Introduction
Clear cell papillary renal cell carcinoma (CCPRCC)
was initially described by Tickoo et al in 2006 as a subtype
of renal tumor of patients with end-stage renal disease (ESRD)
(1). Subsequently, it has been shown
that CCPRCC may also occur in healthy and functional kidneys as
well (2–4). In 2013, CCPRCC was included as a subtype
of renal cell carcinoma (RCC) in the International Society of
Urological Pathology (ISUP) Vancouver Classification of Renal
Neoplasia (5). Formally published
during the spring of 2016, the new version of World Health
Organization (WHO) Classification of Tumors of the Urinary System
and Male Genital Organs described some new entities, including
CCPRCC and 5 other new subtypes of RCC (6).
Up to now, >400 patients with CCPRCC have been
reported (1–4,7–26). By analyzing the published cases, this
tumor is estimated to account for 1–5% of all renal epithelial
neoplasms (5–10,15,18), which
makes CCPRCC the fourth most common RCC (7), just next to clear cell renal cell
carcinoma (CCRCC), papillary renal cell carcinoma (PRCC), and
chromophobe renal cell carcinoma (CRCC). Despite some overlapping
features, CCPRCC is known to be morphologically,
immunohistochemically, and genetically distinct from both CCRCC and
PRCC (27). However, none of
urologists or oncologists has studied this new type of RCC from the
view of clinical diagnosis and treatment. Little is known about
whether CCPRCC has relatively specific characteristics of clinical
epidemiology, clinical laboratory and radiology. Furthermore,
CCPRCC may present often as small masses and its incidence is as
high as 1/15 in RCC of low stage (pT1aN0M0) and low Fuhrman nuclear
grade (1 and 2) (15). Whether the
prognosis of CCPRCC is different from other RCC subtypes is crucial
for treatment of early-stage RCC. Therefore, the purpose of this
study was to (1) determine clinical
features and survival analysis of CCPRCC (2) evaluate similarities and differences with
CCRCC and PRCC to better understand the biologic characteristic of
this newly recognized entity (3)
review its histological morphology and immunohistochemical
expression for correct classification.
Materials and methods
Case selection and clinical data
review
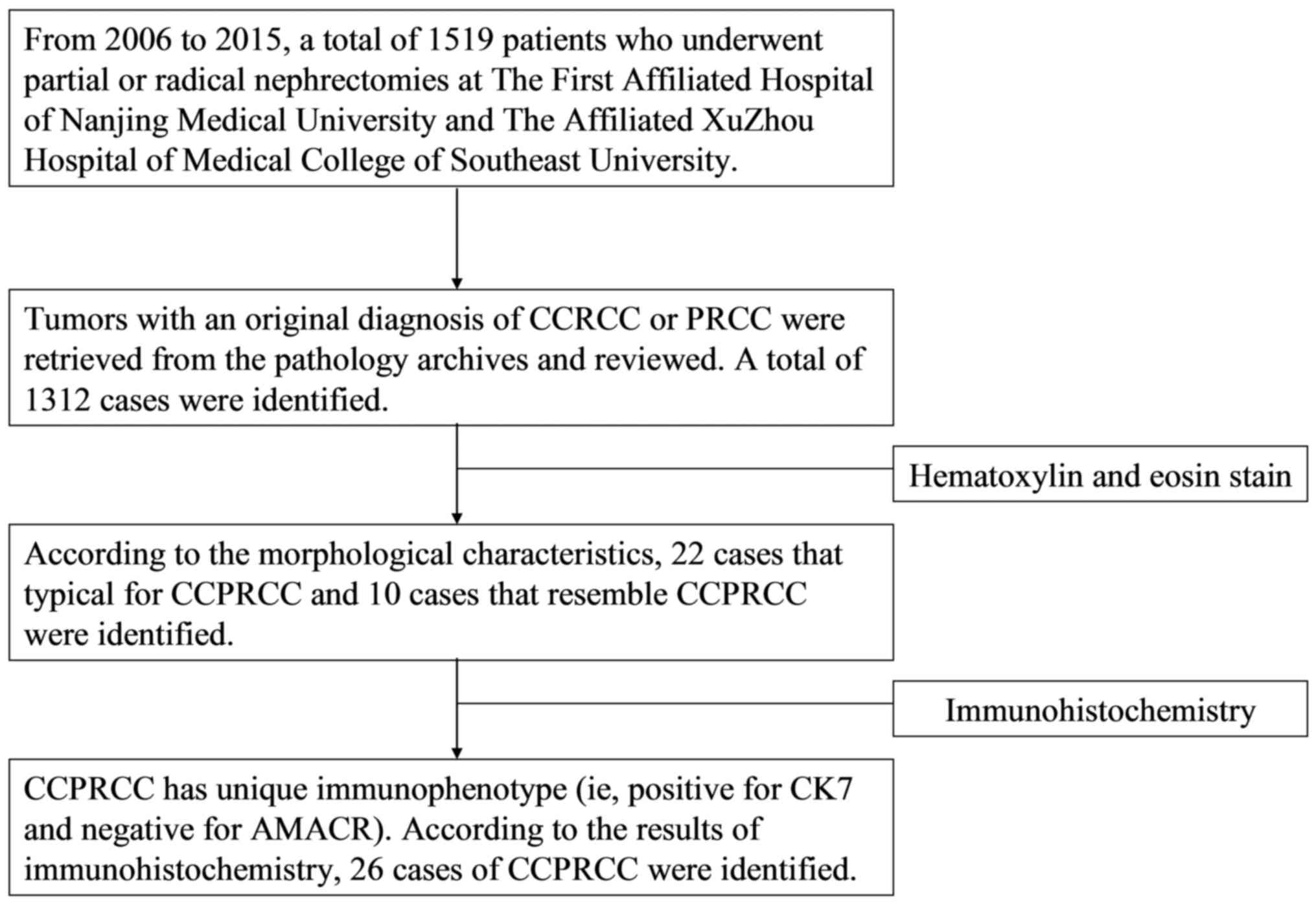
From 2006 to 2015, among the 1,519 RCC patients who
visited the two hospitals (the First Affiliated Hospital of Nanjing
Medical University and the Affiliated Xuzhou Hospital of Medical
College of Southeast University), 26 cases of CCPRCC were
identified and reviewed by three pathologists (Ding, Li and Liu).
The flow diagram explaining patient selection is shown in Fig. 1. Patient data, including age, sex,
height, weight, primary diagnosis, past medical history,
preoperative examination results (including routine blood test,
urinalysis, blood biochemical tests and serum tumor markers) and
tumor characteristics were obtained from the medical records. Body
mass index (BMI) was calculated as the weight in kilograms divided
by the height in meter squared. Commonly accepted BMI ranges in
Chinese people are normal weight (18.5–23.9 kg/m2),
overweight (24–27.9 kg/m2) and obesity (over 28
kg/m2).
Meanwhile, among the cases of RCC with preoperative
multiphasic computed tomography (CT) images data, we randomly
selected 30 cases of CCRCC and PRCC respectively, and their
pathological stages were consistent with CCPRCC (pT1N0M0). We also
collected these patients' information. All patients signed the
informed consent and the research was approved by the Ethics
Committee of the First Affiliated Hospital of Nanjing Medical
University (Ethical approval number: 2016-SRFA-011) and the Ethics
Committee of the Affiliated Xuzhou Hospital of Medical College of
Southeast University (Ethical approval no.
XZXY-LJ-20160111-008).
