Introduction
Ovarian carcinoma is the most lethal of the
gynecologic malignancies in women worldwide (1). Epithelial ovarian carcinoma (EOC), which
accounts for 90% (2) of ovarian
carcinoma and for 4.2% of all cancer deaths in women globally, is a
heterogeneous group of neoplasms (3,4). Due to
several factors of insidious nature, manifesting with little or no
symptoms until the disease progresses to metastasis, along with a
wide diversity of histological subtypes and corresponding clinical
behavior, poses significant therapeutic challenges (5). Although significant progress in the
diagnosis and treatment including surgery, radiotherapy and
chemotherapy have been made in the treatment of ovarian carcinoma,
the 5-year survival rate for all stages is below 45% and is
decreased to 25% among patients with advanced ovarian carcinoma
(6,7).
Inability to prolong patient remission is a critical gap in the
clinical management of ovarian carcinoma (5). The underlying cause of this problem
stems in part from insufficient basic knowledge of the biology and
mechanisms supporting ovarian carcinoma carcinogenesis and
progression. Therefore, it is crucial to find the underlying
molecular mechanisms of ovarian carcinoma and identify the
promising therapeutic targets to improve treatment strategies.
MicroRNAs (miRNAs) are a class of small, highly
conserved, non-coding RNAs with a length of approximately 21–24
nucleotides that bind to the 3′-untranslated region (3′-UTR) of
target messenger RNAs (mRNAs) to induce translational degradation
or repression (8). Given the ability
of miRNAs to regulate gene expression (9), they unsurprisingly became the critical
point in their involvement in cancer. Increasing evidence indicated
that miRNAs are frequently dysregulated in cancers (10–12) where
they have been shown to contribute to disease pathogenesis, as well
as cancer cell death, differentiation, proliferation, metastasis,
and apoptosis (13–15). Previous investigations have identified
the dysregulated expression of various miRNAs involved in human
ovarian carcinoma tumorigenesis and progression, such as cell
proliferation, metastasis, migration and DNA methylation (16,17). Among
such a large number of miRNAs that have been identified as having
significant roles in tumorigenesis, miR-222 was identified as a key
miRNA, located on human chromosome Xp11.3 as a single transcript,
and showing high sequence identity (18). Functional studies showed that miR-222
reinforced cancer cell biological progression (19), promoted tumor cell proliferation,
migration and microtubule formation, and the novel regulatory axis
miR-222-3p/GNAI2/AKT is a potential therapeutic target for EOC
patients in the future (20).
Although the importance of miRNAs in cancer pathological processes
have attracted much attention in recent decades, the pathological
relevance and significance of the majority miRNAs in ovarian
carcinoma remains unclear.
Phosphatase and tensin homolog (PTEN) is well
recognized as tumor suppressor gene and is involved in the
regulation of cancer cell biological behavior. PTEN could
antagonize PI3 kinase (PI3K) activity by converting
phosphatidylinositol (3,4,5)-trisphosphate in the cytoplasm into
phosphatidylinositol (4,5)-bisphosphate. The PI3K dysregulation can
result in Akt hyper-activation, and promote tumor cell
radioresistance, proliferation, migration, and invasion. PTEN has
been identified as one direct target of miR-222 (21), we thus reasoned miR-222 may promote
the invasion and migration by targeting PTEN of ovarian carcinoma,
and be responsible for ovarian carcinoma progression and
tumorigenesis.
In order to explore the regulatory mechanism whereby
miR-222 was involved in ovarian carcinoma cell migration and
invasion, we proved miR-222 could positively regulate PTEN
expression and confirmed miR-222 functioned as a vital part in cell
migration and invasion of ovarian carcinoma regulated by PTEN.
These results may indicate a novel regulatory mechanism for cell
migration and invasion in ovarian carcinoma and provide a new
avenue for exploring ovarian carcinogenesis.
Materials and methods
Clinical specimens
Forty paired tissues and the adjacent normal tissues
(ANT) of ovarian carcinoma were collected at the People's Hospital
of Rizhao (Rizhao, China), from 2013 to 2015. Patients did not
receive radiotherapy or chemotherapy priro to surgery. The Ethics
Committee of People's Hospital of Rizhao approved the present
study. All samples were obtained with written informed consent from
participating patients. All the tissues obtained from biopsy or
surgery were frozen at −80°C. The clinical characteristics are
shown in Table I.
 | Table I.Clinicopathological variables and
miR-200c expression in 40 ovarian carcinoma patients. |
Table I.
