Introduction
Oesophageal cancer is the sixth leading cause of
cancer-associated mortality behind lung, liver, gastric,
colorectal, and breast cancer, worldwide in 2012 (1). In total, ~400,000 mortalities were
attributed to the disease worldwide in 2012 (2). The cancer includes two main sub-types;
oesophageal squamous-cell carcinoma (ESCC) and oesophageal
adenocarcinoma. While oesophageal adenocarcinoma is widespread in
Europe and the USA (2), ESCC is
diagnosed more often in Asia, Africa and South America (3). In Japan in 2009, 90.5% of oesophageal
cancers were squamous-cell carcinomas (4). Cisplatin is used in combination with
fluorouracil as first-line therapy, followed by docetaxel or
paclitaxel as second-line therapy to treat oesophageal cancer in
Japan (5). No treatment strategy has
been defined for patients with oesophageal cancer who are
refractory or intolerant to the standard therapies.
Fibroblast growth factor receptor-like 1 (FGFRL1)
belongs to the FGFR protein family (6). It has an extracellular FGF-binding site,
which is highly homologous with the other members of the family
(7). In contrast to other FGFRs, the
intracellular domain of FGFRL1 lacks the tyrosine kinase domain;
rather, it comprises the tandem tyrosine-based motif and the
histidine-rich region (8). Mice
lacking FGFRL1 expression exhibit malformation of the metanephros
(9,10)
and the diaphragm (11,12), and therefore succumb at birth. Thus,
FGFRL1 serves an essential function in normal development of the
kidney and the diaphragm. However, the mechanism by which FGFRL1
contributes to the development of those tissues remains
unresolved.
The authors of the present study reported previously
that transient inhibition of FGFRL1 expression induces cell cycle
arrest and apoptosis of ESCC cells (13). Expression of FGFRL1 tends to associate
with lymph node metastasis; therefore, high expression of FGFRL1 in
ESCCs suggests poor prognosis of patients (14). FGFRL1 in ESCC cells is frequently
co-expressed with either FGFR1 or FGFR4, and an in situ
proximity ligation assay indicated that FGFRL1 forms a hetero-dimer
with FGFR1 or FGFR4 (15). These
results suggest that FGFRL1 serves a function in the aggressive
behaviour of ESCCs.
Consistent with these results in ESCCs,
downregulation of FGFRL1 expression decreases proliferation of head
and neck squamous carcinoma cells (SCC10A) (16). Furthermore, ovarian tumours exhibit
aberrant expression of FGFRL1 (17),
and FGFRL1 mutation is observed frequently in colorectal tumours
(18). Taken together with the
results of the authors' previous study, the data in these studies
suggest that FGFRL1 serves an important function in cancer
generation and/or expansion.
In the present study, cell lines deficient for
FGFRL1 expression were established from KYSE520 ESCC cells, in
order to investigate the function of FGFRL1 in ESCC cells. FGFRL1
deficiency decreased cell motility and tumour growth in a mouse
xenograft model.
Materials and methods
Materials
Anti-actin (cat. no. ab8226), anti-fibroblast growth
factor binding protein 1 (FGFBP1) (cat. no. ab215353) and
anti-matrix metalloproteinase (MMP)-1 (ab38929) antibodies were
purchased from Abcam (Cambridge, UK). Anti-FGFRL1 (AAB1403271) was
purchased from Merck KGaA (Darmstadt, Germany). Alexa Fluor
488-labelled phalloidin (8878) was purchased from Cell Signalling
Technology, Inc. (Danvers, MA, USA).
Cell culture and genetic depletion of
the FGFRL1 gene using clustered regularly interspaced short
palindromic repeats (CRISPR)-cas9
The KYSE520 ESCC cell line was previously
established from human ESCC by the authors (19). KYSE520 cells were maintained on
collagen I-coated plates in Ham's F12/RPMI-1640 medium (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 5% fetal
bovine serum (FBS; Thermo Fisher Scientific, Inc.). For genetic
depletion of the FGFRL1 gene, KYSE520 cells were cotransfected with
tracrRNA, a plasmid encoding Cas9 (GE Healthcare, Chicago, IL, USA)
and crRNA (Fasmac, Inc., Kanagawa, Japan) for the FGFRL1 gene
(5′-CAGGGGGCUCGGCGUCAUCUGUUUUAGAGCUAUGCUGUUUUG-3′) using
Lipofectamine 3000 (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). At 24 h after the transfection, the cells were harvested with
trypsin and seeded in 10 cm diameter cell culture dishes at a
density of 2×104 cells/dish. Following overnight
culture, the medium was changed to Ham's F12/RPMI-1640 containing
5% FBS and 2 µg/ml puromycin and the cells were cultured for 3
days. The cells were then cultured in Ham's F12/RPMI-1640
containing 5% FBS without puromycin until colonies were visible.
