Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common malignant cancer in the world (1,2). HCC is
also the third most common cause of cancer-related death (3,4). The early
diagnosis of HCC is usually difficult and there is a lack of
effective treatments, so the prognosis of patients with HCC is
usually poor (5). The development of
HCC is a multi-step and multi-stage pathology process with multiple
genes involved (6,7). To date, no specific and sensitive tumor
markers have been clinically used for early diagnosis of HCC.
Therefore, this study was carried out to identify markers for the
diagnosis of HCC.
MicroRNA (miRNA) is a group of endogenous
single-stranded non-coding RNA with a length of about 17–25
nucleotides. miRNAs mainly target the 3′ untranslated region
(3′UTR) of target genes to degrade mRNA or inhibit the translation
of mRNA (8–10). miRNAs also participate in the
formation of organs, cell proliferation and apoptosis, and
tumorigenesis (11,12). It has been found that miRNAs are
closely related to the development of different types of cancer.
Differential expression of some miRNAs may affect the growth and
metastasis of tumors (13).
We screened the differential expression genes of
patients with HCC through the TCGA database and found that
miRNA-299 and miRNA-7706 were differentially expressed in HCC
patients (data not shown). This finding has not been reported
before. Therefore, this study was carried out to investigate the
expression of miRNA-299 and miRNA-7706 in HCC and their effects on
SK-HEP-1 cells, so as to promote the early diagnosis and prognosis
of HCC.
Materials and methods
Sample collection
Tumor tissues and adjacent healthy tissues were
collected from 179 HCC patients who were treated in The Third
Affiliated Hospital of Sun Yet-sen University (Guangzhou, China)
from June 2010 to February 2015. Those patients included 127 males
(70.9%) and 52 females (29.1%) with a mean age of 47.2±8 years.
None of the patients received radiotherapy and chemotherapy before
surgery. TNM staging was performed in accordance with the 2009
seventh edition of the UICC (Union for International Cancer
Control). HCC TNM staging: 36 cases of stage I, 69 cases of stage
II, and 74 cases of stage III–IV. Pathological types of all
specimens were confirmed as HCC. This study was approved by the
Ethics Committee of The Third Affiliated Hospital of Sun Yet-sen
University, and all patients signed informed consent.
Reagents and instruments
SK-HEP-1 cells were purchased from Shanghai
Institutes for Biological Sciences of the Chinese Academy of
Sciences (Shanghai, China). Total RNA extraction reagent TRIzol,
miScript Reverse Transcription kit, PCR kit, fetal bovine serum,
DMEM, PCR amplification primers and transfection reagent were
purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). miRNA-299, miRNA-7706 and negative control scramble
mimics were purchased from GenePharma Co., Ltd. (Shanghai, China).
7900HT fluorescence quantitative PCR instrument was purchased from
ABI (Thermo Fisher Scientific, Inc.).
Primer design
miRNA-199 and miRNA-7706 reverse transcription
primers and PCR specific primers were designed and synthesized by
GenePharma Co., Ltd. (Table I).
 | Table I.Primers of miRNA-199, miRNA-7706 and
endogenous control. |
Table I.
Primers of miRNA-199, miRNA-7706 and
endogenous control.
| Items | Primer sequences |
|---|
| miRNA-299 |
|
| Reverse transcription
primers |
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAGCGGTT-3′ |
| Sense |
5′-TCAAGCTTTGAAGCGCCTGTGC-3′ |
| Antisense |
5′-GCCTAAGGAGGGTCCGAGGTATTC-3′ |
| miRNA-7709 |
|
| Reverse transcription
primers |
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCTCGGCA-3′ |
| Sense |
5′-TCAAGCTTTGAAGCGCCTGTGC-3′ |
| Antisense |
5′-GCCTAAGGAGGGTCCGAGGTATTC-3′ |
| U6 |
|
| Sense |
5′-GGAACGCTTCACGAATTTG −3′ |
| Antisense |
5′-ATTGGAACGATACAGAGAAGATT-3′ |
Experimental methods
Cell culture
SK-HEP-1 cells were cultured with DMEM containing
10% fetal bovine serum and 100 g/l penicillin-streptomycin double
antibiotics in an incubator (37°C, 5% CO2). SK-HEP-1 and
SK-HEP-1 cells were collected at logarithmic growth phase. After
digestion with 0.25% trypsin, 3×105 cells were
transferred to each well of 6-well plates. Cells were cultured in
medium containing no antibiotics before transfection. Transfection
mixture was prepared according to the instructions of
Lipofectamine™ 2000 kit. Transfection mixture was added to each
well of 6-well plate and was shaken. After incubation at 37°C for 6
h, culture medium and transfection reagent were removed. Finally, 2
ml medium containing 10% fetal bovine serum was added into each
well for further culture.
Total RNA extraction
Total RNA was extracted from the cells using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
instructions. After RNA extraction, the concentration of each
sample RNA was determined by ultraviolet spectrophotometer. All RNA
samples were stored at −80°C before use.
Reverse transcription
Reverse transcription was performed using miScript
Reverse Transcription kit and 1 µg of total RNA was used to
synthesize cDNA. Reverse transcription conditions: 42°C for 3 min,
42°C for 60 min and 95°C for 3 min. All cDNA samples were stored at
−80°C before use.