Clinicopathological variables and
miR-200c expression in 40 ovarian carcinoma patients.
|
|
| miR-200c
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Total cases
(n=40) | Low (%) | High (%) | P-value |
|---|
| Age (years) |
|
|
| P>0.05 |
| ≤40 | 18 | 8 (71.43) | 10 (28.57) |
|
|
>40 | 22 | 10 (69.23) | 12 (30.77) |
|
| Tumor size (cm) |
|
|
| P>0.05 |
|
<5 | 18 | 12 (66.67) | 6 (33.33) |
|
| ≥5 | 22 | 6 (72.73) | 16 (27.27) |
|
| Histological
grading |
|
|
| P<0.01 |
| 1–2 | 31 | 13 (90.32) | 18 (9.68) |
|
| 3 | 9 | 5 (55.56) | 4 (44.44) |
|
| Distant
metastasis |
|
|
| P<0.05 |
| Yes | 25 | 9 (90.00) | 16 (10.00) |
|
| No | 15 | 9 (63.33) | 6 (36.67) |
|
| FIGO stage |
|
|
| P<0.01 |
| I–II | 29 | 12 (41.38) | 17 (58.62) |
|
|
III–IV | 11 | 6 (76.47) | 5 (23.53) |
|
Cell culture
Ovarian carcinoma cell lines A2780, SKOV-3 and
OVCAR-3 and human ovarian surface epithelial cell line (HOSEpiC)
were obtained from the Shanghai Institute of Cell Biology
(Shanghai, China). Ovarian carcinoma cell lines were cultured in
Dulbecco's modified Eagle's medium (DMEM; HyClone; GE Healthcare,
Chicago, IL, USA). After the cells adhered, transfection of the
miR-222 mimics/inhibitor/negative control (NC) were performed with
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), which achieved the ectopic expression of miRNA.
The transfection protocol for siRNA was the same as that for
miR-222 mimics/inhibitor. The miR-222 mimics/inhibitor, pGL3-PTEN
3′UTR and pGL3-NC were obtained from Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). The sequences of miR-222 mimics/inhibitor were:
miR-222 mimic, AGCUACAUCUGGCUACUGGGU; and inhibitor,
GCGAUGUAGACCGAUGACCA.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from culture cells and
cancer tissues using the TRIzol reagents (Ambion; Thermo Fisher
Scientific, Inc.). PTEN was relative to β-actin and miR-222 was
normalized relative to U6 endogenous control using the
2−ΔΔCq method. The primers used were: miR-222 forward,
5′-AGCTACATCTGGCTACTGG-3′, and reverse, 5′-GTATCCAGTGCAGGGTCC-3′;
U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-TGGTGTCGTGGAGTCG-3′. PTEN forward 5′-TGGCGGAACTTGCAATCCTCAGT-3′
and reverse, 5′-TCCCGTCGTGTGGGTCCTGA-3′. β-actin forward,
5′-TTGCCGACAGGATGCAGAAGGA-3′ and reverse,
5′-AGGTGGACAGCGAGGCCAGGAT-3′.
Transwell assay
Cell Transwell assays were conducted using chambers
(Costar, NY) with or without 2 mg/ml Matrigel (Clontech
Laboratories, Inc., Mountainview, CA, USA). OVCAR-3 and SKOV-3
cells were transfected with the miR-222 mimic/inhibitor and NC and
isolated to make a final concentration at 2×105/ml, then
placed into the upper chamber. Lower chambers were filled with
medium containing 20% fetal bovine serum as a chemo-attractant. The
cells were fixed for 15 min using 1 ml/well 4% paraformaldehyde,
stained with Giemsa (JRDUN Biotechnology Co. Ltd., Shanghai, China)
for 30 min, and washed three times with 1X phosphate-buffered
saline. Finally, stained cells were counted under a microscope
(Olympus, Tokyo, Japan).
Western blot analysis
Cultured cells were collected and lysed with
radioimmune precipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). Protein samples were transferred to
polyvinyl difluoride membranes (EMD Millipore, Billerica, MA, USA),
and the membranes were incubated in primary antibodies rabbit
monoclonal anti-PTEN (ab109454; 1:1,000; Abcam, Cambridge, MA, USA)
followed by incubation with horseradish peroxidase-coupled
secondary antibody goat anti-rabbit IgG-HRP (sc-2004; 1:3,000;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). β-actin was
used as internal control. ImageJ software (NIH, Bethesda, MD, USA)
was used to quantify the protein bands.
