Introduction
Lung cancer is one of the most common causes of
cancer-associated mortality worldwide (1). As the most common histology of non-small
cell lung cancer (NSCLC), lung adenocarcinoma has a poorer
prognosis compared with squamous cell carcinoma due to its more
rapid development and higher recurrence rate (2). Following surgical resection, even very
early stage patients should be followed closely due to the high
risk of recurrence (3). Molecular
prognostic markers for recurrence have been extensively
investigated (4). Novel prognostic
gene expression signatures in lung adenocarcinoma should be
confirmed using data sets containing information regarding patients
divided into high- and low-risk groups with distinct overall
survival rates. 14-3-3ζ, a subtype of the 14-3-3 protein family, is
widely expressed in a number of types of cancer (5), and serves a critical role in
tumorigenesis and tumor progression by interacting with ~100 key
cellular proteins involved in numerous physiological processes,
including intracellular signaling, cell cycle control, apoptosis
and transcriptional regulation (6).
Further evidence suggests that 14-3-3ζ may be specifically
dysregulated in tumors (7). A number
of cellular proteins targeted by 14-3-3ζ are involved in tumor
development and progression; however, the specific molecular
mechanisms underlying these associations remain unclear. Lu et
al (8) suggested that 14-3-3ζ
overexpression promotes the activation of the transforming growth
factor-β/Smad (TGFβ/Smad) signaling pathway via the upregulation of
Zinc finger homeobox 1B (ZFHX1B). Chen et al (9) identified 14-3-3ζ in complex with
β-catenin as a potential promoter of metastasis in lung cancer. In
addition, downregulation of 14-3-3ζ has been reported to suppress
anchorage-independent growth of lung cancer cells through the
activation of anoikis via the upregulation of Bcl-2-associated
agonist of cell death (Bad) and Bcl-2-like protein 11 coupled with
a decrease of Mcl-1, resulting in the subsequent activation of
Bcl-2-associated X protein (10).
Additional studies have demonstrated that the phosphorylation of
14-3-3ζ by c-Jun N-terminal kinase releases the pro-apoptotic
proteins Bad and Forkhead box protein O3a from 14-3-3ζ, promoting
apoptosis via the mitochondrial apoptotic pathway and antagonizing
the effects of RAC-alpha serine/threonine-protein kinase signaling
(11,12).
Reduced 14-3-3ζ levels have been demonstrated to
increase the G1/G0-phase ratio, and decrease
the S-phase fraction and the rate of DNA synthesis in cell cycle
(13). However, increasing evidence
challenges its putative malignant potential. Clark et al
(14) reported that 14-3-3ζ
interaction with the Raf-1 cysteine-rich domain (Raf-CRD) may serve
in the negative regulation of Raf-1 function. In addition, 14-3-3ζ
was demonstrated to lead to the cytoplasmic enrichment of
β-catenin, caused by a novel mechanism through which chibby (CBY)
acts with 14-3-3ζ proteins to facilitate the nuclear export of
β-catenin, and subsequently repressing the β-catenin-mediated Wnt
signaling pathway (15). Zhu et
al (16) demonstrated that the
binding of 14-3-3ζ and a disintegrin and metalloproteinase (ADAM)
22cyt enhanced cell adhesion by increasing the affinity of ADAM22
disintegrin to its receptor. Therefore 14-3-3ζ interacts with a
number of target proteins ranging from transcription factors to
intracellular signaling molecules.
The present study focused on the potential role of
14-3-3ζ as a candidate proto-oncogene, specifically in lung
adenocarcinoma. Epithelial-cadherin (E-cadherin; encoded by CDH1)
is a classical cadherin that is necessary for effective cell-cell
adhesion (17). Loss of E-cadherin
expression is a hallmark of the epithelial-mesenchymal transition
(EMT), a process responsible for the increased invasiveness of
tumor cells during tumorigenesis (18). Its downregulation during EMT can be
due to promoter methylation or upregulation of transcriptional
repressors, including snail, slug, twist, E12, E47, ZFHX1B and
deltaEF1 (19). E-cadherin,
traditionally known as a tumor suppressor gene, also regulates
β-catenin signaling in the canonical Wnt signaling pathway
(20) and its reduced expression has
been observed in the majority of types of epithelial cancer,
promoting tumor invasiveness and leading to poor patient prognosis
(21). 14-3-3ζ has been proposed to
be a metastatic factor in lung cancer (22); however, the molecular mechanisms
underlying this effect remain unknown. In the present study, the
association between 14-3-3ζ and E-cadherin was investigated in lung
adenocarcinoma.
Materials and methods
Patients and samples
The current retrospective study included 123
patients (67 males and 56 females) with lung adenocarcinoma who
underwent surgical resection between January 2009 and October 2010
in Harbin Medical University Cancer Hospital (Harbin, China).
Clinicopathological data were obtained by reviewing the relevant
medical charts. None of patients received preoperative chemotherapy
or radiotherapy. Primary cancers were classified according to the
TNM staging system (23,24) for lung cancer (American Joint
Committee on Cancer Staging System, 7th Edition). Tumor and normal
alveolar tissue specimens were collected in 10% formalin at room
temperature for 24–48 h and embedded in paraffin for use in all
experiments. The present study was approved by the Hospital Ethics
Committee at the Harbin Medical University Cancer Hospital (Harbin,
China). All investigators involved in the study, apart from the
study statistician, were blinded to patient outcome throughout all
laboratory analyses.
