS (11–13). Therefore, miRNAs participated in the
tumorigenesis of OS, as indicated in previous studies.
Specifically, the decrease of miR-202 expression was identified in
gastric cancer (14), colorectal
carcinoma (15) and endometrial
adenocarcinoma (16). Those studies
indicated that miR-202 also affects the progression of OS. However,
the specific function of miR-202 in OS has yet to be analysed.
Rho-associated coiled-coil containing protein kinase
1 (ROCK1) is considered a direct target gene of many miRNAs in
various kinds of human cancers, such as miR-124 (17), miR-144 (18) and miR-148a (19). Moreover, the carcinogenesis of ROCK1
was identified in glioma (20),
breast cancer (21) and non-small
cell lung cancer (22). In OS, ROCK1
was employed as a potential therapeutic target (23). However, the relationship between ROCK1
and miR-202 in OS still remains unclear.
In the present study, we aimed to investigate the
specific effect of miR-202-5p in OS as well as the interaction
between miR-202-5p and ROCK1. Finally, we examined whether
miR-202-5p weakened the abilities of cell migration and invasion in
OS by inhibiting ROCK1 expression.
Materials and methods
Clinical tissues
Thirty-six surgical tumor specimens and adjacent
tissue samples were obtained from the People's Hospital of Rizhao
(Rizhao, China) after receiving written informed consent. The
patients received no treatment prior to surgery. Human tissue was
frozen in liquid nitrogen and then stored in a refrigerator at
−80°C for further experiments. This experiment was approved by the
Institutional Ethics Committee of People's Hospital of Rizhao.
Cell cultures and cell
transfection
The human OC cell lines U2OS, MG-63, HOS and human
normal osteoblast cell line hFOB1.19 were used for this experiment.
All the cell lines came from the American Type Culture Collection
(ATCC, Manassas, VA, USA). These cells were seeded in DMEM or
RPMI-1640 medium containing 10% fetal bovine serum (FBS) and
cultured at 37°C with 5% CO2.
The miR-202-5p mimic and inhibitor, ROCK1 siRNA
(si-ROCK1) were obtained from GenePharma Co., Ltd. (Shanghai,
China). Then they were transferred into HOS cells using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) based on the manufacturer's protocols.
RT-qPCR
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was applied to extract total RNA containing miRNA
to quantify miR-202-5p expression in OS tissues and cell lines.
RT-qPCR was carried out using the SYBR-Green Master Mix (Toyobo
Co., Ltd., Osaka, Japan) on Applied Biosystems 7500 Sequence
detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). U6 and GAPDH were used as the control for miR-202-5p and
ROCK1. Expression was calculated using the 2−ΔΔCq
method.
Dual luciferase reporter assay
293T cells were incubated in 24-well plates for the
Dual-Luciferase Reporter Assay System (Promega Corporation,
Madison, WI, USA). The wild or mutant type of 3′-untranslated
region (3′-UTR) of ROCK1 was inserted into the pGL3 promoter vector
(Invitrogen; Thermo Fisher Scientific, Inc.) for luciferase
reporter experiments. Then, wild or mutant type of 3′-UTR of ROCK1
and miR-202-5p mimic was transfected into 293T cells. Subsequently,
the Dual Luciferase Reporter Assay System (Promega Corporation) was
applied to measure luciferase activities.
Transwell assay
The Transwell chamber (24-well) was employed to
perform cell migration and invasion assays. HOS cells
(5×104) without serum were placed in the upper chamber
on the non-coated membrane, and the lower chamber was filled with
10% FBS to induce HOS cells to migrate or invade through the
membrane. In addition, the cells were placed in the upper chamber
with the matrigel (BD Biosciences, Franklin Lakes, NJ, USA) for the
invasion assay. Then, the cells were incubated for the migration
and invasion assay. Finally, the cells were stained with crystal
violet. The number of cells was counted using an inverted light
microscope (Zeiss, Oberkochen, Germany).
Western blot analysis
The protein samples were obtained using RIPA lysis
buffer. Proteins were separated through a 10% SDS-PAGE and
incubated with 5% non-fat milk in PVDF membranes at room
temperature. Next, we incubated the membranes overnight at 4°C with
mouse anti-ROCK1 (1:1,000, rabbit polyclonal antibody, ab97592),
anti-GAPDH (1:1,000, rabbit polyclonal antibody, ab9485), which
were subsequently incubated with goat anti-mouse secondary
antibodies. Then, goat anti-rabbit IgG H&L secondary antibodies
(1:1,000, goat polyclonal second antibody, ab150077) protein levels
were measured using enhanced chemiluminescence (ECL; Pierce; Thermo
Fisher Scientific, Inc.).