Luciferase reporter
The PTEN mutant (MT) and wild-type (WT) 3′UTR were
cloned from human genomic DNA. Cells were seeded on 24-well plates
for luciferase assay. miR-222 mimic plus PTEN WT or MT 3′UTR were
transfected into OVCAR-3 and SKOV-3. Then, we used Luciferase
Reporter Assay (Promega Corporation, Madison, WI, USA) according to
the manufacturer's protocols.
Statistical analysis
Statistical results were analyzed by SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA) statistics package. The
results are presented as the mean ± standard deviation (SD). The
Student's t-test was used to analyze experimental data. The
unpaired two group comparison and multiple comparisons were made
with analysis of variance (ANOVA) with Tukey-Kramer post hoc test.
Correlation between mRNA and miRNA was estimated using the
Spearman's correlation method. P<0.05 was considered to indicate
a statistically significant difference.
Results
miR-222 expression is upregulated and
inversely correlates with PTEN in ovarian carcinoma
The miR-222 expression levels were detected in 40
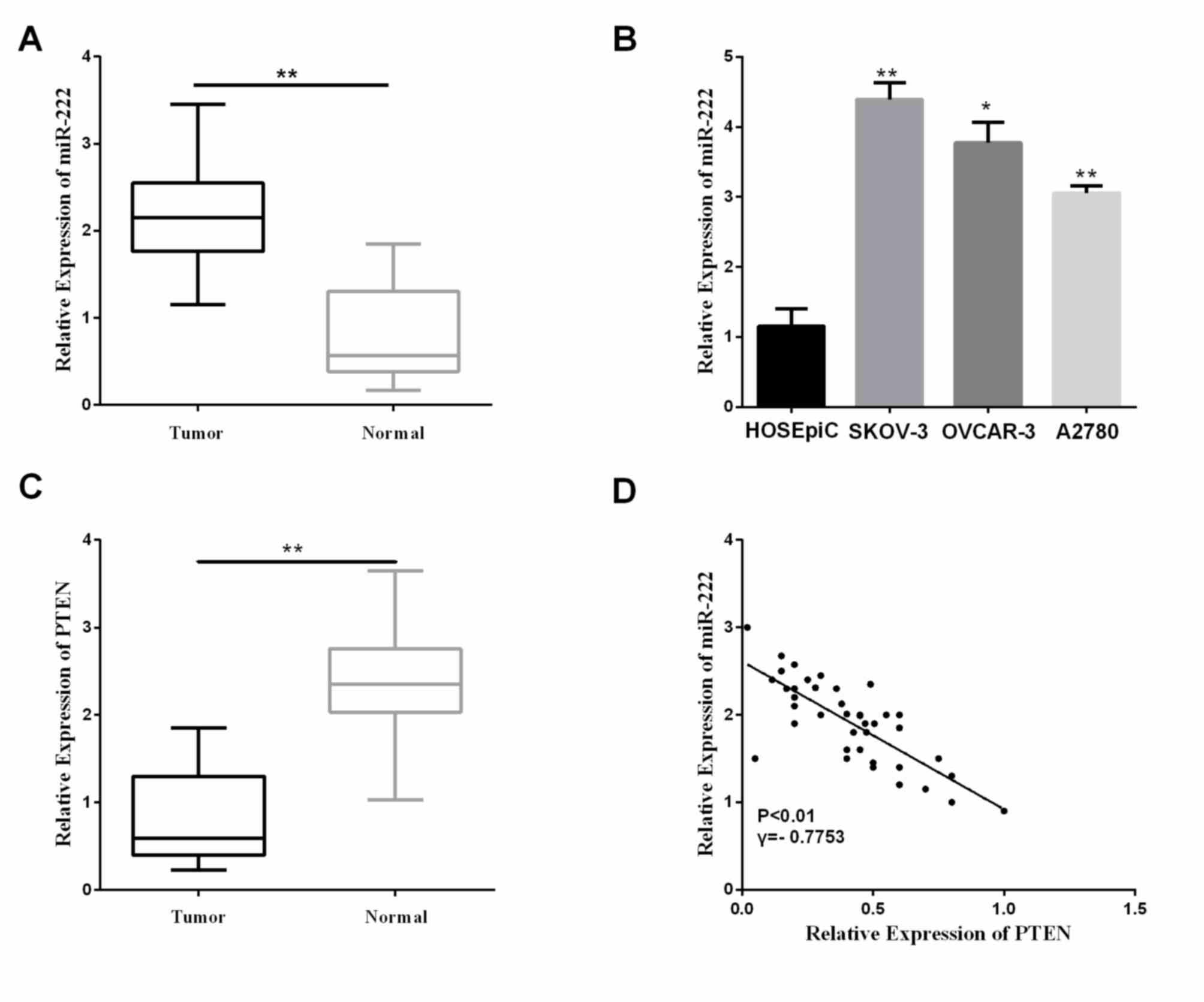
matched pairs of ovarian carcinoma tissues and ANT by RT-qPCR. In
comparison to the normal cervical tissues, miR-222 expression was
significantly higher in the ovarian carcinoma tissues (Fig. 1A; P<0.01). To investigate potential
associations between miR-222 expression and patient's
clinicopathological variables, we divided the patients with ovarian
carcinoma into two groups based on mean value (2.200) of miR-222
expression: High expression (>2,200, n=18), and low expression
(<2,200, n=22) groups. Statistical analysis suggested that a
high miR-222 expression level correlated with histological grading,
distant metastasis, and FIGO stage, whereas no statistical
difference was found in the correlation of miR-222 expression with
age and tumor size (Table I). In
addition to ovarian carcinoma tissue samples, we selected three
cell lines (A2780, SKOV-3 and OVCAR-3) compared with the normal
cells (HOSEpiC) to further detect miR-222 relative expression
levels. The results showed the miR-222 levels in A2780, SKOV-3 and
OVCAR-3 were significantly increased relative to that in the normal
cell HOSEpiC (Fig. 1B,
P<0.05).
