Introduction
Giant-cell tumor of the bone (GCT) is a common
primary bone tumor. According to epidemiological surveys, GCT
accounts for 4–5% of all primary bone tumors and occurs more
frequently in women, and the incidence rate is higher in China than
that in western countries. The lesion is common in the epiphysis,
and the larger lesion can spread to the metaphysis, even the
backbone (1–3). With the development of medical technology, the
detection and treatment rates of GCT have been significantly
increased, but its onset is slow and there are no obvious
clinicopathological features in the early stage; thus, although 80%
of GCT tumors are benign, approximately 20% can still spread to the
lung tissues and other parts, causing deterioration. The preferred
treatment method of GCT is surgery (4–6). Matrix metalloproteinase
(MMP) is a proteolytic enzyme family capable of degrading basement
membrane (BM) and extracellular matrix (ECM). MMP-2 is one of the
important members in the MMP family and is the main enzyme of
degrading type IV collagen. Tumor cells degrade the BM and ECM
under the blood capillary endodermis through the secretion of MMPs,
making the endothelial cells migrate and proliferate outwarda,
ultimately forming new blood vessels and BM (7,8). Tissue
inhibitor of metalloproteinase-3 (TIMP-3) is a widely-distributed
endogenous MMP inhibitor and a new member in the TIMP family, as
well as an MMP-specific full-function inhibitor, which has a
certain inhibitory effect on tumor growth through inhibition of
MMPs (9). However, there is no
previous report on the correlation of the expression levels of
MMP-2 and TIMP-3 with the incidence and prognosis of GCT.
In the present study, the expression levels of MMP-2
and MMP-3 in GCT patients were detected and the correlation of
expression levels of MMP-2 and TIMP-3 with clinicopathological
features and prognosis of GCT was investigated, to determine the
relationship between the occurrence and development of GCT and the
expression levels of MMP-2 and TIMP-3.
Materials and methods
Objects
The 70 samples in the present study were paraffin
samples obtained from the surgical resection of patients treated
and diagnosed as GCT via pathological examination in Dezhou
Hospital (Shandong, China) from September, 2013 to September, 2015.
These patients were aged 38–87 years, including 32 males and 38
females. Other consumptive diseases were eliminated from all the
patients enrolled, and patients signed the informed consent. The
patients had complete clinical and pathological data. At the same
time, the para-carcinoma tissues (>3 cm away from the cancer
tissue) of the above patients were collected as the control group.
Patients enrolled had a 1 year follow-up record and the complete
treatment regimen. The tumor and para-carcinoma tissue samples were
stored in liquid nitrogen at at −196°C.
Instruments and materials
MMP-2 monoclonal antibody, TIMP-3 and GAPDH
monoclonal antibody (cat. nos. 04-1048, AB6000 and MABS819 all from
EMD Millipore, Billerica, MA, USA), TRIzol, immunohistochemistry
and reverse transcription kit (all from Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), inverted fluorescence
microscope (Thermo Fisher Scientific, Inc.), sheep anti-rabbit IgG
secondary antibody (cat. no. AP510, EMD Millipore, Billerica, MA,
USA), pipettor (Eppendorf, Hamburg, Germany), polymerase chain
reaction (PCR) instrument (ABI USA, Vernon, CA, USA), and an
ultraviolet imaging system (Biometra GmbH, Göttingen, Germany) were
used in the present study. The origin and batch number of other
relevant instruments and materials are specified in corresponding
parts.
Detection of expression levels of
MMP-2 and TIMP-3 in cancer tissues of GCT patients via
semi-quantitative PCR
The total RNA was extracted from tissues using the
TRIzol kit according to the manufacturer's instructions after the
tumor and para-carcinoma tissue samples were obtained from GCT
patients and thawed, and the RNA integrity was confirmed via
agarose gel electrophoresis. The electrophoresis results showed
that the 28S, 18S and 5S bands were clear, and the brightness of
the 28S band was about twice that of 18S, indicating that the RNA
was intact and could be used for subsequent experiments. After cDNA
was obtained via reverse transcription using the reverse
transcription kit, the expression of MMP-2 and TIMP-3 in tumor and
para-carcinoma tissues were detected via semi-quantitative PCR with
GAPDH as the internal reference. The reaction conditions
were: 95°C for 30 sec, 64°C for 25 sec and 72°C for 30 sec, a total
of 35 cycles. Primers were produced by Tiangen Biotech Co., Ltd.
(Beijing, China). The sequences are shown in Table I. After the reaction, agarose gel
electrophoresis was performed, followed by observation via the
ultraviolet imaging system.
 | Table I.PCR primers. |
Table I.