Immunohistochemical staining for
14-3-3ζ and E-cadherin
Immunohistochemical staining was performed using the
streptavidin-peroxidase (S-P) method. The dewaxed sections (4 µm)
were baked in an oven (70°C) for 3 h. Endogenous peroxide was
blocked using 3% hydrogen peroxide for 10 min at room temperature.
Following three washes with PBS, the sections were incubated with
blocking serum (OriGene Technologies, Inc., Rockville, MD, USA) for
between 10 and 20 min at room temperature. Sections were washed and
then incubated overnight at 4°C with rabbit polyclonal antibodies
against 14-3-3ζ (dilution, 1:30; sc-732; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and rabbit polyclonal antibodies against
E-cadherin (dilution, 1:30; sc-7,870; Santa Cruz Biotechnology,
Inc.). Following another wash step, the sections were incubated
with horseradish peroxidase-conjugated secondary antibody
(PV-6,000; OriGene Technologies, Inc.) for 30 min at room
temperature and the reaction products were visualized with
diaminobenzidine. The sections were then counterstained with
hematoxylin for 7–10 sec at room temperature. Immunohistochemical
evaluation was performed independently by two pathologists using an
Olympus microscope (BX51; Olympus Corporation, Tokyo, Japan) and
immunohistochemical staining was scored according to the following
criterion: -, 0–5% of the cells stained; +, 6–25% of the cells
stained; ++, 26–50% of the cells stained; +++, 51–75% of the cells
stained; and ++++, 76–100% of the cells stained.
Immunohistochemistry expression scores of 0≤ score ≤1+; and 2+≤
score ≤4+ were classified as low expression and strong expression,
respectively.
Statistical analysis
The data were subjected to statistical analysis
using SPSS software (version 21.0; IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference. The χ2 test was used to compare patient
clinicopathological characteristics with the expression of 14-3-3ζ
and E-cadherin. Correlation between expression levels was studied
using Kendall's tau-b (K). Survival analysis was performed using
the Kaplan-Meier estimator method and Log-rank test. Independent
risk factors of prognosis were analyzed using the multivariate Cox
proportional hazards model. Variables were adopted for their
prognostic significance (P<0.05) in univariate analysis using
forward, stepwise selection (forward likelihood ratio).
Results
Expression of 14-3-3ζ and E-cadherin
in lung adenocarcinoma tissues
The expression of 14-3-3ζ protein in lung
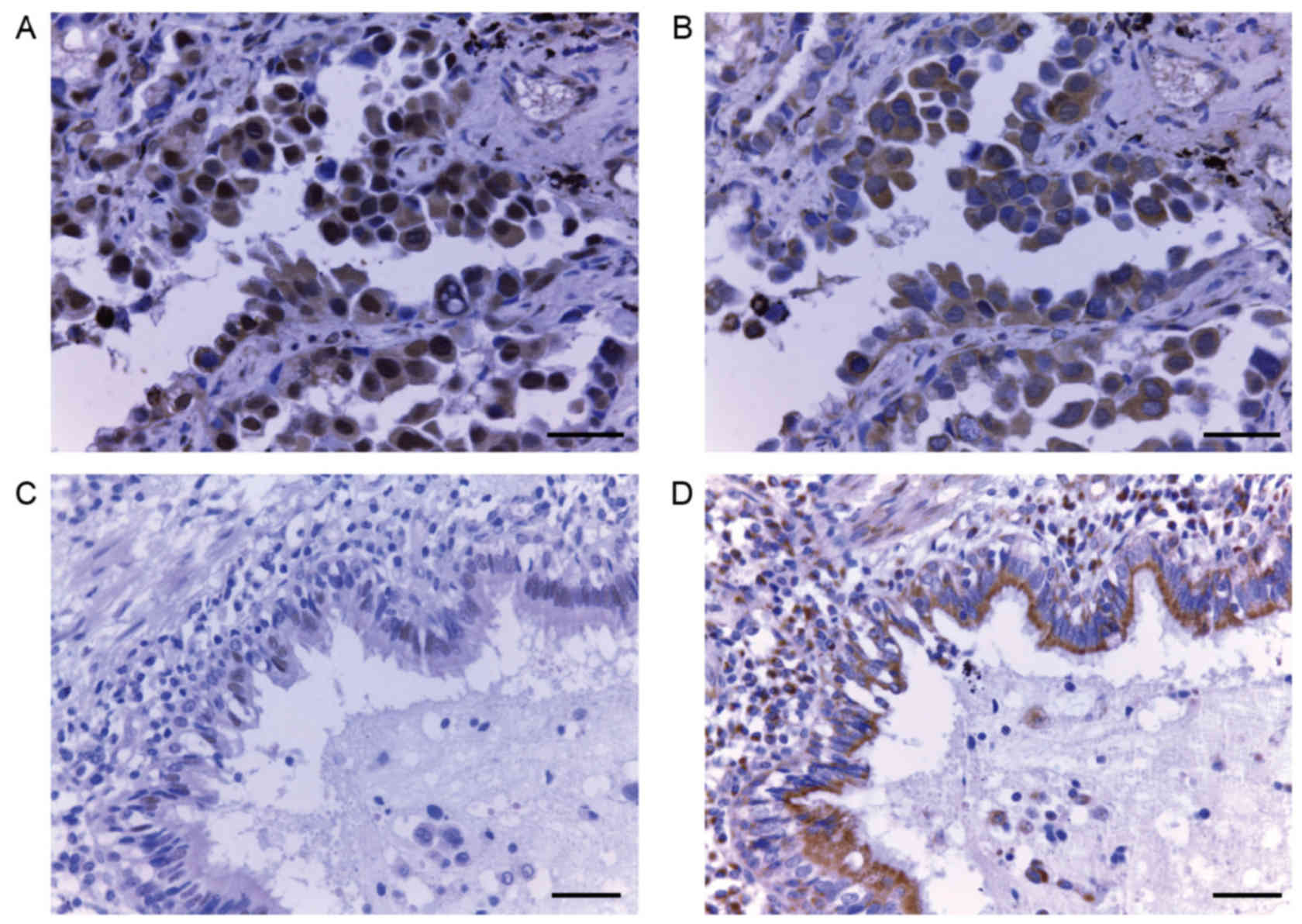
adenocarcinoma samples (Fig. 1A and
B; magnification, ×400) and adjacent normal alveolar tissues
(Fig. 1C and D; magnification, ×200)
was analyzed by immunohistochemistry. A total of 18 14-3-3ζ and 21
E-cadherin samples were excluded due to damage or cytolysis of the
paraffin block. Positive immunohistochemical staining for 14-3-3ζ
was primarily observed in the cytoplasm and membrane (Fig. 1A), but certain tissues also exhibited
nuclear localization of 14-3-3ζ. Cytoplasmic and nuclear expression
were observed concurrently in one specimen. Strong expression of
14-3-3ζ was observed in 55.24% (58/105) of lung adenocarcinoma
tissues, compared with only 31.33% (26/83) of adjacent normal
alveolar tissues (P=0.001). On the contrary, strong expression of
E-cadherin was more prevalent in normal tissues compared with lung
adenocarcinoma tissues (72.44 vs. 67.65%, respectively, P=0.537;
Table I).
