Introduction
The stomach is the most frequent site of malignant
lymphoma involving the gastrointestinal (GI) tract. Among the
subtypes of malignant lymphoma, extranodal marginal zone lymphoma
of mucosa-associated lymphoid tissue (MALT lymphoma) and diffuse
large B-cell lymphoma (DLBCL) account for >90% of all cases
(1). The incidence of Follicular
lymphoma (FL), whether primary or otherwise, has been reported to
account for between 0.8 and 3% of gastric lymphomas (2). FL is one of the most common types of
non-Hodgkin lymphoma in western countries including United States
of America (3) and United Kingdom
(4), whereas the incidence in Asian
countries including Korea (5), Japan
(6) and China (7) is lower than that of western countries.
The incidence of FL is typically low in Korea, occupying ~2.9% of
malignant lymphoma (8).
Histologic features of FL are characterized by
nodular aggregation of two different types of tumor cells, which
are small cleaved centrocytes and large centroblasts, and WHO
histologic grading system is based on the number of centroblasts
per high power field (9). Clinically
FL commonly presents as widely spread systemic disease, and
prognosis varies according to the extent of the disease and
histologic grading at the time of diagnosis (10). In localized early stage diseases,
watchful waiting or radiotherapy is available due to its slowly
progressive nature, while chemotherapy including rituximab is
selected in advanced stage diseases with high tumor burdens
(11). The origin of tumor cells is
germinal center B-cells which are heavily crowded in lymphoid
organs, especially lymph nodes (9).
Therefore, FL has been recognized as primary nodal lymphoma, and
primary extranodal FL has been described in only a few organs
including the skin and duodenum (12). The second part of the duodenum is
known to be the most frequent site of FL of the GI tract followed
by jejunum (10). In the latest
revised fourth edition of WHO classification, duodenal FL was
descried as a distinct variant of FL with typical clinical and
immunophenotypical characteristics. However, in majority of cases,
FL of the GI tract may present through secondary involvement by
widespread nodal FL, therefore, primary gastric FL appears to be
rare (9,10,12,13–17).
The present study describes 3 cases of gastric FL alongside a
comprehensive review of previous cases reported in the literature
and compares their features with those of duodenal FL.
Case report
Case selection
A total of 3 cases of gastric FL were retrieved from
among the cases in the databases of 4 university hospitals in South
Korea (SNU Hospital, SNU Boramae Hospital, SNU Bundang Hospital and
Dongtan Sacred Heart Hospital Hallym University). The cases were
selected from a total of 3,216 cases of non-Hodgkin's lymphoma of
the GI tract, occurring between January 2000 and December 2015, as
the only 3 cases of gastric FL. The pathological features were
reviewed by 3 experienced hematopathologists. Due to the
retrospective nature of the study, ethical approval and patient
consent for publication was waived for the present study by the
Institutional Review Board of Seoul National University Boramae
Hospital (Seoul, South Korea).
Case 1
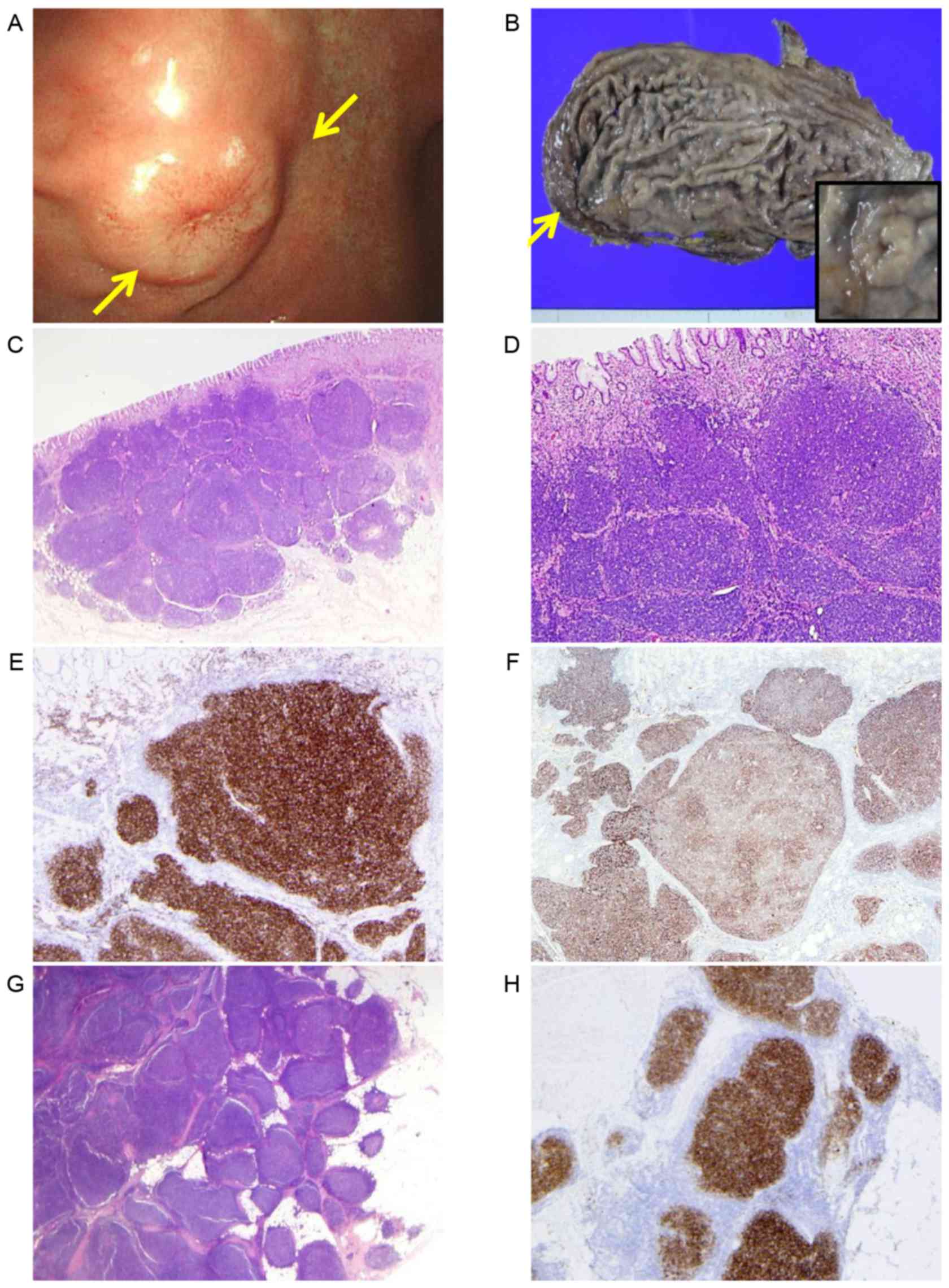
A 61-year-old man was referred to SNU Boramae
Hospital in November, 2015 for the management of a gastric lesion
detected during an annual health check-up program at a local
clinic. Endoscopic examination revealed a well demarcated, polypoid
mass in the anterior wall of the gastric body. Despite three repeat
biopsies, the preoperative pathological results remained unclear,
simply suggesting a malignant lymphoma of germinal center B-cell
origin. Constitutional symptoms, including lymphadenopathy,
organomegaly and anemia, were not exhibited. A partial gastrectomy
was performed and revealed a well demarcated, protruded mass,
measuring 2.0×1.8-cm in the body, as well as an additional
1.5×1.2-cm nodule in the lesser omentum. Specimens were fixed using
10% formaldehyde for 24 h at room temperature and embedded in
