Introduction
Cervical cancer remains one of the most frequent
gynecological malignancies in females around the world, which leads
to the highest morbidity and mortality in young women, particularly
in developing countries (1).
Approximately 98,900 new cases and 30,500 cancer deaths due to
cervical cancer are estimated to occur in China each year (2). Cervical cancers frequently infiltrate
into the neighboring tissue and metastasize to other organs
resulting in a poor prognosis. Although great advanced protocols
including operation, chemotherapy and postoperative radiotherapy
have been established in the therapy of cervical cancer, the
long-term outcomes of clinical therapy remain unsatisfactory and
most patients succumb to metastasis (3). Accumulating research on the molecular
mechanism of progression and carcinogenesis has produced a number
of new potential molecular biomarkers which are likely to be the
targets of cervical cancer (4), but
there are few biomarkers used in predicting of the disease.
Therefore, it is critical to identify specific types of molecular
biomarkers which may accurately predict disease outcome before the
standard treatment. The molecular biomarkers for the early
identification of cervical cancer patients, may contribute to
understanding cervical carcinogenesis and ascertaining diagnostic
and therapeutic strategies, assessing prognosis, and monitoring
response to therapy.
The group of highly conserved, small non-coding RNAs
(18–24 nucleotides) (5) the microRNAs
(miRNAs) have critical roles in modulating in length the
physiological process or pathogenesis by regulating the translation
or degradation of target messenger RNA (6). Accumulating evidence has indicated that
the dysfunction of miRNAs occur in a wide range of human tumors and
can regulate cancer progressions that are involved in cell
differentiation, proliferation, metastasis, and apoptosis (7–9). In
addition, the dysfunctions of miRNAs act as either tumor
suppressors or promoters (10), and
studies have suggested miR-211 could act as boh a suppressor and a
promoter, and it can be considered quite contradictory. miR-211 was
detected at a significantly low level in various types of cancers,
such as hepatocellular carcinoma, melanoma, glioma, epithelial
ovarian cancer (11–14), and on the contrary, miR-211 was
upregulated in other tumors, for example human non-small cell lung
cancer, and colorectal cancer (15,16).
Previous studies have indicated that miR-211 inhibited cervical
cancer cell invasion and epithelial-to-mesenchymal transition (EMT)
through targeting MUC4 (17).
However, the miR-211 expression remains unclear in cervical cancer
tissues. So the regulatory mechanism of miR-211 on cervical cancer
needs further investigation.
Secreted protein acidic and rich in cysteine
(SPARC), as a member of the matricellular family of secreted
proteins, can modulate cell matrix interactions and affect cell
progression, such as cell adhesion, tissue repair and remodeling
(18,19). SPARC is taken to be an oncogene on
account of its high expression in a great range of tumors including
gastric carcinomas, prostate cancer, glioma, and cervical cancer
(20–23). A previous study has demonstrated that
overexpression of SPARC has significant connection with negative
prognostic clinicopathological characteristics and might function
as an important point in EMT of cervical cancer (23).
miR-211 was frequently downregulated and the miR-211
overexpression remarkably suppressed cell proliferation, invasion
and migrationx in vivo as shown in our study. Furthermore,
we confirmed SPARC is a direct and functional target of miR-211.
The newly identified miR-211/SPARC partially elucidates the
cervical cancer molecular mechanism of proliferation, invasion and
migration, and represents a therapeutic point for cervical cancer
treatment.
Materials and methods
Tissue samples
Cervical cancer and the corresponding normal tissues
from 52 patients who underwent surgery were obtained at Yuhuangding
Hospital (Yantai, China) from 2014 to 2016. Histopathological
diagnoses were based on the WHO classification and clinical stages
classification followed the International Federation of Gynecology
and Obstetrics criteria (FIGO). The characteristics of patients
with cervical cancer are described in Table I. The patients signed informed consent
and the study was approved by the Ethics Committee of Yuhuangding
Hospital (Yantai, China).
 | Table I.Clinicopathological variables and
miR-211 expression in 52 cervical cancer patients. |
Table I.
Clinicopathological variables and
miR-211 expression in 52 cervical cancer patients.
|
|
| miR-211
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Cases (n=52) | Low (%) | High (%) | P-value |
|---|
| Age (years) |
|
|
| 0.7232 |
| ≤40 | 16 | 12 (75.0) | 4 (25.0) |
|
|
>40 | 36 | 28 (77.8) | 8 (22.2) |
|
| Tumor size (cm) |
|
|
| 0.4989 |
|
<3 | 26 | 18 (69.2) | 8 (30.8) |
|
| ≥3 | 26 | 16 (61.5) | 10 (38.5) |
|
| Local relapse |
|
|
| 0.0264a |
| Yes | 10 | 5 (50.0) | 5 (50.0) |
|
| No | 42 | 39 (92.9) | 3 (7.1) |
|
| Distant
metastasis |
|
|
| 0.0253a |
| Yes | 22 | 12 (54.5) | 10 (45.5) |
|
| No | 30 | 25 (83.3) | 5 (16.7) |
|
| FIGO stage |
|
|
| 0.0395a |
| IB | 28 | 16 (57.1) | 12 (42.9) |
|
|
IIA | 24 | 16 (66.7) | 8 (33.3) |
|
|
Differentiation |
|
|
| 0.3158 |
|
Well | 14 | 8 (57.1) | 6 (42.9) |
|
|
Moderate/poor | 38 | 26 (68.4) | 12 (31.6) |
|
Cell culture
Primary normal cervical squamous cells (NCSC) and
two human cervical cancer cell lines (HeLa and C33A) were obtained
from ATCC (Manassas, VA, USA). The cells were cultured in RPMI-1640
medium supplemented with 10%, fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cell transfection
The cells were transfected with miR-211
mimics/inhibitor as well as the corresponding control using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), respectively. Cells were used for cell
proliferation, migration and invasion assays after transfection.