Statistical analysis
The obtained data were shown as the mean ± SD. The
data were analyzed with GraphPad Prism 6.0. (GraphPad Software
Inc., La Jolla, CA, USA). The difference was calculated according
to the Chi-square or ANOVA followed by a Tukeys test. P<0.05 was
considered statistically significant.
Results
Low expression of miR-202-5p is
identified in OS tissues and cell lines
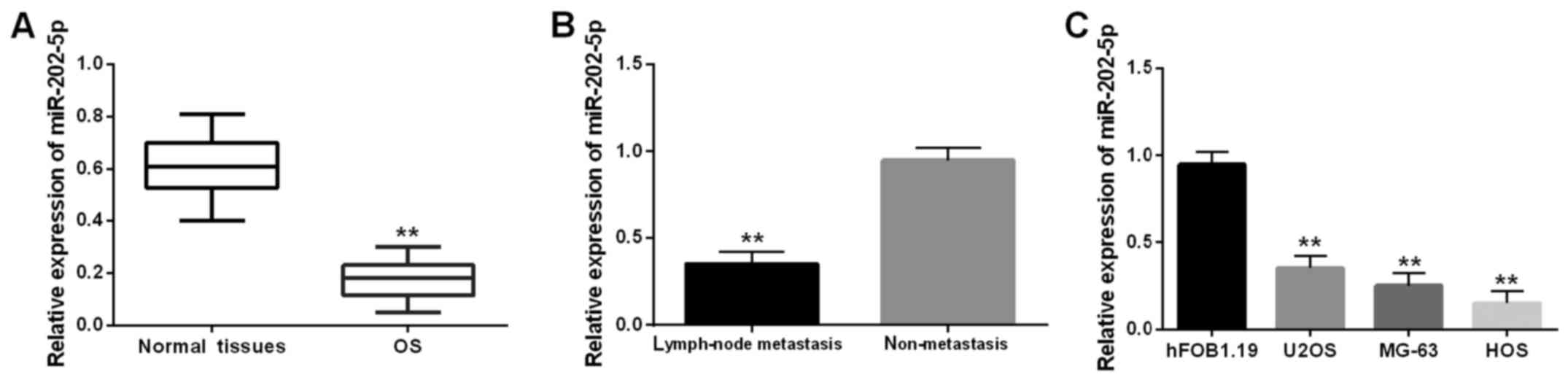
The miR-202-5p level was detected in OS tissues via
RT-qPCR (Fig. 1A). The lower
expression of miR-202-5p was identified in OS tissues in comparison
with the normal tissues. Moreover, downregulation of miR-202-5p was
found in OS that had lymph-node metastasis (Fig. 1B). It indicated that the aberrant
expression was related to lymph-node metastasis. Similarly,
miR-202-5p downregulation was also assessed in U2OS, MG-63 and HOS
cell lines, except for the human osteoblast cell line hFOB1.19
(Fig. 1C). In brief, downregulation
of miR-202-5p may play a vital role in the metastasis of OS.
Overexpression of miR-202-5p exerts an
inhibitory effect on cell migration and invasion in OS