Each colony was isolated and cultured separately. In order to
identify FGFRL1−/− cells, genomic DNA was prepared and used as a
template for PCR (forward primer: 5′-CTCCCAGTTCCACGTGTTAGTGACG-3′
and reverse primer; 5′-CGCCAGAACTCACCTC-3′). The thermocycling
procedure for PCR included 2 min at 98°C, followed by 23 cycles of
30 sec at 97°C, 30 sec at 58°C and 1 min at 72°C. Ex taq (Takara
Bio, Inc., Otsu, Japan) was used for amplification. The PCR
products were directly sequenced.
KYSE520 cell xenografts
All mice were handled and cared for in accordance
with the Guide of Care and Use of Laboratory Animals, and all
experiments were approved by the Ethics Committee of Experimental
Animals of Kyoto University (Kyoto, Japan). All surgical procedures
and postoperative care regimes were reviewed and approved by the
Animal Care and Use Committee of Kyoto University. Wild-type and
FGFRL1-deficient KYSE520 cells were harvested with trypsin and
resuspended in Matrigel (Corning Incorporated, Corning, NY, USA) at
a concentration of 1.5×107 cells/ml. Next, 0.2 ml
(3×106 cells) of the cell suspension was subcutaneously
injected into immunodeficient athymic Balb/c Slc-nu/nu mice (male,
7 weeks old; n=9; Japan SLC, Inc., Nishi-ku, Japan). The mice were
maintained on a 12-h light-dark schedule and given ad libitum
access to food and water. After 0, 2 and 4 weeks major and minor
axes of tumours were measured and tumour mass was calculated using
the formula (major axis) × (minor axis)2/2. As humane
endpoints, two conditions were set. If the major axis of the tumour
exceeded 20 mm, the experiment ended. If animals lost their weight
>15% compared with their age-matched control animals, they were
also removed from experiments. However, neither of these instances
occurred in the present study. At the conclusion of the experiment,
only single tumours were observed, and the maximum tumour volume
observed in the present study was 1,734.1 mm3.
Western blot analysis
Cells were harvested with trypsin and homogenised in
a buffer containing 50 mM Tris-HCl (pH 7.8), 150 mM NaCl, 1 mM
EDTA, 0.5 mM EGTA, 2 mM DTT, 0.5% TritonX-100, 2X PhosSTOP
phosphatase inhibitor mix (Roche Applied Science, Penzberg,
Germany) and 1× Complete Protease Inhibitor Cocktail (Roche Applied
Science). Following centrifugation at 15,000 × g for 15 min at 4°C,
the supernatant was used as the cell extract. Protein
concentrations of the cell extracts were assessed using the
Bradford method. Proteins (40 µg) were separated by SDS-PAGE and
then blotted onto a polyvinylidene difluoride membrane. The
membrane was incubated with blocking buffer containing 5% skimmed
milk and 0.05% Triton X-100 in PBS for 1 h at room temperature,
followed by the aforementioned primary antibodies in blocking
buffer overnight at 4°C and a POD-labelled secondary antibody
matched to the first antibody (1:2,000 dilution; cat. no. 7074 for
anti-rabbit IgG and 7076 for anti-mouse IgG; Cell Signalling, Inc.)
for 1 h at room temperature. Bound antibodies were visualised using
the ECL-Plus reagent (GE Healthcare). Dilutions of primary
antibodies for this study were as follows; anti-FGFRL1 (1:1,000),
anti-actin (1:2,000), anti-FGFR1 (1:100), anti-FGFR3 (1:200),
anti-MMP-1 (1:500) and anti-FGFBP1 (1:500).
Phalloidin staining
Cells were cultured at 37°C with 10% CO2
on a plastic coverslip coated with collagen I (Sumitomo-Bakelite
Co., Ltd., Tokyo, Japan). For FGF2 treatment, cells were incubated
in Ham's F12/RPMI-1640 medium without serum for 5 h at 37°C, after
which the medium was changed for Ham's F12/RPMI-1640 with or
without 20 nM FGF2 and 50 µg/ml heparin (Thermo Fisher Scientific,
Inc.). Following FGF2 treatment, cells were fixed with 4%
paraformaldehyde and 0.5% Triton X-100 in PBS for 30 min at 4°C,
and incubated with 0.15 M phalloidin-Alexa fluor 488 in PBS for 3 h
at 4°C. Images were taken using an Olympus Fluo View 1000 confocal
microscopy system (Olympus Corporation, Tokyo, Japan) with ×40
magnification.