PCR primers.
| Gene name | Sequence |
|---|
| MMP-2 | F:
5′-ATCCACCTTGACGATGCTTTAC-3′ |
|
| R:
5′-TTCAGATGTTCTAAGCCTACGG-3′ |
| TIMP-3 | F:
5′-TGGCCCTCGTAGCCTTGAGGAC-3′ |
|
| R:
5′-CCAGTGCTGCAGGGTCCGAGGT-3′ |
| GAPDH | F:
5′-GATGATTGGCATGGCTTT-3′ |
|
| R:
5′-CACCTTCCGTTCCAGTTT-3′ |
Detection of the expression levels of
MPP-2 and TIMP-3 via western blot analysis
The tumor and para-carcinoma tissue samples of GCT
patients were removed from the liquid nitrogen and cut into pieces
with the scissors. The tissues were homogenized by adding the lysis
buffer (1:20). After centrifugation at 8,000 × g for 15 min, the
supernatant was completely transferred and the total protein was
obtained. The protein loading sample was then prepared into the
loading system at the same concentration via protein
quantification. After SDS-PAGE and membrane transfer, the protein
was washed and sealed, and the target band was cut. Then rabbit
anti-human MPP-2, TIMP-3, GAPDH monoclonal primary antibodies
(1:500) was incubated overnight at 4°C and washed with
Tris-buffered saline with Tween 20 (TBST) three times, and then the
secondary antibody (1:1,000) was incubated at room temperature for
2 h. After being washed with TBST three times, the target protein
band was obtained via color development, the exposure was scanned
and the results were analyzed with GAPDH as the internal reference.
The expression levels of MMP-2 and TIMP-3 in GCT were detected.
Detection of expression levels of
MMP-2 and TIMP-3 via immunohistochemistry
The paraffin sections of tumor tissue and
para-carcinoma tissue samples of GCT patients were soaked in
absolute ethyl alcohol, 95% ethanol, 75% ethanol and distilled
water for 10 min, respectively, washed with phosphate-buffered
saline (PBS) twice (5 min/time), soaked in 3%
H2O2 at room temperature for 10 min and then
washed with PBS for 5 min. The blocking solution was added dropwise
to seal the section for 30 min, and the excess solution was shaken
off before MPP-2 and TIMP-3 primary antibodies were added for
incubation at 4°C overnight. Then the section was washed with PBS
three times (5 min/time), and the secondary antibody was added for
incubation at room temperature for 1 h. After the section was
washed with PBS three times, the horseradish peroxidase was added
for labeling for 30 min, followed by color development via
diaminobenzidine (DAB) for 5 min, and the section was monitored
under the microscope. After the reaction was terminated using
distilled water for 30 min, hematoxylin was added for re-staining
for 6–9 sec, followed by dehydration, transparency, sealing and
microscopic examination. Yellow staining in cytoplasm and
surrounding mesenchyme indicated a positive expression of MMP-2,
while brown yellow staining in nucleus indicated a positive
expression of TIMP-3.
Follow-up
The patients were followed-up during hospitalization
and by telephone according to their medical records. The disease
progression and survival time of patients were recorded. The
patients were followed-up for 1 year, and the detailed follow-up
records, examination and hospitalization records were kept.
Statistical analysis
Data in this study are presented as mean ± standard
deviation. SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA) was
used for data processing. The t-test was used for measurement data,
the Chi-square test was used for the intergroup analysis of
enumeration data, and one-way analysis of variance (ANOVA) was used
for other data. According to the homogeneity test of variance, if
the variance was homogeneous, the Bonferroni method was used for
pairwise comparison; otherwise, the Welch method was used for
analysis. Dunnett's T3 method was used for multiple
comparisons.
Results
Expression of MMP-2 and TIMP-3 in
GCT
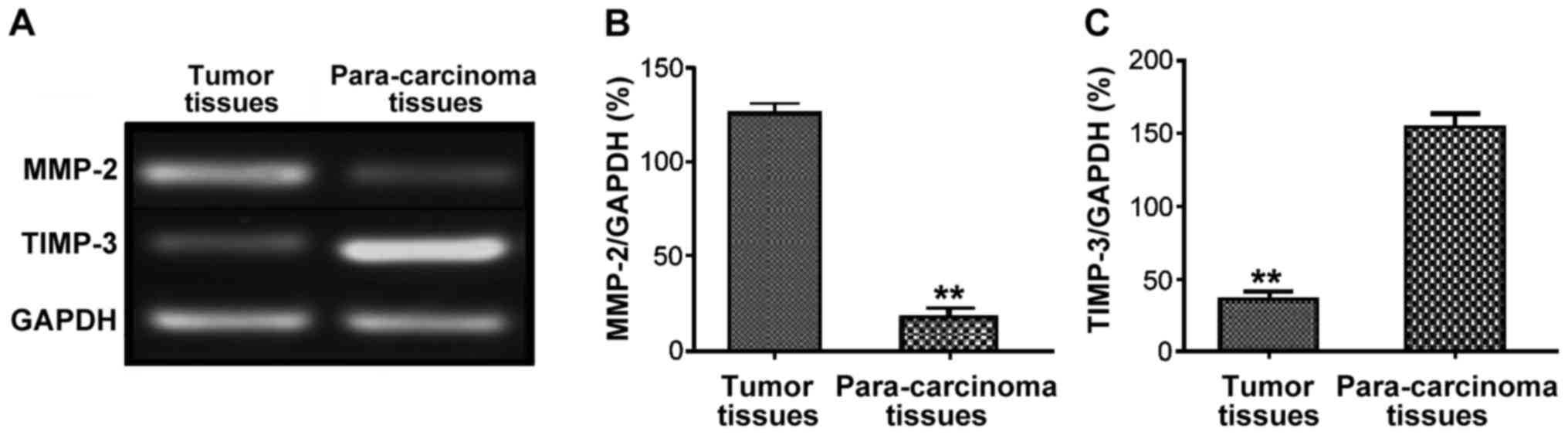
A total of 70 tumor and 70 para-carcinoma tissue
samples were taken from GCT patients, and the expression levels of
MMP-2 and TIMP-3 were detected via semi-quantitative PCR. The
results showed that the relative expression level of MMP-2 in tumor
tissues in GCT patients was significantly higher than that in
para-carcinoma tissues (P<0.01), while the relative expression
level of TIMP-3 was significantly lower than that in para-cancerous
tissues, and the difference was statistically significant
(P<0.01) (Fig. 1).