Immunohistochemistry
Specimens, including 26 CCPRCC, 30 CCRCC and 30
PRCC, were fixed in formalin and embedded in paraffin. The 4-µm
thick sections were stained with the following panel of markers:
CK7 (OV-TL 12/30, 1:200; Dako, Carpinteria, CA, USA); CD10 (56C6,
1:25; Novocastra, Newcastle upon Tyne, UK); AMACR (13H4,
ready-to-use; Dako); CA IX (TH22, 1:100; Novocastra, Buffalo Grove,
IL, USA); vimentin (Vim 3B4, 1:250); Ki67 (MIB-1, 1:200) (both from
Dako, Glostrup, Denmark). Immunoreaction was performed with an
automated immunostainer from Ventana (Ventana Medical Systems,
Tucson, AZ, USA). The immunohistochemistry results (CK7, C10,
AMACR, CA IX and RCC maker) were interpreted as negative, weak
(<30% staining), moderate (30–70% staining) and strong (>70%
staining). Ki67 positive cells showed stained brownish-yellow
granules in the nucleus. According to the literature written by
Delahunt et al (28), the area
with the highest fraction of Ki67-stained cells in section was
chosen at a X10 objective magnification, then it was examined at
X400 objective magnification. Finally, Ki67 labeling index (Ki67
LI) was made through counting 1,000 cancer cells (percentage of
nuclei showing positive staining).
CT examination and image analysis
All multidetector CT examinations were performed by
either 16 detector row helical scanners (Optima CT520) (the First
Affiliated Hospital of Nanjing Medical University) or 64 detector
row helical scanners (Discovery CT750 HD) (both from General
Electric Medical Systems, Milwaukee, WI, USA) (the Affiliated
Xuzhou Hospital of Medical College of Southeast University). For
the acquisition of all images, scans were obtained with the
following parameters: A craniocaudal direction with gantry tilt 0
degrees, a scan time of 0.8 sec, 120 kVp, variable tube current and
a section thickness interval of 2.5–5 mm depending on the protocol
used.
All patients with CCRCC (30 cases) and PRCC (30
cases) underwent four-phase helical renal CT scanning, including
unenhanced phase, corticomedullary phase (CMP), nephrographic phase
(NP) and excretory phase (EP). Among the 25 patients with CCPRCC
(excluding 1 patient with ESRD), 10 underwent four-phase scanning,
4 underwent three-phase (unenhanced phase, CMP and NP) scanning, 4
only underwent unenhanced phase scanning and 7 patients did not
undergo CT. During the four-phase studies, 80–100 ml contrast
medium (nonionic iohexol concentration 300 mgI/ml) (Omnipaque; GE
Healthcare, Logan, UT, USA) was injected intravenously into the
antecubital vein at a rate of 3.0 ml/sec after an unenhanced
helical CT was obtained. Scanning for the CMP, NP and EP was in 30,
90 and 300 sec after contrast injection, respectively. For
three-phase studies, time-delay images were obtained at varied
combinations of corticomedullary and nephrographic phases.
Two experienced abdominal radiologists (Wu and Li),
who were blinded to renal cell carcinoma subtypes, reviewed the CT
images and evaluated the tumor size, enhancement pattern,
calcification, and tumor contour were independently. They measured
the attenuation values of renal lesions with the observer-defined
region of interest (ROI) at a size of ~0.5–1 cm2. The
radiologists kept two ROIs in the center of the tumor lesion or the
most homogenously enhanced part of the lesion, which were
consistent in location during all CT phases. Then the mean of these
2 values was calculated and the measurement was reviewed by two
radiologists (Wu and Li). In addition, the attenuation value of the
aorta was measured as a control in each phase.
Follow-up and evaluation
According to the 2010 TNM staging system and the
Fuhrman grade classification, a total of 955 patients diagnosed
with low stage (pT1N0M0) and low nuclear grade (1 and 2) RCC were
included in this study. We recorded patients' information,
including age, sex, surgical method, pathologic feature and
follow-up data. After excluding the patients with unilateral
multiple RCC tumors, bilateral RCC tumors, other types of malignant
tumors and no follow-up data, 563 patients with CCRCC, 82 patients
with PRCC and 25 patients with CCPRCC were selected. All the
patients received follow-up evaluations every 3 months in the first
year and then twice a year. They received physical examinations,
laboratory tests, and imaging tests (chest X-ray, ultrasonography
or CT when necessary) and were evaluated carefully by urologists
and radiologists. If the patients died, the causes of death were
retrieved from the patients' family members or hospital records.
The duration of follow-up was calculated from the date of the
operation to the date of death or last follow-up before June 2016.
Then cancer-specific survival (CSS) and progression-free survival
(PFS) were estimated.
Statistical analysis
All the statistical analyses were performed with SAS
9.3 (SAS institute Inc., Cary, NC, USA). The results were presented
as means ± standard deviation (SD). The measurement data (such as
age, size of tumor and the expression profiles of Ki67) were
compared using the Kruskal-Wallis test and the data of rate or
constituent ratio (such as patient sex, enhancement pattern, tumor
contour and calcification in the CT image) were compared using the
Chi-square test. We performed independent-samples t test to compare
the attenuation values of CCPRCC with those of CCRCC and PRCC in
four phases (unenhanced phase, CMP, nephrographic phase and EP).
T-tests were also performed to test the magnitude of aortic
attenuation among the four groups in each phase. CSS and PFS were
estimated with the Kaplan-Meier method and were compared with the
log-rank test. Univariate and multivariate Cox regression models
were used to define the risk factors for tumor recurrence and
patient death. Levels of statistical significance were fixed for
P-values <0.05.
Results
Clinical features
A total of 26 patients were diagnosed with CCPRCC in
this study, accounting for 1.7% of all renal cell carcinomas
(26/1519). The clinical features of the patients are summarized in
Table I. The mean age of patients at
diagnosis was 53.3±12.3 years (range 36–74 years), and the mean
tumor size was 2.5±1.5 cm (range 0.5 to 6.5 cm). The fact that 19
patients were male and 7 were female indicates a male preference.
In addition, 18 patients' tumors were located on the left side and
8 on the right side. There were 4 patients with multilocular renal
cyst (MRC), 1 with ESRD, 1 with renal calculus and 1 with bilateral
renal tumors (the right side was CCPRCC and the left side was
CCRCC). We also found that most of the patients with abnormal BMI
(17/26, 65.4%), including 2 obese patients. In terms of clinical
symptoms, most of the patients were found to have tumors by chance
when they were examined in medical centers, and only 8 of 26
patients had flank pain, abdominal pain or hematuria. Some patients
have comorbidities, such as hypertension (11/26, 42.3%), diabetic
mellitus (3/26, 11.5%), benign prostatic hyperplasia (3/26, 11.5%),
atherosclerotic cerebral infarction (2/26, 7.7%), coronary heart
disease (1/26, 3.8%), fatty liver (1/26, 3.8%) and pulmonary
tuberculosis (1/26, 3.8%). Six patients had a smoking history (20
cigarettes per day on average) and 2 had a drinking history (1 kg
liquor per month on average). Eleven patients received radical
nephrectomy (RN) and 15 received partial nephrectomy (PN).
 | Table I.Clinical features of the 26 CCPRCC
patients. |
Table I.
Clinical features of the 26 CCPRCC
patients.