RT-PCR
Real-time fluorescence quantitative PCR was
performed on 7900HT fluorescence quantitative PCR instrument using
cDNA and SYBR premix Ex Taq™ II PCR kit. Reaction system was: 10 µl
of SYBR premix Ex Taq™ II PCR mix, 1 µl of cDNA, 0.5 µl of each
primer and 8 µl of RNase-free water. Reaction conditions were: 95°C
for 5 min, followed by 45 cycles of 95°C for 15 sec, 60°C for 45
sec and 72°C for 25 sec. U6 was used as an endogenous control
(Table II). Results were analyzed
using the 2−ΔΔCt method.
 | Table II.Correlation between the expression of
miRNA-299 and miRNA-7706 and clinical factors. |
Table II.
Correlation between the expression of
miRNA-299 and miRNA-7706 and clinical factors.
| Clinical factors | N (%) | Expression level of
miRNA-299 | P-value | Expression level of
miRNA-7706 | P-value |
|---|
| Age (years) |
|
| 0.684 |
| 0.841 |
|
<60 | 72 (40.2) | 0.94±0.24 |
| 1.12±0.41 |
|
| ≥60 | 107 (59.8) | 0.87±0.31 |
| 1.24±0.47 |
|
| Sex |
|
| 0.714 |
| 0.614 |
| Male | 127 (70.9) | 0.67±0.51 |
| 0.91±0.47 |
|
|
Female | 52 (29.1) | 0.88±0.45 |
| 0.98±0.51 |
|
| Pathological
stage |
|
| 0.039 |
| 0.042 |
| I | 58 (32.4) | 0.94±0.22 |
| 1.22±0.44 |
|
| II | 79 (44.1) | 0.81±0.39 |
| 0.98±0.39 |
|
| III | 42 (23.5) | 0.73±0.16 |
| 0.81±0.33 |
|
| TNM stage |
|
| 0.057 |
| 0.061 |
| I | 36 (20.1) | 0.94±0.29 |
| 1.14±0.41 |
|
| II | 69 (38.5) | 0.92±0.32 |
| 1.07±0.37 |
|
|
III/IV | 74 (41.4) | 0.85±0.34 |
| 0.92±0.46 |
|
| Lymph node
metastasis |
|
| 0.031 |
| 0.044 |
|
Positive | 89 (49.7) | 0.73±0.23 |
| 0.84±0.32 |
|
|
Negative | 90 (50.3) | 0.91±0.18 |
| 1.27±0.39 |
|
| History of
smoking |
|
| 0.062 |
| 0.784 |
|
Yes | 100 (55.8) | 0.82±0.33 |
| 1.09±0.36 |
|
| No | 79 (44.2) | 0.87±0.36 |
| 1.01±0.44 |
|
Cell proliferation assay
miRNA-299, miRNA-7706 and scramble mimics were
transfected into 1×106 SK-HEP-1 cells, followed by
incubation for 24 h. After that, SK-HEP-1 cells were inoculated
into 96-well plates with 6×103 cells per well, followed
by incubation for 3–5 h. DMEM (100 µl) was added after cell
adhesion, followed by incubation in an incubator (37°C, 5%
CO2) for 6 days. CCK-8 (10 µl) was added daily, and OD
values at 450 nm were measured using a microplate reader.
Transwell invasion assay
Matrigel was kept at 4°C overnight (12 h). Matrigel
(50 µl; 1:10 dilution) was added to the upper chamber, followed by
incubation at 37°C for 6 h. After intervention with serum-free
medium for 48 h, cells were digested with trypsin and resuspended
in DMEM containing 1% fetal bovine serum to adjust the cell density
to 1×106/ml. Cell suspension (100 µl) was transferred
into the upper chamber, while the lower chamber was filed with 500
µl of culture medium containing 10% FBS. After incubation for 24 h
in an incubator, Matrigel and the cells that failed to invade were
removed. The membrane was fixed for 30 min, and staining with 0.1%
crystal violet was performed for 15 min. The results were observed
under an inverted microscope. Each experiment was repeated three
times.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS Inc., Chicago, IL, USA). Measurement data were
expressed as mean ± SD. ANOVA was used for comparisons between
multiple groups and SNK test was used for post hoc test. Student's
t-test was used for comparison of clinical factors. Correlation
analyses were performed by Pearson's correlation analysis. Survival
analysis was performed using Kaplan-Meier curve method. P<0.05
was considered to indicate a statistically significant
difference.