paraffin at room temperature for 24 h. Serial 4-µm-thick sections
were sliced and were stained with 0.5% hematoxylin staining
solution for 20 min at room temperature. Subsequently, 1%
hydrochloric acid alcohol was added for 1 min. The slides were
stained with 1% eosin Y staining solution for 1 min at room
temperature and dehydrated at room temperature after applying tap
water at room temperature for 15 min. and immunohistochemistry
(IHC) for CD3 (clone F7.2.38, Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA; 1:600), CD5 (clone 4C7, DAKO; 1:100), CD20
(clone L26, Dako; Agilent Technologies, Inc: 1:200), BCL2 (clone
124, DAKO; Agilent Technologies, Inc.; 1:50), BCL6 (clone LN22,
Leica Microsystems, Ltd., Milton Keynes, UK; 1:60), CD10 (clone
56C6, Leica Microsystems, Ltd; 1:30), CD23 (clone SP23, Thermo
Fisher Scientific, Inc., Waltham, MA, USA; 1:100), CD43 (clone
DF-T1, DAKO; 1:2,500), cyclin D1 (clone SP4, Thermo Fisher
Scientific, Inc: 1:25) and MUM1 (clone EAU32, Leica Microsystems,
Ltd; 1:100). IHC staining was performed using the Ventana Benchmark
XT system (Ventana Medical Systems, Inc., Tucson, AZ, USA) or a
Bond-Max automated immunostainer (Leica Microsystems, Ltd.).
Sections were dried in oven at 60°C for 30 min and deparaffinized
in xylene and rehydrated through a series of graded ethanol
solutions (95, 85, 70 and 55%) at room temperature for 10 min.
Antigen retrieval was performed in a pressure cooker at 95°C for 2
min using 0.01 M citrate buffer (pH 6.0) and incubated overnight at
4°C for all the primary antibodies. Then the slides were washed by
phosphate buffered saline (PBS) four times. After warming to 37°C,
detection involved ultraView Universal DAB Detection kit (cat.
760–500, Ventana Medical Systems, Inc.) that includes a cocktail of
horseradish peroxidase-labeled goat anti-rabbit, mouse IgG
secondary antibodies for 16 min. The complex was then visualized
with 3,3′-diaminobenzidine tetrahydrochloride (DAB) chromogen
according to the manufacturer's protocol. The samples were then
analyzed by a BX51 light microscope (magnification, ×200 and ×400)
(Olympus Corporation, Tokyo, Japan). Histologically, the two tumors
comprised small-to medium-sized lymphoid cells with complete
nodular architecture. Lymphoepithelial lesions and monocytoid or
plasmacytoid features indicative of MALT lymphoma were not
identified. The epicenter of the tumor was the submucosa, however,
the tumor extended to the muscularis propria, with only focal
involvement of the mucosa. Immunohistochemistry (IHC) indicated
that the tumor cells were positive for cluster of differentiation
CD10, CD20, B-cell lymphoma BCL2 and BCL6, and negative for CD3,
CD5, CD23, CD43, cyclin D1 and mutated melanoma-associated antigen
(MUM) 1/interferon regulatory factor (IRF)4. Follicular dendritic
cell (FDC) networks were preserved within the CD21-positive
nodules, demonstrating a predominantly compact pattern with focal
abolishment at the center of the nodule (Fig. 1). The patient was diagnosed with FL of
grade 1–2 with a follicular pattern according to the WHO
classification (10). A fluorescence
in situ hybridization (FISH) test for locus-specific
identifier immunoglobulin heavy chain (IGH)/BCL2 dual fusion
translocation probe (Vysis; Abbott Pharmaceutical Co., Ltd., Lake
Bluff, IL, USA) and BCL2 break-apart probe (Vysis; Abbott
Pharmaceutical Co., Ltd.) revealed no rearrangement of BCL2 at
18q21 (18). The background gastric
mucosa demonstrated prominent lymphoid follicles with Helicobacter
pylori (H. pylori) colonization. As the omental tumor was
interpreted as a multiplicity rather than a metastatic deposit, the
patient's final clinical stage was IE2 using the modified Ann Arbor
(19,20) staging system, or I using the Lugano
(21) staging system. The patient was
treated with 6 cycles of chemotherapy [intravenous (IV) rituximab
375 mg/m2, cyclophosphamide 750 mg/m2 IV,
vincristine 1.4 mg/m2 IV and prednisolone 40
mg/m2 per oral (PO), R-CVP] every 21 days. The patient
came to hospital every month and remained disease-free at the last
follow-up (13 months following diagnosis).