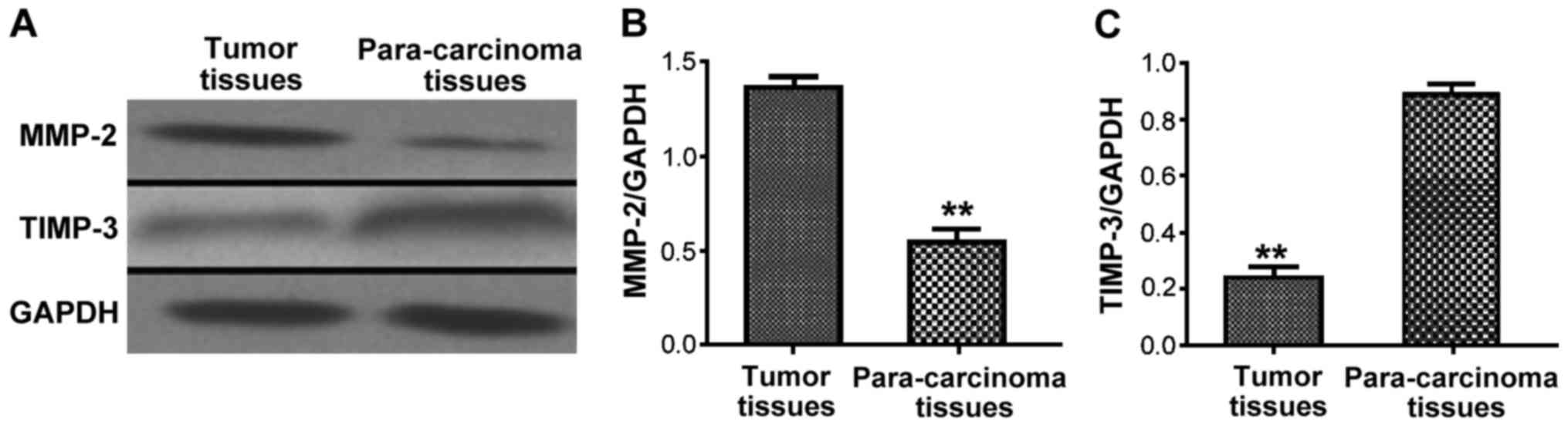
Detection of protein expression of
MMP-2 and TIMP-3 in GCT by western blot analysis
The protein expression levels of MMP-2 and TIMP-3 in
tumor and para-carcinoma tissue samples of GCT patients were
detected via western blot analysis. The results showed that the
protein expression level of MMP-2 in tumor tissues in GCT patients
was obviously higher than that in para-carcinoma tissues
(P<0.01), while the protein expression level of TIMP-3 was
obviously lower than that in para-cancerous tissues, and the
difference was statistically significant (P<0.01) (Fig. 2).