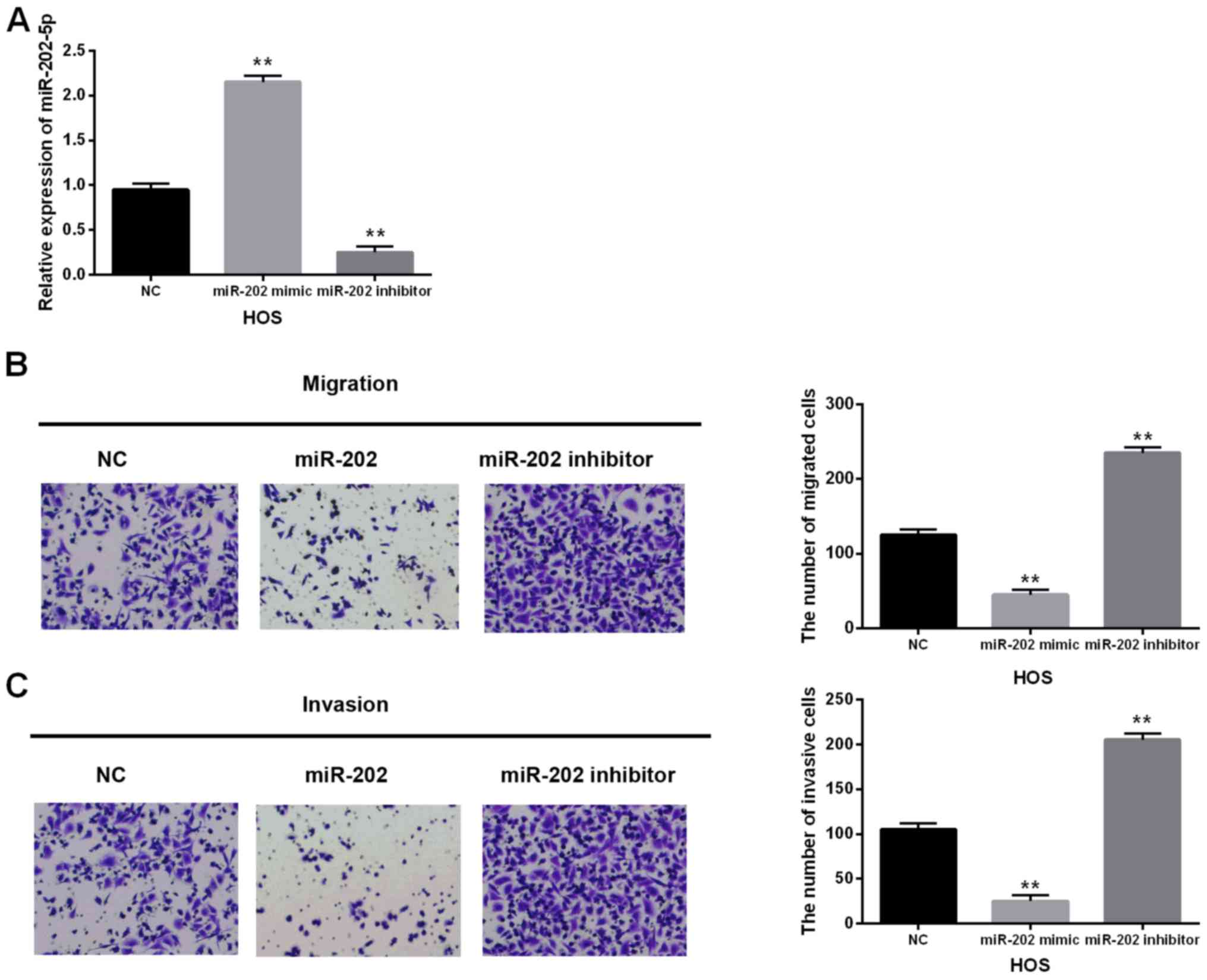
miR-202-5p mimic or inhibitor was transfected into
HOS cells to investigate its function in OS. The miR-202-5p levels
were measured in these transfected cells using RT-qPCR (Fig. 2A). Then the migrating and invasive
abilities in these transfected cells were detected by the Transwell
assay. As expected, the migrating and invasive abilities were
reduced by miR-202-5p mimics but enhanced by miR-202-5p inhibitor
in OS cells (Fig. 2B and C). These
findings showed that miR-202-5p as a suppressive miRNA exerts an
inhibitory effect on cell migration and invasion in OS.
miR-202-5p directly targets ROCK1 and
negatively regulates its expression
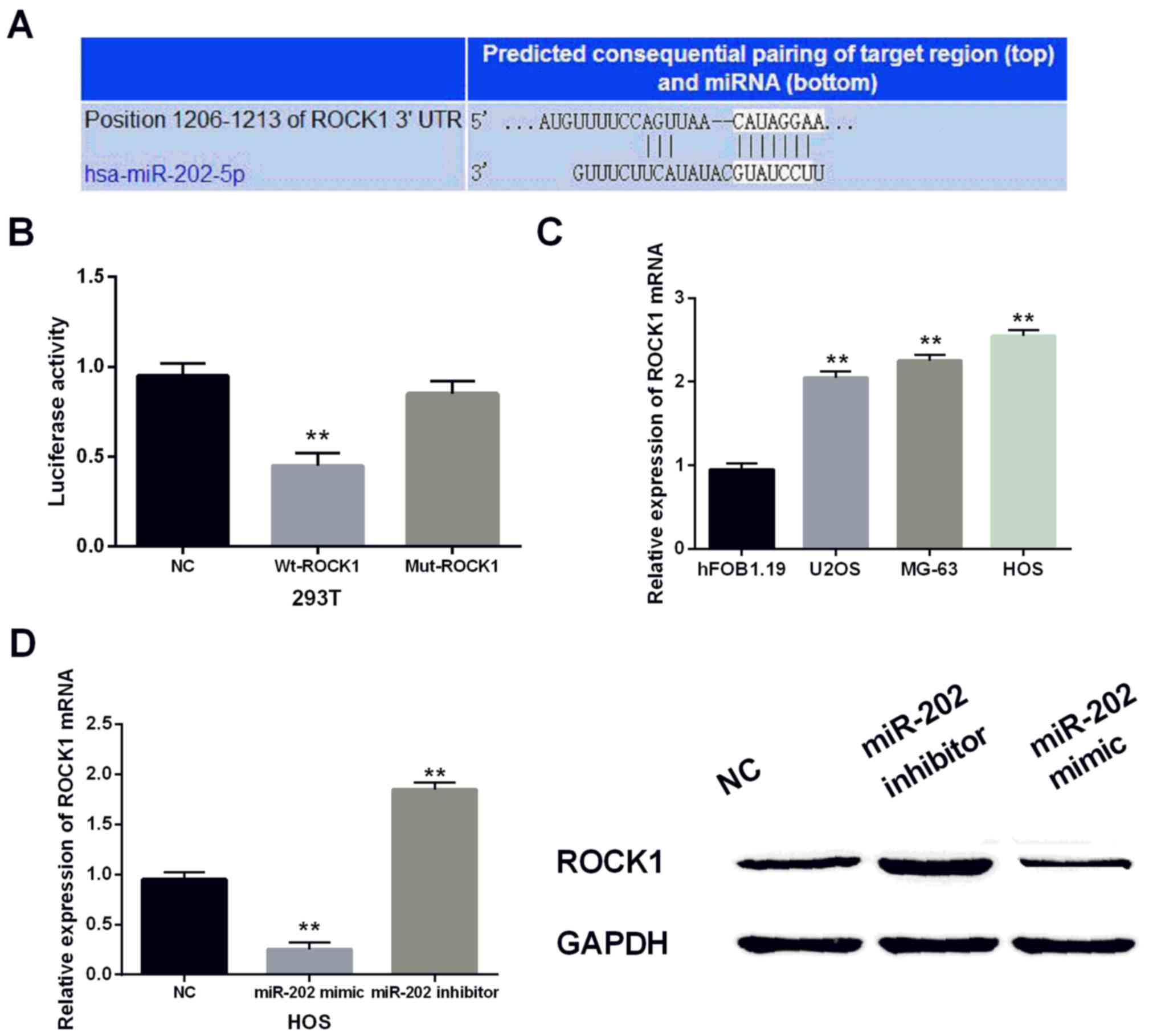
Then we performed luciferase reporter assays to
confirm the prediction that miR-202-5p binds to the 3′-UTR of ROCK1
(Fig. 3A). As predicted,
co-transfection of wild-type of ROCK1 and miR-202-5p mimic in 293T
cells reduced the luciferase activity and no change was found in
cells containing the mutant type of ROCK1 and miR-202-5p mimic
compared to the negative control (Fig.
3B). In addition, the ROCK1 levels in U2OS, MG-63, HOS and