Results
miRNA-299 and miRNA-7706 expression in
HCC tissues and adjacent tissues
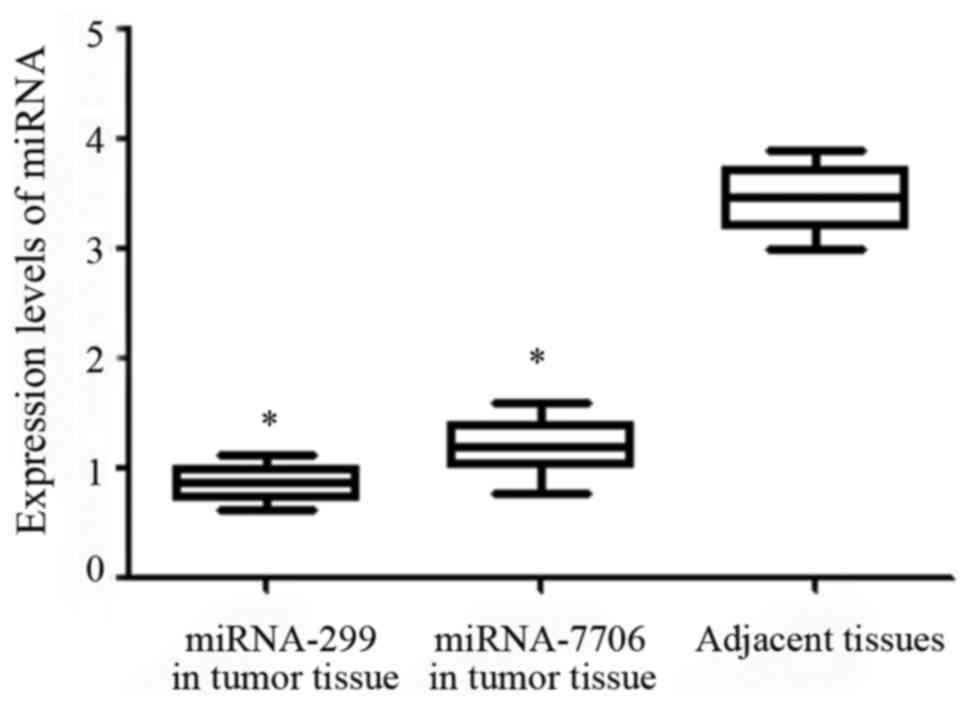
The relative expression levels of miRNA-299 and
miRNA-7706 in tumor tissue were 0.71±0.41 and 1.2±0.57,
respectively, which were significantly lower than those in adjacent
healthy tissue (P<0.05) (Fig.
1).
Correlation between miRNA-299 and
miRNA-7706 expression and clinical factors
Expression levels of miRNA-299 and miRNA-7706 were
correlated with pathological stage and lymph node metastasis
(P<0.05). With the increase in pathological stage, expression
levels of miRNA-299 and miRNA-7706 significantly decreased
(P=0.039; P=0.042). Patients with lymph node metastasis showed
significantly reduced expression levels of miRNA-299 and miRNA-7706
(P=0.031; P=0.044). No significant correlation were found between
expression levels of miRNA-299 and miRNA-7706 and other clinical
and pathological factors including age, sex, TNM stages and history
of smoking (P>0.05) (Table
II).
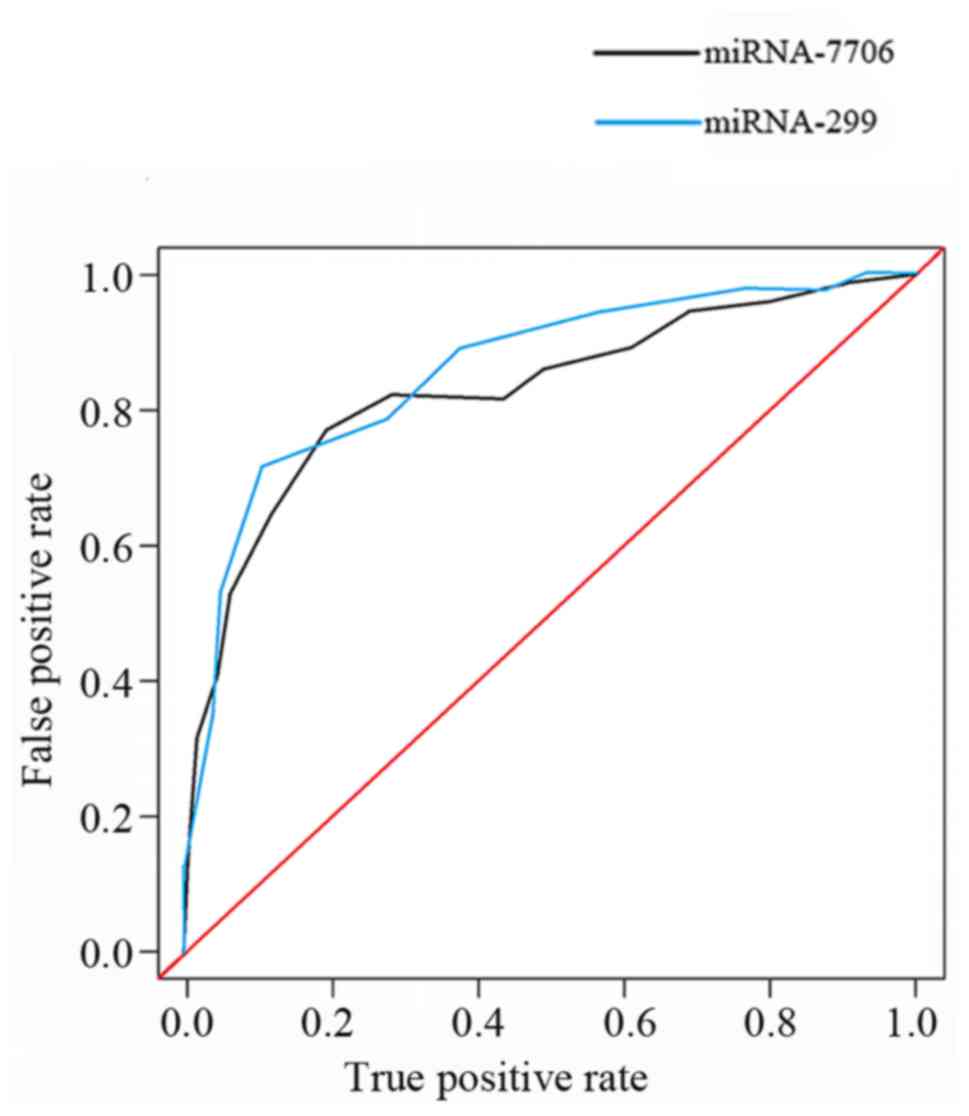
Diagnostic value of miRNA-299 and
miRNA-7706 for HCC
Diagnostic value of miRNA-299 and miRNA-7706 for HCC
was analyzed by ROC curve. As shown in Fig. 2, AUC=0.804, AUC=0.781; confidence
interval 95% CI: 0.724–0.842, 95% CI: 0.754–0.876.