 | Table I.Expression of 14-3-3ζ and E-cadherin
in lung adenocarcinoma tissues. |
Table I.
Expression of 14-3-3ζ and E-cadherin
in lung adenocarcinoma tissues.
|
| 14-3-3ζ |
| E-cadherin |
|
|---|
|
|
|
|
|
|
|---|
| Tissue | Low (n) | High (n) | P-value | Low (n) | High (n) | P-value |
|---|
| Normal | 57 | 26 | 0.001 | 27 | 71 | 0.537 |
| Lung
adenocarcinoma | 47 | 58 |
| 33 | 69 |
|
Correlation between
clinicopathological features and the expression of 14-3-3ζ and
E-cadherin
Data from the correlative analysis of 14-3-3ζ
expression and the clinicopathological characteristics of patients
with lung adenocarcinoma are summarized in Table II. Comparisons of 14-3-3ζ expression
with clinicopathological characteristics demonstrated that 14-3-3ζ
overexpression correlated with differentiation (P<0.001) but not
with any other included clinical parameter. Increased E-cadherin
expression was indicative of smaller tumor size (P=0.018) and
greater differentiation (P=0.028). In addition, the expression of
14-3-3ζ was positively correlated with that of E-cadherin
(P=0.012).
 | Table II.Correlation between
clinicopathological characteristics and the expression of 14-3-3ζ
and E-cadherin. |
Table II.
Correlation between
clinicopathological characteristics and the expression of 14-3-3ζ
and E-cadherin.
|
| 14-3-3ζ
(n=105)c |
| E-cadherin
(n=102)c |
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | Low (n) | Strong (n) | P-value | Low (n) | Strong (n) | P-value |
|---|
| Sex |
|
| 0.249 |
|
| 0.077 |
|
Male | 28 | 28 |
| 21 | 31 |
|
|
Female | 19 | 30 |
| 12 | 38 |
|
| Age |
|
| 0.421 |
|
| 0.627 |
|
≤55 | 19 | 28 |
| 16 | 37 |
|
|
>55 | 28 | 30 |
| 17 | 32 |
|
| Tumor size |
|
| 0.213 |
|
| 0.018b |
| ≤3 | 17 | 28 |
| 9 | 36 |
|
|
>3 | 30 | 30 |
| 24 | 33 |
|
| Node status |
|
| 0.555 |
|
| 0.300 |
|
Negative | 29 | 39 |
| 17 | 43 |
|
|
Positive | 18 | 19 |
| 16 | 26 |
|
|
Differentiation |
|
|
<0.001a |
|
| 0.028b |
|
Good/moderate | 17 | 43 |
| 11 | 39 |
|
|
Poor | 30 | 15 |
| 22 | 30 |
|
| TNM Stage |
|
| 0.805 |
|
| 0.954 |
| I | 15 | 21 |
| 10 | 23 |
|
| II | 13 | 13 |