Case 2
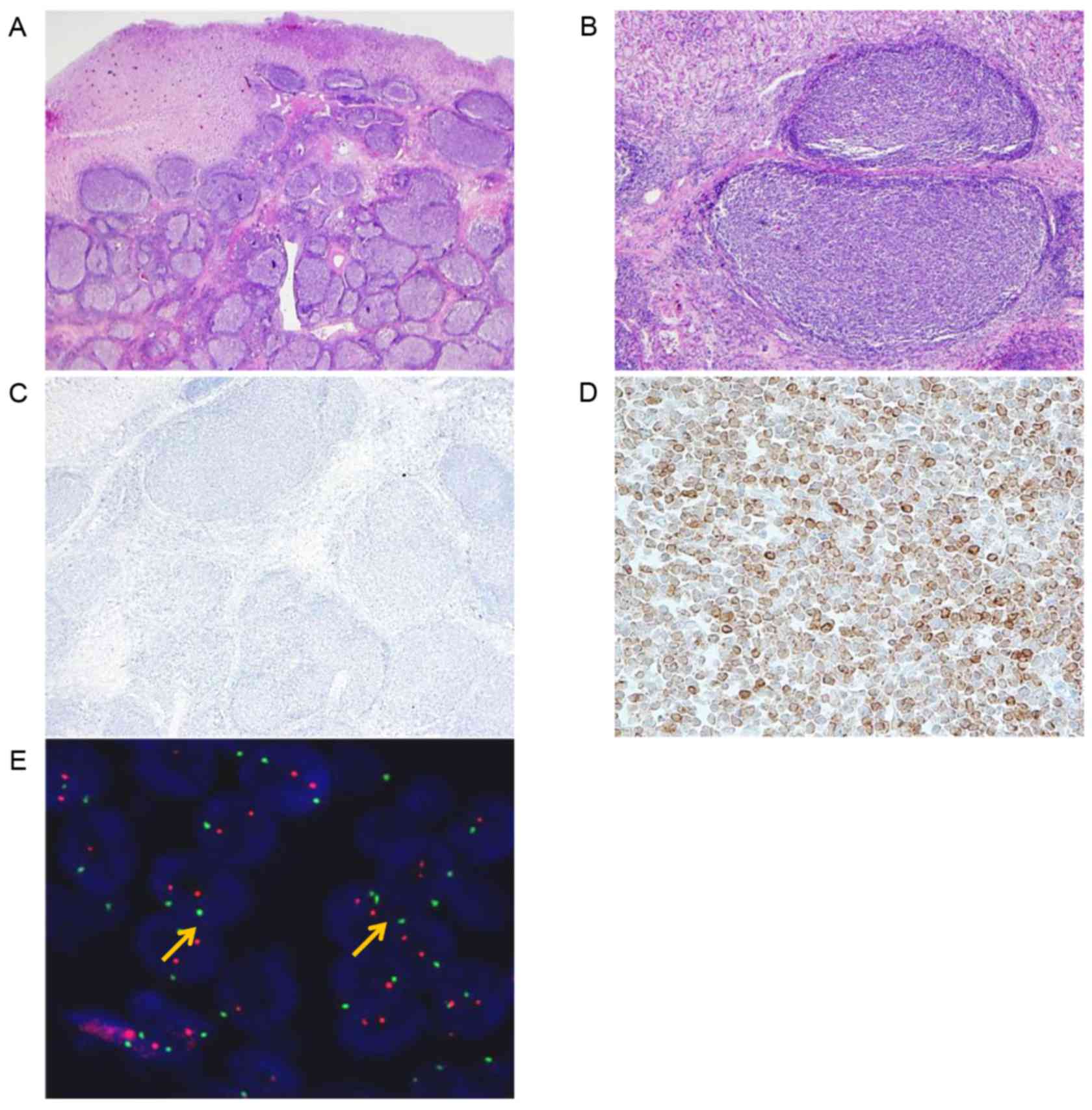
A 50-year-old man underwent gastroscopy for routine
health check-up in Dongtan Sacred Heart Hospital and presented with
a gastric lesion; a single elevated submucosal mass at the fundus,
grossly indicative of a GI stromal tumor. As in the first case, no
additional abnormalities were identified through systemic
examination. The patient underwent a partial gastrectomy following
an endoscopic biopsy that failed to provide a pathological
diagnosis, which revealed a 1.8×1.6-cm submucosal tumor extending
to the muscularis propria. The microscopic features and IHC results
were near identical to those in case 1, with the exception of the
absence of separate tumor nodule in the omentum. Rearrangement of
BCL2 was not detected using the IGH/BCL2 dual fusion probe or BCL2
break-apart probe by FISH (Fig. 2).