| Case no. | Age (years) | Sex | BMI
(kg/m2) | Laterality | Tumor size
(cm) | Clinical
symptom | Creatinine
(µmol/l) | Past medical
history | Smoking/drinking
history | Operative type | Other kidney
disease |
|---|
| 1 | 36 | M | 27.6 | R | 4.5 | Flank pain | 62 | None | Smoking
history | RN | None |
| 2 | 44 | M | 22.2 | L | 2.5 | No symptom | 92 | HTN | NA | PN | None |
| 3 | 36 | M | NA | R | 1.5 | Abdominal pain | 85 | HTN | Smoking
history | PN | None |
| 4 | 54 | F | 24.2 | L | 4.5 | No symptom | 101 | HTN | None | RN | ESRD, MRC |
| 5 | 51 | M | 28.3 | L | 2.2 | No symptom | 96 | HTN | None | PN | MRC |
| 6 | 51 | M | 26.0 | L | 1.8 | Flank pain | 50 | HTN, fatty
liver | None | RN | None |
| 7 | 34 | M | 25.4 | L | 1.8 | No symptom | 75 | None | None | PN | None |
| 8 | 46 | M | 26.4 | L | 1.5 | Flank pain | 55 | HTN | None | PN | Bilateral
tumors |
| 9 | 43 | F | 27.7 | L | 1.2 | No symptom | 42 | None | None | PN | None |
| 10 | 67 | M | NA | L | 2.2 | No symptom | 75 | DM | Smoking
history | PN | None |
| 11 | 68 | M | 24.0 | L | 0.8 | No symptom | 90 | HTN, ACI | Smoking
history | PN | None |
| 12 | 38 | M | 23.8 | L | 5.0 | Flank pain | 68 | None | None | RN | Renal calculus |
| 13 | 45 | M | NA | R | 1.8 | No symptom | 76 | None | Drinking
history | PN | None |
| 14 | 69 | M | 25.2 | R | 4.2 | No symptom | 69 | PTB | Smoking
history | RN | None |
| 15 | 60 | M | 26.3 | L | 3.5 | No symptom | 82 | HTN, DM, ACI | None | RN | None |
| 16 | 67 | M | NA | L | 3.0 | Flank pain | 64 | HTN, BPH | None | RN | MRC |
| 17 | 37 | F | 25.4 | L | 2.0 | No symptom | 67 | None | None | PN | None |
| 18 | 55 | M | 25.6 | L | 1.5 | No symptom | 90 | NA | NA | PN | NA |
| 19 | 61 | M | 26.3 | L | 1.0 | No symptom | 82 | HTN, CHD | Drinking
history | PN | None |
| 20 | 72 | M | 24.6 | L | 2.5 | No symptom | 72 | BPH | None | RN | None |
| 21 | 74 | M | 24.0 | R | 3.0 | No symptom | 72 | BPH | Smoking
history | RN | None |
| 22 | 64 | F | NA | L | 1.5 | Flank pain | 59 | None | None | PN | None |
| 23 | 58 | M | 26.0 | R | 1.2 | No symptom | 74 | HTN | None | RN | None |
| 24 | 60 | F | 31.3 | R | 0.5 | No symptom | 60 | NA | NA | PN | None |
| 25 | 46 | F | NA | L | 6.5 | Hematuria | 65 | NA | NA | RN | None |
| 26 | 50 | F | 26.7 | R | 3.0 | No symptom | 72 | DM | None | PN | MRC |
We also analyzed the results of laboratory tests,
including blood routine, urine routine, blood biochemical and serum
tumor markers. No abnormalities were observed except for high serum
uric acid in 6 patients, mild abnormal glutamic-pyruvic
transaminase in 2 patients and positive urine red blood cell in 1
patient.
Pathologic features
Histopathologically, all tumors were encapsulated by
variably thick fibrous capsules and limited to the renal
parenchyma. Composed of different proportions of papillary,
tubular, cystic, acinar and nested architectures, these tumors
showed several morphologic patterns. The papillae, covered by small
to medium-sized cuboidal cells with abundant clear cytoplasm, were
mostly small, delicate and enclosed in cysts, and occasionally
showed secondary and tertiary hierarchical branching. No
calcification, necrosis or hemosiderin was present in these cases.
All tumors was either Fuhrman nuclear grade 1 (8/26, 30.8%) or
grade 2 (18/26, 69.2%). In most of these tumors, a characteristic
nuclear horizontally linear arrangement away from the basement
membrane was identified. The representative microscopic
illustrations of CCPRCC are shown in Fig.
2A-C. In addition, neither fat invasion nor renal vein
thrombosis was observed and all cases were stage I.
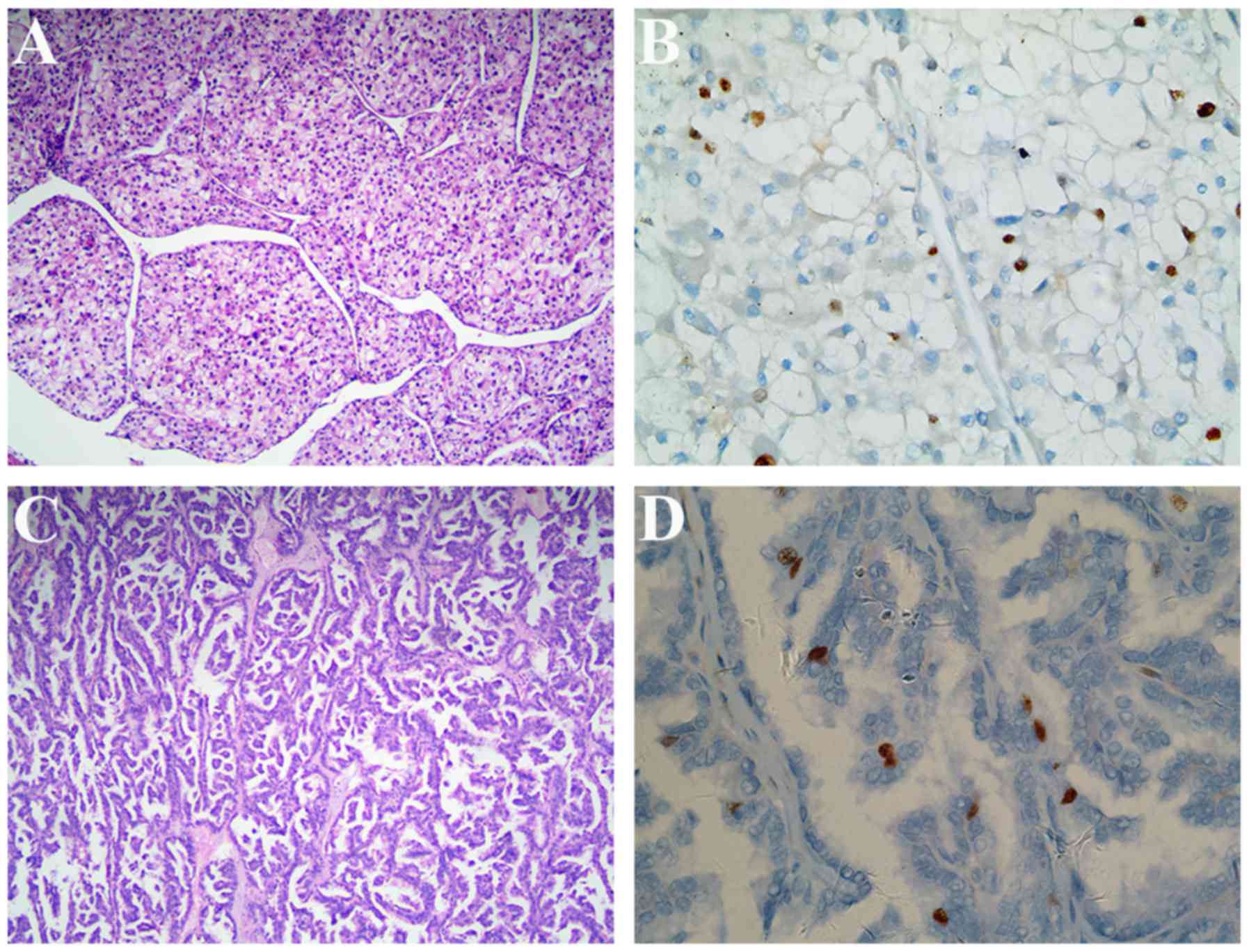
Histomorphological features of CCRCC and PRCC are shown in Fig. 3A and C, respectively.