Correlation of MMP-2 and TIMP-3
expression with clinicopathological features and prognosis of
patients
The relative expression levels of MMP-2 and TIMP-3
in tumor tissue and para-carcinoma tissue samples of GCT patients
were detected by semi-quantitative PCR, and the clinical features
of GCT patients, such as age, tumor size and clinical staging, were
analyzed. The results revealed that the relative expression levels
of MMP-2 and TIMP-3 in the tumor tissues of GCT patients were not
correlated with the age and sex of patients (P>0.05), but
closely related to the tumor diameter, clinical staging, lymphatic
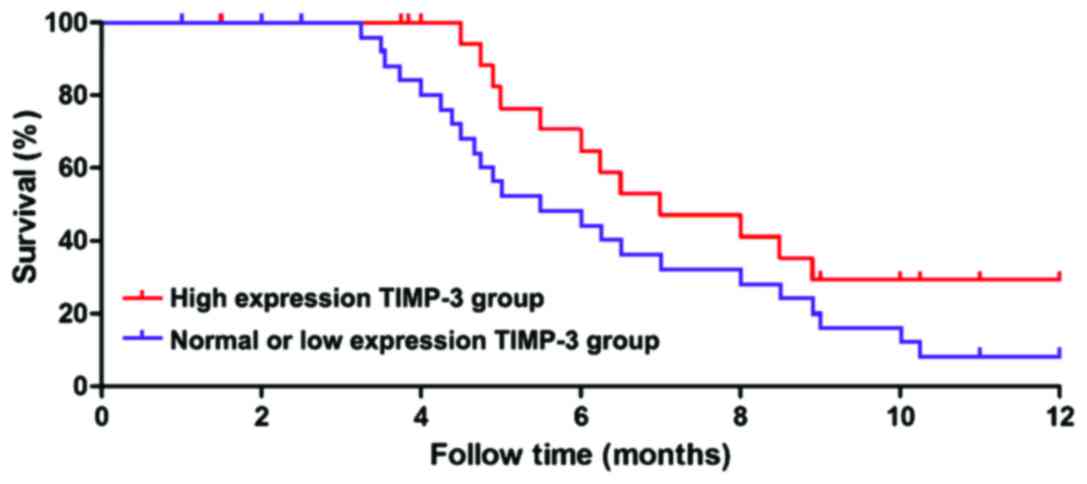
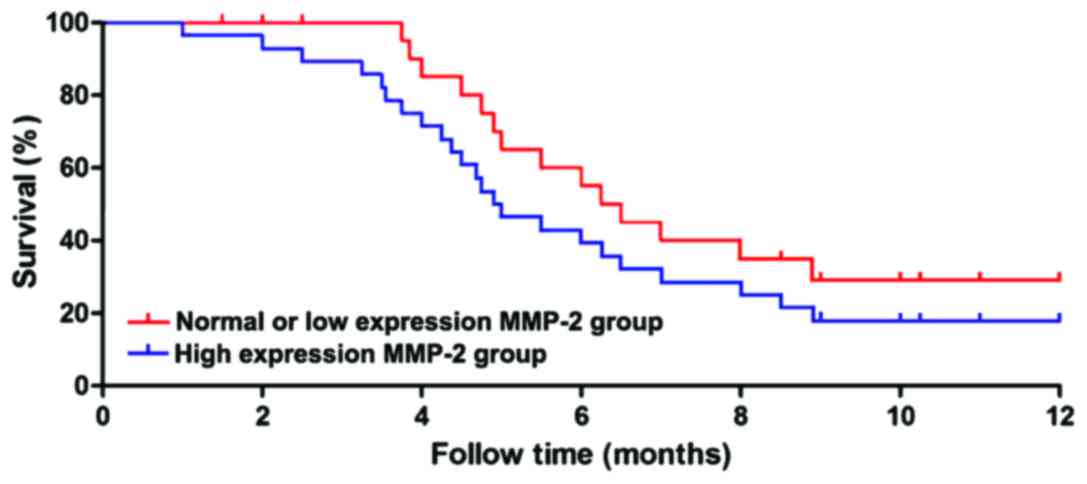
metastasis and recurrence (P<0.05) (Table II). The follow-up data of the above
patients were divided into high-expression group and normal- or
low-expression group according to the relative expression levels of
MMP-2 and TIMP-3. The patients were followed-up for 1 year, and the
survival time of each group was statistically analyzed. The results
showed that the survival time of patients in the high-expression
MMP-2 group was significantly shorter than that in the normal- or
low-expression group (P<0.01), and the survival curve is shown
in Fig. 3. The survival time of
patients in the high-expression TIMP-3 group was significantly
longer than that in the normal- or low-expression group
(P<0.01), and the survival curve is shown in Fig. 4.
 | Table II.Correlation of relative expression
levels of MMP-2 and TIMP-3 with clinical features of patients. |
Table II.
Correlation of relative expression
levels of MMP-2 and TIMP-3 with clinical features of patients.