The colonization of H. pylori was not identified in the surrounding
gastric mucosa. The patient's final stage was IE2 using the
modified Ann Arbor staging system (19,20) and
stage I using the Lugano staging system (21). The patient refused any further
treatment and remained disease-free for 30 months following the
surgery. Subsequently, the patient was lost to but follow-up.
Case 3
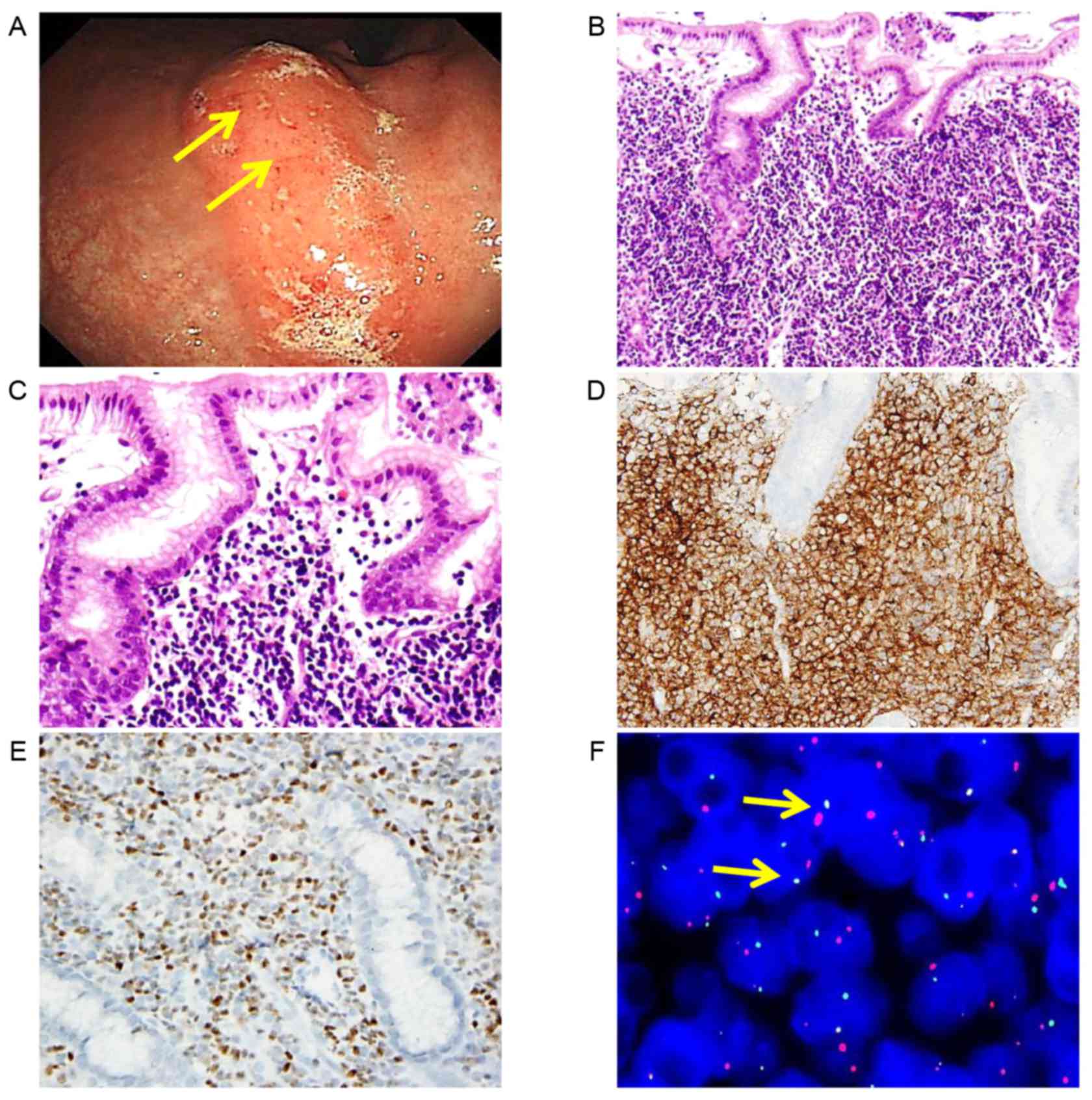
A 40-year-old man presented with epigastric
discomfort for several months. An endoscopy performed on December,
2015 at Seoul National University Hospital (Seoul, Korea) revealed
multiple elevated mass-like lesions at the mid-body of the stomach,
on the lesser curvature side, and a biopsy confirmed FL of grade
1–2 with a predominantly diffuse pattern. Unlike the 2
aforementioned cases, the subepithelial lamina propria was densely
filled with small-to medium-sized lymphoid cells without nodular
aggregation. Despite extensive lymphoid infiltration, definite
lymphoepithelial lesions were not identified. The tumor cells were
positive for CD10, CD20, BCL2 and BCL6, and negative for CD3,
cyclin D1 and MUM1/IRF4, according to the IHC results. The tumor
cells were also positive for IGH/BCL2 translocation, as confirmed
by FISH (Fig. 3). In addition to the
gastric lesion, multiple large hypermetabolic lymph nodes (≤12.0
cm) were identified in the retroperitoneum, mesentery and abdomen.
