Introduction
Intensity-modulated radiation therapy (IMRT) is used
to deliver a high dose of radiation to a large area, particularly
in lung tumors (1,2). This treatment requires high quality
assurance and quality control. Dose verification for individual
patients prior to treatment is required to ensure accurate
treatment (3).
Dose verification of IMRT is performed using single-
or multiple-field synthesis, and a slow photosensitive film or an
ionization chamber matrix needle (4–6). The
ionization chamber matrix method is widely used for its
convenience, high repeatability and high digitalization (7). The ionization chamber matrix is employed
to irradiate the target area vertically and to measure the beam
angle 0° (8). At different gantry
angles, the multileaf collimator (MLC) is affected by gravity,
friction, inertia and other factors (9). This effect results in a difference
between the actual MLC leaf error and that of the planning system
(10). Therefore, in the present
study, the rack angle zero method was used to avoid the effects of
rack accuracy on the actual dose distribution of IMRT (11).
Computed tomography (CT) positioning images used in
radiotherapy planning are static images. However, the respiratory
motion of the patient during scanning alters the position of the
lung tumors and surrounding organs, and affects the irradiation
dose delivered to the target areas and the organs at risk (12,13).
Respiratory motion also affects image acquisition, including that
of cone beam CT (14). Based on the
IMRT of an MLC, the deviation of the target area irradiation dose
caused by respiratory motion was ≤47.8% (15).
The γ analysis method was applied in the present
study (16). The association between
the dynamic IMRT planned γ analysis passing rate and respiratory
amplitude (A), and tumor volume, was investigated using a
semiconductor detector and respiratory motion platform. The
experiments were performed to provide a reference for the design
and evaluation of IMRT plans.
Patients and methods
Patients
A total of 30 patients with lung tumors were
admitted to The Second People's Hospital of Changzhou (Changzhou,
China) and underwent radiation therapy between July 2016 and
December 2016. Patients comprised 18 males and 12 females with a
mean age of 44.5±7.9 years (age range, 36–52 years). The patients
were divided into groups A, B and C, with each group comprising 10
patients. The average volumes of the tumor target area (PTV) were
635, 402 and 213 cm3 in groups A, B and C, respectively.
The volume ratio of the three groups was ~3:2:1. The prescribed
doses of PTV were 60 Gy for all patients, administered in 30
fractions. The whole course of treatment was ~42 days. The present
study was approved by the Medical Ethics Committee of The Second
People's Hospital of Changzhou (Changzhou, China), and all patients
provided written informed consent for participation.
Equipment and materials
An Infinity linear accelerator (Elekta Instrument
AB, Stockholm, Sweden) was used in the present study. The MLC
possessed 80 pairs of leaves, and the width of each leaf was 5 mm.
The algorithm of the Monaco treatment planning system (TPS, version
5.11.01; Elekta Instrument AB) was the Monte Carlo calculation. The
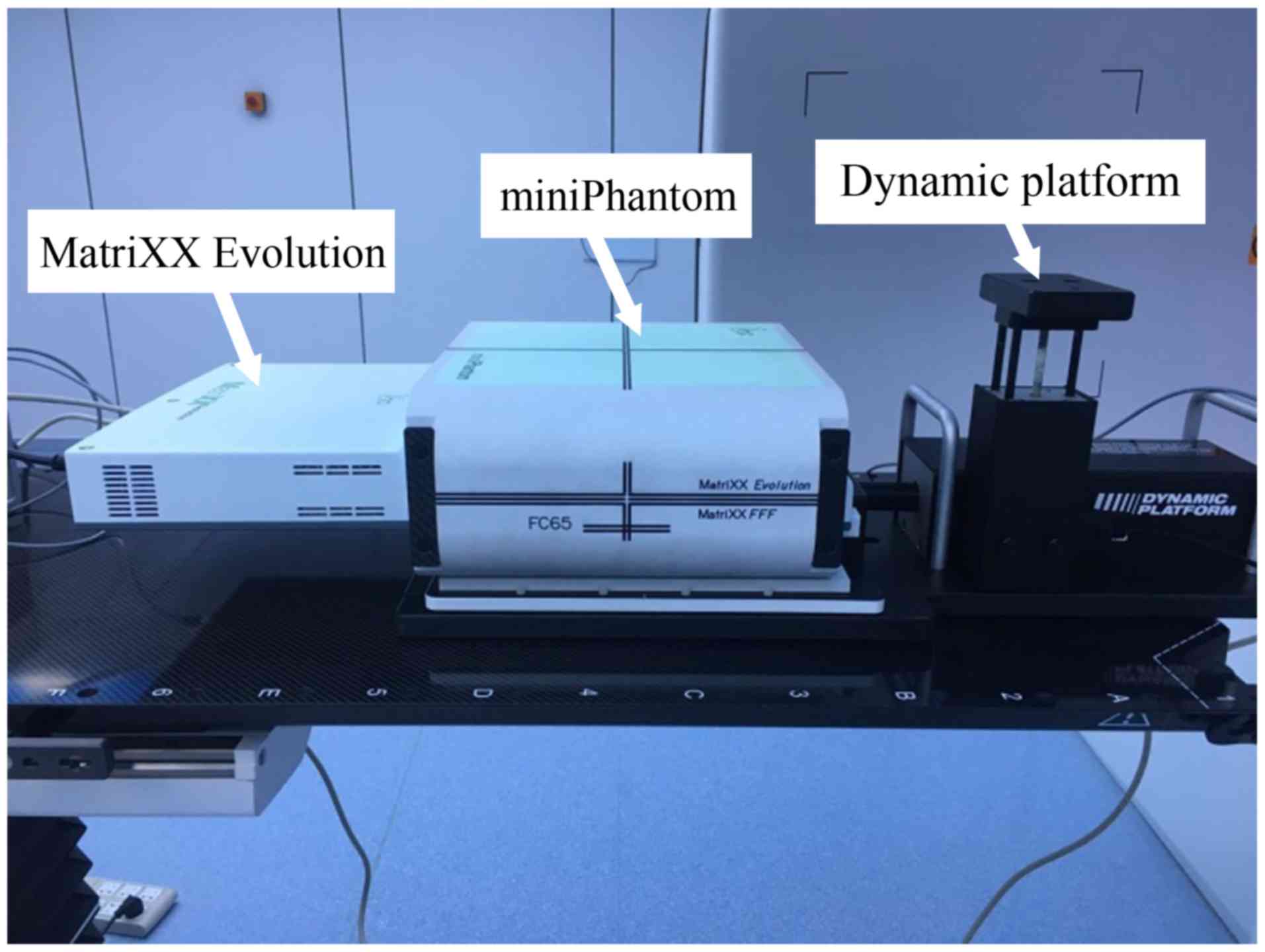
Matrixx evolution dose verification system adopted a 2D ionization
chamber array system (IBA Dosimetry, Bartlett, TN, USA). This
system was composed of 1,020 air ionization chambers and arranged
in a 32×32 matrix. The ionization chamber was 4.5 mm in diameter
and 5 mm in height, with an adjacent spacing of 7.62 mm and a
sensitive volume of 0.08 cm3. The effective measurement
range was 24.4×24.4 cm. The periphery of the matrix system was
wrapped in a 5 cm polymethyl methacrylate material (MiniPhantom;
IBA Dosimetry, Bartlett, TN, USA). The simulated respiratory motion
apparatus was a 008PL dynamic platform (CIRS, Norfolk, VA, USA).