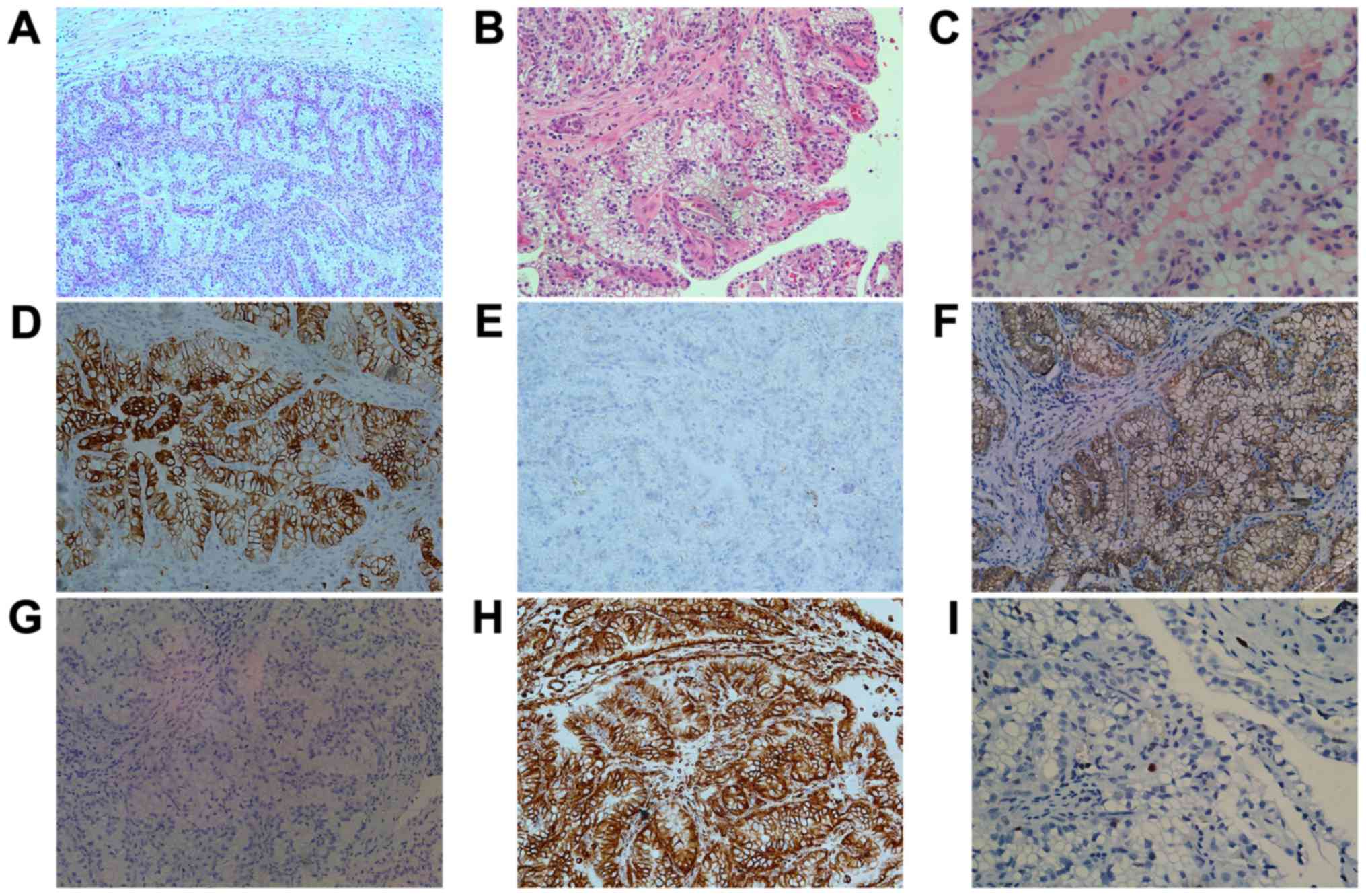 | Figure 2.Histomorphological and
immunohistochemical features of CCPRCC. (A) The tumor is
predominantly papillary encapsulated by a fibrous capsule
(magnification, ×100). (B) The papillae are small and delicate and
are covered by cells with abundant clear cytoplasm (magnification,
×200). (C) The low-grade nuclei located in the luminal side of the
tumor cells (magnification, ×400). (D) CK7 staining shows diffuse
strong positive (magnification, ×200). (E) The expression of AMACR
is negative (magnification, ×200). (F) CA IX staining shows
‘cup-like’ positive with absence of apical staining (magnification,
×200). (G) CD10 is negative in tumor cells (magnification, ×200).
(H) Vimentin staining also shows strong positive (magnification,
×200). (I) A few tumor cells stained brownish-yellow granules in
the nucleus exhibit positive for Ki67 staining (magnification,
×400). CCPRCC, clear cell papillary renal cell carcinoma. |
Immunohistochemically, we assessed the profile of
CK7, AMACR, CA IX, CD10, vimentin and Ki67 in the 26 CCPRCC cases.
All cases were diffusely and moderate to strong cytoplasmic
staining for CK7 (Fig. 2D), CA IX
(Fig. 2F) and vimentin (Fig. 2H), but negative for AMACR (Fig. 1E). The CD10 was negative or focally
positive in tumor cells (Fig. 2G).
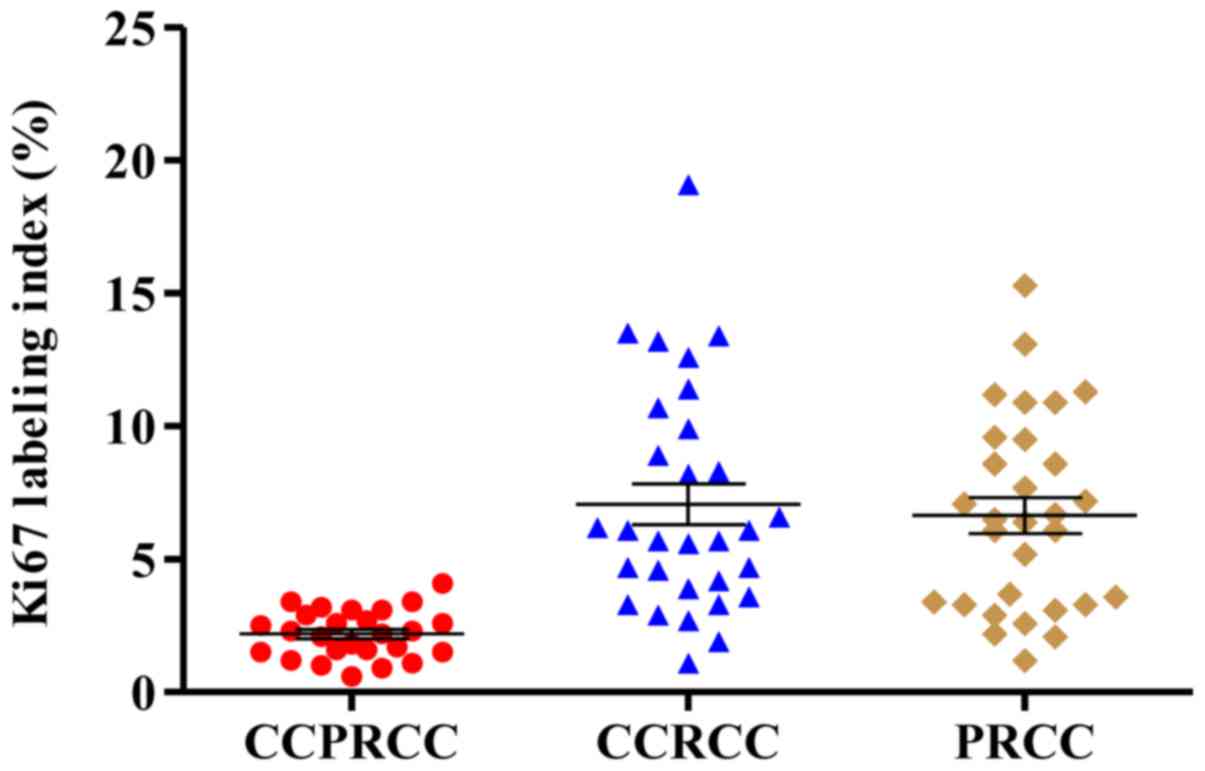
The immunohistochemical staining of Ki67 in different subtypes of
renal cell carcinoma are respectively shown in Figs. 2I, 3B and
D. According to the results of Ki67 LI, the expression of Ki67
in CCPRCC was much lower than that in CCRCC (2.19 vs. 7.07%,
P<0.001; Fig. 4) and that in PRCC
(2.19 vs. 6.65%, P<0.001; Fig. 4).
However, although the expression of Ki67 in CCRCC was still higher
than that in PRCC (7.07 vs. 6.65%, P=0.848; Fig. 4), the difference was not
significant.