Cell migration assay
An i-bidi culture insert (ibidi, Inc.) was set in a
35 mm cell culture dish. To assess cell migration, cells
(5×104) were seeded into a well of an i-bidi culture
insert with Ham's F12/RPMI-1640 containing 5% FBS. Following
overnight culture at 37°C, the insert was removed and the cells
were washed with F12/RPMI-1640 medium once, and then cells were
cultured at 37°C in F12/RPMI-1640 medium without serum for 7 h.
Cells were observed with an Olympus IX-70 microscopy at ×10
magnification.
Cell invasion assay
The CytoSelect 24-well cell invasion assay kit (Cell
BioLabs, Inc., San Diego, CA, USA) was used to assess the invasion
ability of cells. Cells (1.2×105) were seeded on the
culture insert (included in the kit) with Ham's F12/RPMI-1640
containing 5% FBS and incubated for 24 h. Quantification of the
invading cells was performed according to the manufacturers
protocol. Briefly, after removal of non-invasive cells, invasive
cells adhered to the bottom membrane of the insert were quantified
using a colorimetric plate reader according to the manufacturers
protocol.
Microarray
Total RNA was extracted from 6×106 cells
of wild-type and FGFRL1-deficient KYSE520 cells, respectively, with
Isogen (Nippon gene, Inc., Japan). RNA was amplified with Amino
Allyl MessageAMP II kit (Thermo Fisher Scientific, Inc.) and
coupled with Cy5 dye with Cy5 Mono-Reactive Dye Pack (GE
healthcare), and then hybridized to a human Oligo chip (25 k; Toray
Industries, Inc., Tokyo, Japan) according to the manufacturers
protocol.
Statistical analysis
Data are presented as mean ± standard error of the
mean. Statistical analysis was performed using a one-way analysis