Furthermore, the levels of PTEN in the ovarian
carcinoma tissues and the ANT were assessed using RT-qPCR. The PTEN
relative expression level was significantly decreased in the
ovarian carcinoma tissues, as shown in Fig. 1C (P<0.01). miR-222 was negatively
correlated with the PTEN level in these clinical specimens
(Fig. 1D). These results indicated
miR-222 is upregulated in ovarian carcinoma tissues and that the
increase of expression of miR-222 decreased PTEN level, which may
play an essential part in the progression of ovarian carcinomas.
Additionally, miR-222 may be critical in regulating ovarian
carcinoma cell invasion and migration. However, the particular
function of miR-222 ovarian carcinoma is unknown.
miR-222 promotes migration and
invasion of ovarian carcinoma
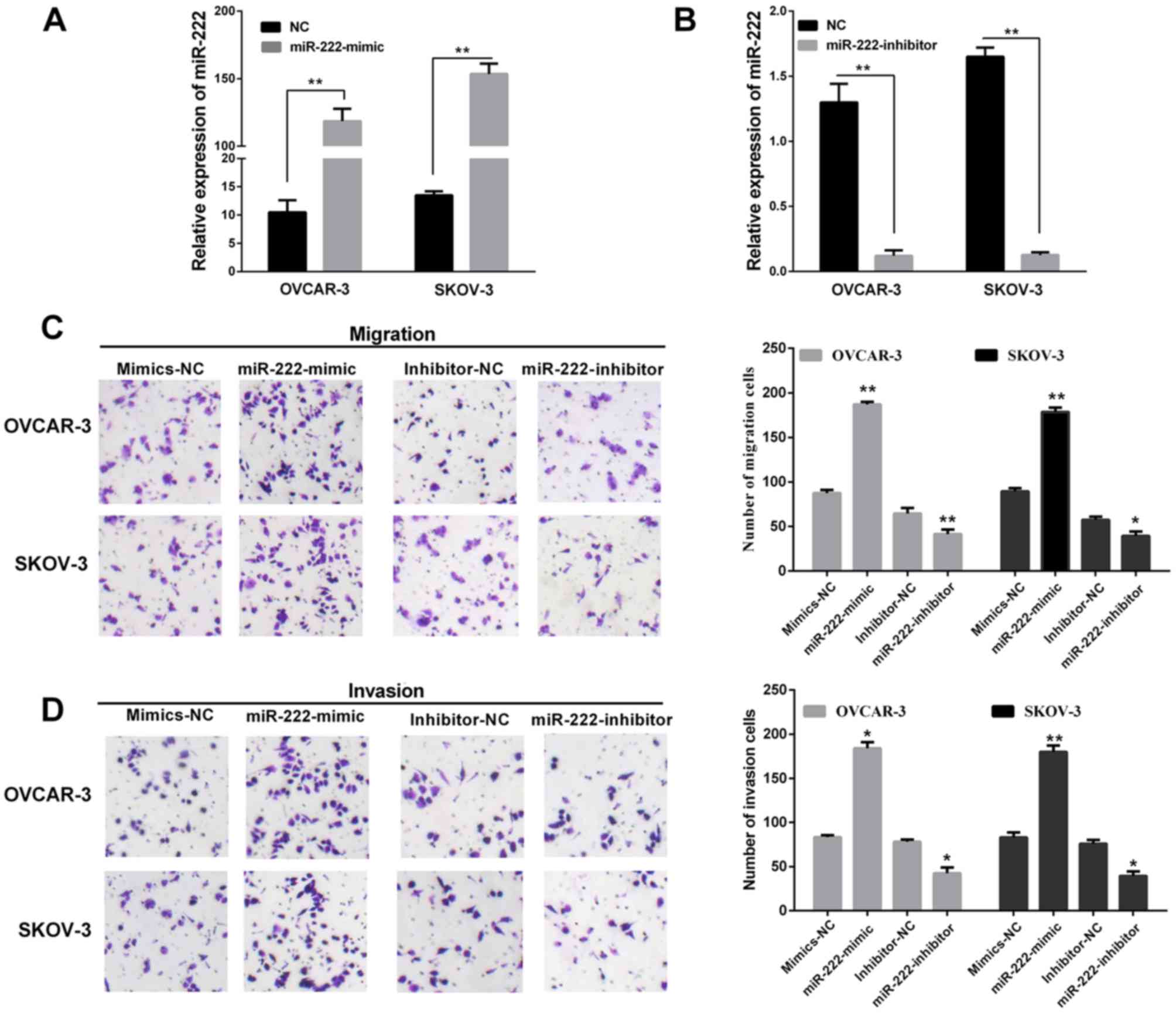
To reveal the functional role of miR-222 in
affecting the migration and invasion of ovarian carcinoma, SKOV-3
and OVCAR-3 cell lines were both transfected with the miR-222
mimic/inhibitor/NC. The results showed SKOV-3 and OVCAR-3 were
transfected with miR-222 mimics both expressed at a relatively high
level compared with cell lines transfected corresponding to
negative control (Fig. 2A). However,
miR-222 expression level of cell lines transfected with miR-222
inhibitor were relatively lower than cells transfected with
corresponding negative control (Fig.
2B). Transwell assays without Matrigel were used to examine the
miR-222 function on the cell migratory potential, and Transwell
assays with Matrigel were used to detect the miR-222 effects on the
cell invasive potential. Overexpression of miR-222 significantly
increased the migration and invasion capacities in the OVCAR-3 and
SKOV-3 cells (Fig. 2C). Similarly,
Fig. 2D showed re-expression of
miR-222 in OVCAR-3 and SKOV-3 cells transfected with the miR-222
mimic/inhibitor compared with corresponding NC. In summary, these
findings indicated that miR-222 may act as an oncomiR and promotes