All transfection was conducted in three times. miR-211 mimic
5′-UUCCCUUUGUCAUCCUUCGCCU-3′, miR-211 inhibitor
5′-AGGCGAAGGAUGACAAAGGGAA-3′, and a negative control
oligonucleotide were designed and synthesized by Guangzhou RiboBio
Co., Ltd. (Guangzhou, China). In order to construct SPARC
overexpressing plasmid, the gene without 3′-UTR was amplified from
cDNA of human normal cervical tissues by polymerase chain reaction
(PCR).
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA of cervical cells and tissues were
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturers instructions.
Then, 5 ng of total RNA was reverse transcribed using a TaqMan
miRNA Reverse Transcription Kit (Applied Biosystems, Foster City,
CA, USA), and the expression levels of mature forms of miR-211 was
determined using an miRNA-specific TaqMan MiRNA Assay Kit. To
determine the mRNA levels of SPARC, total RNA (500 ng) was reverse
transcribed using PrimeScript® RT Master Mix (Takara
Biotechnology Co., Ltd., Dalian, China). reverse transcriptase
according to the manufacturers instructions, and RT-qPCR analyses
were performed using SYBR-Premix Ex Taq™ (Takara Biotechnology Co.,
Ltd., Dalian, China). U6 and GAPDH acted as the internal control
for the expression of miR-211 and SPARC. The primers of SPARC and
GAPDH were synthesized by Invitrogen; Thermo Fisher Scientific,
Inc. The experiments were performed in triplicate. The
transcription primer and PCR primer of miR-211 and U6 were
purchased from Guangzhou RiboBio Co., Ltd. The sequences of the
primers were as follows: miR-211 F, 5′-TCGGCAGGTCCCTTTGTCATCC-3′
and R, 5′-TGCAGGTCAACTGGTGTCGT-3′; U6 F,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and R,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; SPARC F, 5′-AGCACCCCATTGACGGGTA-3′
and R, 5′-GGTCACAGGTCTCGAAAAAGC-3′, GAPDH F,
5′-CTGGGCTACACTGAGCACC-3′ and R, 5′-AAGGGTCGTTGAGGGCAATG-3′.
Western blot analysis
Proteins were isolated from cervical cells with
different transfection using RIPA lysis buffer (Thermo Fisher
Scientific, Inc.) phenylmethylsulfonyl fluoride. The measurement of
protein concentration used bicinchoninic acid assay kit (Beyotime
Institute of Biotechnology, Haimen, China). The protein was
transferred onto polyvinylidene fluoride membrane (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Then, the membranes were
blocked by 5% bovine serum albumin and incubated with specific
primary antibody rabbit polyclonal anti-SPARC antibody (dilution
1:1,000; cat. no. ab209556; Abcam, Cambridge, MA, USA). After that,
the membrane was incubated in the secondary antibody goat
polyclonal anti-rabbit IgG H&L secondary antibody (dilution
1:2,000; cat. no. ab150077; Abcam). The bands were subjected to
quantification using the ImageJ imaging processing program
(National Institutes of Health, Bethesda, MD, USA).
CCK-8 assay
Cell Counting kit-8 (CCK-8) (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was performed to detect cell
proliferation. Cervical cells transfected with miR-211
mimics/inhibitor were seeded into 96-well plates. Then each plate
was added with 10 µl of CCK-8 reagent. The absorbance of each well
at 0, 24, 48, 72 and 96 h was detected at 450 nm. All experiments
were performed three times.
Transwell assay
The Transwell assay with or without Matrigel
(Clontech Laboratories, Inc., Mountainview, CA, USA) was chosen to
measure the migration and invasion. Cervical cells
(5×105) were seeded on the top 24-well Transwell
chamber. RPMI-1640 medium with 20% FBS was added to the
lowerchamber. The cells on the low chambers were removed using
cotton swab. Then the cells on the top chamber were fixed with
methanol, stained with 0.05% crystal violet. Finally, the migrated
or invaded cells were counted under an inverted microscope (IX31;
Olympus Corporation, Tokyo, Japan) and images were captured at ×200
magnification.
Luciferase assay
The bioinformatics analysis software TargerScan
(http://www.targetscan.org/) and miRanda
(http://www.microrna.org/microrna/home.do) were chosen
for predicting the targets of miR-211. The 3′-UTRs of SPARC were
amplified with PCR from genomic DNA. The wild-type (WT) and mutant
type (MT) 3′-UTR of SPARC were cloned into the pMIR-REPORT
luciferase vector (Ambion; Thermo Fisher Scientific, Inc.) and
verified by sequencing. For the luciferase assay, the cells were
co-transfected with miR-211 mimics and WT or MT 3′-UTR of SPARC
luciferase reporter plasmid. Then we used dual-luciferase reporter
assay system (Promega Corporation, Madison, WI, USA) for measuring
the reporter activities. Renilla luciferase activity was used as
normalization.
Statistical analysis
The results are presented as the mean ± standard
deviation (SD). Statistical analysis was conducted using the SPSS
19.0 statistical software (IBM Corp., Armonk, NY, USA). Each
experiment was repeated at least three times. Student's t-test or
Tukey-Kramer post hoc test after one-way analysis of variance
(ANOVA) in SPSS were used to analyze the differences between the
groups. Correlation between mRNA and miRNA were estimated using the
Spearman's correlation method. P<0.05 was considered to be a
significant difference.