| Type | n | MMP-2 expression
level | P-value | TIMP-3 expression
level | P-value |
|---|
| Age (years) |
|
|
|
| 0.0782 |
| ≤60 | 34 | 3.98±1.33 | 0.0692 | 5.53±1.08 |
|
|
>60 | 36 | 4.16±1.28 |
| 5.85±1.12 |
|
| Sex |
|
|
|
| 0.0873 |
| Male | 33 | 4.07±1.08 | 0.0619 | 4.63±1.62 |
|
|
Female | 37 | 3.96±1.22 |
| 4.57±1.29 |
|
| Tumor size |
|
|
|
| 0.0098 |
| <5
cm | 28 | 2.29±1.39 | 0.0072 | 6.08±1.19 |
|
| ≥5
cm | 42 | 6.89±1.87 |
| 3.95±2.01 |
|
| Lymphatic
metastasis |
|
|
|
| 0.0097 |
| Yes | 46 | 6.76±2.01 | 0.0078 | 2.17±1.78 |
|
| No | 24 | 3.58±1.96 |
| 6.53±1.05 |
|
| Clinical staging |
|
|
|
| 0.0097 |
| Limited
stage disease | 53 | 2.76±0.83 | 0.0086 | 5.92±1.25 |
|
| Extensive
stage disease | 17 | 5.37±1.06 |
| 2.57±1.03 |
|
| Relapse |
|
|
|
| 0.019 |
| Yes | 49 | 5.68±1.86 | 0.026 | 3.87±1.37 |
|
| No | 21 | 4.52±1.36 |
| 5.96±1.69 |
|
Detection of MMP-2 and TIMP-3
expressions in GCT by immunohistochemistry
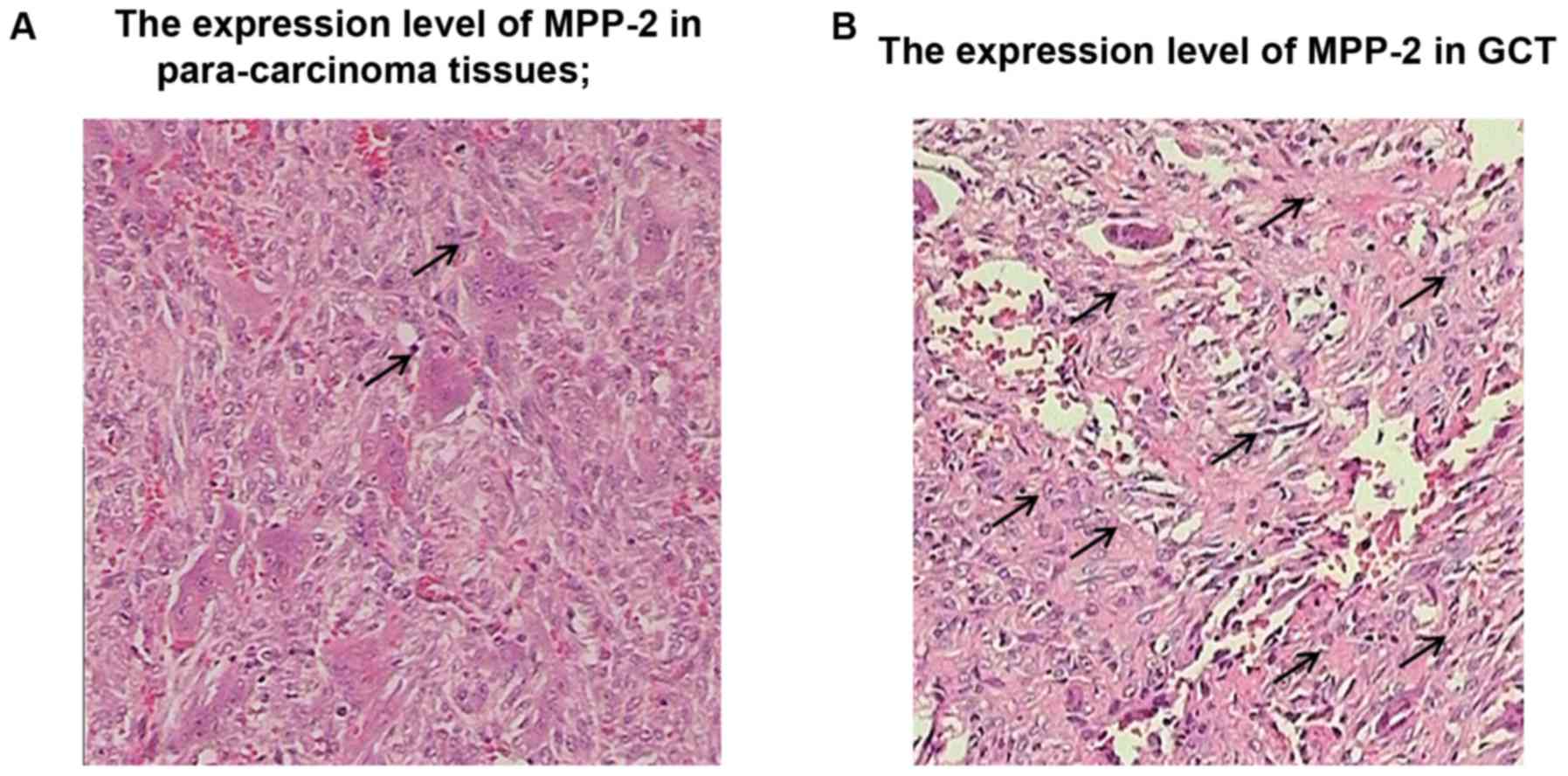
The expression levels of MMP-2 and TIMP-3 in GCT
were detected by immunohistochemistry. Yellow staining in cytoplasm
and surrounding mesenchyme indicated a positive expression of