cell migration and invasion during ovarian carcinoma
progression.
PTEN is a target and downregulated by
miR-222
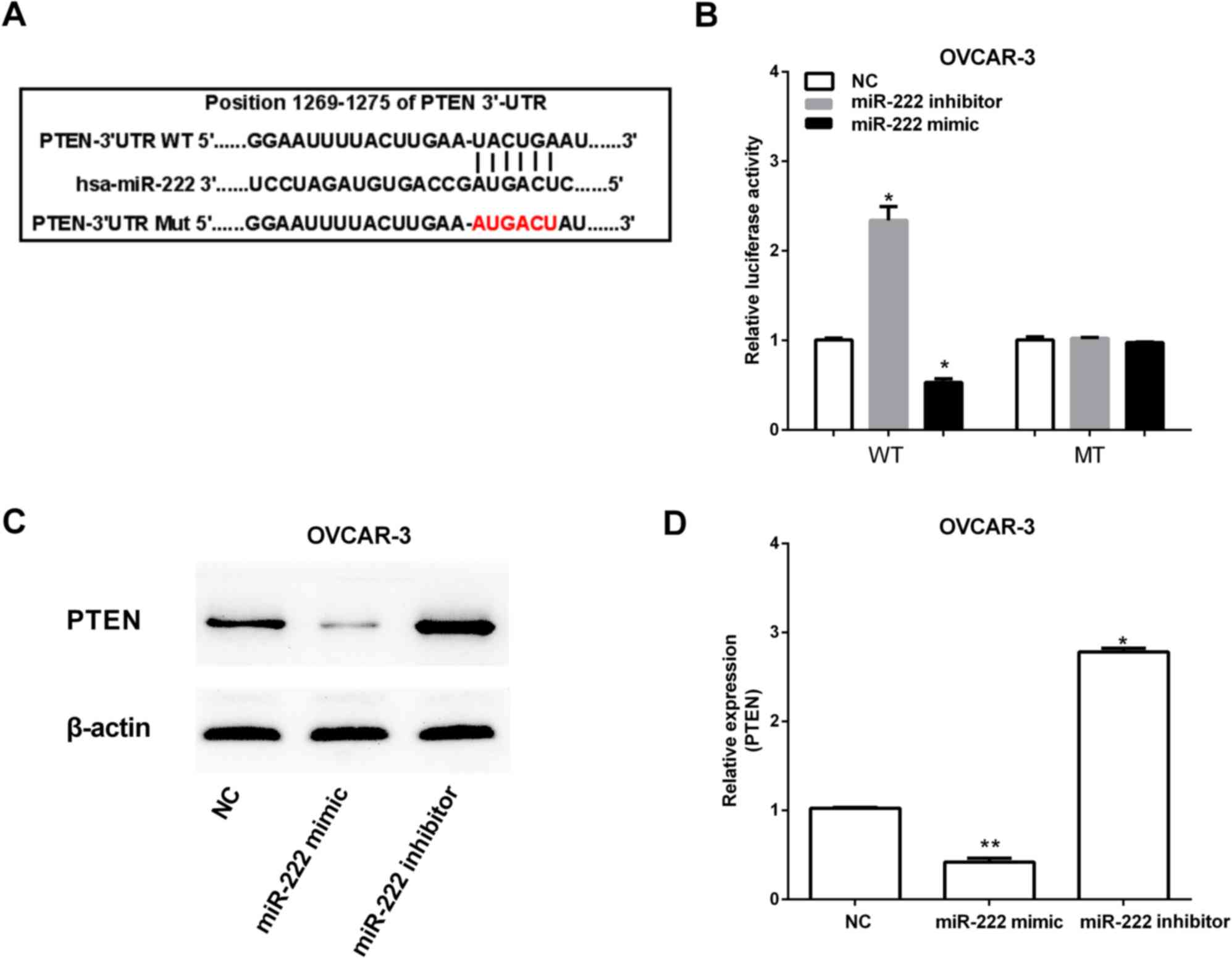
Predictive tools (TargetScan Huma http://www.targetscan.org/vert_71/) were used to
find target genes of miR-222. The miR-222 putative binding sites
were found in the 3′-UTR of PTEN at 1269–1275 bp (Fig. 3A). To determine whether miR-222 can
regulate PTEN expression, the WT plasmids pGL-UTR and MT plasmid
pGL-mUTR of PTEN 3′-UTR were constructed based on luciferase
reporter assay, which were then both transfected into OVCAR-3 with
or without miR-222 overexpression. Decreased expression of PTEN was
observed after transfecting by miR-222 mimic. However, miR-222
inhibitor increased PTEN expression (Fig.
3B). Furthermore, the PTEN mRNA and protein expression were
increased by miR-222 inhibitor, and miR-222 mimic had the opposite
effect on PTEN expression (Fig. 3C and
D).
Overexpression of PTEN promotes
migration and invasion of ovarian carcinoma
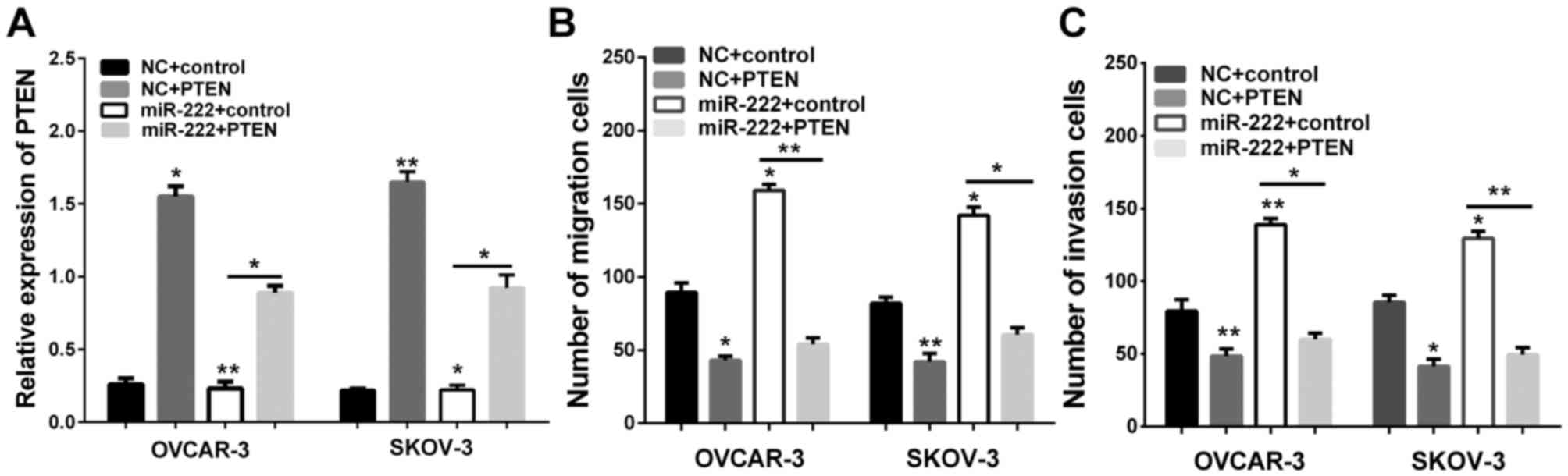
Having confirmed that miR-222 acts to reduce PTEN
levels in ovarian carcinoma, we then determined whether PTEN is
involved in the migration and invasion of ovarian carcinoma. We
therefore transfected the control group and PTEN plasmid into the
miR-222 cells and NC for Transwell assay. The successful
overexpression of PTEN was verified by RT-qPCR (Fig. 4A). We further analyzed the migration
and invasion of OVCAR-3 and SKOV-3. We found that an increase of
the expression of PTEN could effectively reverse the promotion
effect on cell migration and invasion induced by miR-222
overexpression in OVCAR-3 and SKOV-3 (Fig. 4B and C). These findings suggest that
PTEN is responsible for the tumorigenic effects in ovarian
carcinoma cells, and PTEN may be important in migration and
invasion of ovarian carcinoma cells.
Discussion
A number of miRNAs have been proved to be encoded in