A bone marrow examination revealed the involvement of FL. The
patient's final stage was IVA using the Ann Arbor staging system
(10,18). It was concluded that the gastric
lesion was possibly secondary to nodal FL, due to the presence of
massive lymphadenopathy. The patient underwent 6 cycles of R-CVP
(rituximab 375 mg/m2 IV, cyclophosphamide 750
mg/m2 IV, vincristine 1.4 mg/m2 IV and
prednisolone 40 mg/m2 PO) every 21 days without surgical
intervention, demonstrating partial response on computed tomography
(CT) scan. Following R-CVP, he received 9 cycles of rituximab
maintenance therapy (750 mg/m2 IV every 2 months) and
showed complete response. At time of publication, he is planning to
undergo 3 more cycles of maintenance chemotherapy (26 months
post-diagnosis).
Discussion
FL is a basic nodal-based neoplasm, although several
cases of primary extranodal FL have previously been described as
specific variants (10,12,13–15).
Involvement of the GI tract by advanced nodal FL may be uncommon,
although accurate incidence rates have not been reported (22). Among extranodal FL, primary gastric FL
is rare. According to a nationwide survey of malignant lymphoma
among Koreans released in 2011 (8),
there was no single case of gastric FL in the 1,112 recorded cases
of GI lymphoma. To date, <20 cases of primary gastric FL with
well documented pathological results have been reported in the
literature, although the overall incidence should be slightly
higher (Table I) (13,16,23–25).
Due to the higher incidence of MALT lymphoma in stomach, and the
similar cytologic morphology of tumor cells of MALT lymphoma and
FL, primary gastric FL may have been misdiagnosed as MALT lymphoma,
especially in small biopsy specimens. Based on a review of previous
studies, the majority of gastric FL presented as single- or
multiple-polypoid submucosal masses, which could hinder the
accurate diagnosis through endoscopic mucosal biopsy.
Histologically, the tumor cells were of low grade, consistent with
grade 1–2 and exhibited a predominantly follicular pattern, similar
to those observed in 2 of the cases described in the present study.
In contrast to cases 1 and 2, case 3 was confirmed as FL by
endoscopic biopsy due to diffuse tumor cell infiltration in the
lamina propria. This diffuse mucosal involvement may be a useful
feature to differentiate secondary involvement by nodal FL from
primary gastric FL. Takata et al (13,14)
suggested that an FDC pattern can help differentiate primary
duodenal FL from nodal FL by performing IHC for FDC markers (CD23
or CD43); loss of FDC network in the center, with remnant
peripheral FDC exhibiting a ‘hollow pattern’ is identified in
duodenal FL, whereas a compact nodular network of FDCs is exhibited
in nodal FL. The FDCs are reported to provide a growth advantage to
FL cells by interacting with tumor cells. The hollow pattern may be
associated with relatively lower histologic grade and the indolent
clinical nature of duodenal FL (15).
In contrast, case 1 of the present study exhibited preserved FDC
nodules, while case 2 revealed near complete loss of FDCs without
peripheral accentuation; these patterns differ from the typical
hollow pattern of duodenal FL previously reported (13–15). Case
3 also exhibited an absence of an FDC network, similar to case 2.
Therefore, the ‘hollow pattern’ does not appear to be specific to
primary gastric FL. Rather, a preserved FDC network may be
interpreted as a phenomenon associated with disease
progression.
 | Table I.Summarized clinicopathological
parameters of cases of gastric follicular lymphoma in the
literature. |
Table I.
Summarized clinicopathological
parameters of cases of gastric follicular lymphoma in the
literature.
| First author,
year | Age, years | Sex | Multiplicity | Grade | t(14:18) | H.
pylori | Stagea | Treatment | Outcome,
months | (Refs.) |
|---|
| Present case | 61 | M | No | 1–2 | No | Yes | I | R-CVP | NED, 8 |
|
|
| 50 | M | No | 1–2 | No | No | I | Surgery only | NED, 30 |
|
|
| 40 | M | Yes | 3a | Yes | Yes | IV | R-CVP | PR, 6 |
|
| Takata et
al, 2013 | 51–81 | M (n=4), F
(n=4) | Unknown | 1–2 | 3/8 | Unknown | I (n=5), II (n=2),
IV (n=1) | Unknown | Unknown | (9) |
| Tzankov et
al, 2002 | 72 | F | No | 1–2 | Yes | Unknown | I | CHOP | NED, 13 | (24) |
| Huang et al,
2002 | 48 | F | No | 3a, b | Unknown | Unknown | IIE | CEOP | NED, 70 | (23) |
| Kanda et al,
2002 | 57 | F | Yes | 1–2 | Yes | Unknown | I | Surgery only | NED, 15 | (16) |
| Iwamuro et
al, 2002 | 73 | M | No | 1 | No | Yes | IIE | Surgery and
R-CHOP | NED, 30 | (25) |
| Misdragi et
al, 2002 | 67 | F | Yes | 1–2 | No | No | I | Rituximab | MOC, 48 | (27) |
The majority of reported primary gastric FL cases
presented with a localized extent of disease. In the present case
report, defining the pathological staging of case 1 was difficult
due to a concomitant omental tumor nodule without regional lymph
node involvement. According to the Tumor-Node-Metastasis staging of
the American Joint Committee on Cancer, an omental nodule may be
interpreted as a regional lymph node metastasis (N1) or a
subserosal invasion (T3) (26).
However, there was little evidence of extrafollicular invasion, as
observed in the CD10 IHC results in the gastric and omental
lesions. Therefore, the omental tumor may be interpreted as
multiplicity rather than as a metastatic deposit. Multifocality is
one of the well-known characteristics in duodenal FL (27). It was concluded that case 3 of the
present study was most likely a secondary involvement of the
stomach based on Dawson's criteria of primary GI lymphoma (28), and the final stage was designated
stage IV due to bone marrow involvement. Unlike primary gastric
FLs, secondary gastric involvement may occur in the advanced stage
of nodal FL.