| 7 | 14 |
|
|
III | 19 | 24 |
| 16 | 32 |
|
| Chemotherapy |
|
| 0.296 |
|
| 0.106 |
| No | 18 | 16 |
| 14 | 17 |
|
|
Yes | 29 | 42 |
| 19 | 52 |
|
| E-cadherin
expressiond |
|
| 0.012e |
|
|
|
| Low or
negative | 22 | 14 |
|
|
|
|
|
Strong | 25 | 44 |
|
|
|
|
Prediction of survival in lung
adenocarcinoma patients by 14-3-3ζ and E-cadherin
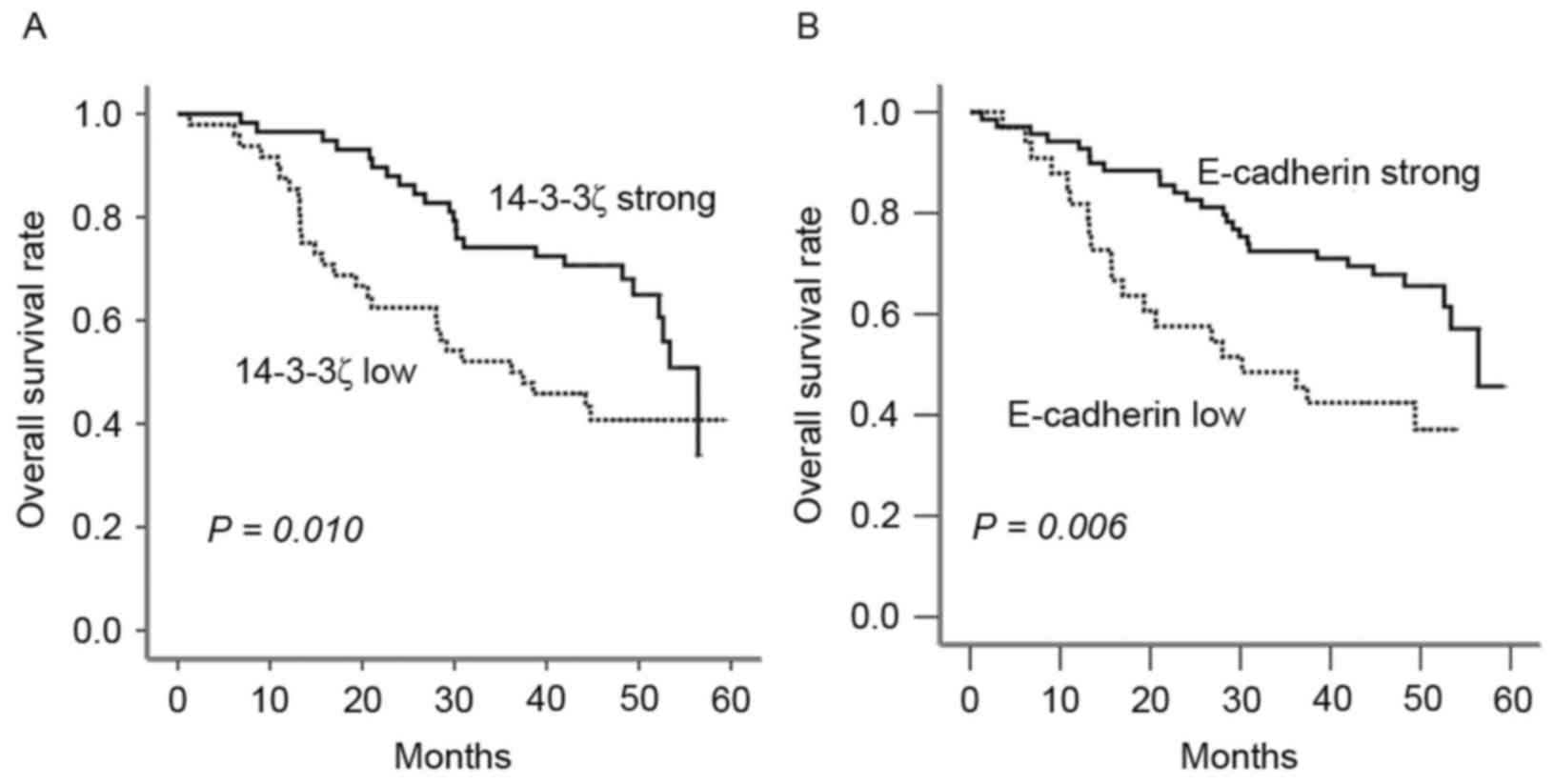
To elucidate the prognostic role of 14-3-3ζ in
patients with lung adenocarcinoma, overall survival (OS) rates were
estimated using Kaplan-Meier survival curves. Patients with high
expression of 14-3-3ζ were observed to have a longer OS period
compared with those with low expression (P=0.010; Fig. 2). Similarly, increased E-cadherin was
indicative of an improved prognosis P=0.006).
14-3-3ζ expression as an independent
prognostic factor in resected lung adenocarcinoma patients
Univariate and multivariate analyses were performed
using the Cox proportional hazards model to evaluate the impact of
14-3-3ζ expression and pathological factors on the prognosis of
patients with lung adenocarcinoma (Table III). Multivariate analyses
demonstrated that 14-3-3ζ expression (P=0.026) and TNM stage
(P<0.001) were associated with OS, which suggests that 14-3-3ζ
may act as a prognostic indicator for OS of patients with lung
adenocarcinoma (Table IV).
 | Table III.Univariate analysis of overall
survival in patients with lung adenocarcinoma. |
Table III.
Univariate analysis of overall
survival in patients with lung adenocarcinoma.
|
Characteristics | HR (95%
CI)a | P-value |
|---|
| Age | 0.694 (0.415,
1.163) | 0.165 |
| Gender | 0.550 (0.322,
0.938) | 0.028 |
| TNM stage | 1.733 (1.259,
2.386) | 0.001 |
| Node status | 3.552 (1.938,
6.510) | <0.001 |
| Tumor size | 1.430 (0.829,
2.468) | 0.199 |
|
Differentiation | 0.692 (0.481,
0.996) | 0.048 |
| 14-3-3ζ
expression | 0.754 (0.575,
0.990) | 0.042 |
| E-cadherin
expression | 0.729 (0.546,
0.974) | 0.032 |
| Chemotherapy | 0.651 (0.388,
1.091) | 0.103 |
 | Table IV.Multivariate analysis of overall
survival in patients with lung adenocarcinoma. |
Table IV.