MMP-2, while brown yellow staining in nucleus indicated a positive
expression of TIMP-3. The immunohistochemical results of MMP-2 in
GCT tissues and para-carcinoma tissues showed that the positive
rate of MMP-2 in GCT tissues was obviously higher than that in
para-carcinoma tissues, and the difference was statistically
significant (P<0.01) (Fig. 5). The
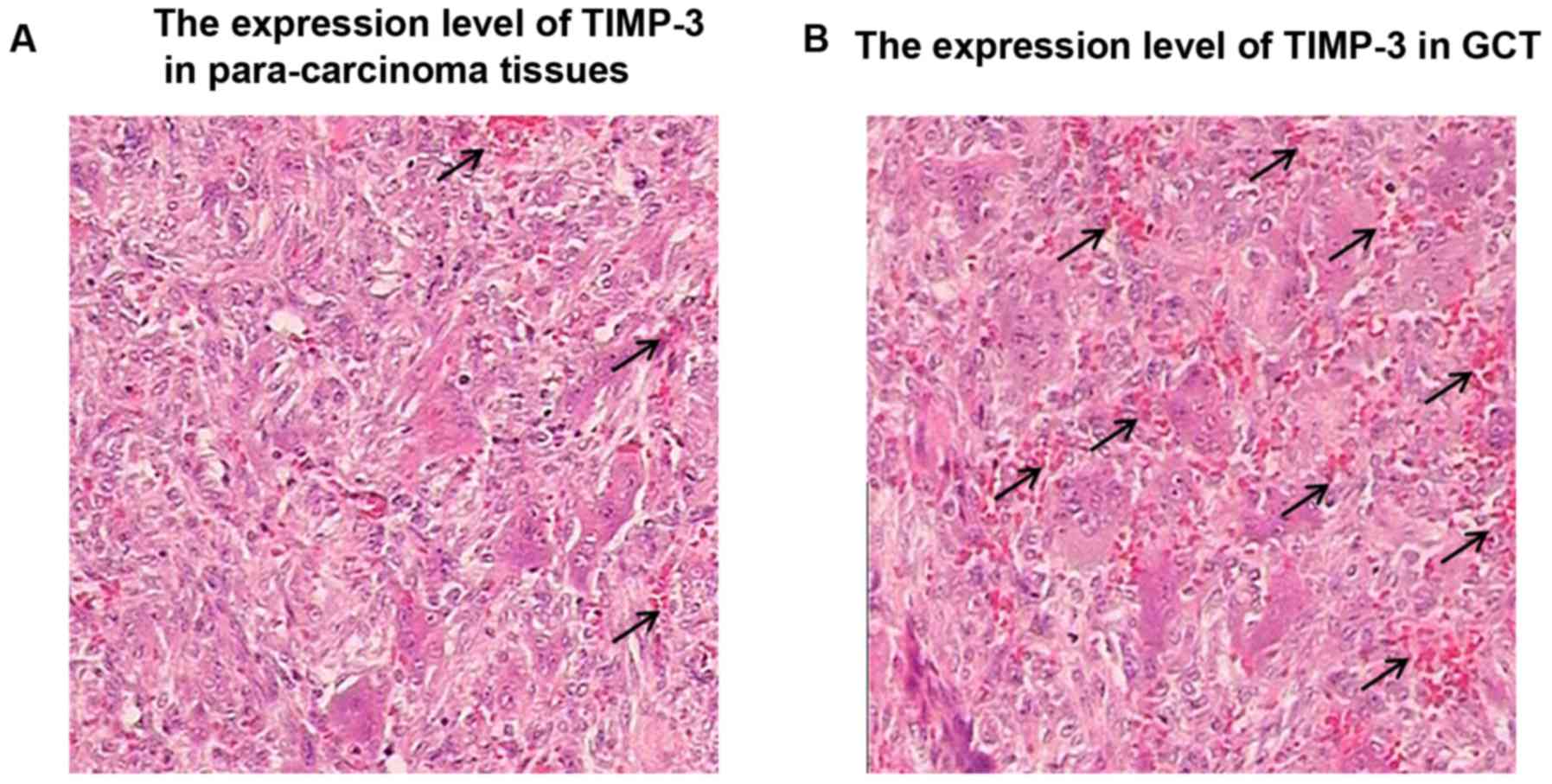
immunohistochemical results of TIMP-3 in GCT tissues and
para-carcinoma tissues revealed that the positive rate of TIMP-3 in
GCT tissues was obviously lower than that in para-carcinoma
tissues, and the difference was statistically significant
(P<0.01) (Fig. 6).
Correlation between MMP-2 and TIMP-3
expression in GCT
The expression levels of MMP-2 and TIMP-3 in GCT
patients were statistically analyzed. The results showed that the
expression level of TIMP-3 in GCT tissues of patients with high
MMP-2 expression was lower (P<0.05). The correlation between
MMP-2 and TIMP-3 expression levels revealed that there was a
negative correlation between MMP-2 and TIMP-3 expression levels
(r=−0.258, P<0.05) (Table
III).
 | Table III.Correlation of expression of MMP-2 and
TIMP-3 in GCT. |
Table III.