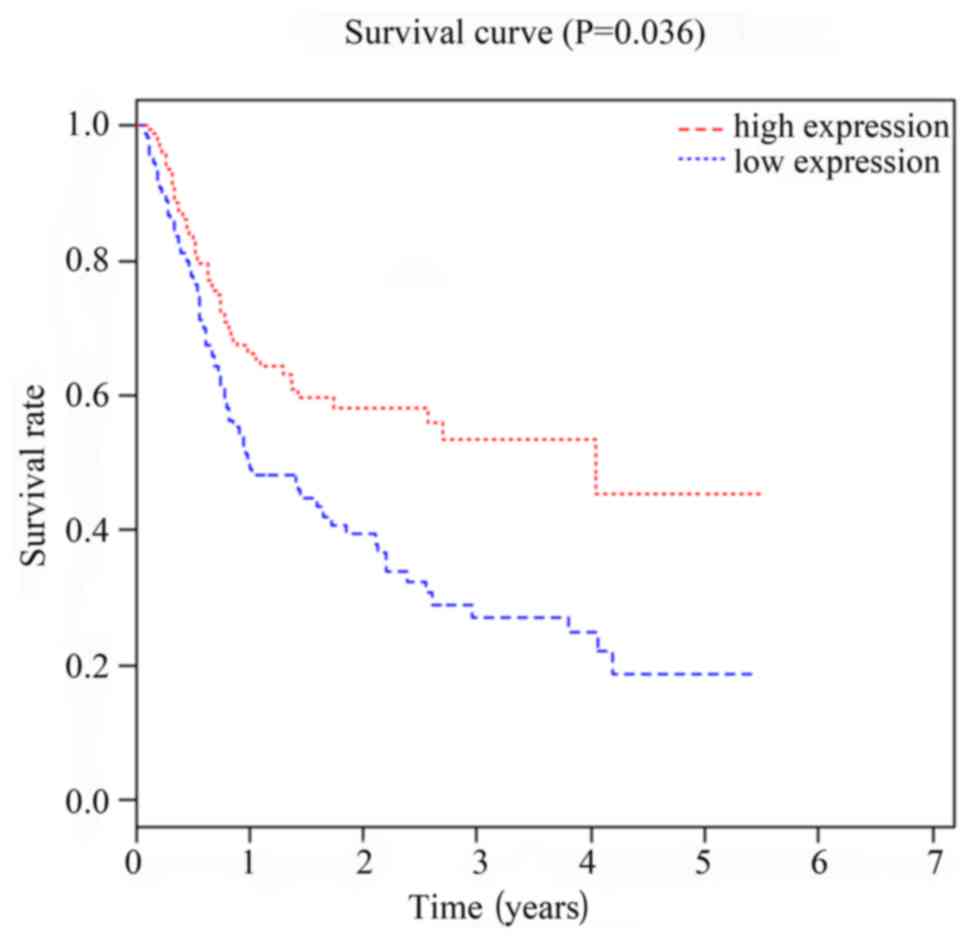
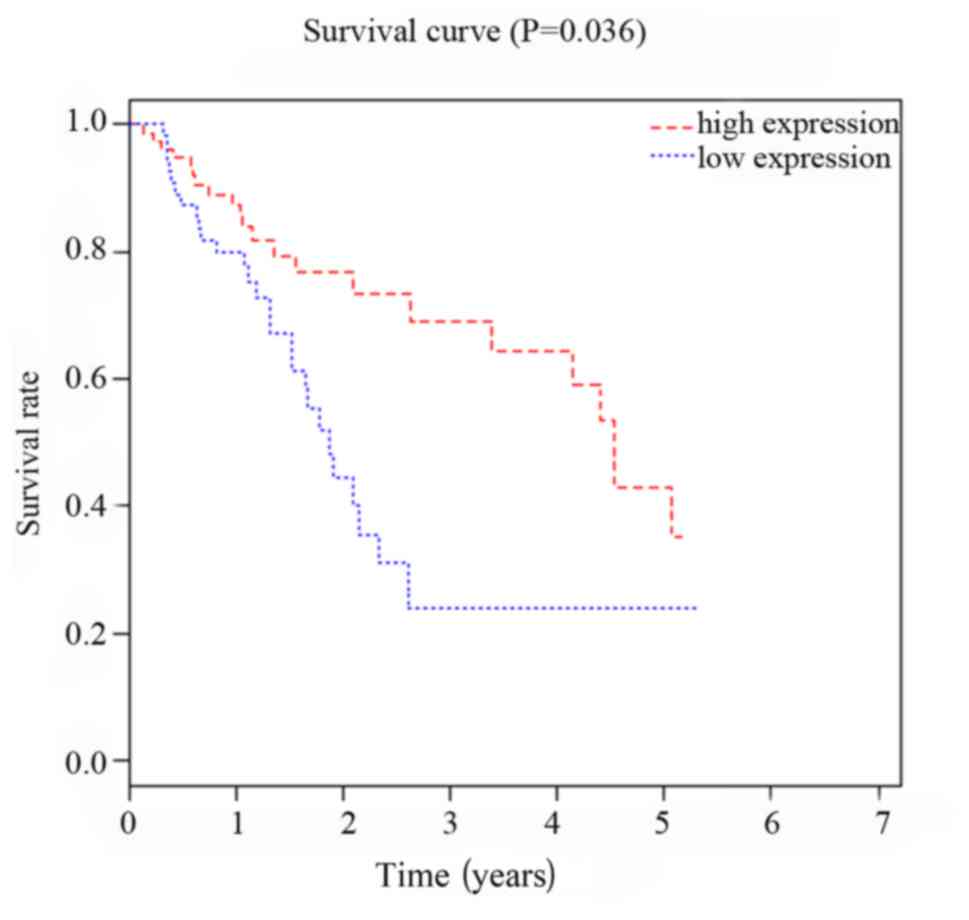
Correlation between expression of
miRNA-299 and miRNA-7706 and prognosis of HCC patients
Patients were divided in to two groups according to
the median value of the expression levels of miRNA-299 and
miRNA-7706. Follow-up study showed that survival rate of miRNA-299
and miRNA-7706 high expression group was significantly higher than
that of low expression group (P<0.05, P=0.027). As shown in
Figs. 3 and 4, Kaplan-Meier survival curve analysis
showed that expression levels of miRNA-299 and miRNA-7706 were
correlated with the prognosis of patients with HCC.
Effects of miRNA-299 mimics and
miRNA-7706 mimics transfection on expression of miRNA-299 and
miRNA-7706 in HCC cells
After transfection of miRNA-299-mimics-SK-HEP-1 and