Results
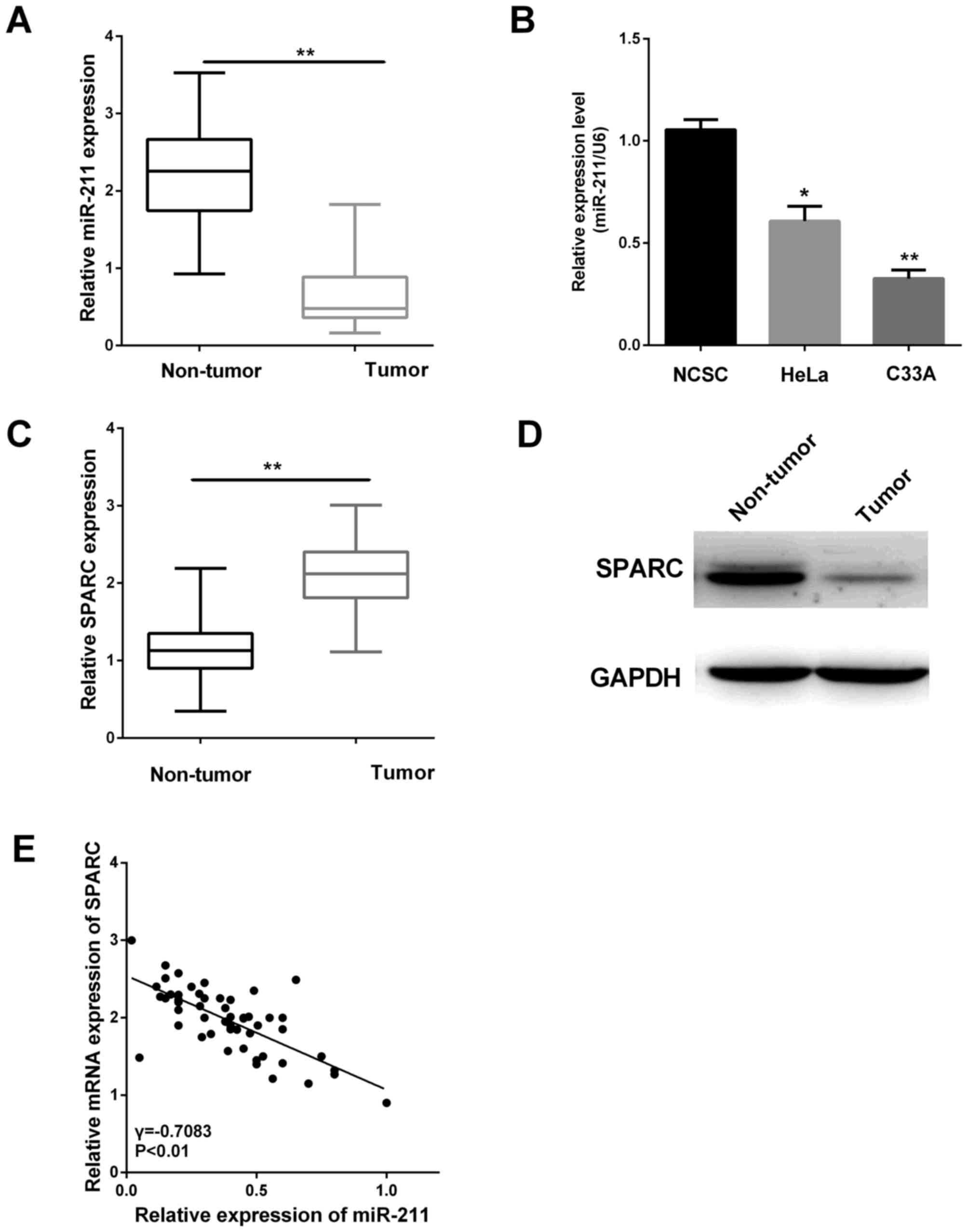
miR-211 expression is downregulated
and inversely correlates with SPARC expression
To investigate whether the miR-211 expression was
altered in cervical tissues, RT-qPCR was performed in 52 pairs of
cervical tissues. The result suggested the miR-211 level was lower
in cervical tissues (Fig. 1A,
P<0.01). We further measured miR-211 expression in cervical
cancer cell lines (HeLa and C33A) compared with the normal NCSC
cells. The results showed the miR-211 expression in HeLa and C33A
were significantly reduced relative to that in the normal NCSC
cells (Fig. 1B, P<0.05,
P<0.01).
Furthermore, the SPARC expression levels in 52 pairs
of cervical tissues were also assessed by RT-qPCR and western blot
analysis. The relative expression of SPARC was higher in cancer
tissues as shown in Fig. 1C and D
(P<0.01). Then, we found there was an inverse correlation
between miR-211 expression and the SPARC level in these clinical
specimens (Fig. 1E). This correlation
may play an essential part in the cervical cancer progression.