hFOB1.19 cell lines were identified via RT-qPCR. Upregulation of
ROCK1 was found in U2OS, MG-63, and HOS cells apart from hFOB1.19
(Fig. 3C). Furthermore, we observed
the ROCK1 level in cells with miR-202-5p mimic or inhibitor to
further explore their relationship. We found that ROCK1 level was
declined by the miR-202-5p mimic and enhanced by the miR-202-5p
inhibitor by RT-qPCR and western blot analysis (Fig. 3D). Collectively, miR-202-5p directly
targeted ROCK1 and negatively regulated its expression.
ROCK1 has a carcinogenic effect on OS
cells
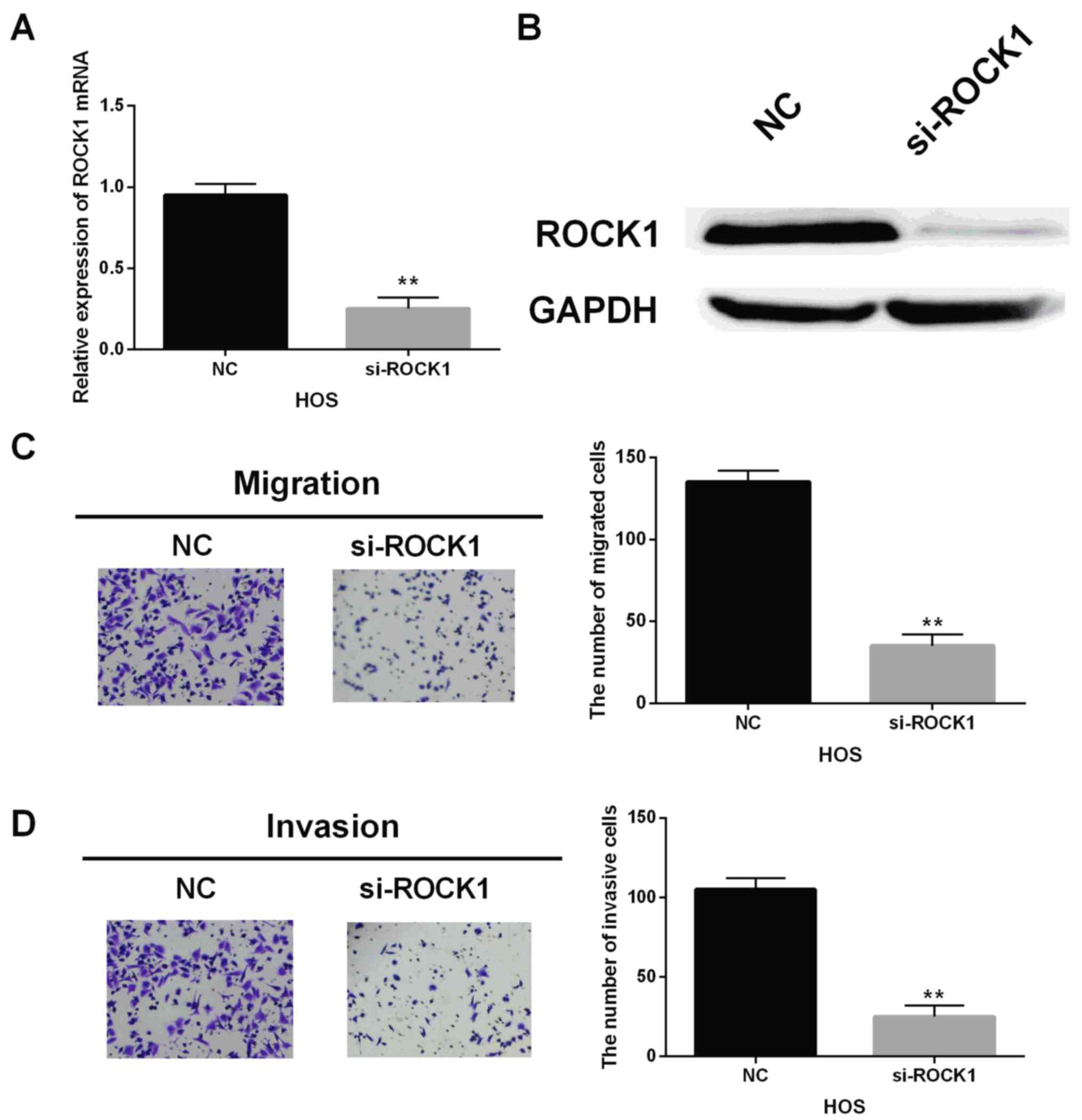
Next, ROCK1 siRNA was transfected into HOS cells to
block its expression. Moreover, the mRNA and protein level of ROCK1
were declined by ROCK1 siRNA in HOS cells (Fig. 4A and B). Similarly, the ROCK1 siRNA
also impaired the migrating and invasive abilities in HOS cells,
which was the same as the inhibitory action of miR-202-5p
overexpression (Fig. 4C and D). In
brief, ROCK1 had a carcinogenic effect on OS.
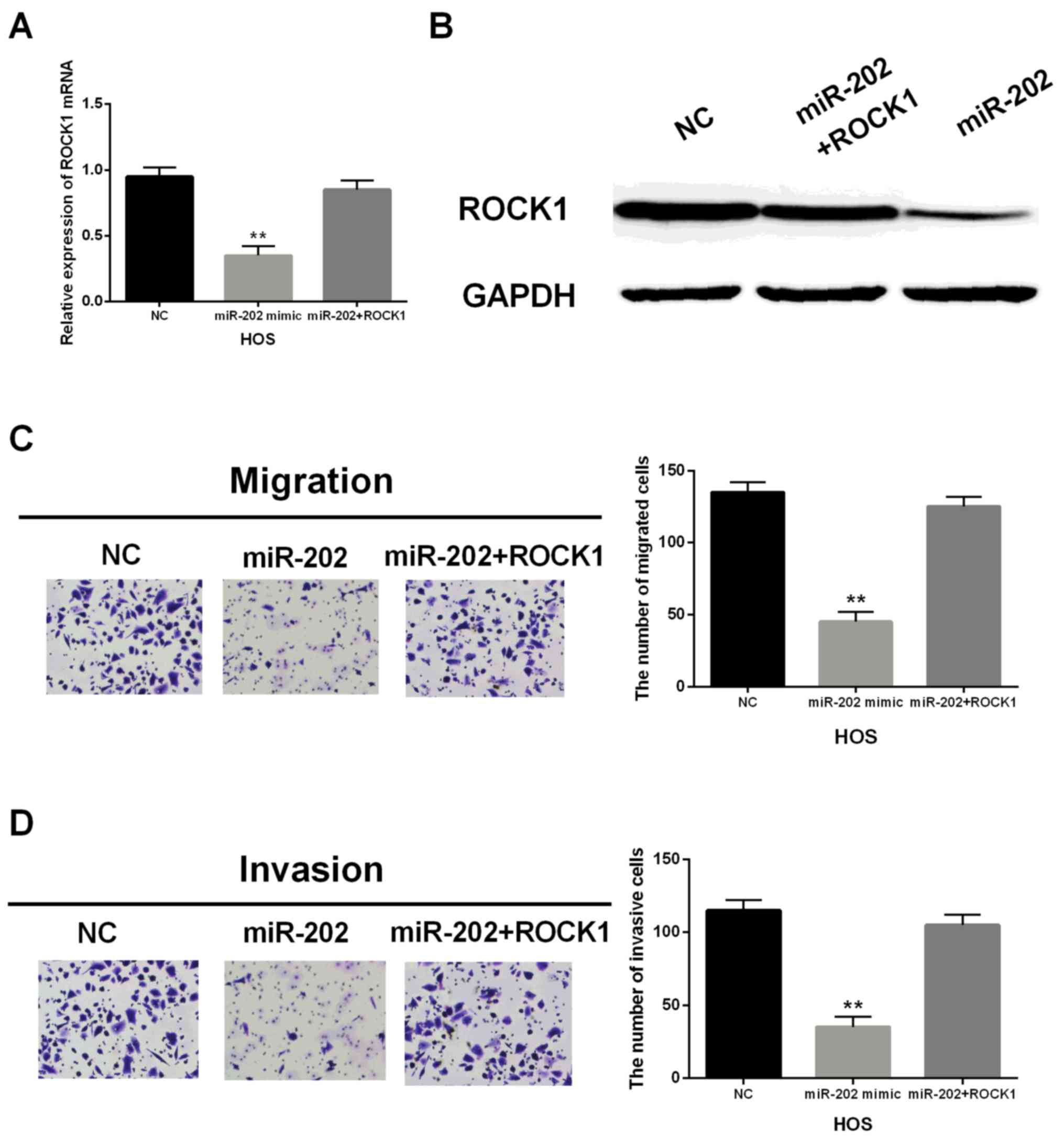
Upregulation of ROCK1 restores the
suppressive effect of miR-202-5p in OS