Multivariate analysis of overall
survival in patients with lung adenocarcinoma.
|
Characteristics | HR (95%
CI)a | P-value |
|---|
| Gender |
| 0.146 |
| TNM stage | 2.209 (1.469,
3.320) | <0.001 |
| Node status |
| 0.151 |
|
Differentiation |
| 0.057 |
| 14-3-3ζ
expression | 0.692 (0.500,
0.956) | 0.026 |
| E-cadherin
expression |
| 0.598 |
Discussion
E-cadherin is a tumor suppressor protein that is
used as a prognostic marker for cancer. Downregulation of
E-cadherin during cancer progression correlates with aggressive
tumor behavior and a poor prognosis (25,26).
Conversely, high expression of E-cadherin has been demonstrated to
reduce tumor progression and invasiveness in addition to the
formation of metastases (21,27–30). The
results from the present study demonstrated that the overexpression
of 14-3-3ζ was significantly associated with positive E-cadherin
expression in tissue specimens from patients with lung
adenocarcinoma and was a prognostic factor, which is in agreement
with a number of previous studies that have suggested this
association (14,15,31,32).
Notably, Raf-1 is a critical effector of Ras signaling and
transformation. Activating Rac1 and the subsequent phosphorylation
of Raf and mitogen-activated protein kinase kinase results in
constitutive, anchorage-independent activation of the
Ras/mitogen-activated protein kinase/extracellular signal-regulated
kinase signaling pathway, which elevates the expression of the
master regulator Snail 1, thereby inducing transcriptional
repression of E-cadherin mRNA (33).
Clark et al (14) indicated
that 14-3-3ζ interaction with the Raf-CRD may serve a role in the
negative regulation of Raf-1 function by facilitating the
dissociation of 14-3-3ζ from the NH2 terminus of Raf-1, thus
leading to enhanced E-cadherin expression. Additionally, when
β-catenin translocates into the nucleus, it interacts with the
Tcf/Lef family to activate the canonical Wnt signaling pathway,
switching on the transcription of certain target genes and
resulting in the proliferation and metastasis of tumor cells
(34,35). Previously, 14-3-3ζ was demonstrated to
cause the cytoplasmic enrichment of β-catenin, potentially through
interaction with CBY1, and to subsequently repress the
β-catenin-mediated Wnt signaling pathway, which is responsible for
activating EMT and is characteristic of E-cadherin loss (15). Therefore, 14-3-3ζ appears to inhibit
tumor cell invasion and metastasis indirectly (15). In addition, 14-3-3ζ overexpression has
been demonstrated to promote TGFβ/Smad signaling pathway activation
(8), although a reduction of
E-cadherin expression and induction of EMT is not always observed,
according to certain studies (36,37). Ahn
et al (38) indicated that
Smad3 regulates, at the transcriptional level, miR-200 family
members, which themselves regulate ZEB1 and ZEB2, transcriptional
repressors of E-cadherin, at the posttranscriptional level. Mise
et al (39) identified that
Smad3-mediated TGF-β signaling targeted the zyxin gene, mediating
cancer cell motility and EMT during lung cancer progression by
regulating cell-cell junctions, integrin 5 expression and
cell-extracellular matrix adhesion. Therefore, data from the
present study provides novel insights into the indirect regulation
of E-cadherin by 14-3-3ζ via TGFβ/Smad-mediated cancer cell
adhesion and motility. Notably, Snail 1, one of the major
transcriptional suppressors of E-cadherin mRNA, exhibits a
repressive effect on the expression of 14-3-3 family members,
including the zeta form (31), which
is in agreement with the previously suggested role of 14-3-3ζ in
promoting E-cadherin expression (32). Therefore, the positive correlation of
14-3-3ζ with E-cadherin observed in the present study implies a
role for 14-3-3ζ as a metastatic inhibitor.
Nevertheless, contradictory reports do exist. Zhao
et al (40) demonstrated that
that 14-3-3ζ and Hsp27 negatively correlate with E-cadherin
expression in NSCLC. In addition, 14-3-3ζ overexpression has been
observed to reduce cell adhesion by activating the TGFβ/Smad
signaling pathway, leading to ZFHX1B/SIP-1 upregulation, E-cadherin
loss and EMT in breast cancer (8). In
accordance with the presumed role of 14-3-3ζ as a metastatic
inhibitor, western blot analysis of human lung giant cell carcinoma
cells revealed that 14-3-3ζ expression was more prevalent in poorly
metastatic cells than in highly metastatic cells, and that cells
that overexpress 14-3-3ζ were reported to exhibit a lower
proliferative ability, higher adhesive ability and lower migratory
ability compared with cells transfected with control vector
(41). In addition, 14-3-3ζ
expression was observed to be increased in lung cancer cells
treated with Rg3, a metastasis suppressor, leading to the
inhibition of cell invasiveness and metastasis (42). Metastasis is not the only malignant
characteristic of lung cancer, but it is the primary cause of
worsening prognosis and high mortality rate (43).
The results from the present study demonstrated that
strong 14-3-3ζ staining was associated with greater differentiation
and longer OS, and was determined to be an independent prognostic
factor for OS according to multivariate analysis. Supporting the
data from the present study, the positive interaction between
14-3-3ζ and another metastasis-associated adhesion molecule ADAM22
has been reported (16). Zhu et
al (16) identified, for the
first time, a novel interaction between 14-3-3ζ and ADAM22, a
transmembrane protein containing disintegrin-like and
metalloproteinase-like domains (44)
and demonstrated that the binding of 14-3-3ζ to ADAM22cyt enhances
cell adhesion by increasing the affinity of ADAM22 disintegrin to
its receptor, therefore implying a potential role of the 14-3-3ζ
protein in the ADAM22-associated regulation of cell adhesion.