of variance with Microsoft Excel for Mac 2011 (version 14.7.7;
Microsoft Corporation, Redmond, WA, USA). As a post hoc test,
Dunnett's test was used. P<0.05 was considered to indicate a
statistically significant difference. The Tukey-Kramer method was
used to compare tumour mass with the wild type mice. P<0.01 was
considered to indicate a statistically significant difference.
Results
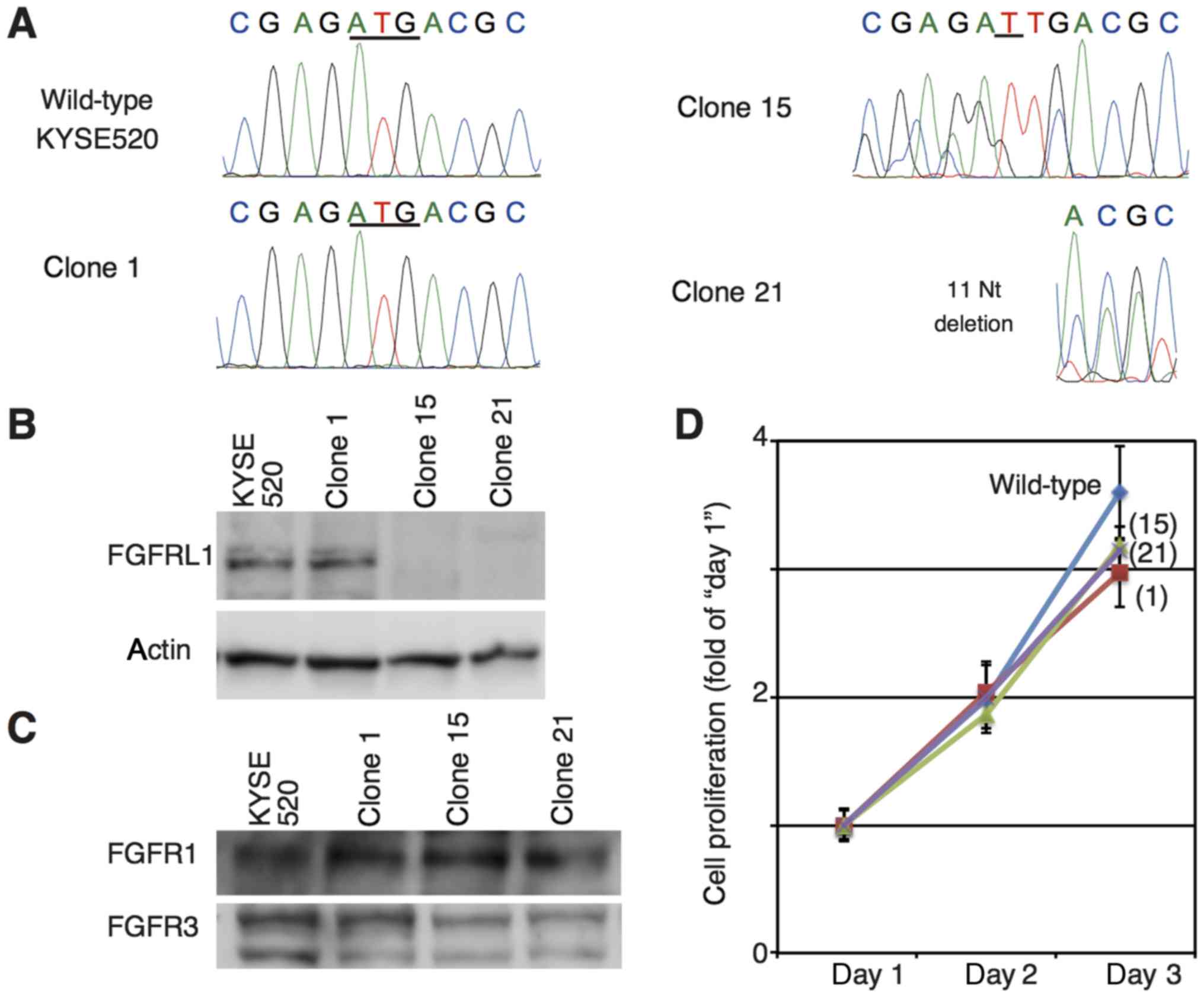
Mutagenesis of FGFRL1 gene
Two cell lines deficient for FGFRL1 expression,
clones 15 and 21, were established from an ESCC cell line (KYSE520)
using the CRISPR-Cas9 method (Fig.
1A). In the clone 15 line, a single thymidine was inserted into
the first ATG site of the FGFRL1 gene. A total of 11 nucleotides
(AGGCCGAGATG, which includes the first ATG site of FGFRL1) were
deleted from the clone 21. Clone 1 was also established from
KYSE520 cells treated by the CRISPR-Cas9 method; however, the
method failed to produce a mutation at the FGFRL1 gene. No
expression of FGFRL1 was detectable in clones 15 and 21, however,
its expression was detected in samples derived from parental
KYSE520 and clone 1 (Fig. 1B).
KYSE520 cells expressed FGFR1 and FGFR3 (Fig. 1C). Neither expression of FGFR2 nor
FGFR4 was detectable with western blotting under the conditions
used in the present study. Genetic depletion of FGFRL1 had no
obvious effects on the expression of other FGF receptors (Fig. 1C). Cell proliferation curves of those
cells were comparable (Fig. 1D).
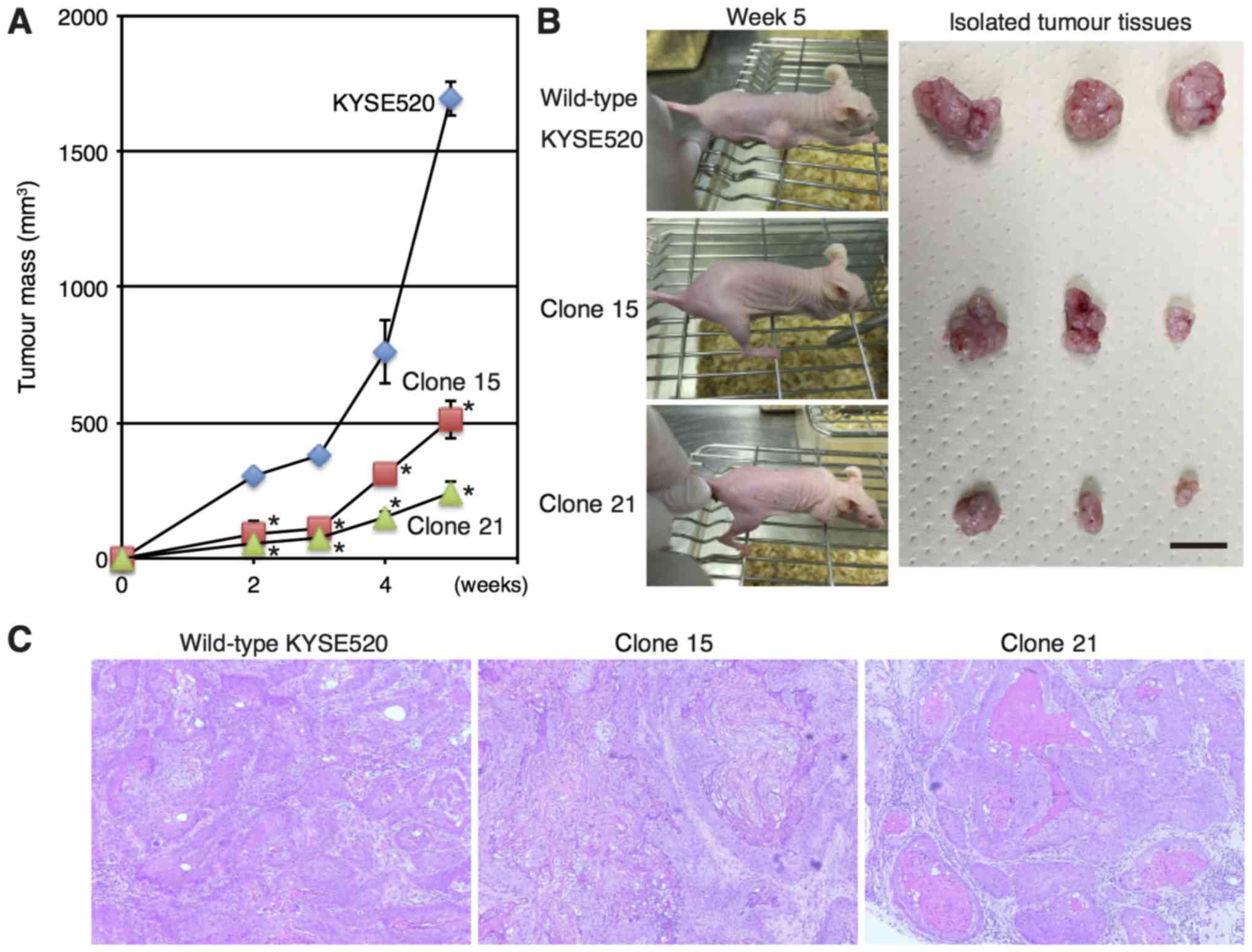
Effects of FGFRL1 gene depletion on
tumour growth in a xenograft model
To examine the effects of FGFRL1 depletion in