miR-202-5p mimic and ROCK1 vector were transfected
into HOS cells to further investigate their suppressive function.
Overexpression of ROCK1 recovered the decrease of ROCK1 mRNA and
protein levels induced by miR-202-5p mimic (Fig. 5A and B). Functionally, the abilities
of migration and invasion in transfected HOS cells containing
miR-202-5p mimic and ROCK1 vector were regained in comparison with
the cells only miR-202-5p mimic (Fig. 5C
and D). Generally speaking, upregulation of ROCK1 restored the
suppressive effect of miR-202-5p in OS cells, which further
indicated that miR-202-5p repressed cell migration and invasion in
OS by regulating ROCK1.
Discussion
Previous studies have demonstrated that
identification of miRNAs can be used as a biomarker for the
diagnosis and prognosis of OS (24).
In our study, downregulation of miR-202-5p and the upregulation of
ROCK1 were identified in OS. Overexpression of miR-202-5p impaired
the migrating and invasive abilities in OS, which was similar to
the knockout of ROCK1. Furthermore, the upregulation of ROCK1
restored the inhibitory effect of miR-202-5p in OS.
Many studies have shown that miR-202 was usually
downregulated and participated in the formation of many human
cancers. For instance, the miR-202 level was reduced and suppressed
tumor progression in esophageal squamous cell carcinoma (25). In hepatocellular carcinoma, the
miR-202 overexpression suppressed cell proliferation (26). Additionally, miR-202 was found to
function as a suppressive miRNA in non-small cell lung cancer
(27). The miR-202 downregulation was
frequently identified in human cancers which was in agreement with
our results in OS. More importantly, miR-202 was reported to be
significantly declined. Moreover, it repressed cell growth and
promoted cell apoptosis in OS (28).
This study also confirmed our findings of miR-202 in OS. However,
to the best of our knowledge, there was no study about the role of
miR-202 for migration and invasion in OS cells. In addition, we
demonstrated that the miR-202-5p overexpression had an inhibitory
effect on cell migration and invasion in OS. Therefore, miR-202 may
be used as an indicator for the diagnosis and prediction of OS,
which was helpful in the treatment of OS patients.