Correlation of expression of MMP-2 and
TIMP-3 in GCT.
|
| TIMP-3 |
|
|
|---|
|
|
|
|
|
|---|
| MMP-2 | High expression | Normal or low
expression | r | P-value |
|---|
| High expression | 12 | 58 |
|
|
| Normal or low
expression | 49 | 21 | −0.258 | 0.021 |
Discussion
GCT, also known as osteoclastoma, is a common and
potentially malignant tumor in the bone, characterized by
infiltrative growth, easy recurrence and the risk of metastasis to
lung tissues (10,11). There are rich blood vessels, including
capillary-like blood vessels, in GCT. Previous findings have shown
that both microvessel density and VEGF expression level in GCT are
very high; in other words, the occurrence and recurrence of GCT are
closely related to tumor angiogenesis (12). GCT is often in a more advanced stage
when found clinically. Different from other epithelial cell tumors,
pathological cells of GCT are difficult to identify in the bone
marrow. It has been shown that the incidence of GCT may be closely
related to the induction of chromosomal aberrations (13).
MMP is a kind of calcium and zinc atom-dependent
protease family, which can degrade almost all the components of
ECM. In previous years, it has been shown that the changes and
degradation of ECM and BM components are one of the important
factors of tumor invasion, metastasis and recurrence (14). TIMP-3, as a widely-distributed
endogenous MMP inhibitor, and a non-soluble protein, can closely
bind with the ECM components and competitively bind with MMP
receptor. Thus, it is a fully functioning MMP inhibitor that can
inhibit MMPs and matrix lysin and block the activity of all
activated MMPs (15,16). Moreover, TIMP-3 inhibits the formation
of tumor vessel by inhibiting the response of microvascular
endothelial cells to growth factors, and inhibits the tumor
progression by regulating cell proliferation and promoting cell
apoptosis (17). The analysis of
correlation of MMP-2 and TIMP-3 expression with clinicopathological
data can effectively explain the pathogenesis of related diseases.
Previous findings have shown that MMP-2 can affect tumor
angiogenesis, suggesting that MMP-2 is associated with the
occurrence and development of a variety of tumors (18,19). Liu
et al (20) found that MMP-2
is highly expressed in a variety of solid tumors, indicating that
MMP-2 is closely related to the occurrence and metastasis of solid
tumors. In the present study, semi-quantitative PCR, western blot
analysis and immunohistochemistry were performed to study the
expression level of MMP-2 in GCT tissues. The results showed that
MMP-2 was highly expressed in GCT at the gene and protein levels,
which was closely related to the tumor size, clinical staging,
metastasis, recurrence, and the results were also consistent with
the report on pulmonary metastasis of GCT. The survival curve of
GCT patients during the 1 year follow-up also revealed that the
survival time of patients with a high MMP-2 expression was
significantly shorter than that with normal or low expression, and
the difference was statistically significant (P<0.01). The above
results suggested that MMP-2 is involved in the occurrence and
development of GCT, which may lead to metastasis and recurrence of
GCT possibly by degrading the BM and ECM. In the present study, it
was found that the expression level of TIMP-3 in GCT was
significantly lower than that in para-carcinoma tissues, which was
closely related to the clinicopathological features, such as the
diameter, metastasis and recurrence of GCT. The immunohistochemical
results also showed that the expression level of TIMP-3 in
para-carcinoma tissues was obviously higher than that in GCT
(P<0.01). The study on the correlation between MMP-2 and TIMP-3
expression levels showed that there was a negative correlation
between them. The above results indicated that TIMP-3 may inhibit
angiogenesis in GCT and inhibit the activity of MMP-2, thus
inhibiting the GCT.
In conclusion, both MMP-2 and TIMP-3 are closely
related to the occurrence and development of GCT, which can be used
as one of the indexes in the clinical examination of GCT. However,
there were still some shortcomings in this study. The pathogenesis
was not studied in depth, the sample size in the experiments was
small, there were no healthy volunteers for the comparative study,
and the results remain to be further confirmed. However, the
research value of MMP-2 and TIMP-3 in GCT is unquestionable, which
can bring new breakthroughs to the clinical treatment of GCT.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nicoli TK, Saat R, Kontio R, Piippo A,
Tarkkanen M, Tarkkanen J and Jero J: Multidisciplinary approach to
management of temporal bone giant cell tumor. J Neurol Surg Rep.
77:144–149. 2016. View Article : Google Scholar
|
|
2
|
Hu P, Zhao L, Zhang H, Yu X, Wang Z, Ye Z,
Wu S, Guo S, Zhang G, Wang J, et al: Recurrence rates and risk
factors for primary giant cell tumors around the knee: A
multicentre retrospective study in China. Sci Rep. 6:363322016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gong HS, Liu GJ, Huang QS, Chen FH and
Zhao HB: Surgical management of sacrococcygeal region giant tumors
by use of balloon occlusion abdominal aorta. Turk Neurosurg.