ovarian carcinoma progression and development through effect on
cancer cell proliferation, migration, apoptosis, and invasion
(16,17). For example, microRNA-223-3p promoted
cell growth and invasion through targeting SOX11 expression of
ovarian carcinoma (22). Xu et
al demonstrated that miR-28-5p promotes the progression and
development of ovarian carcinoma through inhibition of N4BP1
(23). Thus, identification of
cancer-specific miRNAs and their involved targets is pivotal for
comprehending their function in tumor migration and invasion, and
to provide critical clues for diagnosis and therapy of ovarian
carcinoma.
Previous studies have suggested miR-222 was altered
in ovarian carcinoma and its functional role was extremely tangled
as it could act as promising target for therapeutic purposes
(20). miR-222 was significantly
increased in ovarian carcinoma cell lines and tissues, and its
expression was positively correlated with PTEN. Further studies
revealed that miR-222 promoted ovarian carcinoma cell migration and
invasion. The quantity of cell invasion and migration of miR-222
with/without overexpression group showed by Transwell assay was
increased compared with negative control group, and the number of
cell invasion and migration of miR-222 without overexpression group
was decreased. These outcomes suggested that miR-200 might serve as
a novel biomarker or therapeutic target for ovarian carcinoma.
PTEN, which is well recognized as a tumor
suppressor gene in human cancer, has been found involved in the
regulation of cancer cell biological behavior and playing an
important part in the progression and development of various kinds
of human cancer. PTEN has been reported as a target of miR-222
(21), Shen et al (24) reported that miR-222/PTEN/Akt/FOXO1
axis mediated ADR-resistance and prognosis of breast cancer
patients. It was also suggested that miR-222 could decrease the
sensitivity of breast cancer cells to adriamycin through