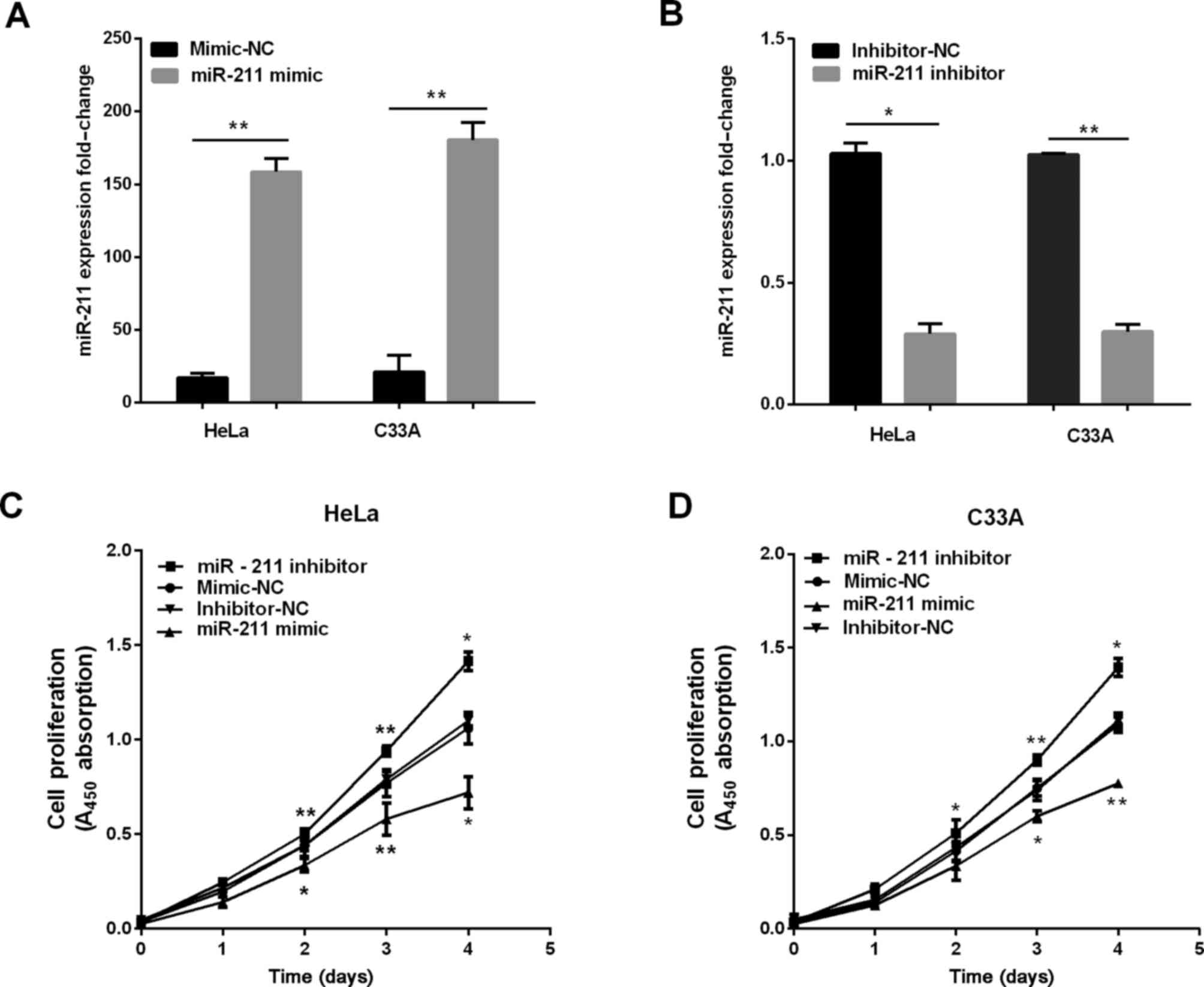
miR-211 inhibits cervical cell
proliferation, migration and invasion
We established stable cells to investigate the
effect of miR-211 in the progression of cervical cancer in both
HeLa and C33A cells, and the overexpression of miR-211 was detected
using RT-qPCR. As shown in Fig. 2A and
B, the miR-211 expression was higher when cells were
transfected with miR-211 mimics, and the expression of miR-211 was
lower when transfected with miR-211 inhibitor. Subsequently, CCK-8
was used to measure the potential of miR-211 in cervical cell
proliferation. The results demonstrated overexpression of miR-211
inhibited cervical cell proliferation, and knockdown of miR-211
dramatically promoted proliferative abilities as determined by
CCK-8 assay (Fig. 2D).