26:904–911. 2016.PubMed/NCBI
|
|
4
|
Dabak N, Göçer H and Çıraklı A: Advantages
of pressurized-spray cryosurgery in giant cell tumors of the bone.
Balkan Med J. 33:496–503. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rekhi B, Verma V, Gulia A, Jambhekar NA,
Desai S, Juvekar SL, Bajpai J and Puri A: Clinicopathological
features of a series of 27 cases of post-denosumab treated giant
cell tumors of bones: A single institutional experience at a
tertiary cancer referral centre, India. Pathol Oncol Res.
23:157–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parra-Herran C1, Schoolmeester JK, Yuan L,
Dal Cin P, Fletcher CD, Quade BJ and Nucci MR: Myxoid
leiomyosarcoma of the uterus: A clinicopathologic analysis of 30
cases and review of the literature with reappraisal of its
distinction from other uterine myxoid mesenchymal neoplasms. Am J
Surg Pathol. 40:285–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang L, Zheng Z, Zhou Q, Bai X, Fan L,
Yang C, Su L and Hu D: miR-155 promotes cutaneous wound healing
through enhanced keratinocytes migration by MMP-2. J Mol Histol.
48:147–155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adhikari N, Mukherjee A, Saha A and Jha T:
Arylsulfonamides and selectivity of matrix metalloproteinase-2: An
overview. Eur J Med Chem. 129:72–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rother S, Samsonov SA, Moeller S,
Schnabelrauch M, Rademann J, Blaszkiewicz J, Köhling S,
Waltenberger J, Pisabarro MT, Scharnweber D and Hintze V: Sulfate
hyaluronan alters endothelial cell activation in vitro by
controlling the biological activity of the angiogenic factors
vascular endothelial growth factor-A and tissue inhibitor of
metalloproteinase-3. ACS Appl Mater Interfaces. 9:9539–9550. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shooshtarizadeh T, Rahimi M and
Movahedinia S: P63 expression as a biomarker discriminating giant
cell tumor of bone from other giant cell-rich bone lesions. Pathol
Res Pract. 212:876–879. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qi DW, Wang P, Ye ZM, Yu XC, Hu YC, Zhang
GC, Yan XB, Zheng K, Zhao LM and Zhang HL: Clinical and
radiographic results of reconstruction with fibular autograft for
distal radius Giant Cell Tumor. Orthop Surg. 8:196–204. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Y, Zhao L, Zhang H, Yu X, Wang Z, Ye Z,
Wu S, Guo S, Zhang G, Wang J and Ning X: Sex differences in the
recurrence rate and risk factors for primary giant cell tumors
around the knee in China. Sci Rep. 6:281732016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pujani M, Bahadur S, Jairajpuri ZS, Jetley
S and Jameel J: Giant cell tumor bone in an elderly male - an
unusual case misdiagnosed on MRI as a malignant sarcoma. Indian J
Surg Oncol. 6:285–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Almalki SG, Valle Llamas Y and Agrawal DK:
MMP-2 and MMP-14 silencing inhibits VEGFR2 cleavage and induces the
differentiation of porcine adipose-derived mesenchymal stem cells
to endothelial cells. Stem Cells Transl Med. 6:1385–1398. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yadav PK, Yadav BS, Panigrahi PN, Tripathi
V, Chaturvedi N and Kataria M: Molecular characterization and
in-silico analysis of the tissue inhibitor of metalloproteinases-3
(TIMP-3) gene of canine mammary tumor. Comb Chem High Throughput
Screen. 60:686–691. 2017.
|
|
16
|
Su CW, Su BF, Chiang WL, Yang SF, Chen MK
and Lin CW: Plasma levels of the tissue inhibitor matrix
metalloproteinase-3 as a potential biomarker in oral cancer
progression. Int J Med Sci. 14:37–44. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sharma K, Tyagi R, Singh R, Sharma SK and
Anand A: Serum levels of TIMP-3, LIPC, IER3 and SLC16A8 in CFH
negative AMD cases. J Cell Biochem. 118:2087–2095. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin HF, Hsi E, Huang LC, Liao YC, Juo SH
and Lin RT: Methylation in the matrix metalloproteinase-2 gene is
associated with cerebral ischemic stroke. J Investig Med.
65:794–799. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pillai SS, Yukawa H, Onoshima D, Biju V
and Baba Y: Förster resonance energy transfer mediated
photoluminescence quenching in stoichiometrically assembled
CdSe/ZnS quantum dot-peptide labeled black hole quencher conjugates
for matrix metalloproteinase-2 sensing. Anal Sci. 33:137–142. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu D, Zhang R, Wu J, Pu Y, Yin X, Cheng
Y, Wu J, Feng C, Luo Y and Zhang J: Interleukin-17A promotes
esophageal adenocarcinoma cell invasiveness through ROS-dependent,
NF-κB-mediated MMP-2/9 activation. Oncol Rep. 37:1779–1785. 2017.
View Article : Google Scholar : PubMed/NCBI
|