Kobayashi et al (45)
demonstrated that 14-3-3ζ secreted by ascite monocytes/macrophages,
which characteristically release a number of inflammatory cytokines
and chemokines, is present in the malignant ascites of patients
with epithelial ovarian carcinoma and could be taken up by tumor
cells, implying a potential role for secreted 14-3-3ζ in the
inhibition of tumor growth and proliferation. While these reports
are in accordance with the data from the present study, much of the
current literature proposes that the upregulated expression of
14-3-3ζ is associated with high histological grade, lymph node
metastasis and poor clinical outcome in certain types of cancer.
Fan et al (46) suggested that
increased 14-3-3ζ expression is positively correlated with a more
advanced pathologic stage and grade of NSCLC, and was associated
with worse overall and cancer-specific survival. Niemantsverdriet
et al (7) reported that strong
14-3-3ζ staining was associated with reduced OS and was determined
to be an independent prognostic factor for disease-free survival by
multivariate analysis. Further studies are required to explain why
contradictory data exists regarding 14-3-3ζ in tumorigenesis and
tumor progression. The present study specifically focused on the
metastatic impact of 14-3-3ζ in relatively early stages of
resectable lung adenocarcinoma without distant metastasis, which is
unlike the majority of the previous studies performed in lung
cancer, which included a number of pathologies and advanced stage
cancers (46,47). 14-3-3ζ overexpression exerts complex
biological effects via its interaction with a number of different
proteins in a spatial and a temporal fashion, emphasizing
individual functions in diverse signaling pathways in various tumor
types (48,49). In addition, 14-3-3ζ exhibits multiple,
opposing roles, making the analysis of its effects in complex
systems challenging. It should therefore be studied with a focus on
controlled study parameters.
In conclusion, the increased expression of 14-3-3ζ
was observed more frequently in patients with resectable lung
adenocarcinoma with an improved prognosis and was positively
associated with E-cadherin. Therefore, 14-3-3ζ may be a novel
marker for predicting the prognosis of resectable lung
adenocarcinoma. To identify the molecular mechanisms underlying
this observation, a longitudinal study of a large population is
required.
Acknowledgements
The authors of the present study would like to thank
Ms. Xiaohui He for providing patient data and Dr. Jingshu Geng of
the Medical Record Room and the Department of Pathology of Harbin
Medical University Cancer Hospital respectively, for collecting
tissue specimens.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 30772540 and
81172214 to L.C. and 81301991 to Y.Z.).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML and LC contributed to the conception and design
of the study. XL, HL, YZ, XC, JH and WL performed the experiment.
QM and XL performed the statistical analysis. ML wrote the
manuscript. QM revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Harbin Medical University Cancer Hospital. All patients provided
written informed consent.
Consent for publication
As all data was anonymized, this study does not
contain any individual person's data in any form (including
individual details, images, or videos) and accordingly consent for
publication was exempted.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial mesenchymal transition
|
|
OS
|
overall survival
|
|
NSCLC
|
non-small cell lung cancer
|
|
E-cadherin
|
epithelial-cadherin
|
|
Tcf/Lef
|
T-cell factor/lymphoid enhancer
factor
|
|
TGFβ
|
transforming growth factor-β
|
|
ZFHX1B
|
Zinc finger homeobox 1B
|
|
Raf-CRD
|
Raf-1 cysteine-rich domain
|
|
ADAM
|
a disintegrin and
metalloproteinase
|
|
EOC
|
epithelial ovarian carcinoma
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brundage MD, Davies D and Mackillop WJ:
Prognostic factors in non-small cell lung cancer: A decade of
progress. Chest. 122:1037–1057. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi PJ, Jeong SS and Yoon SS: Prediction
and prognostic factors of post-recurrence survival in recurred
patients with early-stage NSCLC who underwent complete resection. J
Thorac Dis. 8:152–160. 2016.PubMed/NCBI
|
|
4
|
Botling J, Edlund K, Lohr M, Hellwig B,
Holmberg L, Lambe M, Berglund A, Ekman S, Bergqvist M, Pontén F, et
al: Biomarker discovery in non-small cell lung cancer: Integrating
gene expression profiling, meta-analysis, and tissue microarray
validation. Clin Cancer Res. 19:194–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang X, Cao W, Zhang L, Zhang W, Zhang X
and Lin H: Targeting 14-3-3zeta in cancer therapy. Cancer Gene
Ther. 19:153–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
La Porta S, Roth L, Singhal M, Mogler C,
Spegg C, Schieb B, Qu X, Adams RH, Baldwin HS, Savant S and
Augustin HG: Endothelial Tie1-mediated angiogenesis and vascular
abnormalization promote tumor progression and metastasis. J Clin
Invest. 128:834–845. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niemantsverdriet M, Wagner K, Visser M and
Backendorf C: Cellular functions of 14-3-3 zeta in apoptosis and
cell adhesion emphasize its oncogenic character. Oncogene.