PTEN/Akt/p27 kip1 signaling pathway (25). In the present study, we determined
PTEN as the target of miR-222 in ovarian carcinoma, a significantly
increased expression of PTEN was observed after transfection by
miR-222 mimic. However, miR-222 inhibitor decreased PTEN
expression. Furthermore, to the best of our knowledge, for the
first time, interference of PTEN was shown to promote migration and
invasion. These studies together demonstrated that miR-222 might be
a powerful anti-ovarian carcinoma candidate.
In conclusion, we revealed that miR-222 could
promote ovarian carcinoma cell migration and invasion. PTEN was
identified as a functional target of miR-222. The newly identified
miR-222/PTEN may provide new insight into pathogenesis and
represents a potential therapeutic target for ovarian
carcinoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LG and WZ contributed to the conception of the
study, YY contributed significantly to data analysis and manuscript
preparation, XX performed the data analyses and wrote the
manuscript, HL and GZ helped perform the analysis with constructive
discussions. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
People's Hospital of Rizhao (Rizhao, China). All patients signed
the informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim
JH, Kim JW and Kim S: MicroRNA expression profiles in serous
ovarian carcinoma. Clin Cancer Res. 14:2690–2695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cittelly DM, Dimitrova I, Howe EN,
Cochrane DR, Jean A, Spoelstra NS, Post MD, Lu X, Broaddus RR,
Spillman MA, et al: Restoration of miR-200c to ovarian cancer
reduces tumor burden and increases sensitivity to paclitaxel. Mol
Cancer Ther. 11:2556–2565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pal MK, Jaiswar SP, Dwivedi VN, Tripathi
AK, Dwivedi A and Sankhwar P: MicroRNA: A new and promising
potential biomarker for diagnosis and prognosis of ovarian cancer.