miRNA-299-scramble-mimics-SK-HEP-1 into SK-HEP-1 cells, expression
levels of miRNA-299 were 7.89±2.87 and 1.17±0.45, respectively.
After transfection of miRNA-7706-mimics-SK-HEP-1 and
miRNA-7706-scramble-mimics-SK-HEP-1 into SK-HEP-1 cells, expression
levels of miRNA-7706 were 11.58±2.25 and 1.53±0.57. After
transfection of miRNA-299-mimics-SK-HEP-1 and
miRNA-7706-mimics-SK-HEP-1, expression levels of miRNA-299 and
miRNA-7706 in SK-HEP-1 cells were significantly increased (t=4.01,
P<0.05; t=7.50, P<0.05).
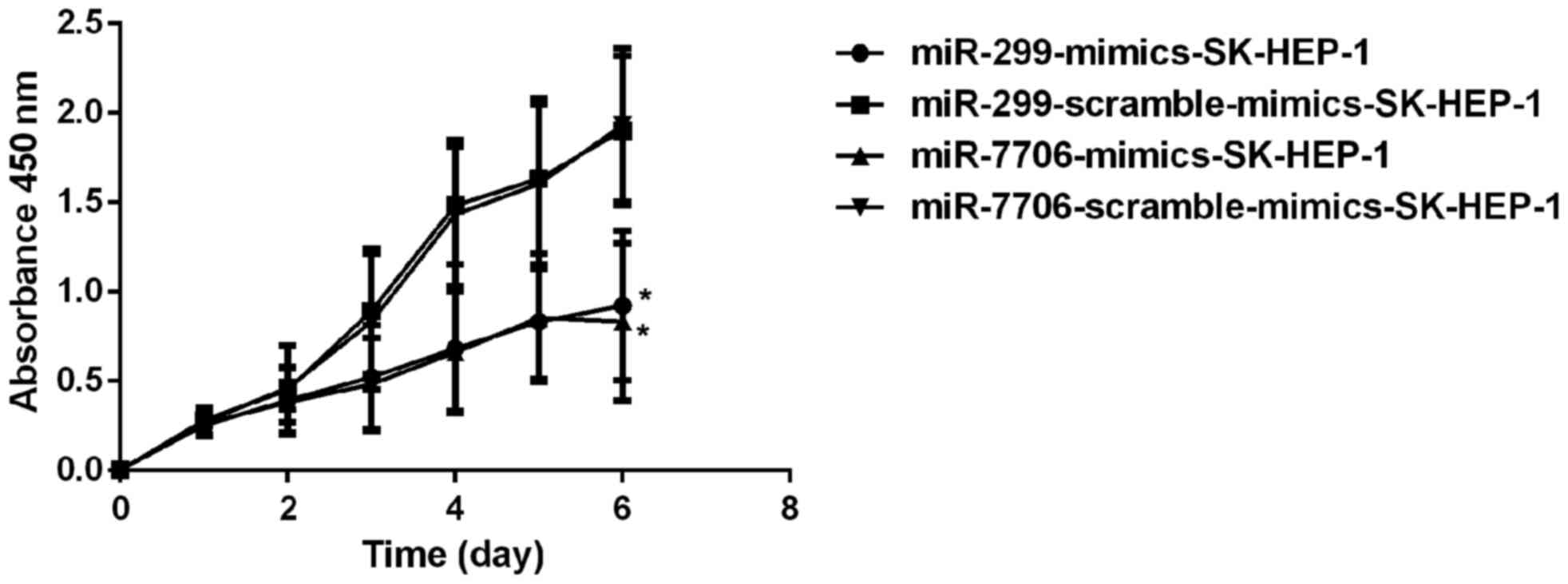
Effects of miRNA-299 and miRNA-7706 on
proliferation of SK-HEP-1 cells
miRNA-299-mimics-SK-HEP-1,
miRNA-7706-mimics-SK-HEP-1, miRNA-299-scramble-mimics -SK-HEP-1 and
miRNA-7706-scramble-mimics-SK-HEP-1 were transfected into