27:1315–1319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu J, Guo H, Treekitkarnmongkol W, Li P,
Zhang J, Shi B, Ling C, Zhou X, Chen T, Chiao PJ, et al: 14-3-3zeta
Cooperates with ErbB2 to promote ductal carcinoma in situ
progression to invasive breast cancer by inducing
epithelial-mesenchymal transitition. Cancer Cell. 16:195–207. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen CH, Chuang SM, Yang MF, Liao JW, Yu
SL and Chen JJ: A novel function of YWHAZ/β-catenin axis in
promoting epithelial-mesenchymal transition and lung cancer
metastasis. Mol Cancer Res. 10:1319–1331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Zhao J, Du Y, Park HR, Sun SY,
Bernal-Mizrachi L, Aitken A, Khuri FR and Fu H: Down-regulation of
14-3-3zeta suppresses anchorage-independent growth of lung cancer
cells through anoikis activation. Proc Natl Acad Sci USA.
105:162–167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neal CL, Yao J, Yang W, Zhou X, Nguyen NT,
Lu J, Danes CG, Guo H, Lan KH, Ensor J, et al: 14-3-3zeta
overexpression defines high risk for breast cancer recurrence and
promotes cancer cell survival. Cancer Res. 69:3425–3432. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi JE, Hur W, Jung CK, Piao LS, Lyoo K,
Hong SW, Kim SW, Yoon HY and Yoon SK: Silencing of 14-3-3ζ
over-expression in hepatocellular carcinoma inhibits tumor growth
and enhances chemosensitivity to cis-diammined dichloridoplatium.
Cancer Lett. 303:99–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao W, Yang X, Zhou J, Teng Z, Cao L,
Zhang X and Fei Z: Targeting 14-3-3 protein, difopein induces
apoptosis of human glioma cells and suppresses tumor growth in
mice. Apoptosis. 15:230–241. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clark GJ, Drugan JK, Rossman KL, Carpenter
JW, Rogers-Graham K, Fu H, Der CJ and Campbell SL: 14-3-3 zeta
negatively regulates raf-1 activity by interactions with the Raf-1
cysteine-rich domain. J Biol Chem. 272:20990–20993. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li FQ, Mofunanya A, Harris K and Takemaru
K: Chibby cooperates with 14-3-3 to regulate beta-catenin
subcellular distribution and signaling activity. J Cell Biol.
181:1141–1154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu Pc, Sun Y, Xu R, Sang Y, Zhao J, Liu
G, Cai L, Li C and Zhao S: The interaction between ADAM 22 and
14-3-3zeta: Regulation of cell adhesion and spreading. Biochem
Biophys Res Commun. 301:991–999. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Daugaard I, Sanders KJ, Idica A,
Vittayarukskul K, Hamdorf M, Krog JD, Chow R, Jury D, Hansen LL,
Hager H, et al: miR-151a induces partial EMT by regulating
E-cadherin in NSCLC cells. Oncogenesis. 6:e3662017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li S, Xu F, Zhang J, Wang L, Zheng Y, Wu
X, Wang J, Huang Q and Lai M: Tumor-associated macrophages
remodeling EMT and predicting survival in colorectal carcinoma.
Oncoimmunology. 7:e13807652017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peinado H, Portillo F and Cano A:
Transcriptional regulation of cadherins during development and
carcinogenesis. Int J Dev Biol. 48:365–375. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taddei ML, Chiarugi P, Cirri P, Buricchi
F, Fiaschi T, Giannoni E, Talini D, Cozzi G, Formigli L, Raugei G
and Ramponi G: Beta-catenin interacts with low-molecular-weight
protein tyrosine phosphatase leading to cadherin-mediated cell-cell
adhesion increase. Cancer Res. 62:6489–6499. 2002.PubMed/NCBI
|
|
21
|
Chen JJ, Peck K, Hong TM, Yang SC, Sher
YP, Shih JY, Wu R, Cheng JL, Roffler SR, Wu CW and Yang PC: Global
analysis of gene expression in invasion by a lung cancer model.
Cancer Res. 61:5223–5230. 2001.PubMed/NCBI
|
|
22
|
Guo Y, Yin J, Zha L and Wang Z:
Clinicopathological significance of platelet-derived growth factor
B, platelet-derived growth factor receptor-β and E-cadherin
expression in gastric carcinoma. Contemp Oncol (Pozn). 17:150–155.
2013.PubMed/NCBI
|
|
23
|
Chassagnon G, Bennani S and Revel MP: New
TNM classification of non-small cell lung cancer. Rev Pneumol Clin.
73:34–39. 2017.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harms A, Kriegsmann M, Fink L, Länger F
and Warth A: The new TNM classification for lung tumors: Changes
and the assessment of multiple tumor foci. Pathologe. 38:11–20.
2017.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang D, Xu S, Zhang Q and Zhao W: The
expression and clinical significance of the androgen receptor and
E-cadherin in triple-negative breast cancer. Med Oncol. 29:526–533.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao L, Antic T, Hyjek E, Gong C, Mueller
J, Waxman I, DeMay RM and Reeves W: Immunohistochemical analysis of
E-cadherin and zeste homolog 2 expression in endoscopic
ultrasound-guided fine-needle aspiration of pancreatic
adenocarinoma. Cancer Cytopathol. 121:644–652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elzagheid A, Buhmeida A, Laato M,
El-Faitori O, Syrjänen K, Collan Y and Pyrhönen S: Loss of
E-cadherin expression predicts disease recurrence and shorter
survival in colorectal carcinoma. APMIS. 120:539–548. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhai B, Yan HX, Liu SQ, Chen L, Wu MC and
Wang HY: Reduced expression of E-cadherin/catenin complex in
hepatocellular carcinomas. World J Gastroenterol. 14:5665–5673.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ling C, Raasch JL and Welham NV:
E-cadherin and transglutaminase-1 epithelial barrier restoration
precedes type IV collagen basement membrane reconstruction
following vocal fold mucosal injury. Cells Tissues Organs.