The maximum A value of the simulated respiratory motion range was
~25 mm. The respiratory frequency and A value were set using the
program control. In the present study, the sine wave function sin
was used to simulate the human respiratory waveform. The quality
assurance (QA) phantom center (tumor center) was set at the center
of the accelerator when A=0 (17).
The overall QA experimental device is demonstrated in Fig. 1, with the Matrixx evolution device
placed on the dynamic platform.
Plan design
The QA method used (18) is briefly described as follows: The CT
scan image was transmitted to the Monaco planning system for 3D
reconstruction. The radiologist outlined the patients' target area
in accordance with the ICRU62 report (19), clinical examination and imaging
technology. Radiation was administered using a 6-MV X-ray with a
prescription dose of 60 Gy, which reached 95% PTV. The irradiation
was conducted 30 times. The dynamic IMRT plans of the 30 patients
were designed using the Monaco planning system. Using the Monte
Carlo algorithm, a computational grid of 0.3×0.3 cm was obtained.
Each patient plan was transferred to the QA module and the rack
angle was returned to 0. The QA phantom coronal plane isocenter
dose was outputted. The average γ analysis passing rate of the
patients was 98% in the simulated static state.
Data acquisition
The respiratory apparatus was horizontally placed on
the treatment bed to ensure that the forward and backward
directions of movement were parallel with the bed. The QA phantom
was placed on the respiratory apparatus in order that the effective
measurement point of the 2D matrix was located at the isocenter