1×106 SK-HEP-1 cells, followed by incubation for 24 h.
After that, SK-HEP-1 cells were inoculated into 96-well plates with
6×103 cells per well, followed by incubation in an
incubator (37°C, 5% CO2) for 6 days. CCK-8 (10 µl) was
added daily, and OD values at 450 nm were measured using a
microplate reader. GraphPad Prism 5 software (GraphPad Software,
Inc., La Jolla, CA, USA) was used to plot the proliferation curve
of SK-HEP-1 HCC cells after transfection (Fig. 5).
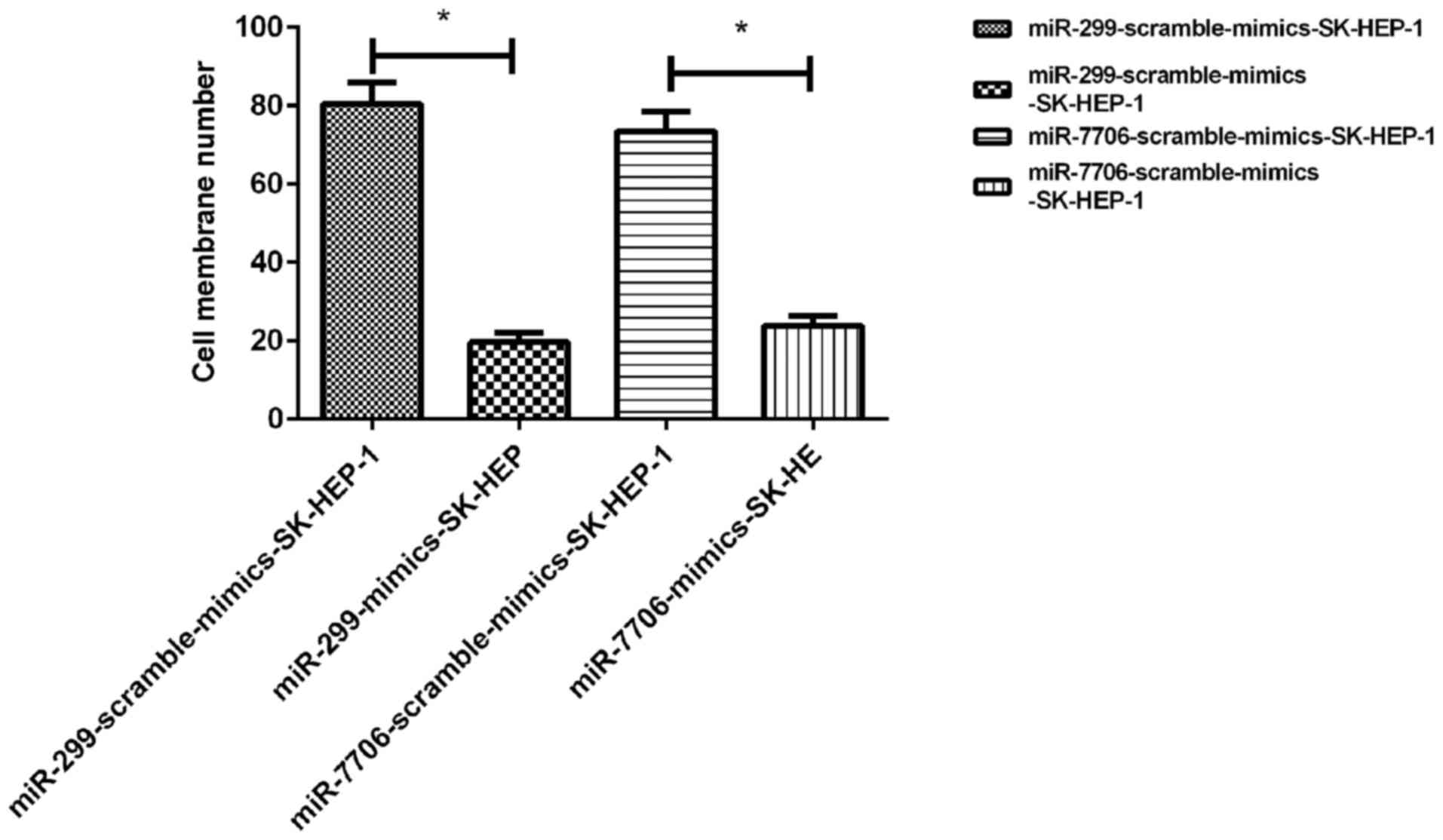
Effects of miRNA-299 and miRNA-7706 on
invasion of SK-HEP-1 cells
Compared with SK-HEP-1 cells transfected with
scramble mimics, invasion ability of SK-HEP-1 cells was
significantly weakened after transfection with miRNA-299 and
miRNA-7706 mimics (P<0.05) (Fig.