193:158–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Molina-Ortiz I, Bartolomé RA,
Hernández-Varas P, Colo GP and Teixidó J: Overexpression of
E-cadherin on melanoma cells inhibits chemokine-promoted invasion
involving p190RhoGAP/p120ctn-dependent inactivation of RhoA. J Biol
Chem. 284:15147–15157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Larriba MJ, Casado-Vela J, Pendás-Franco
N, Peña R, de Herreros García A, Berciano MT, Lafarga M, Casal JI
and Muñoz A: Novel snail1 target proteins in human colon cancer
identified by proteomic analysis. PLoS One. 5:e102212010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tong S, Xia T, Fan K, Jiang K, Zhai W, Li
JS, Wang SH and Wang JJ: 14-3-3ζ promotes lung cancer cell invasion
by increasing the Snail protein expression through atypical protein
kinase C (aPKC)/NF-κB signaling. Exp Cell Res. 348:1–9. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Campbell PM and Der CJ: Oncogenic Ras and
its role in tumor cell invasion and metastasis. Semin Cancer Biol.
14:105–114. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee JY, Lowell CA, Lemay DG, Youn HS, Rhee
SH, Sohn KH, Jang B, Ye J, Chung JH and Hwang DH: The regulation of
the expression of inducible nitric oxide synthase by Src-family
tyrosine kinases mediated through MyD88-independent signaling
pathways of Toll-like receptor 4. Biochem Pharmacol. 70:1231–1240.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pautz A, Art J, Hahn S, Nowag S, Voss C
and Kleinert H: Regulation of the expression of inducible nitric
oxide synthase. Nitric Oxide. 23:75–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hollestelle A, Peeters JK, Smid M,
Timmermans M, Verhoog LC, Westenend PJ, Heine AA, Chan A, Sieuwerts
AM, Wiemer EA, et al: Loss of E-cadherin is not a necessity for
epithelial to mesenchymal transition in human breast cancer. Breast
Cancer Res Treat. 138:47–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen A, Beetham H, Black MA, Priya R,
Telford BJ, Guest J, Wiggins GA, Godwin TD, Yap AS and Guilford PJ:
E-cadherin loss alters cytoskeletal organization and adhesion in
non-malignant breast cells but is insufficient to induce an
epithelial-mesenchymal transition. BMC Cancer. 14:5522014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ahn SM, Cha JY, Kim J, Kim D, Trang HT,
Kim YM, Cho YH, Park D and Hong S: Smad3 regulates E-cadherin via
miRNA-200 pathway. Oncogene. 31:3051–3059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mise N, Savai R, Yu H, Schwarz J, Kaminski
N and Eickelberg O: Zyxin is a transforming growth factor-β
(TGF-β)/Smad3 target gene that regulates lung cancer cell motility
via integrin α5β1. J Biol Chem. 287:31393–31405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao GY, Ding JY, Lu CL, Lin ZW and Guo J:
The overexpression of 14-3-3ζ and Hsp27 promotes non–small cell
lung cancer progression. Cancer. 120:652–663. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen HH, Wang Y, Xu YJ, Du ZY, Hao HJ, Xu
DY, Tan XG, Lin HY and Lu YL: Inhibition of metastasis of lung
cancer cells by 14-3-3ζ protein:an experimental investigation. Bull
Acad Milit. 2005.
|
|
42
|
An N, Zhu W, Feng Z, Ye S, Yu C and Cai C:
Effect of 20(R) ginsenoside Rg3 on protein expression of lung
cancer cell line. Zhongguo Fei Ai Za Zhi. 11:311–320. 2008.(In
Chinese). PubMed/NCBI
|
|
43
|
Schneider BJ and Kalemkerian GP:
Personalized therapy of small cell lung cancer. Adv Exp Med Biol.
890:149–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu P, Sang Y, Xu H, Zhao J, Xu R, Sun Y,
Xu T, Wang X, Chen L, Feng H, et al: ADAM22 plays an important role
in cell adhesion and spreading with the assistance of 14-3-3.
Biochem Biophys Res Commun. 331:938–946. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kobayashi R, Deavers M, Patenia R,
Rice-Stitt T, Halbe J, Gallardo S and Freedman RS: 14-3-3 zeta
protein secreted by tumor associated monocytes/macrophages from
ascites of epithelial ovarian cancer patients. Cancer Immunol
Immunother. 58:247–258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fan T, Li R, Todd NW, Qiu Q, Fang HB, Wang
H, Shen J, Zhao RY, Caraway NP, Katz RL, et al: Up-regulation of
14-3-3zeta in lung cancer and its implication as prognostic and
therapeutic target. Cancer Res. 67:7901–7906. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Khorrami A, Bagheri Sharif M, Tavallaei M
and Gharechahi J: The functional significance of 14-3-3 proteins in
cancer: Focus on lung cancer. Horm Mol Biol Clin Investig. 32:pii:
/j/hmbci.2017.32.issue-3/hmbci-2017-0032/hmbci-2017-0032.xml.
2017.PubMed/NCBI
|
|
48
|
Cau Y, Valensin D, Mori M, Draghi S and
Botta M: Structure, function, involvement in diseases and targeting
of 14-3-3 proteins: An update. Curr Med Chem. 25:5–21. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ko S, Kim JY, Jeong J, Lee JE, Yang WI and
Jung WH: The role and regulatory mechanism of 14-3-3 sigma in human
breast cancer. J Breast Cancer. 17:207–218. 2014. View Article : Google Scholar : PubMed/NCBI
|