vivo, mice were injected subcutaneously with wild-type and
FGFRL1-deficient KYSE520 cells. As presented in Fig. 2A and B, tumours derived from
FGFRL1-deficient cell lines (clones 15 and 21) exhibited reduced
growth in vivo. The haematoxylin-eosin staining indicated
that FGFRL1-deficient cells formed well-differentiated squamous
cell carcinomas in a mouse xenograft model (Fig. 2C), whereas wild-type cells formed
moderately differentiated squamous cell carcinomas, as originally
described (19). These results
indicated that FGFRL1 may contribute to tumorigenesis in
vivo.
Effects of FGFRL1-depletion on mRNA
expression profiles
In order to determine the underlying molecular
mechanism of the deceleration of tumour growth resulting from
FGFRL1-deficiency, mRNA levels were compared between wild-type and
FGFRL1-deficient cells using microarrays (Fig. 3A). Microarray demonstrated that the
expression MMP-1, C-X-C chemokine receptor type 7, keratin 14,
FGFBP1 and keratin 6A was markedly downregulated in
FGFRL1-deficient cells (Fig. 3B). In
the five genes, reduced protein expression of MMP-1 and FGFBP1 were
confirmed using western blotting (Fig.
3C).
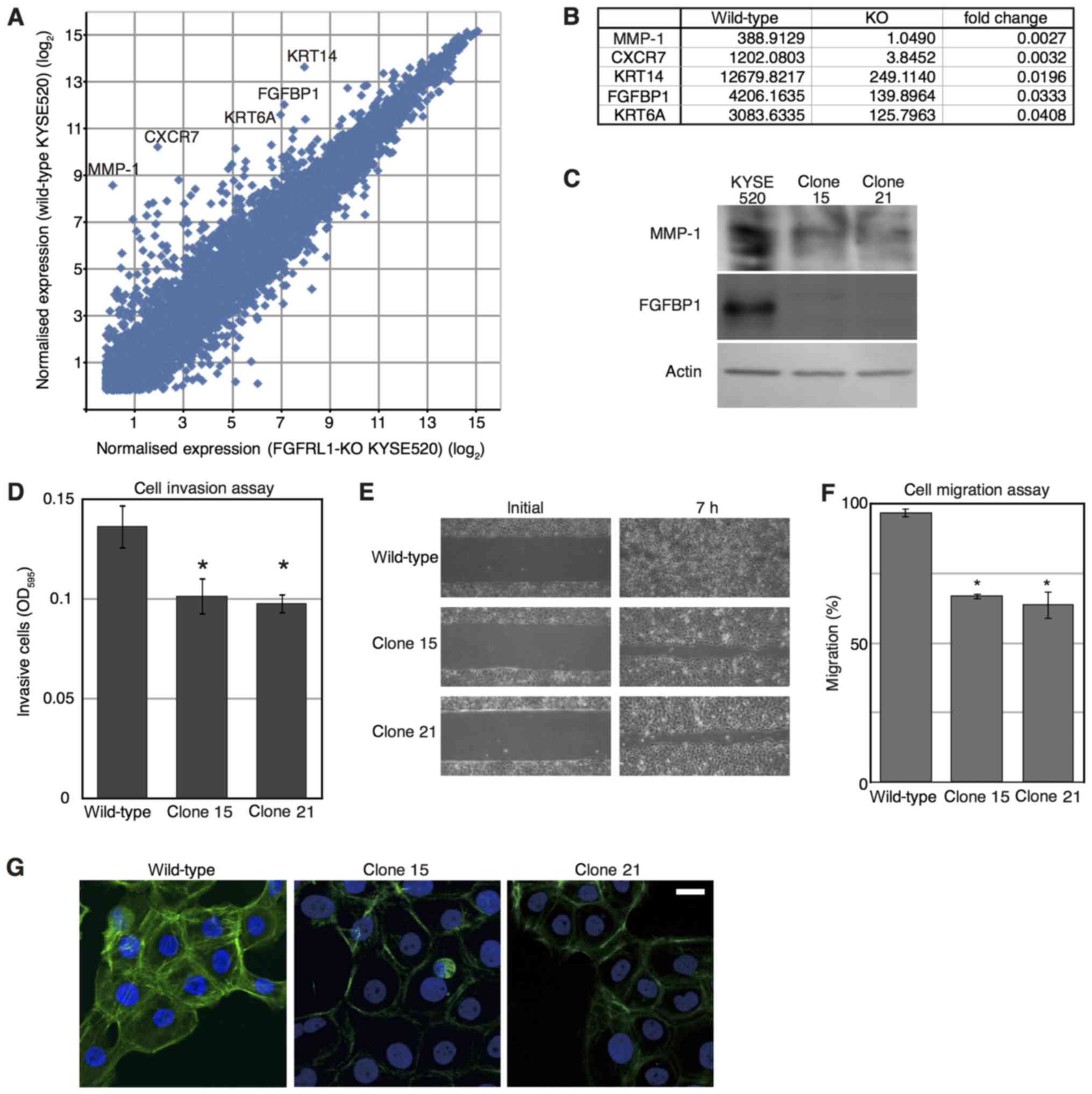 | Figure 3.Microarray analysis of
FGFRL1-deficient cells. (A) Identification of transcripts
differentially expressed in wild-type and FGFRL1-deficient cells.
Mean of expression levels in parental KYSE520 cells and the clone
1, and those in clones 15 and 21, were plotted as ‘wild-type
KYSE520’ and ‘FGFRL1-KO KYSE520’, respectively. Each dot represents
an individual gene. Transcripts markedly downregulated in
KYSE520-deficient cells are denoted by their gene symbol. (B) Genes