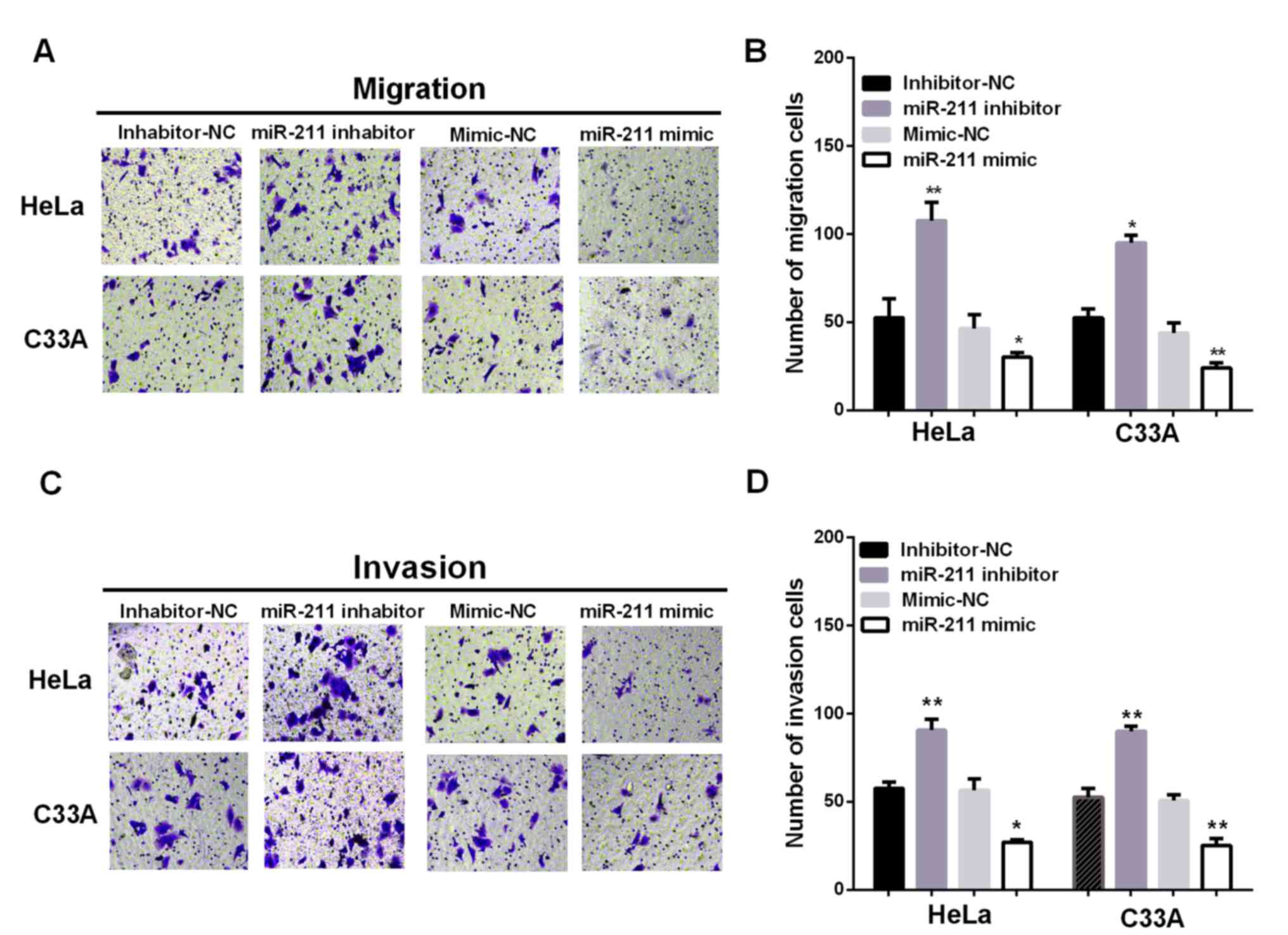
Additionally, to investigate the effect of miR-211
in metastasis, Transwell chambers were conducted to test the
migration and invasion ability of cervical cells with different
transfection. Ectopic expression of miR-211 significantly
suppressed HeLa and C33A cell migration, whereas inhibiting miR-211
expression promoted HeLa and C33A cell migration (Fig. 3A and B; P<0.05). In the cell
invasion assay, overexpression of miR-211 dramatically suppressed
HeLa and C33A cell invasion, whereas inhibition of miR-211
expression significantly promoted HeLa and C33A cell invasion
(Fig. 3C and D; P<0.05). These
results suggested miR-211 can inhibit the migratory and invasive
ability of cervical cancer cells.
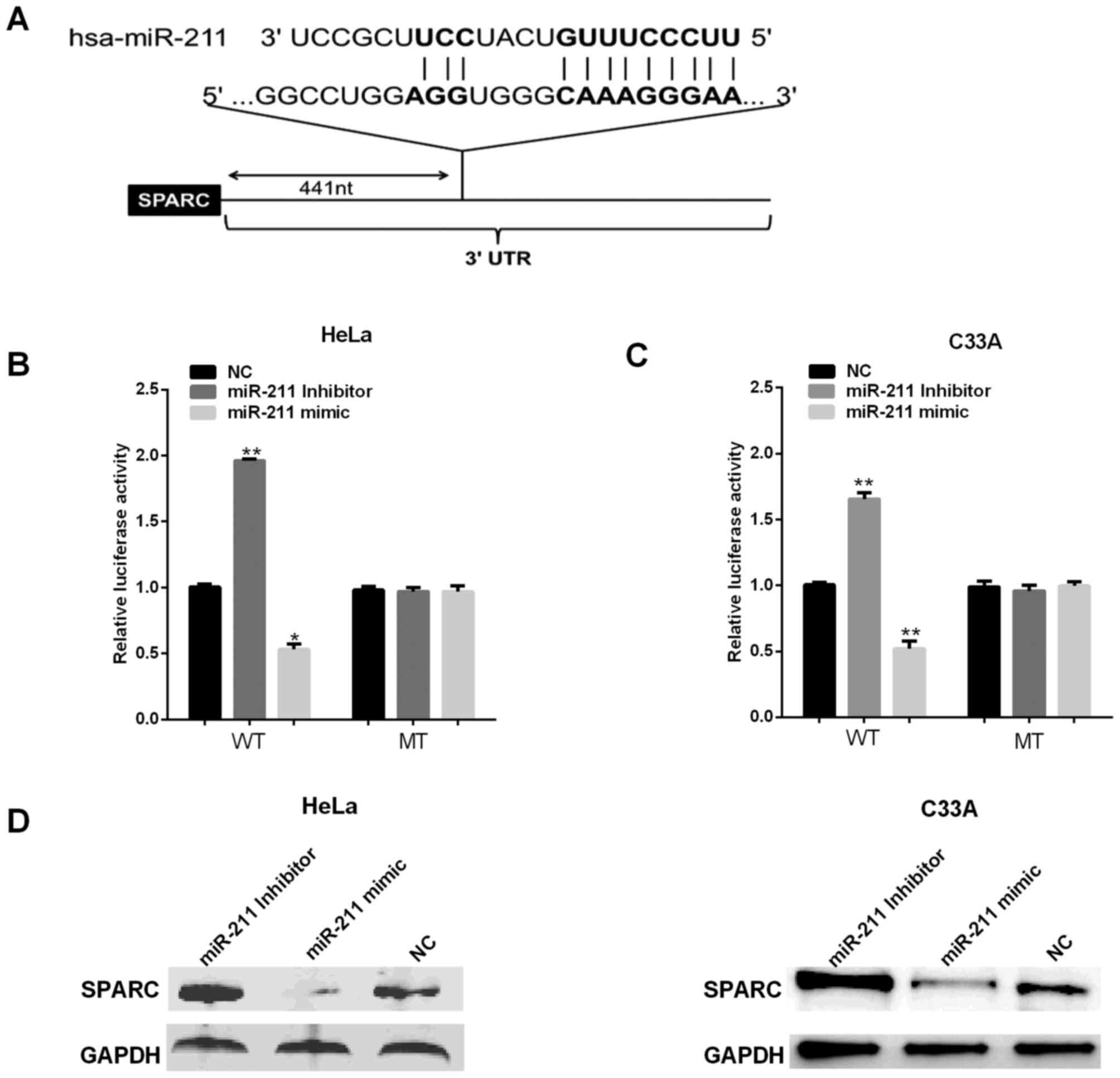
SPARC is the direct target of miR-211
in cervical cells
Bioinformatics analysis software TargetScan and
miRanda were used for predicting the targets of miR-211. The
putative binding sites for miR-211 were found at the 3′-UTR of
SPARC (Fig. 4A). To further confirm
whether the 3′-UTR of SPARC can be directly targeted by miR-211, WT
pmirGLO-UTR of SPARC 3′-UTR or MT pmirGLO-UTR of SPARC 3′-UTR
vector was co-transfected into two cell lines with either miR-211
mimic/inhibitor or negative controls (NC), followed by the
measurement with luciferase reporter assays. Fig. 4B and C shows that reductions were
approximately 40% in HeLa cell and 45% in C33A cells of the
luciferase activity in all cells transfected with the miR-211 mimic
and SPARC 3′-UTR WT vector, whereas the repressive effect of
miR-211-inhibitor increased luciferase activity in WT SPARC by
approximately 46 and 35%, but the luciferase activity had no
significant change in cells transfected with SPARC 3′UTR-containing
mutant miR-211-binding sites. We next assessed the miR-211 effect
on its potential target SPARC expression at protein level. To this
end, miR-211 mimics/inhibitor were transfected into HeLa and C33A
cells, respectively, SPARC expression was measured using western