CT features
Major CT findings and characteristics for each of
the groups are presented in Table
II. The mean lesion size was 2.4±1.4 cm for CCPRCC, 3.1±0.9 cm
for CCRCC and 2.9±1.0 cm for PRCC (P=0.086). There were
statistically significant differences in the enhancement patterns
among all subtypes (P=0.001), most CCPRCC (10/14, 71.4%) and CCRCC
(21/30, 70.0%) cases had a mixed enhancement pattern while PRCC
commonly had a homogeneous enhancement pattern (22/30, 73.3%). In
terms of tumor contour, the proportion of smooth tumor in CCPRCC
was higher than that in CCRCC (100 vs. 50.0%, P=0.001) and that in
PRCC (100 vs. 90.0%, P=0.220). Calcification within the tumor was
noted in 1 patient (6.7%) with CCRCC, in 1 patient (6.7%) with
PRCC, and none in patients with CCPRCC. These differences, however,
were not significant (P>0.05).
 | Table II.Imaging characteristics of patients
and renal lesions. |
Table II.
Imaging characteristics of patients
and renal lesions.
| Feature | CCPRCC (n=14) | CCRCC (n=30) | PRCC (n=30) | P-value |
|---|
| Age (year) | 51.3±13.4 | 54.5±10.7 | 58.6±11.1 | 0.154 |
| Lesion size
(cm) | 2.4±1.2 | 3.1±0.9 | 2.9±1.0 | 0.086 |
| Enhancemen pattern
(%) |
|
Homogeneous | 4 (28.6) | 9 (30.0) | 22 (73.3) | 0.001 |
|
Heterogeneous | 10 (71.4) | 21 (70.0) | 8 (26.7) |
|
| Tumor contour
(%) |
|