exhibiting notably decreased expression in FGFRL1-deficient cells.
(C) Western blot analysis of cell extracts prepared from the
indicated cells. (D) Cell invasion through a porous type-I collagen
membrane. After 24 h, invaded cells were stained with a blue dye
and permeabilised in 200 µl of buffer. The experiment was repeated
three times. Data represent the mean values. Error bars indicate
the standard error of the mean. *P<0.05 (Dunnett's test) vs. the
wild-type. (E) Cell migration assay. (F) The invaded area presented
in (E) was assessed. The experiment was repeated three times. The
data in the graph are expressed as mean values. Error bars indicate
the standard error of the mean. *P<0.05 (Dunnett's test) vs. the
wild-type. (G) Phalloidin staining of cell lines. Scale bar, 10 µm.
FGFRL1, fibroblast growth factor receptor-like 1; OD, optical
density; MMP, matrix metalloproteinase; CXCR7, C-X-C chemokine
receptor type 7; KRT14, keratin 14; FGFBP1, fibroblast growth
factor binding protein 1; KRT6A, keratin 6A. |
MMP-1 digests collagen in a metal ion-dependent
manner and is associated with cell invasion (20). Fig. 3D
presents the invasion of cells through a collagen I membrane. The
invasiveness of FGFRL1-deficient cells was decreased compared with
that of wild-type KYSE520 cells.
FGFBP1 is a secreted FGF-binding protein that is
able to promote cell motility (21).
As presented in Fig. 3E and F,
wild-type KYSE520 cells sealed a 400 µm wide wound within 7 h,
however, FGFRL1-deficient cells did not. Thus, FGFRL1-deficiency
may have decreased the cell motility of KYSE520 cells compared with
controls.