layer of the accelerator. The respiratory motion was in the
head-to-foot direction, which is the most common direction of
motion of lung tumors (20). The
motion function was the sinusoidal function. In a normal resting
state, respiratory frequency is 16–20 breaths/min (21). Shimizu et al (22) demonstrated a lung tumor marker
movement range of 6.8–15.9 mm. The A values used were 0, 5, 10, 15,
20 and 25 mm. The T values were set as 4, 5 and 6 sec. The
respiratory motion simulation period was ≥4.9 sec in accordance
with the dynamic platform. Therefore, the 25 mm/4 sec groups were
absent. Thus, a total of 17 control experiments were performed for
A, B and C groups. The passing rates were measured in the A, B and
C groups, with 3%/3 mm (dose deviation, 3%; distance deviation, 3
mm) as the standard.
Statistical analysis
Data are expressed as the mean ± standard deviation
and were analyzed using the SPSS statistical software package
version 20.0 software (IBM Corp., Armonk, NU, USA). All experiments
were performed in triplicate. The A values and γ analysis passing
rates of different tumor volumes were analyzed using one-way
analysis of variance (ANOVA). An unpaired Student's t-test was used
for two group comparisons and one-way ANOVA followed by Tukey's
test was used for three or more group comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Comparison of the measured dose
fluxgraph and the output dose fluxgraph
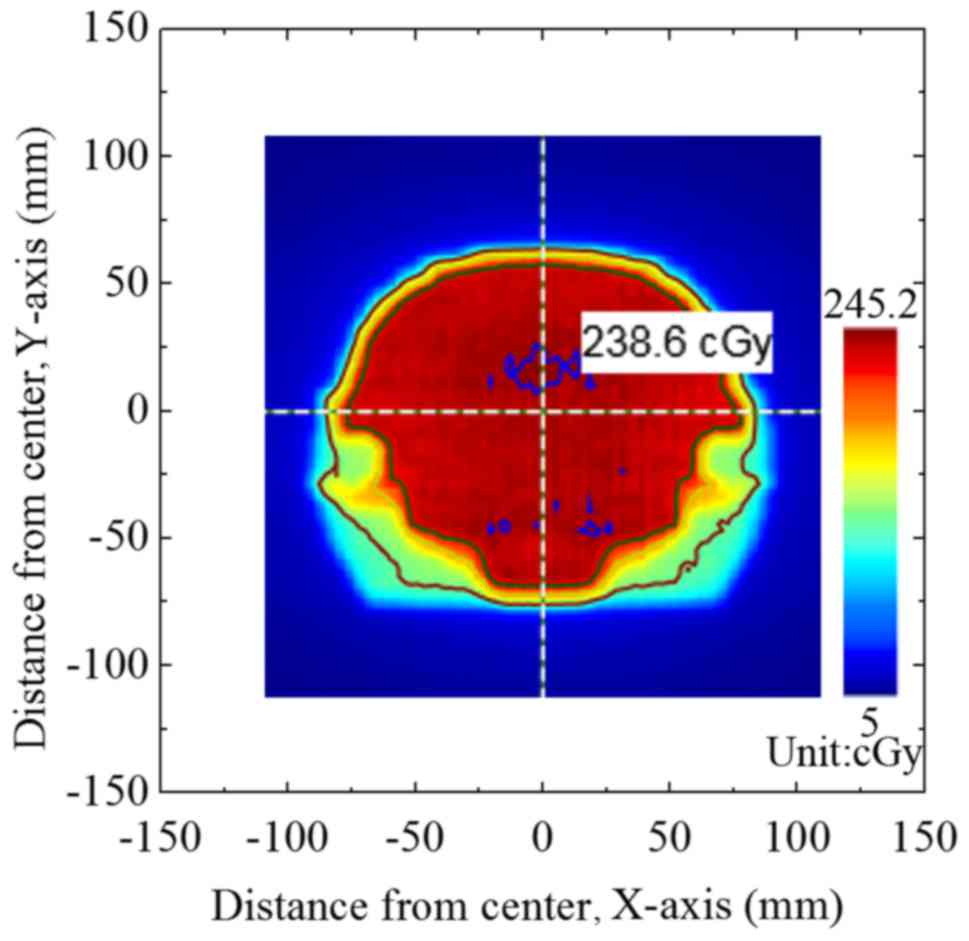
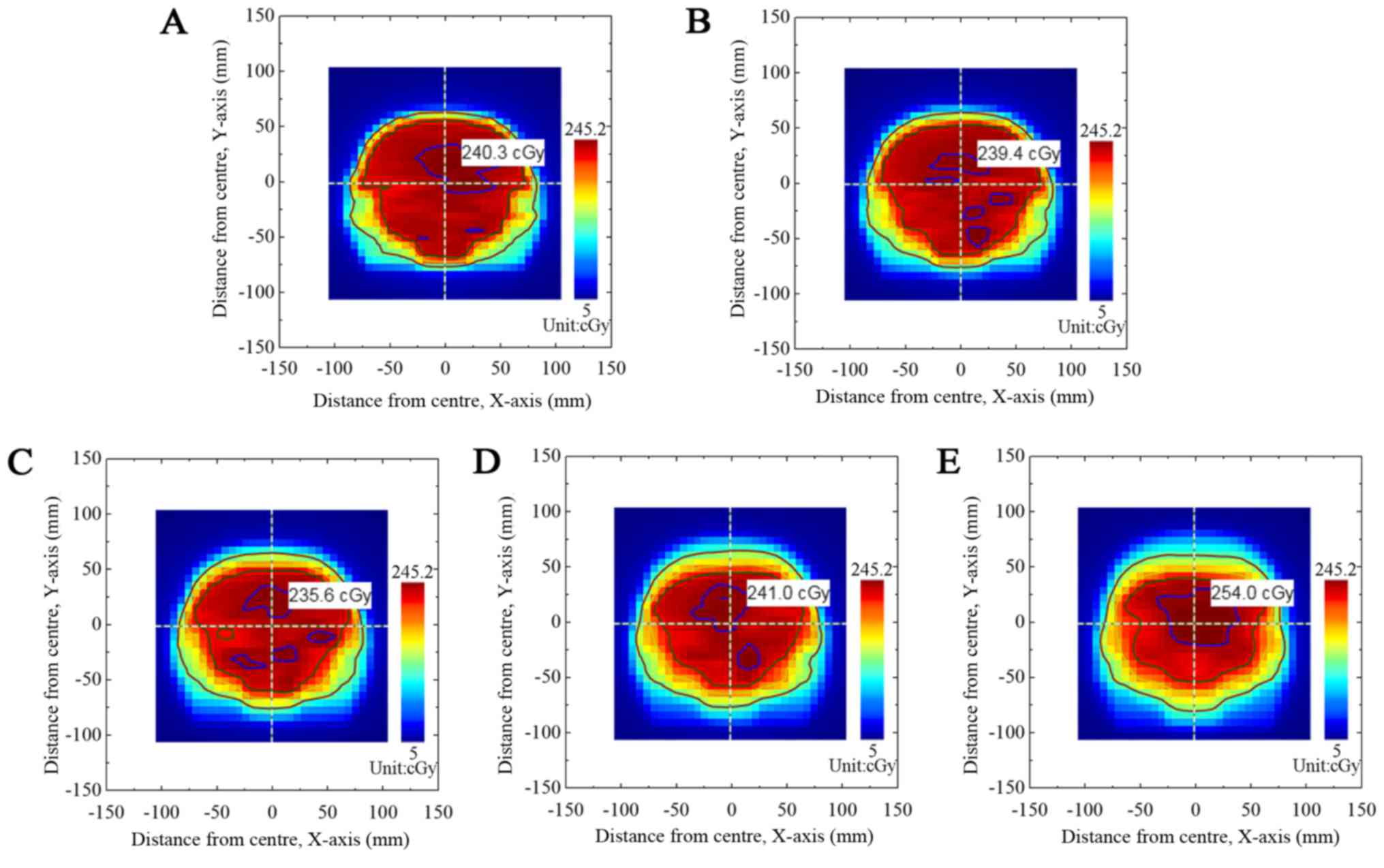
Fig. 2 presents the
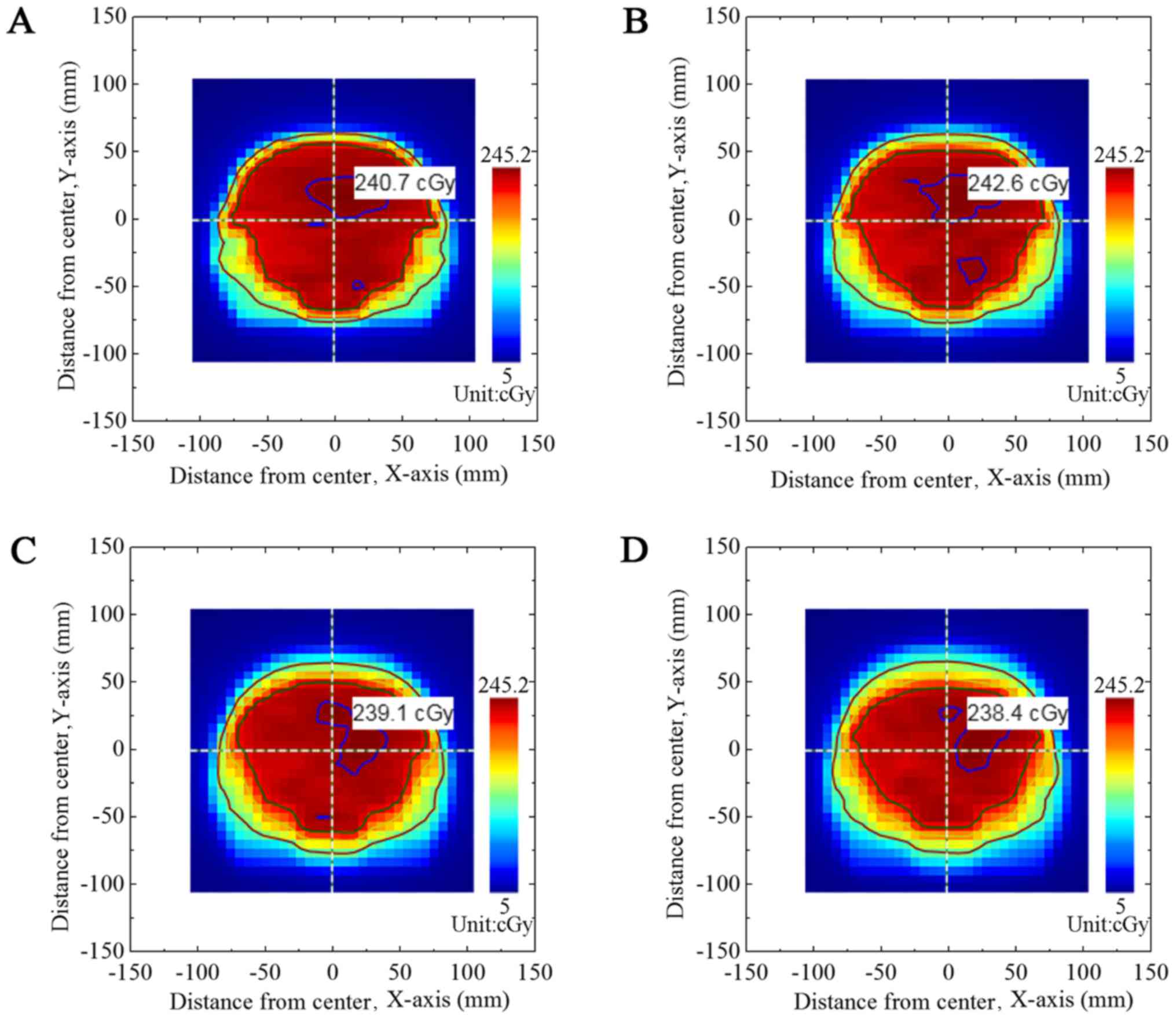
output dose fluxgraph from the TPS plan of group A. Fig. 3 presents the dose fluxgraph in group A
at A=5, 10, 15 or 20 mm (T=4 sec). Figs.