Certain studies have identified the lack of BCL2
translocation in primary cutaneous FL as a distinctive feature that
differentiates it from nodal FL, suggesting distinct tumorigenic
processes in extranodal sites (29).
This conclusion was not supported in GI FL; a number of previous
studies have identified BCL2 rearrangement in primary intestinal FL
(12,23). However, the incidence of BCL2
translocation in primary gastric FL was decreased compared with
that in nodal FL, or even duodenal FL, although the expression of
BCL2 protein was consistently observed (2). The 2 primary gastric FL cases described
in the present study were negative for BCL2 translocation, whereas
the case of secondary involvement of nodal FL exhibited
translocation t(14;18). It is possible that the absence of
translocation t(14;18) may serve as a diagnostic indicator,
differentiating primary gastric FL from nodal FL. However, this
decreased tendency for BCL2 translocation may be as a result of the
under-recognition of FL disguised as MALT lymphoma. Nevertheless,
translocation-negative cases also revealed an overexpression of
BCL2 protein, similar to previous studies, suggesting an
alternative genetic mechanism rather than an IGH/BCL2 rearrangement
(30).
To the best of our knowledge, there has been no
proven etiological agent for gastric FL identified to date,
although a number of studies presume that local immunity will
contribute to the pathogenesis of FL of the GI tract, just as H.
pylori participates in the development of MALT lymphoma
(31). Local antigen-experienced
B-cells are suggested to be the origin of primary FL of the GI
tract based on the frequent expression of IgA and the α4β7 mucosal
homing receptor in the tumor cells (32). Colonization of H. pylori was
identified in case 1 and 3 of the present case report, and Hatano
et al (17) revealed an ~50%
incidence of H. pylori in gastric FLs in patients of
Japanese origin. Considering that the seropositivity for H.
pylori is ~54% in patients of Korean origin (33), the causal association of H.
pylori in gastric FL is not robust enough to be conclusive.
However, in case 1 of the present study, follicular gastritis was
present in the background mucosa, suggesting the involvement of
H. pylori in the tumorigenesis of gastric FL. Although
antibiotics targeting H. pylori in FL failed in a
considerable proportion of patients, there were also a limited
number of patients that achieved complete remission (34). Nevertheless, there may be a subgroup
of gastric FL associated with a local antigen-presenting
environment such as H. pylori infection, however, further
studies are required to elucidate such cases.
Although the vast majority of GI FLs demonstrate a
limited disease extent at the time of diagnosis unlike nodal FLs,
the prognosis of gastric FLs does not appear to differ from that of
nodal FLs considering the relatively indolent behavior of nodal
FLs, even in advanced stages reported in previous studies (1,2,10,16,24,25,35).
Based on a review of gastric FLs, including 2 cases from the
present study, no disease relapses or disease-associated
mortalities have been reported (13,16,23–25).
Due to this indolent behavior, despite heterogeneous treatment
modalities, therapeutic interventions are recommended only in
symptomatic or progressive cases (36). In contrast to the majority of nodal
FLs that acquire additional genetic alterations leading to
widespread disease and high-grade transformation, FL cells of the
GI tract tend to reside in the primary site, potentially explaining
the indolent nature of the tumor. There are several reports of
gastric FLs with a co-existing diffuse large B-cell lymphoma
component, yet such patients generally had favorable disease
courses (23,37).
The main differential diagnosis of FL in the GI
tract includes florid lymphoid hyperplasia, MALT lymphoma, mantle
cell lymphoma and secondary involvement of nodal FL. A distinction
from reactive lymphoid hyperplasia may be made in the same context
as nodal lesions, where monomorphic composition, absence of
tangible body macrophages and architectural disruption favor FL.
Occasionally, MALT lymphoma or mantle cell lymphoma is dominated by
small lymphocytes with a nodular architecture. Tumor cells in MALT
lymphoma present a characteristic lymphoepithelial lesion, which is
uncommon in the majority of FL cases (2,10,23,27,36).
However, there has been one study of gastric FL with a
lymphoepithelial lesion resembling MALT lymphoma (24). Furthermore, certain cases of gastric
FL with diffuse mucosal involvement lacking BCL translocation may
easily be misinterpreted as MALT lymphoma, particularly in small
biopsy specimens (38). Under these
circumstances, IHC results usually provide clear distinctions
between these types of tumor cell. Neoplastic cells in MALT
lymphoma present with negative BCL6 and CD10 IHC test results. As
for mantle cell lymphoma, CD5 and cyclin D1 may assist in forming
the correct diagnosis. For determining the secondary involvement of
nodal FL, Dawson's criteria for primary GI lymphoma should be
applied (1).
In conclusion, primary gastric FL is a rare,
under-recognized disease. Although the general prognosis is
indolent, similar to that of nodal FL, a lack of BCL2
rearrangement, low-grade histology with a follicular pattern and
sparse mucosal involvement may serve as diagnostic indicators for
excluding secondary involvement by nodal FL.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the Basic
Science Research Program through the National Research Foundation
of Korea funded by the Ministry of Education (no.
2017R1A2B4005052).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conception and design, JK, YK, JC and SM.
Administrative support, JK, YK and JH. Provision of clinical data
including patient history, endoscopic findings and images of case
1, JS, SM, JC and JH. Provision and interpretation of
histopathologic, immunohistochemical and molecular analysis of case
1, JS, SM, JC and JH. Provision of clinical data including patient
history, endoscopic findings and images of case 2, YK, HK, CL and
SS. Provision and interpretation of histopathologic,
immunohistochemical and molecular analysis of case 2, YK, HK, CL
and SS. Provision of clinical data including patient history,
endoscopic findings and images of case 3, CL, SS and HK. Provision
and interpretation of histopathologic, immunohistochemical and
molecular analysis of case 3, CL, SS and HK. Collection and
assembly of data, HN and YK. Reviewing the slides for
histopathological and immunohistochemical features, HN, YK and JK.