The structure of actin filaments was examined, since
the filaments are required for cell motility (22). Visualization of actin filaments via
phalloidin staining identified that actin filaments around the
nucleus were sparse in FGFRL1-deficient cells compared with
controls, whereas the filaments along the plasma membrane were
observed as frequently as those of wild type cells (Fig. 3G). These results suggest that FGFRL1
may contribute to the regulation of actin filament assembly.
Discussion
While FGFRL1 expression tends to be high in ESCC
patients with a poor prognosis (14),
its function in the generation/expansion of ESCCs remains
unresolved. In the present study, genetic depletion of FGFRL1 in
ESCC KYSE520 cells indicated that FGFRL1 may contribute to tumour
growth in vivo. Tumour growth in a mouse xenograft model
involves multiple processes, including cell proliferation,
migration, adhesion and angiogenesis. Genetic depletion of the
FGFRL1 gene failed to inhibit cell proliferation, although
transient inhibition of FGFRL1 expression by siRNA induced cell
cycle arrest (13,16). This may suggest that FGFRL1-deficiency
is able to affect cell proliferation transiently, but not
permanently. This, in turn, suggests that genetic depletion of
FGFRL1 is able to decrease cell motility and invasiveness, rather
than cell proliferation.
In the present study, it was identified that
FGFRL1-deficency decreased the expression level of proteins
regulating cell motility and invasiveness, FGFBP1 and MMP-1. In
vivo, FGFBP1 acts as a carrier protein that releases FGFs from
the extracellular matrix, thereby increasing the proliferation,
migration and angiogenesis induced by FGFs (21,23).
Consistently, the present study indicated that FGFRL1-deficiency
reduced tumorigenic potential in a xenograft mouse model and
decreased cell motility in vitro. Moreover, MMP-1 serves a
critical function in local invasion of oesophageal carcinomas and
is an independent prognostic factor (24). It was demonstrated in a previous study
that high expression of FGFRL1 is associated with lymph node
metastasis of ESCCs (14). In
concurrence with those data, the present study demonstrated that
FGFRL1-deficiency decreased the invasion of KYSE520 ESCC cells.
Furthermore, it was demonstrated in the present study that actin
filaments around the nucleus were sparse in FGFRL1-deficient cells.
FGFRL1 may be able to promote tumour invasion via the regulation of
protein expression and actin filament assembly, since the assembly
is essential for motility and invasion of cells (22). The results of the present study
suggest a reason for the association of FGFRL1 expression with
lymph node metastasis of ESCCs. The underlying molecular mechanism
by which FGFRL1 regulates the assembly of actin filaments remains
unresolved.
The expression levels of MMP-1 and FGFBP1 were not
decreased in normal tissues of FGFRL1-knockout mice (10,12). The
present results suggest that gene editing is useful for studying
the function of a protein not only in normal development, but also
in tumour generation/development.
To conclude, FGFRL1-knockout cell lines were
established from ESCC KYSE520 cells. FGFRL1-deficiency decreased
cell motility, invasion and tumorigenic potential of KYSE520 cells.
FGFRL1 deficiency in KYSE520 cells also decreased the expression of
proteins promoting cell motility and invasion, MMP-1 and FGFBP1.
The present study may provide an explanation for the clinical
observation that high expression of FGFRL1 in ESCCs is often
associated with lymph node metastasis and poor prognosis of
patients.
Acknowledgements
We would like to thank Ms. Takako Murai (Department
of Nanobio Drug Discovery, Graduate School of Pharmaceutical
Science, Kyoto University, Japan) for technical assistance.
Funding
The present study was supported by The Japan Society
for the Promotion of Science [the Grant-In Aid for Scientific
Research (B) (JSPS Kakenhi number 26293302)].
Availability of data and materials
The GEO accession number of the microarray results
used in the present study is GSE96956. The datasets used and/or
analysed during the current study are available from the
corresponding author on reasonable request.
Authors' contributions
YT, KS and YS designed the study. YT, TM, KW and TS
performed research. YT and YS analysed data and wrote the
paper.
Ethics approval and consent to
participate
All mice were handled and cared for in accordance
with the Guide of Care and Use of Laboratory Animals, and all
experiments were approved by the Ethics Committee of Experimental
Animals of Kyoto University (Kyoto, Japan). All surgical procedures
and postoperative care regimes were reviewed and approved by the
Animal Care and Use Committee of Kyoto University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E586. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thrift AP: The epidemic of oesophageal
carcinoma: Where are we now? Cancer Epidemiol. 41:88–95. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tachimori Y, Ozawa S, Numasaki H, Ishihara
R, Matsubara H, Muro K, Oyama T, Toh Y, Udagawa H, Uno T, et al:
Comprehensive registry of esophageal cancer in Japan, 2010.