6).
Discussion
In this study, expression of miRNA-299 and
miRNA-7706 in tumor tissue and adjacent healthy tissue of HCC
patients was determined by qRT-PCR. Studies have found that the
expression level of miRNA-299 is low in a variety of tumor tissues,
such as endometrial rectal, colon and ovarian cancers (14–16).
However, studies on miRNA-7706 are relatively insufficient.
Expression pattern and function of two miRNAs in HCC still haven't
been reported. Analysis on the correlation between expression
levels of miRNA-299 and miRNA-7706 and patients' clinical data
showed that expression levels of miRNA-299 and miRNA-7706 were
significantly reduced in patients with lymph node metastasis. Other
clinical and pathological factors such as age, sex, TNM grade and
history of smoking showed no significant correlation with miRNA-299
and miRNA-7706 expression. Expression levels of miRNA-299 and
miRNA-7706 in tumor tissues were significantly lower than that in
adjacent tissues. At the same time, expression levels of those two
miRNAs in HCC cells SK-HEP-1 were also lower than that in control
cells. These results indicate that miRNA-299 and miRNA-7706 can
inhibit tumor cell proliferation. This is consistent with the
finding of Göhring et al (17)
that miRNA-299 can inhibit the proliferation and invasion of tumor
cells. Based on those studies, we speculate that miRNA-299 and
miRNA-7706 can also regulate cell proliferation and invasion in
HCC. In this study, results of CCK-8 cell proliferation assay and
Transwell cell invasion assay showed that miRNA-299 and miRNA-7706
could significantly inhibit the proliferation and invasion of
SK-HEP-1 cells. Many studies (18–20) found
that inhibition of tumor cell proliferation and invasion can
significantly inhibit tumor growth, which is consistent with the
results in our study. ROC curve analysis showed that AUCs of
miRNA-299 and miRNA-7706 AUC were 0.804 and 0.781, respectively,
which is consistent with the finding reported by Ren et al
(21), indicating that two miRNAs can
be used as markers for clinical diagnoses. Besides that, analyses
on the correlation between the expression of miRNA-299 and
miRNA-7706 and clinical factors showed that expression levels of
miRNA-299 and miRNA-7706 have no significant correlations with age,
sex, TNM stage and history of smoking of patients (P<0.05),
indicating that miRNA-299 and miRNA-7706 can potentially be new
markers for HCC. Kaplan-Meier survival curve analysis showed that
the survival rate of patients in miRNA-299 and miRNA-7706 high
expression group was significantly higher than that of low
expression group, indicating that patients with low expression
levels of those two miRNAs tend to have short-term survival. In
this study, we detected the expression of miRNA-299 and miRNA-7706
in HCC. Correlation analysis was performed to explore the
correlations between the expression of those two miRNAs and
clinical factors, which were indicated by the P-value. Chi-square
test showed that pathological stage and lymph node metastasis were
significantly correlated with the expression of those two miRNAs in
different stages. Those data may provide new indexes for clinical
staging and prediction of lymph node metastasis. In order to
provide references for clinical practice, effects of those two
miRNAs on proliferation and invasion of HCC cells were explored. We
found that those two miRNAs could effectively inhibit the
proliferation and invasion of cancer cells. ROC analysis showed
that those two miRNAs has promising clinical diagnostic value for
HCC, which is expected to be applied clinically in future.
Results of our study showed that miR-299 and
miR-7706 have similar functionality in hepatoma carcinoma cell line
SK-HEP-1. However, our study also has some shortcomings. Most
patients in this study were from local region. Therefore regional
difference may affect the results of our study. In addition, this
study is also limited by the small sample size. The interaction
between miRNA-299 and miRNA-7706 is still unknown. Therefore, our
future studies will focus on the interactions between those two
miRNAs and their roles in other diseases.
In conclusion, expression levels of miRNA-299 and
miRNA-7706 were significantly reduced in tumor tissue of HCC
patients, suggesting that they may play important roles in the
development of HCC. Detection of expression levels of miRNA-299 and
miRNA-7706 may provide references for the diagnosis of HCC. Our
study provided new insights for the diagnosis and treatment of
HCC.