Reviewing molecular analysis of cases, JH and HK. Reviewing
literature, HN, CL and SS. Manuscript writing and revising, HN and
YK. Adding valuable scientific comments in the discussion section,
JC, SM, HK and JH. All authors reviewed the manuscript and approved
the final article.
Ethics approval and consent to
participate
This study has been granted an exemption from
requiring ethics approval by the Institutional Review Board of
Seoul National University Boramae Hospital (IRB number,
10-2018-15).
Consent for publication
Patients' written consent was waived with removal of
all identifying information in this retrospective case study under
the approval of the Institutional Review Board of Seoul National
University Boramae Hospital.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ghimire P, Wu GY and Zhu L: Primary
gastrointestinal lymphoma. World J Gastroenterol. 17:697–707. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoshino T, Miyake K, Ichimura K, Mannami
T, Ohara N, Hamazaki S and Akagi T: Increased incidence of
follicular lymphoma in the duodenum. Am J Surg Pathol. 24:688–693.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Teras LR, DeSantis CE, Cerhan JR, Morton
LM, Jemal A and Flowers CR: 2016 US lymphoid malignancy statistics
by World Health Organization subtypes. Ca Cancer J Clin: Sep 12,
2016 (Epub ahead of print).
|
|
4
|
Smith A, Crouch S, Lax S, Li J, Painter D,
Howell D, Patmore R, Jack A and Roman E: Lymphoma incidence,
survival and prevalence 2004–2014: sub-type analyses from the UK's
haematological malignancy research network. Br J Cancer.
112:1575–1584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee H, Park HJ, Park E, Ju HY, Oh CM, Kong
HJ, Jung KW, Park BK, Lee E, Eom HS and Won YJ: Nationwide
statistical analysis of lymphoid malignancies in Korea. Cancer Res
Treat. 50:222–238. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chihara D, Ito H, Matsuda T, Shibata A,
Katsumi A, Nakamura S, Tomotaka S, Morton LM, Weisenburger DD and
Matsuo K: Differences in incidence and trends of haematological
malignancies in Japan and the United States. Br J Haematol.
164:536–545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gross SA, Zhu X, Bao L, Ryder J, Le A,
Chen Y, Wang XQ and Irons RD: A prospective study of 728 cases of
non-Hodgkin lymphoma from a single laboratory in Shanghai, China.
Int J Hematol. 88:165–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JM, Ko YH, Lee SS, Huh J, Kang CS, Kim
CW, Kang YK, Go JH, Kim MK, Kim WS, et al: WHO Classification of
malignant lymphomas in Korea: Report of the third nationwide study.
J Pathol Transl Med. 45:254–260. 2011.
|
|
9
|
Takata K, Miyata-Takara T, Sato Y and
Yoshino T: Pathology of follicular lymphoma. J Clin Exp Hematop.
54:3–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sweldlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H and Thiele J: WHO Classification of Tumours of
Haematopoietic and Lymphoid Tissues. Revised. 4th edition. volume.
2. IARC Lyon; pp. 266–273. 2017
|
|
11
|
Dreyling M, Ghielmini M, Rule S, Salles G,
Vitolo U and Ladetto M; ESMO Guidelines Committee: Newly diagnosed
and relapsed follicular lymphoma: ESMO clinical practice guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 27(Suppl 5):
v83–v90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goodlad JR, MacPherson S, Jackson R,
Batstone P and White J; Scotland and Newcastle Lymphoma Group:
Extranodal follicular lymphoma: A clinicopathological and genetic
analysis of 15 cases arising at non-cutaneous extranodal sites.
Histopathology. 44:268–276. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takata K, Sato Y, Nakamura N, Tokunaka M,
Miki Y, Yukie Kikuti Y, Igarashi K, Ito E, Harigae H, Kato S, et
al: Duodenal follicular lymphoma lacks AID but expresses BACH2 and
has memory B-cell characteristics. Mod Pathol. 26:22–31. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takata K, Sato Y, Nakamura N, Kikuti YY,
Ichimura K, Tanaka T, Morito T, Tamura M, Oka T, Kondo E, et al:
Duodenal and nodal follicular lymphomas are distinct: The former
lacks activation-induced cytidine deaminase and follicular
dendritic cells despite ongoing somatic hypermutations. Mod Pathol.
22:940–949. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takata K, Okada H, Ohmiya N, Nakamura S,
Kitadai Y, Tari A, Akamatsu T, Kawai H, Tanaka S, Araki H, et al:
Primary gastrointestinal follicular lymphoma involving the duodenal
second portion is a distinct entity: A multicenter, retrospective
analysis in Japan. Cancer Sci. 102:1532–1536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanda M, Ohshima K, Suzumiya J, Haraoka S,
Kawasaki C, Sakisaka S and Kikuchi M: Follicular lymphoma of the
stomach: Immunohistochemical and molecular genetic studies. J
Gastroenterol. 38:584–587. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hatano B, Ohshima K, Tsuchiya T, Yamaguchi
T, Kawasaki C and Kikuchi M: Clinicopathological features of
gastric B-cell lymphoma: A series of 317 cases. Pathol Int.