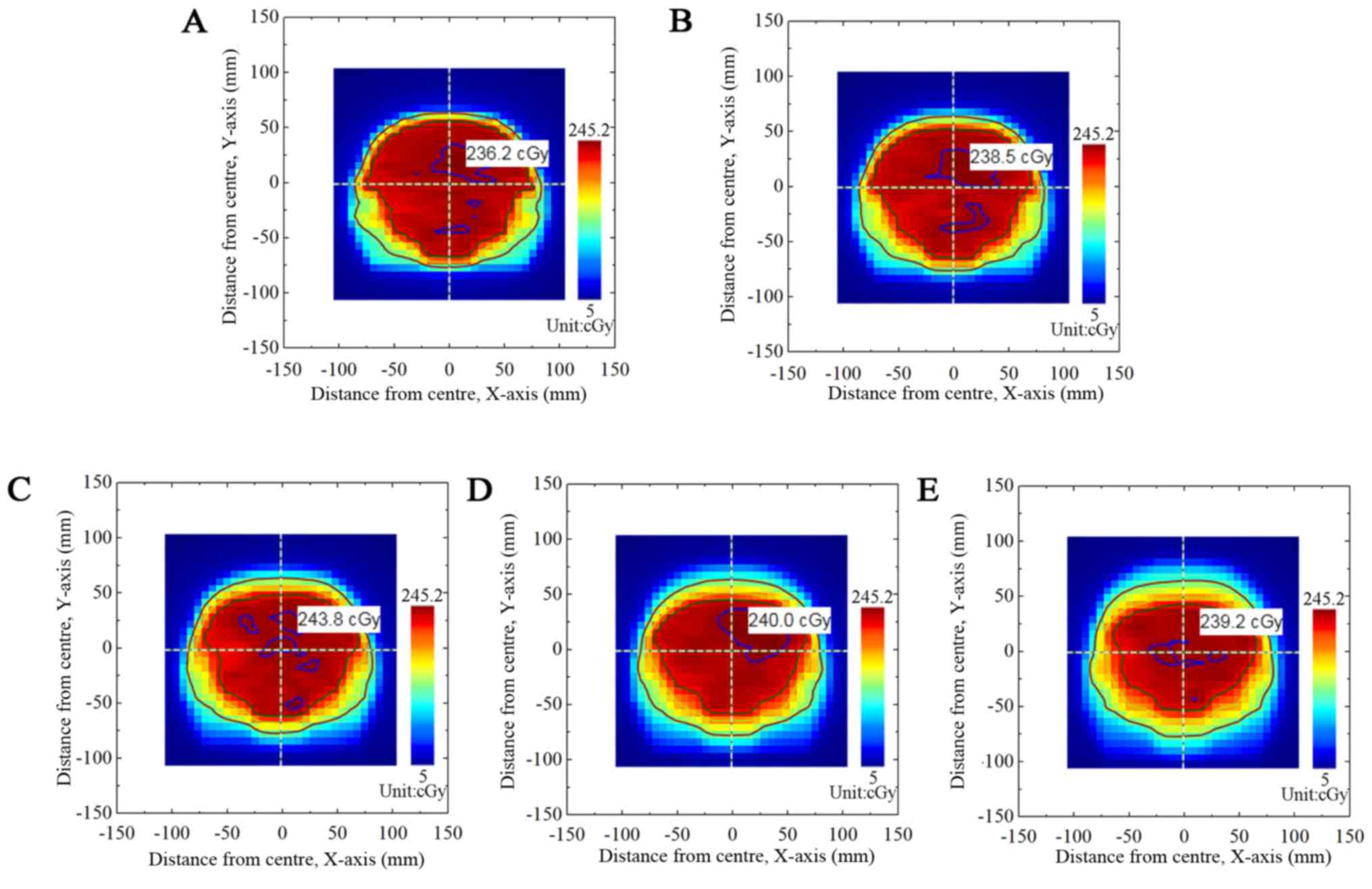
4 and 5 present the corresponding
dose fluxgraphs in the B group (T=5 and 6 sec). Figs. 3, 4 and
5 demonstrate that the high-dose area
(where dose was >90% of the maximal dose) decreased with
increased A values. The low-dose area (where dose was <60% of
the maximal dose) remained constant.