Acknowledgements
Not applicable.
Funding
This study was funded by Special Scientific
Expenditure for Business Construction of State TCM Clinical
Research Base, State Administration of TCM of the People's Republic
of China. State TCM Scientific Task [2016] 20, no. JDZX2015173.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MD and FW wrote the manuscript and was responsible
for cell culture. HC collected the sample. YL performed reverse
transcription. JZ contributed to RT-PCR. ZZ and HY helped cell
proliferation assay and Transwell assay. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Third Affiliated Hospital of Sun Yet-sen University (Guangzhou,
China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yoshimoto S, Loo TM, Atarashi K, Kanda H,
Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, et
al: Obesity-induced gut microbial metabolite promotes liver cancer
through senescence secretome. Nature. 499:97–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mittal S and El-Serag HB: Epidemiology of
hepatocellular carcinoma: consider the population. J Clin
Gastroenterol. 47:S2–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang YC, Xu Z, Zhang TF and Wang YL:
Circulating microRNAs as diagnostic and prognostic tools for
hepatocellular carcinoma. World J Gastroenterol. 21:9853–9862.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kokudo N, Hasegawa K, Akahane M, Igaki H,
Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y, et al:
Evidence-based clinical practice guidelines for hepatocellular
carcinoma: The Japan society of hepatology 2013 update (3rd JSH-HCC
guidelines). Hepatol Res. 45:452015. View Article : Google Scholar
|
|
7
|
Chen Y, Zhang J, Wang H, Zhao J, Xu C, Du
Y, Luo X, Zheng F, Liu R, Zhang H, et al: miRNA-135a promotes
breast cancer cell migration and invasion by targeting HOXA10. BMC
Cancer. 12:1112012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsu SD, Tseng YT, Shrestha S, Lin YL,
Khaleel A, Chou CH, Chu CF, Huang HY, Lin CM, Ho SY, et al:
miRTarBase update 2014: An information resource for experimentally
validated miRNA-target interactions. Nucleic Acids Res. 42(D1):
D78–D85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee YJ, Kim V, Muth DC and Witwer KW:
Validated microRNA target databases:an evaluation. Drug Dev Res.
76:389–396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kudo M, Matsui O, Izumi N, Iijima H,
Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, et
al: Liver Cancer Study Group of Japan: JSH consensus-based clinical
practice guidelines for the management of hepatocellular carcinoma:
2014 update by the Liver Cancer Study Group of Japan. Liver Cancer.
3:458–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reddy KB: MicroRNA (miRNA) in cancer.
Cancer Cell Int. 15:382015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shenoy A and Blelloch RH: Regulation of
microRNA function in somatic stem cell proliferation and
differentiation. Nat Rev Mol Cell Biol. 15:565–576. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang H, Li RP, Liang P, Zhou YL and Wang
GW: miR-125a inhibits the migration and invasion of liver cancer
cells via suppression of the PI3K/AKT/mTOR signaling pathway. Oncol
Lett. 10:681–686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu L, Chen X, Zhang Y, Hu Y, Shen X and
Zhu W: Long non-coding RNA TUG1 promotes endometrial cancer
development via inhibiting miR-299 and miR-34a-5p. Oncotarget.
8:31386–31394. 2017.PubMed/NCBI
|
|
15
|
Fateh A, Feizi MAH, Safaralizadeh R and
Azarbarzin S: Importance of miR-299-5p in colorectal cancer. Ann
Gastroenterol. 30:322–326. 2017.PubMed/NCBI
|
|
16
|
Xing Y, Cui D, Wang S, Wang P, Xing X and
Li H: Oleuropein represses the radiation resistance of ovarian
cancer by inhibiting hypoxia and microRNA-299-targetted heparanase
expression. Food Funct. 8:2857–2864. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Göhring AR, Reuter S, Clement JH, Cheng X,
Theobald J, Wölfl S and Mrowka R: Human microRNA-299-3p decreases
invasive behavior of cancer cells by downregulation of Oct4
expression and causes apoptosis. PLoS One. 12:e01749122017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim HS, Lee KS, Bae HJ, Eun JW, Shen Q,
Park SJ, Shin WC, Yang HD, Park M, Park WS, et al: MicroRNA-31
functions as a tumor suppressor by regulating cell cycle and
epithelial-mesenchymal transition regulatory proteins in liver
cancer. Oncotarget. 6:8089–8102. 2015.PubMed/NCBI
|
|
19
|
Li WF, Dai H, Ou Q, Zuo GQ and Liu CA:
Overexpression of microRNA-30a-5p inhibits liver cancer cell
proliferation and induces apoptosis by targeting MTDH/PTEN/AKT
pathway. Tumour Biol. 37:5885–5895. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan Q, Loya K, Rani B, Möbus S,
Balakrishnan A, Lamle J, Cathomen T, Vogel A, Manns MP, Ott M, et
al: MicroRNA-221 overexpression accelerates hepatocyte
proliferation during liver regeneration. Hepatology. 57:299–310.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren S, Xin Z, Xu Y, Xu J and Wang G:
Construction and analysis of circular RNA molecular regulatory
networks in liver cancer. Cell Cycle. 6:2204–2211. 2017. View Article : Google Scholar
|