blot analysis. The results show miR-211 mimics decreased the
protein level of SPAR (Fig. 4C and
D), while miR-211 inhibitor enhanced SPARC expression.
Together, these results demonstrated miR-211 negatively regulated
endogenous SPARC expression in HeLa and C33A cells.
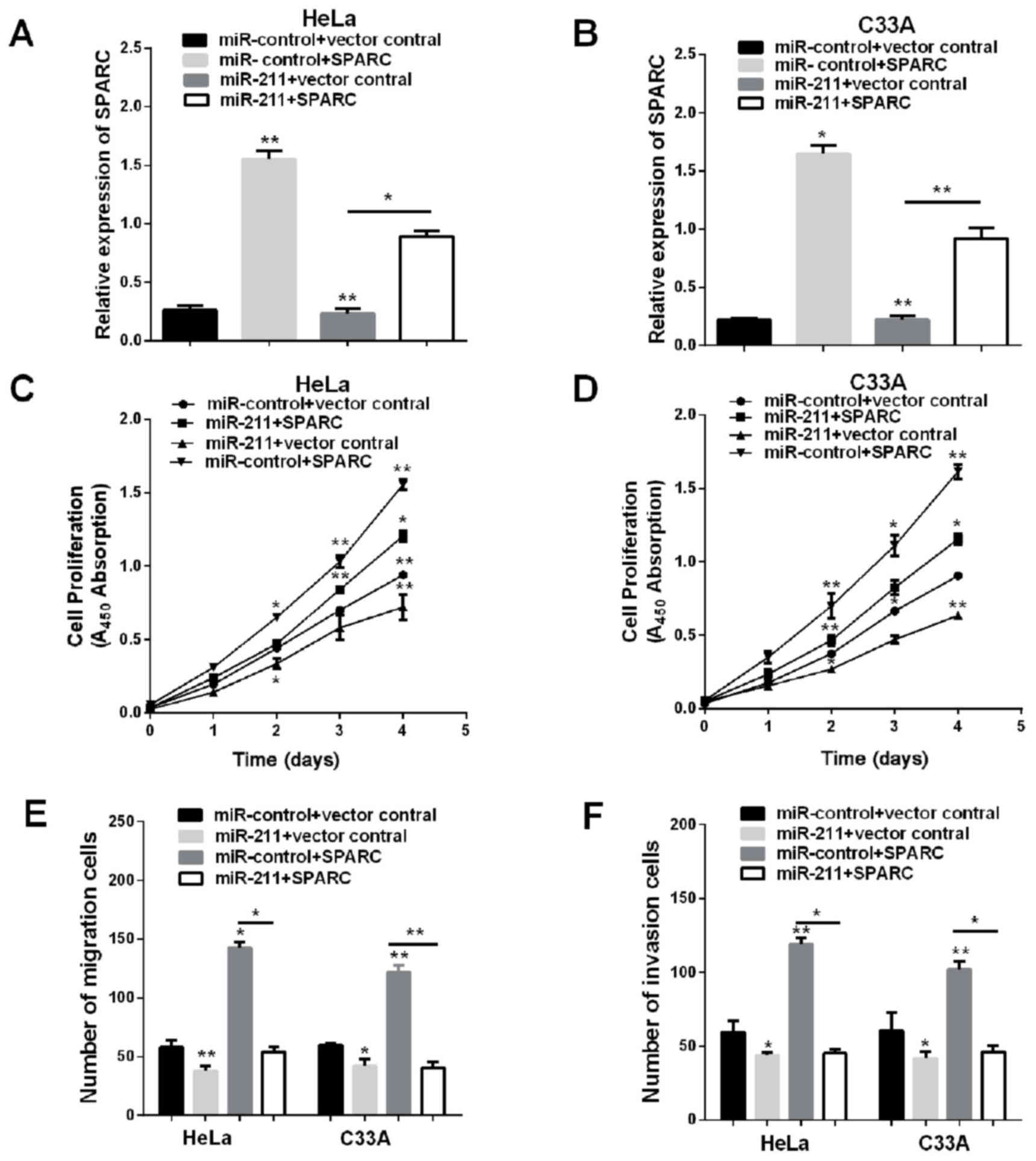
Overexpression of SPARC restores the
inhibited proliferation, migration and invasion function of
miR-211
The evidence given indicates that miR-211 may have
various types of potential targets in HeLa and C33A cells, the
anti-proliferative effect of miR-211 may not be limited to
repression of SPARC. To detect whether overexpression of SPARC
would simulate miR-211-mediated effects, transfection of
pmirGLO-SPARC and co-transfection of pmirGLO-SPARC and miR-211
increased the mRNA expression of SPARC in HeLa and C33A cells,
co-transfection of pmirGLO-SPARC and miR-211 abrogated the effects
of miR-211 on SPARC expression (Fig. 5A
and B), and then we calculated the capabilities of cell
proliferation, migration and invasion. Restoration of SPARC
eliminated cell viability, invasion and migration that was reduced
by miR-211 mimic (Fig. 5C-F). These
results demonstrated that SPARC is a direct functional target gene
of miR-211 and that miR-211 functions as a tumor suppressor through
SPARC.
Discussion
Mounting evidence has demonstrated that miR-211 acts
as an inhibitor in the progression of cancer cell growth, invasion
and migration in many kinds of cancers (12,14,24,25).
However, the molecular mechanism by which miR-211 regulates the
progression and development of cervical cancer has not been fully
investigated. This investigation demonstrated the synthesized
analysis of miR-211 effects on cervical cancer. The miR-211
expression is downregulated in cervical cancer in vivo, and
reduced miR-211 levels have inverse correlation with SPARC
expression. The results indicated miR-211 might act as a suppressor
and play a significantly important role of cervical cancer growth
and metastasis.
There is increasing evidence demonstrating that