Association of the γ analysis passing
rate, and the A and T values
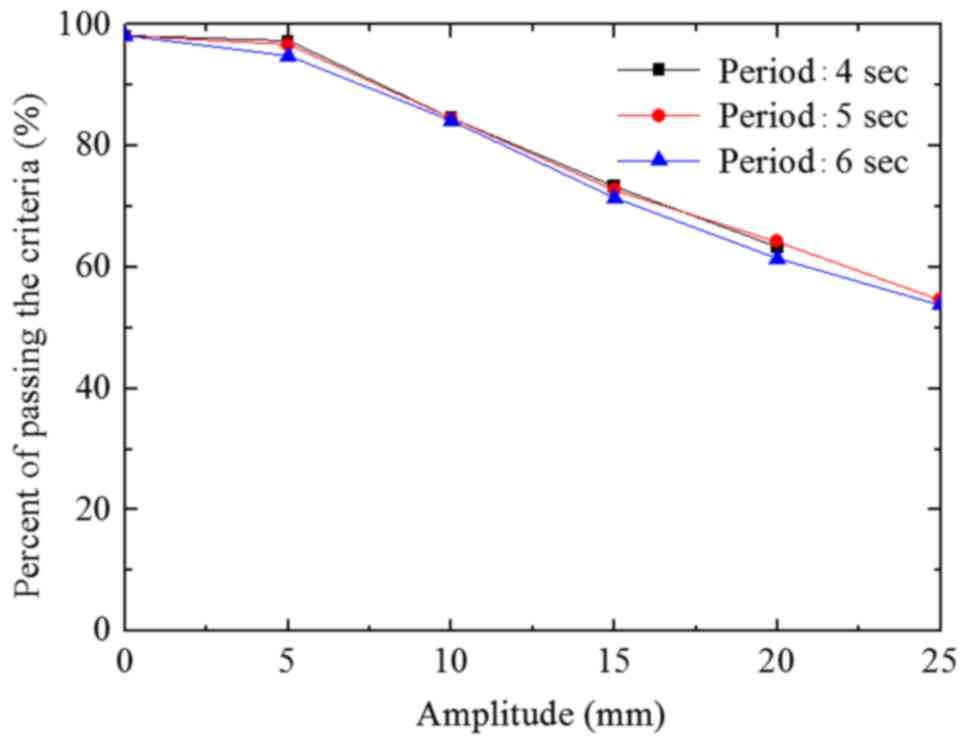
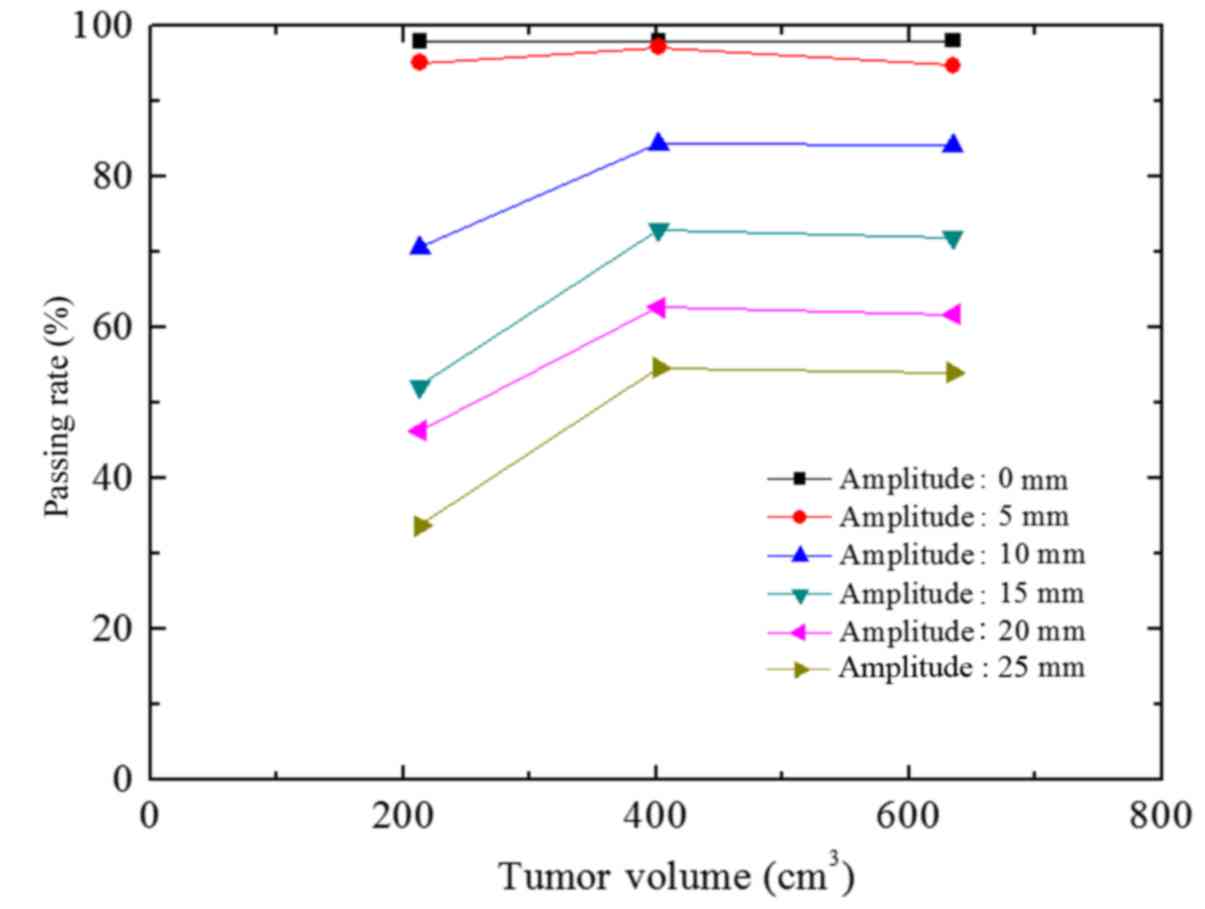
The passing rate deviation was <3.3% when A=5 mm
(T=4, 5 and 6 sec) compared with the passing rate (static) in the A
group (Fig. 6). However, this was not
statistically significant (P>0.05). The passing rate decreased
when the A value increased from 5 mm. When the A value exceeded 10
mm, the passing rates significantly differed from that of the
static state (P<0.05). When the A values were 10, 15, 20 and 25
mm, the passing rate deviations were 13.9, 30.7, 40.7 and 48.3%,
respectively (T=6 sec). The passing rate deviation among the 3
groups was <2.5% at the same A value (T=4, 5 and 6 sec).