Smooth | 14 (100) | 15 (50.0) | 27 (90.0) | <0.0001 |
|
Other | 0 | 15 (50.0) | 3 (10.0) |
|
| Calcification
(%) | 0 | 1 (6.7) | 1 (6.7) | 0.787 |
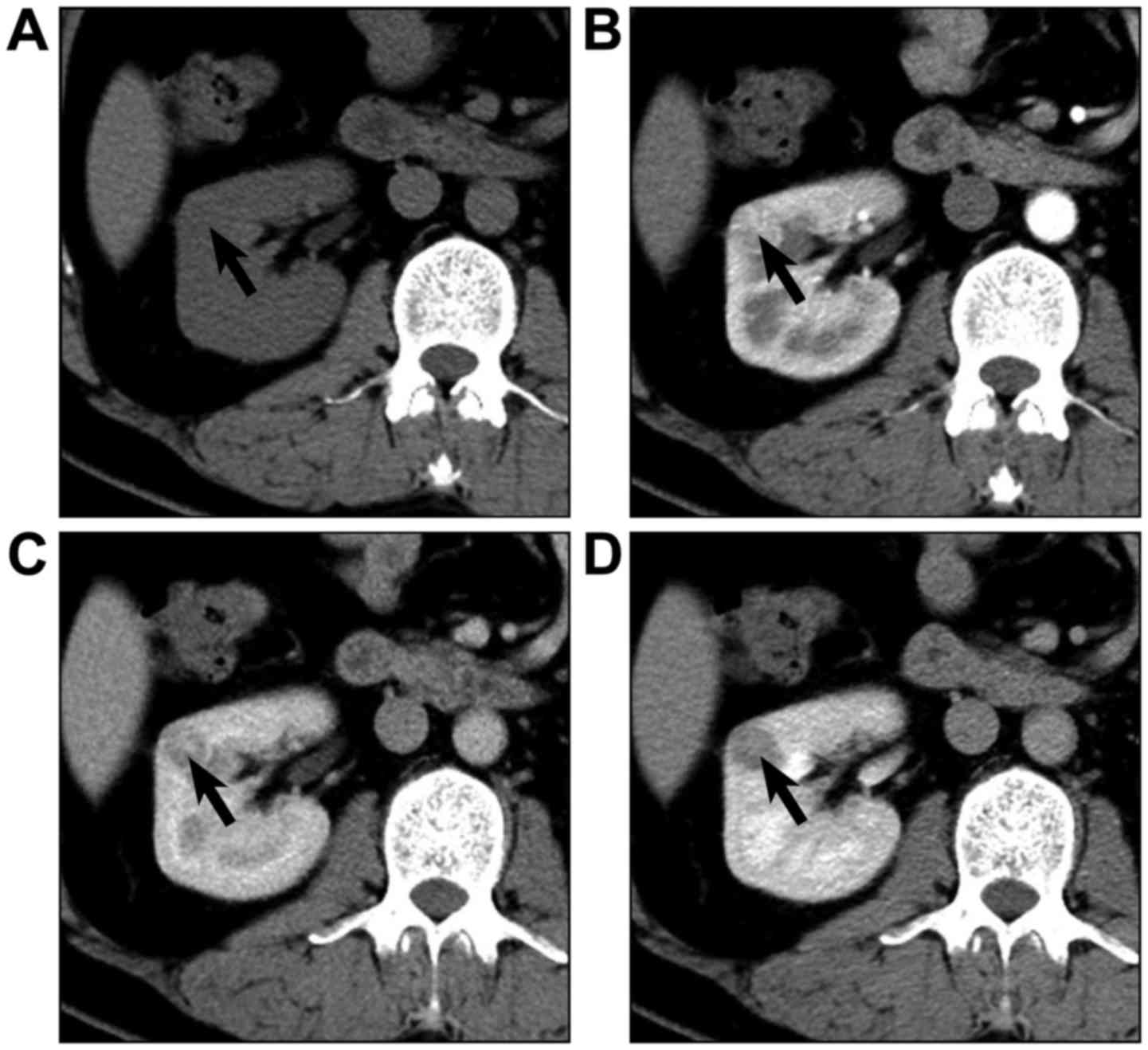
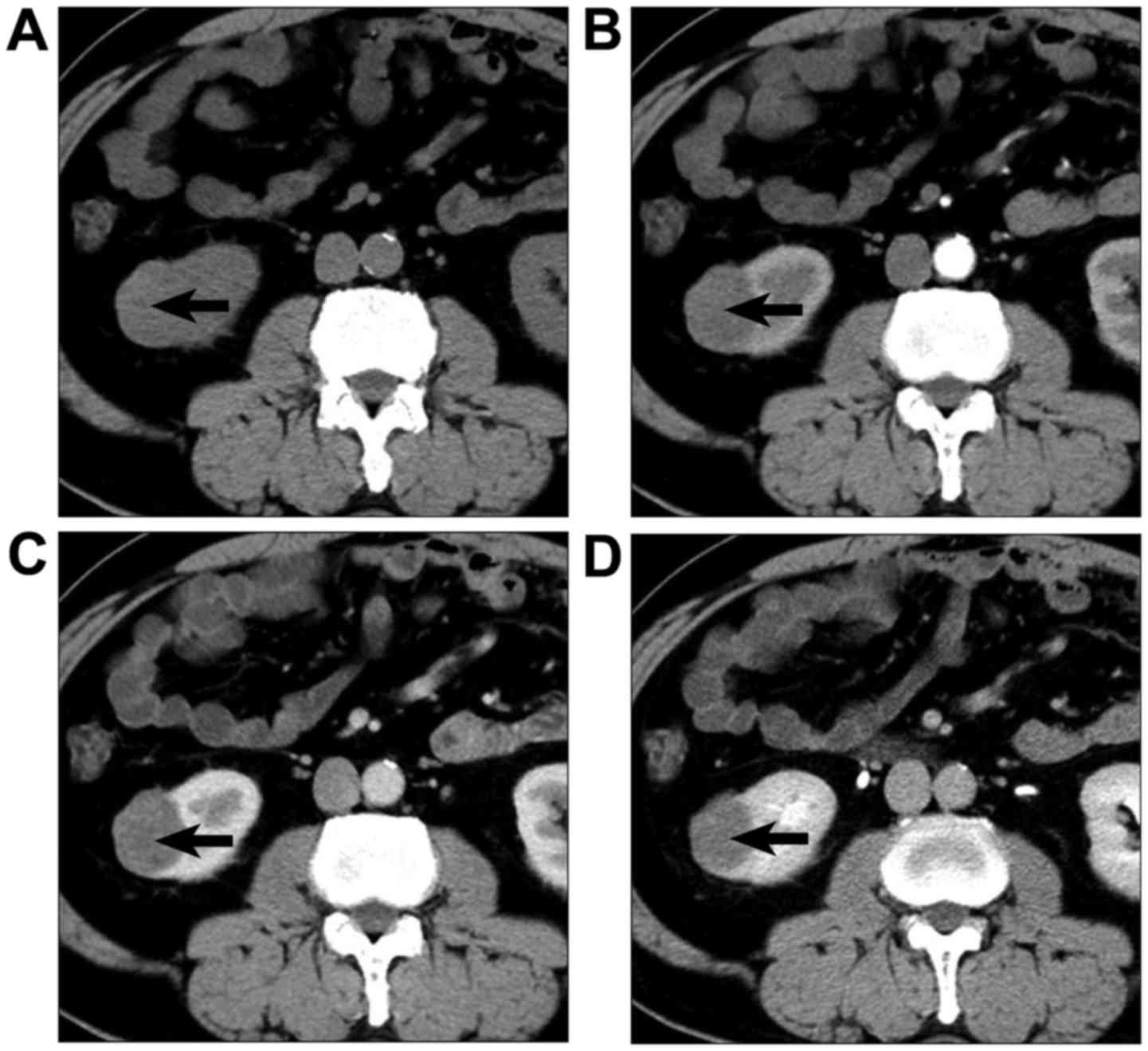
Typical lesions from each group are presented in
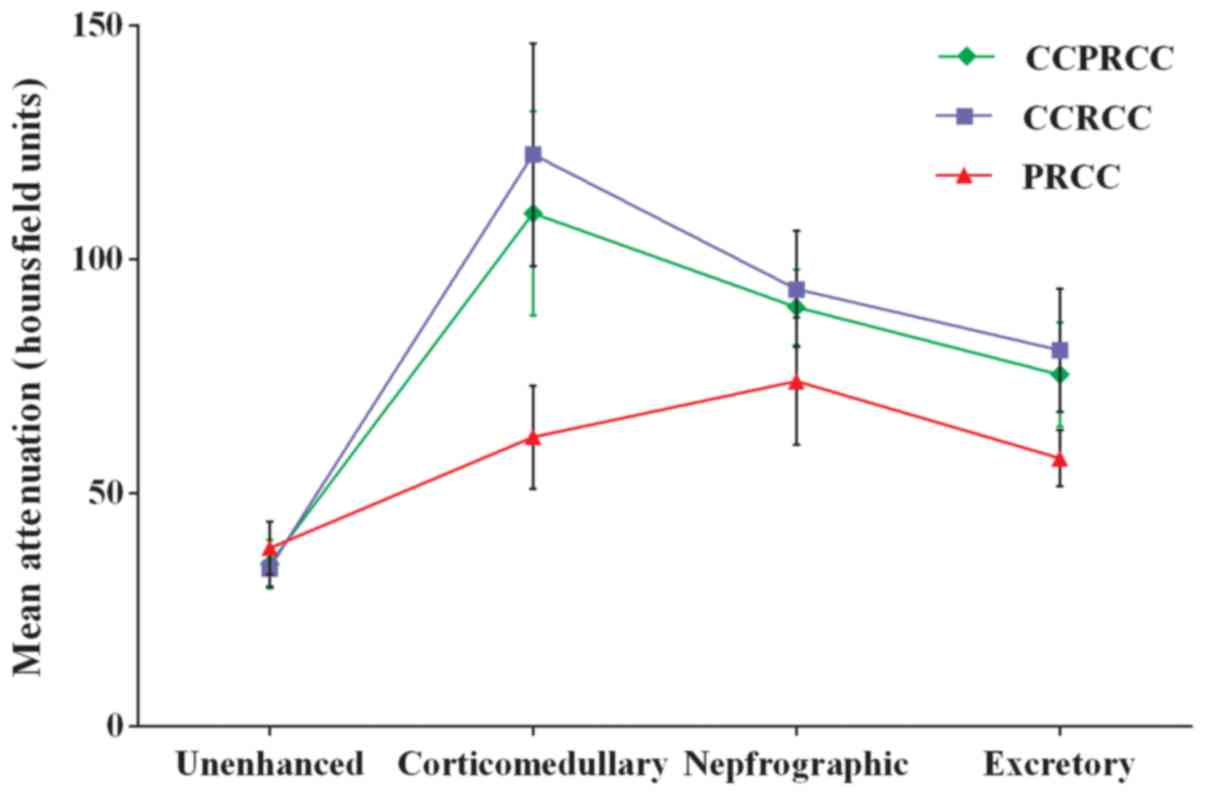
Figs. 5–7. Mean attenuation values for CCPRCC, CCRCC,
and PRCC in each phase are shown in Table III and Fig. 8. The mean pre-enhancement attenuation
values of all 3 subtypes did not differ from each other. Although
the magnitude of CCRCC enhancement was greater than that of CCPRCC
in CMP (122.4 HU vs. 109.8 HU), NP (93.6 HU vs. 89.7 HU) and EP
(80.5 HU vs. 75.3 HU), all these differences were not significant
(P>0.05). Different from mean enhancement of these 2 subtypes of
RCC peaked in the CMP, the peak height of enhancement for PRCC was
in the NP. However, the magnitude of enhancement of PRCC was
significantly lower than that of CCPRCC in the CMP (61.9 HU vs.
109.8 HU, P=0.009), NP (73.9 HU vs. 89.7 HU, P=0.038), and EP (57.4
HU vs. 75.3 HU, P=0.028). Comparing the CCPRCC group with the CCRCC
and PRCC groups, there were no significant differences in aortic
attenuation in any of the phases.
 | Table III.Attenuation of renal lesions on the
basis of histologic subtype. |
Table III.
Attenuation of renal lesions on the
basis of histologic subtype.