52:677–682. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choe JY, Yun JY, Na HY, Huh J, Shin SJ,
Kim HJ, Paik JH, Kim YA, Nam SJ, Jeon YK, et al: MYC overexpression
correlates with MYC amplification or translocation, and is
associated with poor prognosis in mantle cell lymphoma.
Histopathology. 68:442–449. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carbone PP, Kaplan HS, Musshoff K,
Smithers DW and Tubiana M: Report of the committee on Hodgkin's
disease staging classification. Cancer Res. 31:1860–1861.
1971.PubMed/NCBI
|
|
20
|
Radaszkiewicz T, Dragosics B and Bauer P:
Gastrointestinal malignant lymphomas of the mucosa-associated
lymphoid tissue: Factors relevant to prognosis. Gastroenterology.
102:1628–1638. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rohatiner A, D'amore F, Coiffier B,
Crowther D, Gospodarowicz M, Isaacson P, Lister TA, Norton A, Salem
P, Shipp M, et al: Report on a workshop convened to discuss the
pathological and staging classifications of gastrointestinal tract
lymphoma. Ann Oncol. 5:397–400. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Herrmann R, Panahon AM, Barcos MP, Walsh D
and Stutzman L: Gastrointestinal involvement in non-Hodgkin's
lymphoma. Cancer. 46:215–222. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang WT, Hsu YH, Yang SF and Chuang SS:
Primary gastrointestinal follicular lymphoma: A clinicopathologic
study of 13 cases from Taiwan. J Clin Gastroenterol. 42:997–1002.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tzankov A, Hittmair A, Müller-Hermelink
HK, Rüdiger T and Dirnhofer S: Primary gastric follicular lymphoma
with parafollicular monocytoid B-cells and lymphoepithelial
lesions, mimicking extranodal marginal zone lymphoma of MALT.
Virchows Arch. 441:614–617. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iwamuro M, Imagawa A, Kobayashi N, Kubota
Y, Miyatani K, Takata K and Okada H: Synchronous adenocarcinoma and
follicular lymphoma of the stomach. Intern Med. 52:907–912. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Edge S, Byrd D, Compton C, Fritz A, Greene
F and Trotti A: AJCC cancer staging manual. Springer; New York;
2010
|
|
27
|
Misdraji J, Harris NL, Hasserjian RP,
Lauwers GY and Ferry JA: Primary follicular lymphoma of the
gastrointestinal tract. Am J Surg Pathol. 35:1255–1263. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dawson IM, Cornes JS and Morson BC:
Primary malignant lymphoid tumours of the intestinal tract. Report
of 37 cases with a study of factors influencing prognosis. Br J
Surg. 49:80–89. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goodlad JR, Krajewski AS, Batstone PJ,
McKay P, White JM, Benton EC, Kavanagh GM and Lucraft HH; Scotland
and Newcastle Lymphoma Group: Primary cutaneous follicular
lymphoma: A clinicopathologic and molecular study of 16 cases in
support of a distinct entity. Am J Surg Pathol. 26:733–741. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Horsman DE, Okamoto I, Ludkovski O, Le N,
Harder L, Gesk S, Siebert R, Chhanabhai M, Sehn L, Connors JM and
Gascoyne RD: Follicular lymphoma lacking the t(14;18)(q32;q21):
identification of two disease subtypes. Br J Haematol. 120:424–433.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hussell T, Isaacson PG, Crabtree JE and
Spencer J: Helicobacter pylori-specific tumour-infiltrating T cells
provide contact dependent help for the growth of malignant B cells
in low-grade gastric lymphoma of mucosa-associated lymphoid tissue.
J Pathol. 178:122–127. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bende RJ, Smit LA, Bossenbroek JG, Aarts
WM, Spaargaren M, de Leval L, Boeckxstaens GE, Pals ST and van
Noesel CJ: Primary follicular lymphoma of the small intestine:
Alpha4beta7 expression and immunoglobulin configuration suggest an
origin from local antigen-experienced B cells. Am J Pathol.
162:105–113. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lim SH, Kwon JW, Kim N, Kim GH, Kang JM,
Park MJ, Yim JY, Kim HU, Baik GH, Seo GS, et al: Prevalence and
risk factors of Helicobacter pylori infection in Korea: Nationwide
multicenter study over 13 years. BMC Gastroenterol. 13:1042013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakamura S, Matsumoto T, Umeno J, Yanai S,
Shono Y, Suekane H, Hirahashi M, Yao T and Iida M: Endoscopic
features of intestinal follicular lymphoma: The value of
double-balloon enteroscopy. Endoscopy. 39(Suppl 1): E26–E27. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luminari S, Bellei M, Biasoli I and
Federico M: Follicular lymphoma-treatment and prognostic factors.
Rev Bras Hematol Hemoter. 34:54–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schmatz AI, Streubel B, Kretschmer-Chott
E, Püspök A, Jäger U, Mannhalter C, Tiemann M, Ott G, Fischbach W,
Herzog P, et al: Primary follicular lymphoma of the duodenum is a
distinct mucosal/submucosal variant of follicular lymphoma: A
retrospective study of 63 cases. J Clin Oncol. 29:1445–1451. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maeshima AM, Omatsu M, Nomoto J, Maruyama
D, Kim SW, Watanabe T, Kobayashi Y, Tobinai K and Matsuno Y:
Diffuse large B-cell lymphoma after transformation from low-grade
follicular lymphoma: Morphological, immunohistochemical, and FISH
analyses. Cancer Sci. 99:1760–1768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Burke JS: Lymphoproliferative disorders of
the gastrointestinal tract: A review and pragmatic guide to
diagnosis. Arch Pathol Lab Med. 135:1283–1297. 2011. View Article : Google Scholar : PubMed/NCBI
|