miRNAs can function as an crucial point in gene expressions, and
then influence the tumor development and procession (26). The miR-211 expression was
downregulated in various tumors, for example glioma (13), and ovarian cancer (14). However, the biological effect of
miR-211 remains poorly understood in cervical cancer. In the study,
the findings showed the miR-211 can inhibit growth, migration and
invasion, and indicated the crucial point of miR-211 in cervical
cancer progression.
This research revealed the relative expression level
of SPARC was higher in cervical cancer tissues. SPARC can be found
widely expressed in tumors and could function as an important
member in regulating cell invasiveness, survival and tumor-stroma
interactions to expedite cancer progression. Two different
bioinformatics analysis software were used for predicting the
targets of miR-211. SPARC was looked for as the potential gene
effector which may participate in the biological function of
miR-211. We confirmed that SPARC was a direct target, and the
higher SPARC expression was confirmed taking significant part in
tumor invasion and metastasis in certain cancers, such as gastric
cancer (20), melanoma (27), and hepatocellular carcinoma (28). In our study, overexpression of SPARC
restored the inhibited proliferation, migration and invasion
function of miR-211, these results demonstrated that SPARC is a
direct functional target gene of miR-211 and that miR-211 functions
as a tumor suppressor through SPARC.
In conclusion, miR-211 acts as a tumor suppressor by
reducing cell growth, migration and invasion in cervical cancer.
Furthermore, we noted that miR-211 has an inverse correlation with
SPARC and targets it by binding to its 3′-UTR. This newly
identified miR-211 may provide further insight into progression and
offers a promising therapeutic target for the treatment of cervical
cancer. Further study to investigate the function of miR-211/SPARC
in tumorigenesis and progression of cervical cancer is needed.
Acknowledgements
Not applicable.