| Imaging phase | CCPRCC | CCRCC | PRCC |
|---|
| Lesion
attenuation |
|
Unenhanced | 34.8±5.2 | 33.9±3.9 | 38.2±5.7 |
|
P-value | – | 0.596 | 0.418 |
|
Corticomedullary | 109.8±21.8 | 122.4±23.8 | 61.9±11.0 |
|
P-value | – | 0.675 | 0.009 |
|
Nephrographic | 89.7±8.1 | 93.6±12.4 | 73.9±13.6 |
|
P-value | – | 0.422 | 0.038 |
|
Excretory | 75.3±11.2 | 80.5±13.2 | 57.4±6.0 |
|
P-value | – | 0.508 | 0.028 |
| Aortic
attenuation |
|
Unenhanced | 44.7±3.0 | 43.7±3.3 | 44.2±3.6 |
|
P-value | – | 0.581 | 0.895 |
|
Corticomedullary | 221.3±37.8 | 227.2±27.4 | 236.8±29.8 |
|
P-value | – | 0.314 | 0.437 |
|
Nephrographic | 126.5±14.1 | 119.7±13.4 | 123.8±11.6 |
|
P-value | – | 0.848 | 0.353 |
|
Excretory | 94.9±8.1 | 91.5±7.68 | 91.5±7.2 |
|
P-value | – | 0.880 | 0.938 |
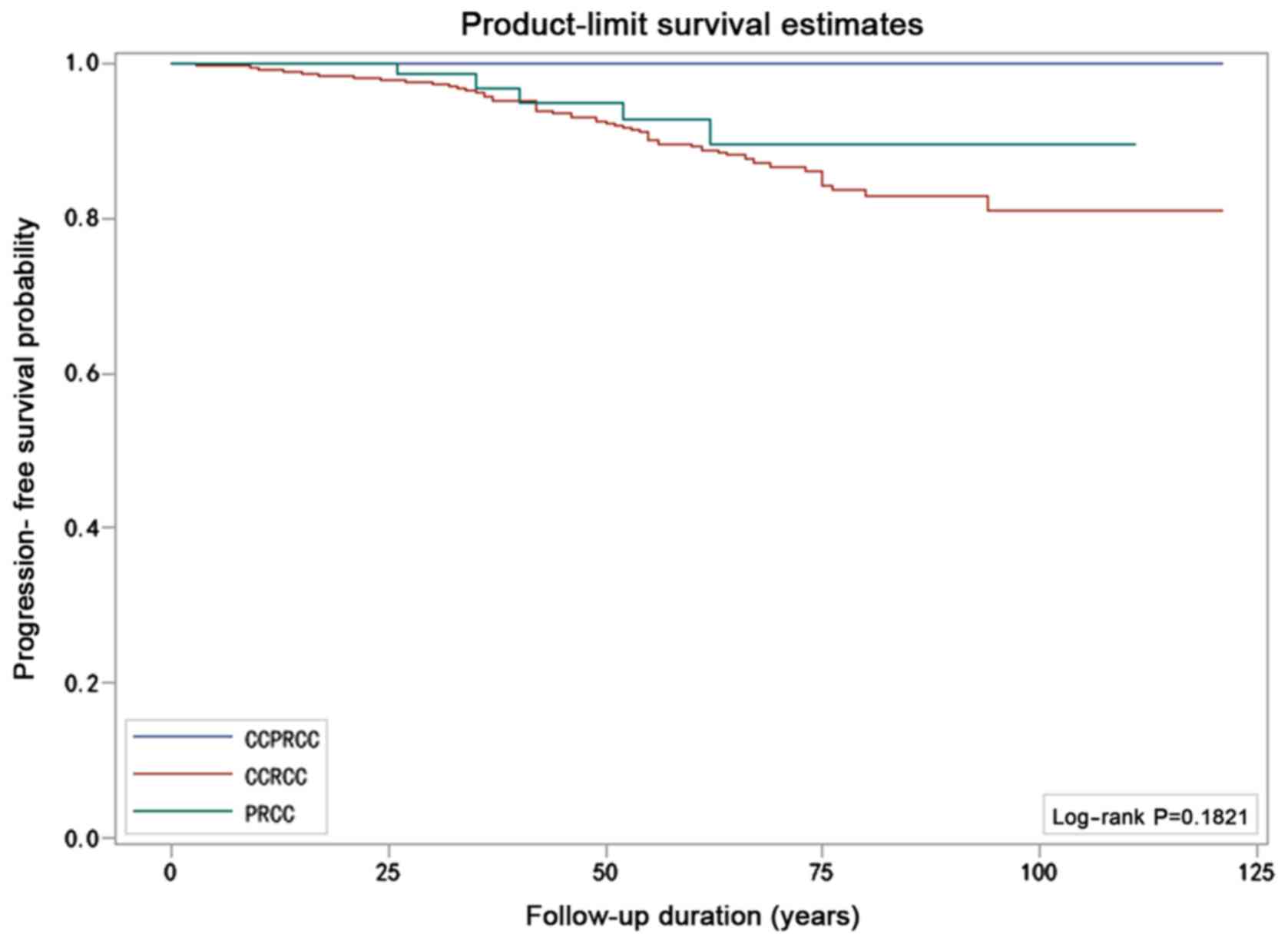
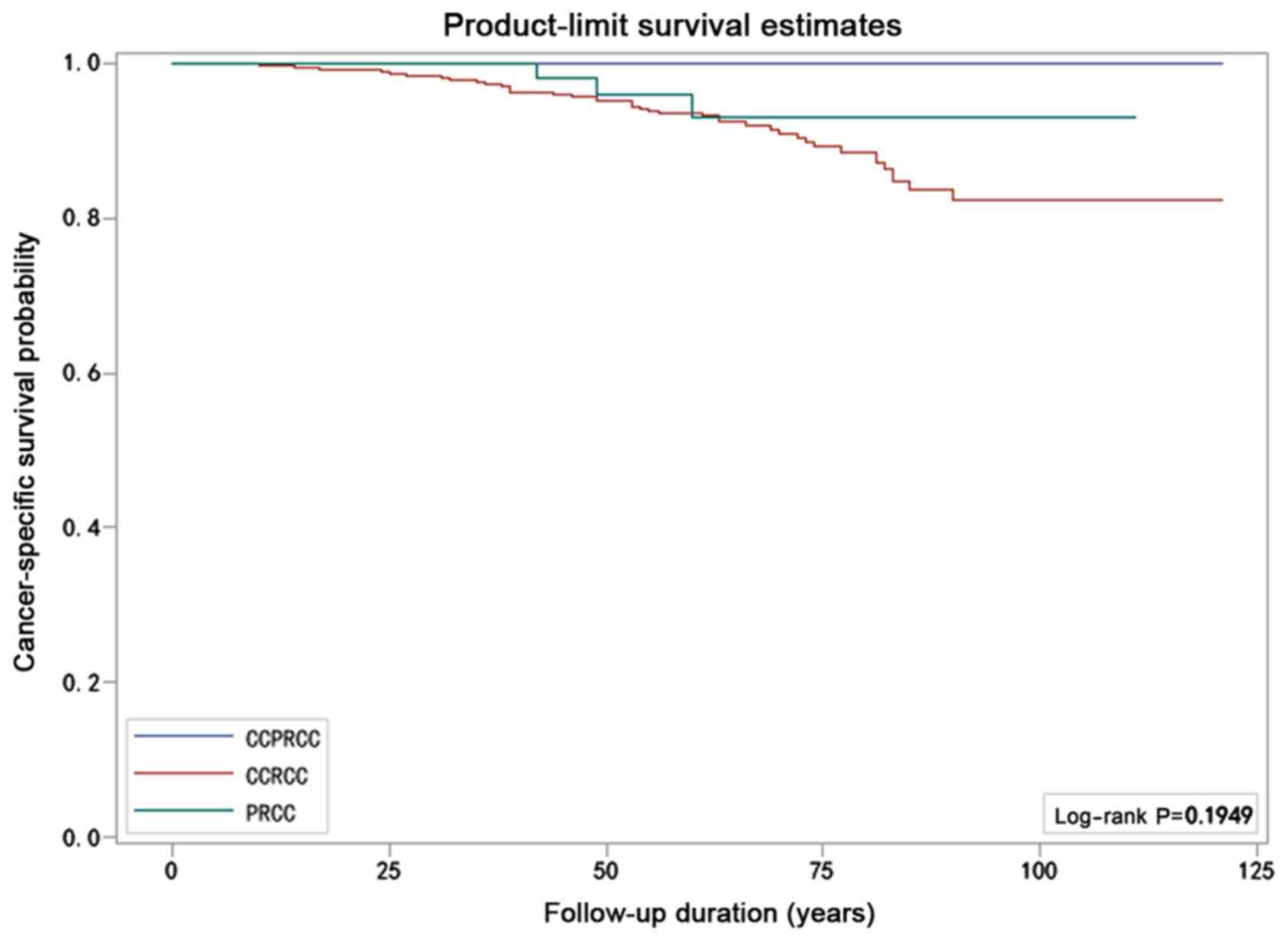
Survival analysis
The patients' clinical and pathological
characteristics are listed in Table
IV by histologic subtype. The median follow-up among the
patients with CCPRCC, CCRCC, and PRCC was 50, 57 and 51.5 months,
respectively. None of the 25 patients with CCPRCC died of the
disease or demonstrated disease progression. Among the 563 patients
with CCRCC, 46 died due to the disease and 59 were observed to have
tumor recurrence. The PFS rate was 89.5% and the CSS rate was 91.8%
at 10 years. Meanwhile, in all 82 patients with PRCC, 3 patients
died of it and 5 patients had tumor recurrence. The PFS rate was
93.9% and the CSS rate was 96.3% at 10 years. Although the results
suggested that the prognosis of CCPRCC was better than that in the
other two groups, the Kaplan-Meier curves did not show significant
differences in either the CSS (P=0.195, log-rank test) or PFS rates
(P=0.182, log-rank test) for the three subtypes (Figs. 9 and 10).
 | Table IV.Clinical and pathologic
characteristics of follow-up patients. |
Table IV.