To further explore the function of miR-202, ROCK1
was verified as a direct target of miR-202 in OS. To date, ROCK1 as
a direct target gene has been reported to bind to miR-300 (29), miR-340 (30), miR-584 (31) and miR-1280 (32). However, the relationship between
miR-202 and ROCK1 has not been analysed thus far. In the present
study, upregulation and carcinogenic effects of ROCK1 were observed
in OS. The same findings of ROCK1 were also reported in OS induced
by miR-145 (33), miR-198 (34) and miR-335 (35). Findings of those studies were in
agreement with our results. In addition, miR-202-5p was negatively
associated with ROCK1 expression. Upregulation of ROCK1 restored
the suppressive effect of miR-202-5p in OS. Collectively,
miR-202-5p impaired the migrating and invasive abilities in OS
partly by inhibiting ROCK1. Therefore, understanding the role of
miR-202 is significant for the treatment of OS.
In conclusion, downregulation of miR-202-5p and
upregulation of ROCK1 were found in OS. miR-202-5p was verified to
directly target ROCK1. More importantly, miR-202-5p was identified
to inhibit the migrating and invasive abilities in OS cells by
inhibiting ROCK1.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL as the first author contributed significantly in
the analysis of and wrote the manuscript. DM as the second author
performed the data analyses and wrote the manuscript. XL as the
fourth author helped perform the analysis with constructive
discussions. BC as the fifth author sorted out experimental data.
JY as the corresponding author contributed to the conception of the
study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the People's Hospital of Rizhao (Rizhao, China). Signed written
informed consents were obtained from the patients and/or
guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Allison DC, Carney SC, Ahlmann ER,
Hendifar A, Chawla S, Fedenko A, Angeles C and Menendez LR: A
meta-analysis of osteosarcoma outcomes in the modern medical era.
Sarcoma. 2012:7048722012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JA, Kim DH, Lim JS, Park KD, Song WS,
Lee SY and Jeon DG: The survival of osteosarcoma patients 10 years
old or younger is not worse than the survival of older patients: A
retrospective analysis. Cancer Res Treat. 39:160–164. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu B, Peng XC, Zheng XL, Wang J and Qin
YW: MiR-126 restoration down-regulate VEGF and inhibit the growth
of lung cancer cell lines in vitro and in vivo. Lung Cancer.
66:169–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu W, Zhao X, Zhang YJ, Fang GW and Xue
Y: MicroRNA-375 as a potential serum biomarker for the diagnosis,
prognosis, and chemosensitivity prediction of osteosarcoma. J Int
Med Res. 46:975–983. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie Y, Sun W, Deng Z, Zhu X, Hu C and Cai
L: miR-302b suppresses osteosarcoma cell migration and invasion by
targeting Runx2. Sci Rep. 7:133882017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ji Q, Xu X, Li L, Goodman SB, Bi W, Xu M,
Xu Y, Fan Z, Maloney WJ, Ye Q, et al: miR-216a inhibits
osteosarcoma cell proliferation, invasion and metastasis by
targeting CDK14. Cell Death Dis. 8:e31032017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun Z, Liu Q, Hong H, Zhang H and Zhang T:
miR-19 promotes osteosarcoma progression by targeting SOCS6.
Biochem Biophys Res Commun. 495:1363–1369. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu Q, Zhou W, Feng Q, Liu X, Xiong Y and
Li H: MicroRNA-92b promotes cell proliferation and invasion in
osteosarcoma by directly targeting Dickkopf-related protein 3. Exp
Ther Med. 15:173–181. 2018.PubMed/NCBI
|
|
13
|
Zhou C, Jiang CQ, Zong Z, Lin JC and Lao
LF: miR-146a promotes growth of osteosarcoma cells by targeting
ZNRF3/GSK-3β/β-catenin signaling pathway. Oncotarget.
8:74276–74286. 2017.PubMed/NCBI
|
|
14
|
Zhao Y, Li C, Wang M, Su L, Qu Y, Li J, Yu
B, Yan M, Yu Y, Liu B, et al: Decrease of miR-202-3p expression, a
novel tumor suppressor, in gastric cancer. PLoS One. 8:e697562013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Q, Huang Z, Guo W, Ni S, Xiao X, Wang
L, Huang D, Tan C, Xu Q, Zha R, et al: microRNA-202-3p inhibits
cell proliferation by targeting ADP-ribosylation factor-like 5A in
human colorectal carcinoma. Clin Cancer Res. 20:1146–1157. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng X, Hou C, Liang Z, Wang H, Zhu L and
Xu H: miR-202 suppresses cell proliferation by targeting FOXR2 in
endometrial adenocarcinoma. Dis Markers. 2017:28274352017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu X, Li S, Lin Y, Chen H, Hu Z, Mao Y, Xu
X, Wu J, Zhu Y, Zheng X, et al: MicroRNA-124-3p inhibits cell
migration and invasion in bladder cancer cells by targeting ROCK1.