Esophagus. 14:189–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuwano H, Nishimura Y, Oyama T, Kato H,
Kitagawa Y, Kusano M, Shimada H, Takiuchi H, Toh Y, Doki Y, et al:
Guidelines for diagnosis and treatment of carcinoma of the
esophagus April 2012 edited by the Japan Esophageal Society.
Esophagus. 12:1–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wiedemann M and Trueb B: Characterization
of a novel protein (FGFRL1) from human cartilage related to FGF
receptors. Genomics. 69:275–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stauber DJ, DiGabriele AD and Hendrickson
WA: Structural interactions of fibroblast growth factor receptor
with its ligands. Proc Natl Acad Sci USA. 97:49–54. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trueb B: Biology of FGFRL1, the fifth
fibroblast growth factor receptor. Cell Mol Life Sci. 68:951–964.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gerber SD, Steinberg F, Beyeler M,
Villiger PM and Trueb B: The murine Fgfrl1 receptor is essential
for the development of the metanephric kidney. Dev Biol.
335:106–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gerber SD, Amann R, Wyder S and Trueb B:
Comparison of the gene expression profiles from normal and Fgfrl1
deficient mouse kidneys reveals downstream targets of Fgfrl1
signaling. PLoS One. 7:e334572012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baertschi S, Zhuang L and Trueb B: Mice
with a targeted disruption of the Fgfrl1 gene die at birth due to
alterations in the diaphragm. FEBS J. 274:6241–6253. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Amann R, Wyder S, Slavotinek AM and Trueb
B: The FgfrL1 receptor is required for development of slow muscle
fibers. Dev Biol. 394:228–241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsuchiya S, Fujiwara T, Sato F, Shimada Y,
Tanaka E, Sakai Y, Shimizu K and Tsujimoto G: MicroRNA-210
regulates cancer cell proliferation through targeting fibroblast
growth factor receptor-like 1 (FGFRL1). J Biol Chem. 286:420–428.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimada Y, Okumura T, Nagata T, Hashimoto
I, Sawada S, Yoshida T, Fukuoka J, Shimizu K and Tsukada K:
Expression analysis of fibroblast growth factor receptor-like 1
(FGFRL1) in esophageal squamous cell carcinoma. Esophagus.
11:48–53. 2014. View Article : Google Scholar
|
|
15
|
Shimada Y, Okumura T, Takei Y, Watanabe K,
Nagata T, Hori T, Tsuchiya S, Tsukada K and Shimizu K: Role of
fibroblast growth factor receptors in esophageal squamous cell
carcinoma. Esophagus. 13:30–41. 2016. View Article : Google Scholar
|
|
16
|
Zuo J, Wen M, Lei M, Peng X, Yang X and
Liu Z: MiR-210 links hypoxia with cell proliferation regulation in
human Laryngocarcinoma cancer. J Cell Biochem. 116:1039–1049. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schild C and Trueb B: Aberrant expression
of FGFRL1, a novel FGF receptor, in ovarian tumors. Int J Mol Med.
16:1169–1173. 2005.PubMed/NCBI
|
|
18
|
Donnard E, Asprino PF, Correa BR, Bettoni
F, Koyama FC, Navarro FC, Perez RO, Mariadason J, Sieber OM,
Strausberg RL, et al: Mutational analysis of genes coding for cell
surface proteins in colorectal cancer cell lines reveal novel
altered pathways, druggable mutations and mutated epitopes for
targeted therapy. Oncotarget. 5:9199–9213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimada Y, Imamura M, Wagata T, Yamaguchi
N and Tobe T: Characterization of 21 newly established esophageal
cancer cell lines. Cancer. 69:277–284. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pulukuri SM and Rao JS: Matrix
metalloproteinase-1 promotes prostate tumor growth and metastasis.
Int J Oncol. 32:757–765. 2008.PubMed/NCBI
|
|
21
|
Tassi E, McDonnell K, Gibby KA, Tilan JU,
Kim SE, Kodack DP, Schmidt MO, Sharif GM, Wilcox CS, Welch WJ, et
al: Impact of fibroblast growth factor-binding protein-1 expression
on angiogenesis and wound healing. Am J Pathol. 179:2220–2232.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bugyi B and Carlier MF: Control of actin
filament treadmilling in cell motility. Annu Rev Biophys.
39:449–470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abuharbeid S, Czubayko F and Aigner A: The
fibroblast growth factor-binding protein FGF-BP. Int J Biochem Cell
Biol. 38:1463–1468. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamashita K, Mori M, Kataoka A, Inoue H
and Sugimachi K: The clinical significance of MMP-1 expression in
oesophageal carcinoma. Br J Cancer. 84:276–282. 2001. View Article : Google Scholar : PubMed/NCBI
|