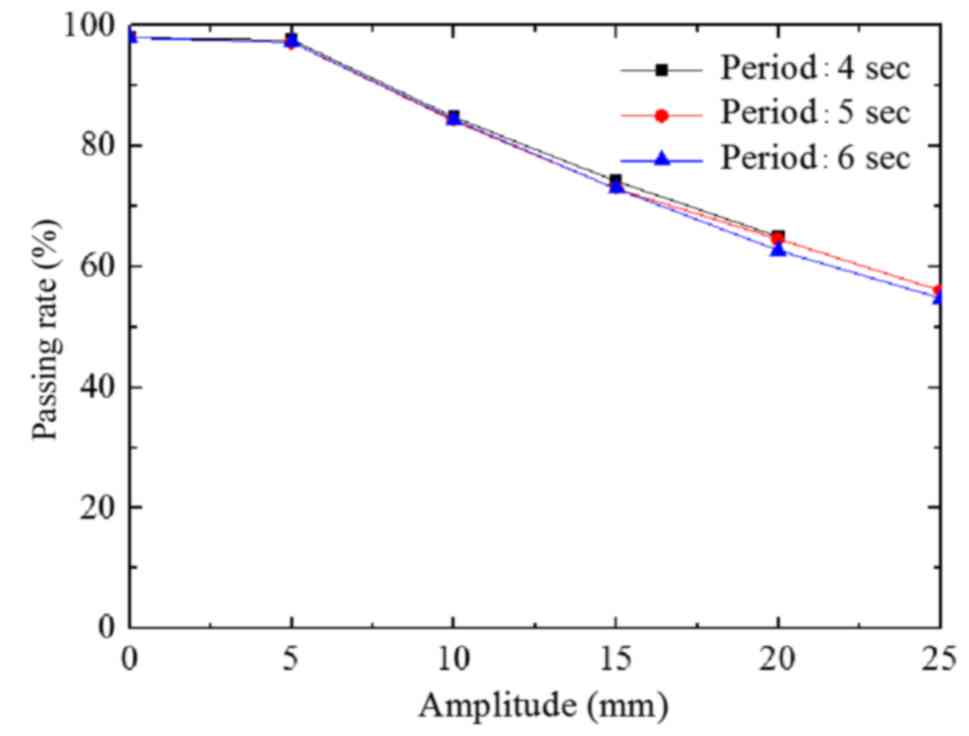
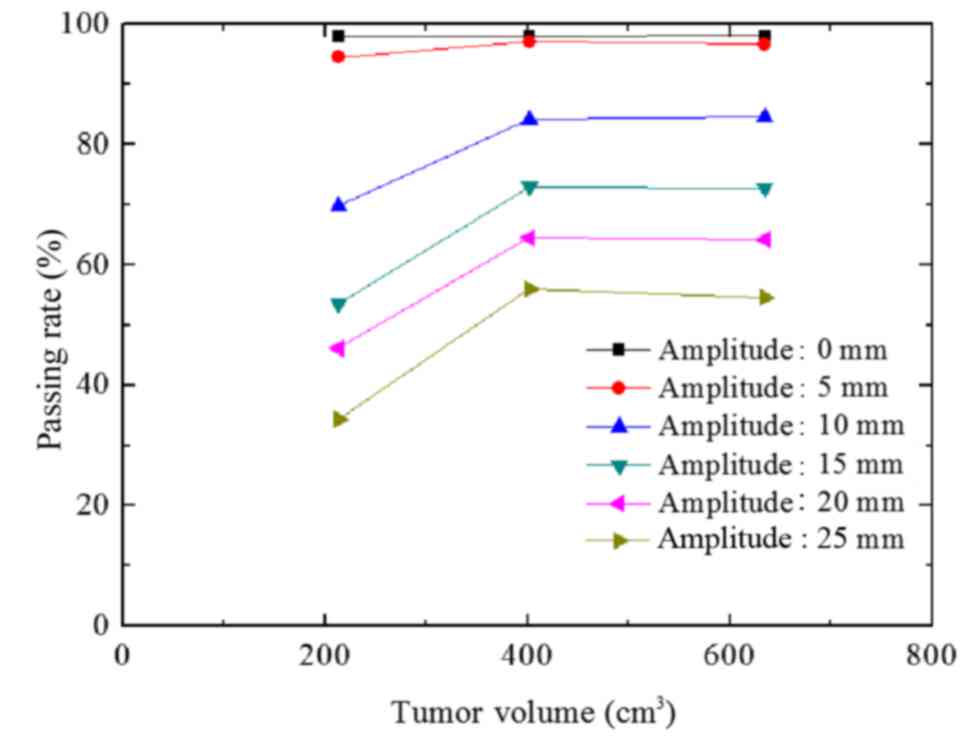
The passing rate of group B decreased with increased
A value (Fig. 7), reflecting the
results of group A. The maximum passing rate deviation among the 3
groups was 2.3% at the same A value (P>0.05).
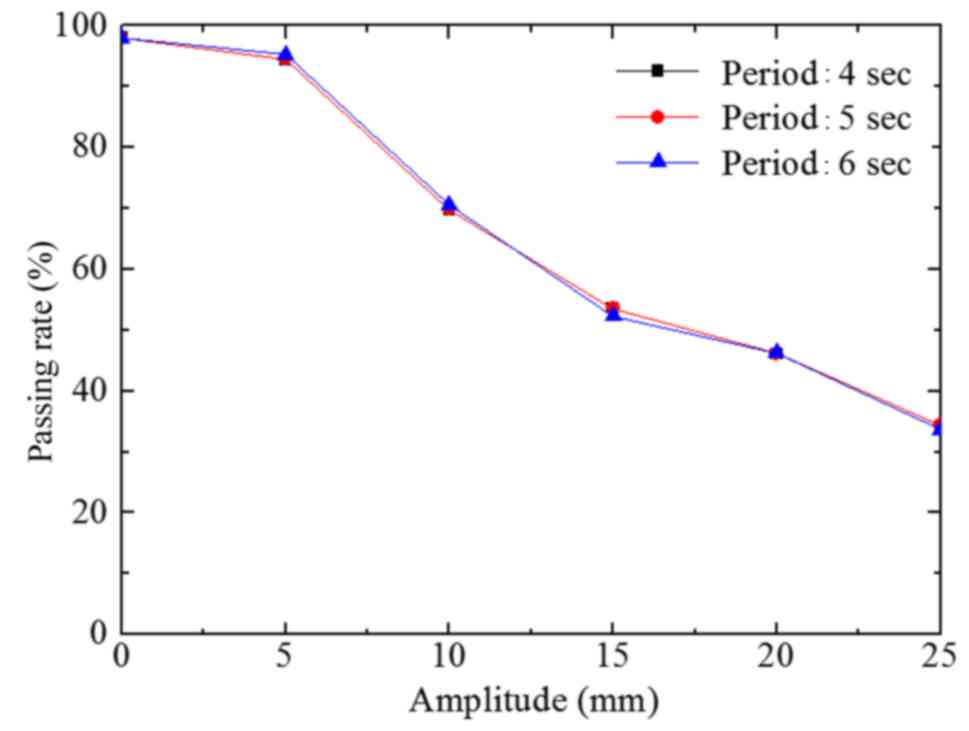
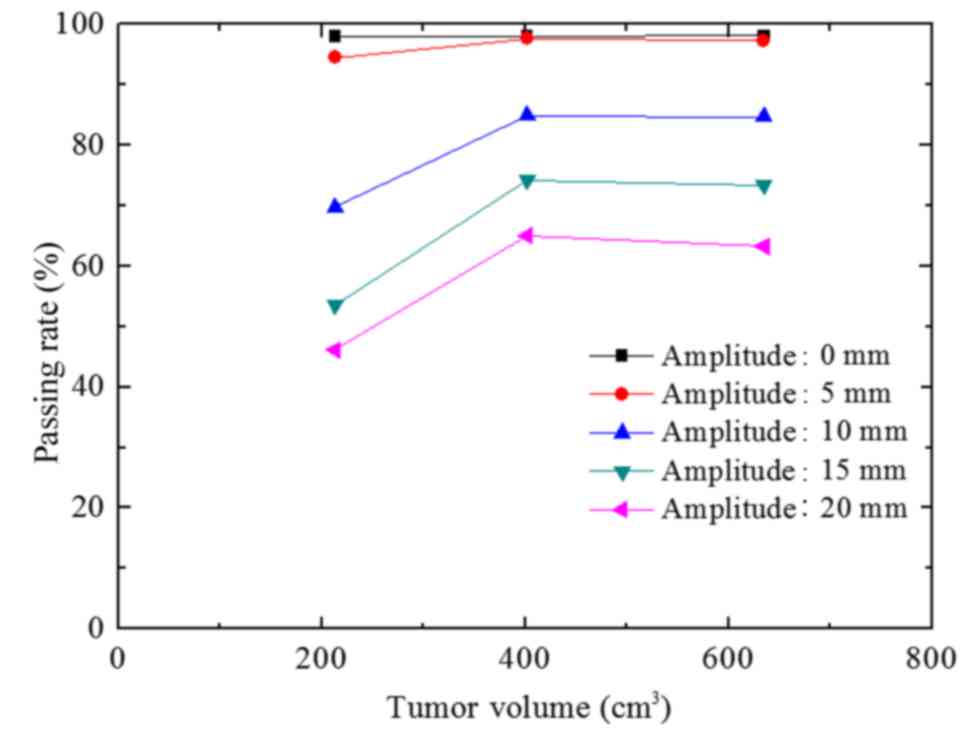
The passing rate of group C was similar to that of
group B (Fig. 8). However, with
increasing A values, the descending trend of group C was more
evident compared with that of group B.
Association between the tumor volume
and passing rate
The association between respiratory amplitude,
volume and passing rate under different breathing cycles was
analyzed. When the average tumor volume decreased from 635
cm3 (group A) to 402 cm3 (group B), the
maximum passing rate deviation was 1.7%, (P>0.05; Fig. 9). However, when the average tumor
volume decreased from 402 cm3 (group B) to 213
cm3 (group C), the passing rate significantly decreased,
with a maximum deviation of 20.6% (T=4 sec). The association
between the passing rate and tumor volume at T=5 and 6 sec were
similar to that at T=4 sec (Figs. 10
and 11).
Discussion
The correct implementation of IMRT is not achievable
without assessment of the radiation field output dose. Tumor motion
affects the accuracy of the dose distribution (23). IMRT plan conformal indices may predict
the effects of respiratory motion on the dose distribution
(24).
Comparison of the TPS dose fluxgraph and the
measured dose fluxgraph revealed that the increase in A value
reduced high-dose target areas, enlarged low-dose target areas and
blurred the isodose margin. The respiratory motion caused dose
perturbation and resulted in a blurred isodose margin (25).
Increased A value reduced the passing rate (Figs. 6–8). The
A value deviation was statistically significant at 10 mm (P=0.001).
When the A value was <5 mm, the passing rate of the irradiation
field was >90%. Schaefer et al (26) investigated the dose distribution at an
A value of 8 mm. The results demonstrated that the deviation was
usually <5%. This is likely due to the respiratory motion caused
by movement of the target area, suggesting that the irradiation
field and target area moved in association with each other. When
the A value decreased, the ratio of the deviation area accounting
for the target diminished. This effect resulted in the ascending
trend of the passing rate. When the size of the irradiation field
was increased, the ratio of the deviation area accounting for the
target area also increased. This effect resulted in the descending
trend of the passing rate.
Figs. 6–8 demonstrate the lack of statistical
significance of the association between the passing rate and T
value (P>0.05). This result was likely because the respiratory
period was relatively short compared with the overall treatment