Funding
This study did not receive any specific grant from
funding agencies in the public, commercial, or not-for-profit
sectors.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XQ and DG contributed to the conception of the
study. QR contributed significantly to the data analysis and study
preparation. XJ and JB performed the data analyses and wrote the
study. LS helped perform the analysis with constructive
discussions. All authors have read and approved the final
study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Yuhuangding Hospital (Yantai, China) and the patients signed the
informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pimple S, Mishra G and Shastri S: Global
strategies for cervical cancer prevention. Curr Opin Obstet
Gynecol. 28:4–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun Y, Yang X, Liu M and Tang H: B4GALT3
up-regulation by miR-27a contributes to the oncogenic activity in
human cervical cancer cells. Cancer Lett. 375:284–292. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ojesina AI, Lichtenstein L, Freeman SS,
Pedamallu CS, Imaz-Rosshandler I, Pugh TJ, Cherniack AD, Ambrogio
L, Cibulskis K, Bertelsen B, et al: Landscape of genomic
alterations in cervical carcinomas. Nature. 506:371–375. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ambros V and Lee RC: Identification of
microRNAs and other tiny noncoding RNAs by cDNA cloning. Methods
Mol Biol. 265:131–158. 2004.PubMed/NCBI
|
|
6
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saumet A and Lecellier CH: microRNAs and
personalized medicine: Evaluating their potential as cancer
biomarkers. Adv Exp Med Biol. 888:5–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Srivastava SK, Arora S, Averett C, Singh S
and Singh AP: Modulation of microRNAs by phytochemicals in cancer:
Underlying mechanisms and translational significance. BioMed Res
Int. 2015:8487102015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Virant-Klun I, Ståhlberg A, Kubista M and
Skutella T: MicroRNAs: From female fertility, germ cells, and stem
cells to cancer in humans. Stem Cells Int. 2016:39849372016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ventura A and Jacks T: MicroRNAs and
cancer: Short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mazar J, Qi F, Lee B, Marchica J,
Govindarajan S, Shelley J, Li JL, Ray A and Perera RJ: MicroRNA 211
functions as a metabolic switch in human melanoma cells. Mol Cell
Biol. 36:1090–1108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng B, Qu L, Li J, Fang J, Yang S, Cao Z,
Mei Z and Sun X: MiRNA-211 suppresses cell proliferation, migration
and invasion by targeting SPARC in human hepatocellular carcinoma.
Sci Rep. 6:266792016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Lv J, Zhang F, Che H, Liao Q,
Huang W, Li S and Li Y: MicroRNA-211 expression is down-regulated
and associated with poor prognosis in human glioma. J Neurooncol.
133:553–559. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia B, Yang S, Liu T and Lou G: miR-211
suppresses epithelial ovarian cancer proliferation and cell-cycle
progression by targeting Cyclin D1 and CDK6. Mol Cancer. 14:572015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye L, Wang H and Liu B: miR-211 promotes
non-small cell lung cancer proliferation by targeting SRCIN1.
Tumour Biol. 37:1151–1157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sümbül AT, Göğebakan B, Bayram S, Batmacı
CY and Öztuzcu S: MicroRNA 211 expression is upregulated and
associated with poor prognosis in colorectal cancer: A case-control
study. Tumour Biol. 36:9703–9709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu D, Liu S, Zhang L and Song L: MiR-211
inhibits invasion and epithelial-to-mesenchymal transition (EMT) of
cervical cancer cells via targeting MUC4. Biochem Biophys Res
Commun. 485:556–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bornstein P and Sage EH: Matricellular
proteins: Extracellular modulators of cell function. Curr Opin Cell
Biol. 14:608–616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen J, Shi D, Liu X, Fang S, Zhang J and
Zhao Y: Targeting SPARC by lentivirus-mediated RNA interference
inhibits cervical cancer cell growth and metastasis. BMC Cancer.
12:4642012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao ZS, Wang YY, Chu YQ, Ye ZY and Tao
HQ: SPARC is associated with gastric cancer progression and poor
survival of patients. Clin Cancer Res. 16:260–268. 2010.https://doi.org/10.1158/1078-0432.CCR-09-1247
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Derosa CA, Furusato B, Shaheduzzaman S,
Srikantan V, Wang Z, Chen Y, Seifert M, Ravindranath L, Young D,
Nau M, et al: Elevated osteonectin/SPARC expression in primary
prostate cancer predicts metastatic progression. Prostate Cancer
Prostatic Dis. 15:150–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi Q, Bao S, Maxwell JA, Reese ED,
Friedman HS, Bigner DD, Wang XF and Rich JN: Secreted protein
acidic, rich in cysteine (SPARC), mediates cellular survival of
gliomas through AKT activation. J Biol Chem. 279:52200–52209. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi D, Jiang K, Fu Y, Fang R, Liu XI and
Chen J: Overexpression of SPARC correlates with poor prognosis in
patients with cervical carcinoma and regulates cancer cell
epithelial-mesenchymal transition. Oncol Lett. 11:3251–3258. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang CY, Hua L, Sun J, Yao KH, Chen JT,
Zhang JJ and Hu JH: MiR-211 inhibits cell proliferation and
invasion of gastric cancer by down-regulating SOX4. Int J Clin Exp
Pathol. 8:14013–14020. 2015.PubMed/NCBI
|
|
25
|
Wang L, Shen YF, Shi ZM, Shang XJ, Jin DL
and Xi F: Overexpression miR-211-5p hinders the proliferation,
migration, and invasion of thyroid tumor cells by downregulating
SOX11. J Clin Lab Anal e22293. 2017.
|
|
26
|
Manikandan J, Aarthi JJ, Kumar SD and
Pushparaj PN: Oncomirs: The potential role of non-coding microRNAs
in understanding cancer. Bioinformation. 2:330–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
An XJ, Li YQ, Qu XY, Zhang J, Zhang LY,
Wang M, Zhu L, Chen SY, Chen HX, Tu YT, et al: Silencing
endothelin-3 expression attenuates the malignant behaviors of human
melanoma cells by regulating SPARC levels. J Huazhong Univ Sci
Technolog Med Sci. 33:581–586. 2013.https://doi.org/10.1007/s11596-013-1162-3
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin ZY and Chuang WL: Genes responsible
for the characteristics of primary cultured invasive phenotype
hepatocellular carcinoma cells. Biomed Pharmacother. 66:454–458.
2012. View Article : Google Scholar : PubMed/NCBI
|