J Transl Med. 11:2762013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai SD, Chen JS, Xi ZW, Zhang LJ, Niu ML
and Gao ZY: MicroRNA 144 inhibits migration and proliferation in
rectal cancer by downregulating ROCK 1. Mol Med Rep. 12:7396–7402.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng B, Liang L, Wang C, Huang S, Cao X,
Zha R, Liu L, Jia D, Tian Q, Wu J, et al: MicroRNA-148a suppresses
tumor cell invasion and metastasis by downregulating ROCK1 in
gastric cancer. Clin Cancer Res. 17:7574–7583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
An L, Liu Y, Wu A and Guan Y: microRNA-124
inhibits migration and invasion by down-regulating ROCK1 in glioma.
PLoS One. 8:e694782013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Song Y, Wang Y, Luo J and Yu W:
MicroRNA-148a suppresses epithelial-to-mesenchymal transition by
targeting ROCK1 in non-small cell lung cancer cells. Mol Cell
Biochem. 380:277–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng M, Sun X, Li Y and Zuo W:
MicroRNA-145 inhibits growth and migration of breast cancer cells
through targeting oncoprotein ROCK1. Tumour Biol. 37:8189–8196.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu X, Choy E, Hornicek FJ, Yang S, Yang
C, Harmon D, Mankin H and Duan Z: ROCK1 as a potential therapeutic
target in osteosarcoma. J Orthop Res. 29:1259–1266. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cong C, Wang W, Tian J, Gao T, Zheng W and
Zhou C: Identification of serum miR-124 as a biomarker for
diagnosis and prognosis in osteosarcoma. Cancer Biomark.
21:449–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meng X, Chen X, Lu P, Ma W, Yue D, Song L
and Fan Q: MicroRNA-202 inhibits tumor progression by targeting
LAMA1 in esophageal squamous cell carcinoma. Biochem Biophys Res
Commun. 473:821–827. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Zheng D, Xiong Y, Xue C, Chen G,
Yan B and Ye Q: miR-202 suppresses cell proliferation in human
hepatocellular carcinoma by downregulating LRP6
post-transcriptionally. FEBS Lett. 588:1913–1920. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao Z, Lv B, Zhang L, Zhao N and Lv Y:
miR-202 functions as a tumor suppressor in non-small cell lung
cancer by targeting STAT3. Mol Med Rep. 16:2281–2289. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun Z, Zhang T, Hong H, Liu Q and Zhang H:
miR-202 suppresses proliferation and induces apoptosis of
osteosarcoma cells by downregulating Gli2. Mol Cell Biochem.
397:277–283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou F, Li Y, Hao Z, Liu X, Chen L, Cao Y,
Liang Z, Yuan F, Liu J, Wang J, et al: MicroRNA-300 inhibited
glioblastoma progression through ROCK1. Oncotarget. 7:36529–36538.
2016.PubMed/NCBI
|
|
30
|
Maskey N, Li D, Xu H, Song H, Wu C, Hua K,
Song J and Fang L: MicroRNA-340 inhibits invasion and metastasis by
downregulating ROCK1 in breast cancer cells. Oncol Lett.
14:2261–2267. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xue H, Guo X, Han X, Yan S, Zhang J, Xu S,
Li T, Guo X, Zhang P, Gao X, et al: MicroRNA-584-3p, a novel tumor
suppressor and prognostic marker, reduces the migration and
invasion of human glioma cells by targeting hypoxia-induced ROCK1.
Oncotarget. 7:4785–4805. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Majid S, Dar AA, Saini S, Shahryari V,
Arora S, Zaman MS, Chang I, Yamamura S, Chiyomaru T, Fukuhara S, et
al: MicroRNA-1280 inhibits invasion and metastasis by targeting
ROCK1 in bladder cancer. PLoS One. 7:e467432012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lei P, Xie J, Wang L, Yang X, Dai Z and Hu
Y: microRNA-145 inhibits osteosarcoma cell proliferation and
invasion by targeting ROCK1. Mol Med Rep. 10:155–160. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang S, Zhao Y and Wang L: MicroRNA-198
inhibited tumorous behaviors of human osteosarcoma through directly
targeting ROCK1. Biochem Biophys Res Commun. 472:557–565. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Y, Wang N, Zeng X, Sun J, Wang G, Xu
H and Zhao W: MicroRNA-335 and its target Rock1 synergistically
influence tumor progression and prognosis in osteosarcoma. Oncol
Lett. 13:3057–3065. 2017. View Article : Google Scholar : PubMed/NCBI
|