time.
When the tumor volume was decreased from 635
cm3 (group A) to 402 cm3 (group B), the
passing rate decreased to 5.6%, and when the tumor volume was
decreased from 402 cm3 (group B) to 213 cm3
(group C), the passing rate significantly decreased (P=0.004).
These results indicate that the passing rate was closely associated
with tumor volume. This result supports that of decreased passing
rate with increased A value, as the proportion of the tumor
exceeding the irradiation field due to respiratory motion would
increase. These results suggest that the effect of respiratory
motion on the dose distribution should be considered more carefully
for smaller tumors (26).
In lung tumor radiotherapy, the deviation in dose
distribution caused by respiratory motion requires careful
consideration. Respiratory gating and autonomous respiration
control methods are often used to reduce the tumor dose (12,27). An
internal target volume treatment plan based on the 4D CT images
should be designed for additional effectiveness (28).
To conclude, at constant A and T values, lung tumor
volume was demonstrated to be proportional to dose verification γ
analysis passing rate. Large tumors achieved high passing rates,
and small tumors achieved low passing rates. At a constant tumor
volume, a descending trend in passing rate with increasing A value
was revealed. The respiratory period demonstrated no association
with passing rate. Therefore, A values should be carefully
controlled in clinical radiotherapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Jiangsu Province Research of China (grant no.
BK20151181), the High-Level Medical Talents Training Project of
Changzhou (grant no. 2016CZLJ004) and the Municipal Social
Development Project of the Changzhou City (grant no.
CJ20160029).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KX and XN designed the study. HS and TL collected
the data. LG and JS performed the statistical analysis and helped
to draft the manuscript. All authors approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Clinical
Ethics Committee of Second People's Hospital of Changzhou of
Nanjing Medical University (Changzhou, China). All patients
provided written informed consent for their inclusion in the
current study.
Consent for publication
All patients consented to publication.
Competing interests
The authors declare that there are no competing
interests.
Authors' information
KX, HS, LG, TL, JS and XN are affiliated with the
Radiotherapy Department, Second People's Hospital of Changzhou,
Nanjing Medical University, Changzhou, Jiangsu 213003, P.R.
China
References
|
1
|
Shi HS, Gao X, Li D, Zhang QW, Wang YS,
Zheng Y, Cai LL, Zhong RM, Rui A, Li ZY, et al: A systemic
administration of liposomal curcumin inhibits radiation pneumonitis
and sensitizes lung carcinoma to radiation. Int J Nanomed.