Cancer Biol Med. 12:328–341. 2015.PubMed/NCBI
|
|
4
|
Lupia M and Cavallaro U: Ovarian cancer
stem cells: Still an elusive entity? Mol Cancer. 16:642017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koutsaki M, Libra M, Spandidos DA and
Zaravinos A: The miR-200 family in ovarian cancer. Oncotarget.
8:66629–66640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Xu C, Wang Y and Zhang X:
MicroRNA-365 inhibits ovarian cancer progression by targeting
Wnt5a. Am J Cancer Res. 7:1096–1106. 2017.PubMed/NCBI
|
|
7
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chan E, Prado DE and Weidhaas JB: Cancer
microRNAs: From subtype profiling to predictors of response to
therapy. Trends Mol Med. 17:235–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hata A and Lieberman J: Dysregulation of
microRNA biogenesis and gene silencing in cancer. Sci Signal.
8:re32015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saumet A and Lecellier CH: MicroRNAs and
personalized medicine: Evaluating their potential as cancer
biomarkers. Adv Exp Med Biol. 888:5–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Srivastava SK, Arora S, Averett C, Singh S
and Singh AP: Modulation of microRNAs by phytochemicals in cancer:
Underlying mechanisms and translational significance. BioMed Res
Int. 2015:8487102015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Virant-Klun I, Ståhlberg A, Kubista M and
Skutella T: MicroRNAs: From female fertility, germ cells, and stem
cells to cancer in humans. Stem Cells Int. 2016:39849372016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kinose Y, Sawada K, Nakamura K and Kimura
T: The role of microRNAs in ovarian cancer. BioMed Res Int.
2014:2493932014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Kim S and Kim IM: Regulation of
metastasis by microRNAs in ovarian cancer. Front Oncol. 4:1432014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garofalo M, Quintavalle C, Romano G, Croce
CM and Condorelli G: miR221/222 in cancer: Their role in tumor
progression and response to therapy. Curr Mol Med. 12:27–33. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei Y, Yang J, Yi L, Wang Y, Dong Z, Liu
Z, Ou-Yang S, Wu H, Zhong Z, Yin Z, et al: MiR-223-3p targeting
SEPT6 promotes the biological behavior of prostate cancer. Sci Rep.
4:75462014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fu X, Li Y, Alvero A, Li J, Wu Q, Xiao Q,
Peng Y, Hu Y, Li X, Yan W, et al: MicroRNA-222-3p/GNAI2/AKT axis
inhibits epithelial ovarian cancer cell growth and associates with
good overall survival. Oncotarget. 7:80633–80654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li B, Lu Y, Wang H, Han X, Mao J, Li J, Yu
L, Wang B, Fan S, Yu X, et al: miR-221/222 enhance the
tumorigenicity of human breast cancer stem cells via modulation of
PTEN/Akt pathway. Biomed Pharmacother. 79:93–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fang G, Liu J, Wang Q, Huang X, Yang R,
Pang Y and Yang M: MicroRNA-223-3p regulates ovarian cancer cell
proliferation and invasion by targeting SOX11 expression. Int J Mol
Sci. 18(pii): E12082017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu J, Jiang N, Shi H, Zhao S, Yao S and
Shen H: miR-28-5p promotes the development and progression of
ovarian cancer through inhibition of N4BP1. Int J Oncol.
50:22362017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen H, Wang D, Li L, Yang S, Chen X, Zhou
S, Zhong S, Zhao J and Tang J: MiR-222 promotes drug-resistance of
breast cancer cells to adriamycin via modulation of PTEN/Akt/FOXO1
pathway. Gene. 596:110–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang DD, Yang SJ, Chen X, Shen HY, Luo LJ,
Zhang XH, Zhong SL, Zhao JH and Tang JH: miR-222 induces adriamycin
resistance in breast cancer through PTEN/Akt/p27kip1 pathway.
Tumour Biol. 37:15315–15324. 2016. View Article : Google Scholar : PubMed/NCBI
|