7:2601–2611. 2012.
|
|
2
|
Li Y, Wang J, Tan L, Hui B, Ma X, Yan Y,
Xue C, Shi X, Drokow EK and Ren J: Dosimetric comparison between
IMRT and VMAT in irradiation for peripheral and central lung
cancer. Oncol Lett. 15:3735–3745. 2018.PubMed/NCBI
|
|
3
|
Sumida I, Yamaguchi H, Das IJ, Kizaki H,
Aboshi K, Tsujii M, Yamada Y, Suzuki O, Seo Y, Isohashi F and Ogawa
K: Intensity-modulated radiation therapy dose verification using
fluence and portal imaging device. J Appl Clin Med Phys.
17:259–271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park JY, Lee JW, Choi KS, Lee JS, Kim YH,
Hong S and Suh TS: Development of a novel quality assurance system
based on rolled-up and rolled-out radiochromic films in volumetric
modulated arc therapy. Med Phys. 38:6688–6696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanooka M, Doi H, Miura H, Inoue H, Niwa
Y, Takada Y, Fujiwara M, Sakai T, Sakamoto K and Kamikonya N:
Three-dimensional radiochromic film dosimetry for volumetric
modulated arc therapy using a spiral water phantom. J Radiat Res.
54:1153–1159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bucciolini M, Buonamici FB and Casati M:
Verification of IMRT fields by film dosimetry. Med Phys.
31:161–168. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gloi AM, Buchana RE, Zuge CL and Goettler
AM: RapidArc quality assurance through MapCHECK. J Appl Clin Med
Phys. 12:39–47. 2011. View Article : Google Scholar
|
|
8
|
Li QL, Deng XW, Chen LX, Huang XY and
Huang SM: The angular dependence of a 2-dimensional diode array and
the feasibility of its application in verifying the composite dose
distribution of intensity-modulated radiation therapy. Chin J
Cancer. 29:617–620. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xin-Ye N, Ren L, Yan H and Yin FF:
Sensitivity of 3D dose verification to multileaf collimator
misalignments in stereotactic body radiation therapy of spinal
tumor. Technol Cancer Res Treat. 15:NP25–NP34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He R and Yang C: SU-FF-T-359: MapPhan with
MapCHECK and Im'RT MatriXX for real gantry angle IMRT QA. Medical
Physics. 36:2604. 2009. View Article : Google Scholar
|
|
11
|
García-Vicente F, Fernández V, Bermúdez R,
Gómez A, Pérez L, Zapatero A and Torres JJ: Sensitivity of a
helical diode array device to delivery errors in IMRT treatment and
establishment of tolerance level for pretreatment QA. J Appl Clin
Med Phys. 13:36602012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Webb S: Motion effects in (intensity
modulated) radiation therapy: A review. Phys Med Biol.
51:R403–R425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Starkschall G, Britton K, McAleer MF,
Jeter MD, Kaus MR, Bzdusek K, Mohan R and Cox JD: Potential
dosimetric benefits of four-dimensional radiation treatment
planning. Int J Radiat Oncol Biol Phys. 73:1560–1565. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Keall PJ, Mageras GS, Balter JM, Emery RS,
Forster KM, Jiang SB, Kapatoes JM, Low DA, Murphy MJ, Murray BR, et
al: T The management of respiratory motion in radiation oncology
report of AAPM task group 76. Med Phys. 33:3874–3900. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duan J, Shen S, Fiveash JB, Popple RA and
Brezovic IA: Dosimetric and radiobiological impact of dose
fractionation on respiratory motion induced IMRT delivery errors: A
volumetric dose measurement study. Med Phys. 33:1380–1387. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Low DA, Harms WB, Mutic S and Purdy JA: A
technique for the quantitative evaluation of dose distributions.
Med Phys. 25:656–661. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang SB, Pope C, Al Jarrah KM, Kung JH,
Bortfeld T and Chen GT: An experimental investigation on
intra-fractional organ motion effects in lung IMRT treatments. Phys
Med Biol. 48:1773–1784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Court L, Wagar M, Berbeco R, Reisner A,
Winey B, Schofield D, Ionascu D, Allen AM, Popple R and Lingos T:
Evaluation of the interplay effect when using RapidArc to treat
targets moving in the craniocaudal or right-left direction. Med
Phys. 37:4–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Newhauser W: ICRU PRESCRIBING, recording
and reporting photon beam therapy. international commissions on
radiation units and measurements (Supplement to ICRU report 50):
Bethesda, MD, USA. Report 62. Radiation Protection Dosimetry.
133:60–62. 2009. View Article : Google Scholar
|
|
20
|
Langen KM and Jones DT: Organ motion and
its management. Int J Radiat Oncol Biol Phys. 50:265–278. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ozhasoglu C and Murphy MJ: Issues in
respiratory motion compensation during external-beam radiotherapy.
Int J Radiat Oncol Biol Phys. 52:1389–1399. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimizu S, Shirato H, Ogura S,
Akita-Dosaka H, Kitamura K, Nishioka T, Kagei K, Nishimura M and
Miyasaka K: Detection of lung tumor movement in real-time
tumor-tracking radiotherapy. Int J Radiat Oncol Biol Phys.
51:304–310. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seco J, Sharp GC, Turcotte J, Gierga D,
Bortfeld T and Paganetti H: Effects of organ motion on IMRT
treatments with segments of few monitor units. Med Phys.
34:923–934. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Happersett L, Mageras GS, Zelefsky MJ,
Burman CM, Leibel SA, Chui C, Fuks Z, Bull S, Ling CC and Kutcher
GJ: A study of the effects of internal organ motion on dose
escalation in conformal prostate treatments. Radiother Oncol.
66:263–270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bortfeld T, Jiang SB and Rietzel E:
Effects of motion on the total dose distribution. Semin Radiat
Oncol. 14:41–51. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schaefer M, Münter MW, Thilmann C,
Sterzing F, Haering P, Combs SE and Debus J: Influence of
intra-fractional breathing movement in step-and-shoot IMRT. Phys
Med Biol. 49:N175–N179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Korreman SS: Motion in radiotherapy:
Photon therapy. Phys Med Biol. 57:R161–R191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoon MS, Jeong JU, Nam TK, Ahn SJ, Chung
WK and Song JY: Evaluation of dose distribution in intensity
modulated radiosurgery for lung cancer under condition of
respiratory motion. PLoS One. 11:e01631122016. View Article : Google Scholar : PubMed/NCBI
|