Clinical and pathologic
characteristics of follow-up patients.
| Characteristic | CCPRCC (n=25) | CCRCC (n=563) | PRCC (n=82) |
|---|
| Age (month) |
| Mean ±
SD | 53.6±13.4 | 56.7±12.3 | 58.3±13.9 |
|
Range | 34–74 | 20–86 | 20–83 |
| Sex (%) |
|
Male | 18 (72.0) | 361 (64.1) | 68 (82.9) |
|
Female | 7 (28.0) | 202 (35.9) | 14 (17.1) |
| Tumor size
(cm) |
| Mean ±
SD | 2.5±1.5 | 4.1±1.7 | 3.7±1.6 |
|
Range | 0.5–6.5 | 1.0–7.0 | 0.5–7.0 |
| Nuclear grade
(%) |
| 1 | 9 (36.0) | 123 (21.8) | 15 (18.3) |
| 2 | 16 (64.0) | 440 (78.2) | 67 (81.7) |
| Operative type
(%) |
| PN | 14 (56.0) | 99 (17.6) | 26 (31.7) |
| RN | 11 (44.0) | 464 (82.4) | 56 (68.3) |
| Follow-up time
(month) |
| Mean ±
SD | 57.3±33.2 | 57.8±24.0 | 53.5±24.8 |
|
Range | 12–121 | 6–121 | 13–111 |
Among all the investigated prognostic factors, tumor
size (P<0.0001, HR=6.16), Fuhrman's nuclear grade (P=0.01,
HR=3.29), and operative type (P=0.02, HR=2.84) had a significant
impact on tumor recurrence in the univariate analysis. Multivariate
analysis subsequently showed that only tumor size (P<0.0001,
HR=6.22), as an independent factor, had an impact on tumor
recurrence (Table V). Both univariate
analysis and multivariate analysis could not detect the results of
RCC subtype because the sample numbers were not balanced in the 3
groups and CCPRCC accounted for a rather small proportion. Similar
results were noted in the univariate and multivariate analyses for
CSS (Table VI).
 | Table V.Univariate and multivariate analyses
of patient and tumor characteristics with regard to their
prognostic impact on progression-free survival (Cox regression
analysis). |
Table V.
Univariate and multivariate analyses
of patient and tumor characteristics with regard to their
prognostic impact on progression-free survival (Cox regression
analysis).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (year) |
| 0.58 |
| 0.87 |
|
≤60 | 1.00
(reference) |
| 1.00
(reference) |
|
|
>60 | 0.86 (0.52,
1.44) |
| 1.04 (0.62,
1.75) |
|
| Sex |
| 0.82 |
| 0.95 |
|
Female | 1.00
(reference) |
| 1.00
(reference) |
|
|
Male | 1.06 (0.63,
1.81) |
| 1.02 (0.60,
1.73) |
|
| T stage |
| <0.0001 |
| <0.0001 |
|
T1a | 1.00
(reference) |
| 1.00
(reference) |
|
|
T1b | 6.16 (3.29,
11.54) |
| 6.22 (2.91,
13.30) |
|
| Nuclear grade |
| 0.01 |
| 0.10 |
| 1 | 1.00
(reference) |
| 1.00
(reference) |
|
| 2 | 3.29 (1.32,
8.19) |
| 2.16 (0.86,
5.47) |
|
| Operative type |
| 0.02 |
| 0.59 |
| RN | 1.00
(reference) |
| 1.00
(reference) |
|
| PN | 2.84 (1.22,
6.59) |
| 0.76 (0.27,
2.08) |
|
| RCC subtype |
| NA |
| NA |
|
CCPRCC | 1.00
(reference) |
| 1.00
(reference) |
|
|
CCRCC | NA | 0.984 | NA | 0.986 |
|
PRCC | NA | 0.985 | NA | 0.986 |
 | Table VI.Univariate and multivariate analyses
of patient and tumor characteristics with regard to their
prognostic impact on cancer-specific survival (Cox regression
analysis). |
Table VI.
Univariate and multivariate analyses
of patient and tumor characteristics with regard to their
prognostic impact on cancer-specific survival (Cox regression
analysis).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (year) |
| 0.62 |
| 0.87 |
|
≤60 | 1.00
(reference) |
| 1.00
(reference) |
|
|
>60 | 0.86 (0.48,
1.55) |
| 1.05 (0.58,
1.90) |
|
| Sex |
| 0.53 |
| 0.72 |
|
Female | 1.00
(reference) |
| 1.00
(reference) |
|
|
Male | 1.18 (0.63,
2.21) |
| 1.12 (0.60,
2.10) |
|
| T stage |
| <0.0001 |
| <0.0001 |
|
T1a | 1.00
(reference) |
| 1.00
(reference) |
|
|
T1b | 8.38 (3.76,
18.67) |
| 9.15 (3.39,
24.67) |
|
| Nuclear grade |
| 0.02 |
| 0.11 |
| 1 | 1.00
(reference) |
| 1.00
(reference) |
|
| 2 | 4.2 |
| 2.61 (0.80,
8.54) |
|
| Operative type |
| 0.03 |
| 0.45 |
| RN | 1.00
(reference) |
| 1.00
(reference) |
|
| PN | 0.32 (0.12,
0.89) |
| 0.60 (0.17,
2.12) |
|
| RCC subtype |
| NA |
| NA |
|
CCPRCC | 1.00
(reference) |
| 1.00
(reference) |
|
|
CCRCC | NA | 0.986 | NA | 0.988 |
|
PRCC | NA | 0.987 | NA | 0.988 |
Discussion
Accumulated data have demonstrated that CCPRCC is a
distinct entity of renal epithelial neoplasm (7). In this study, we provided a clinical
view to understand this tumor. The frequency of CCPRCC was 1.7%
among 1,519 kidney resections of RCC and the mean age of patients
with CCPRCC was 53.3 years. The disease was more common in men than
in women, which is consistent with previous studies (13,15,18). Of
the 26 CCPRCC cases, the majority were asymptomatic. Only 7
patients suffered from abdominal pain or flank pain and 1 patient
from hematuria, which reveals that patients with CCPRCC have no
typical clinical symptoms. We also observed whether these patients
were complicated with other kidney diseases. Multilocular renal
cyst (MRC), the most common kidney disease, occurred in 4 patients,
although CCPRCC was initially thought to have association with ESRD
(1,11). In our study, ESRD occurred with CCPRCC
in only 1 case. In addition, there was 1 patients with bilateral
renal tumors (the right side was CCPRCC and the left side was
CCRCC), which is rare in the sporadic setting.
The patient's height, weight, past medical history
and smoking history were recorded. Through analyzing the results,
we find 17 patients (65.4%) having body mass index (BMI) greater
than 23.9 kg/m2, 11 patients (42.3%) having hypertension
occurred in, and 6 patients (23.1%) being current smokers or former
smokers. Chow et al examined the health records of 363 992
Swedish men who underwent at least one physical examination from
1971 to 1992, and they found that increasing blood pressure levels,
obesity and cigarette smoking were independent risk factors of
kidney cancer (29). This conclusion
was further confirmed by Sanfilippo et al (30), through observational studies of 156
774 participants over 10.8 years. The risk rose with increasing
blood pressures and BMI. Furthermore, we explored the biologic
mechanisms underlying these risk factors. Obesity and hypertension
are associated with increased glomerular filtration rate and renal
plasma flow (30,31). This may render kidneys more
susceptible to damage and increase in the oxygen demands of tubular
cells, which in turn provokes an imbalance between oxygen delivery
and oxygen demand (32). Meanwhile,
obesity, hypertension and cigarette smoke are associated with
oxidative stress and lipid peroxidation, which may cause an
increase in oxygen consumption as well (30,32,33). In
addition, the combustion of tobacco produces carbon monoxide, which
can be combined with hemoglobin to form carboxyhemoglobin. Finally,
chronic hypoxia in the kidney potentiates the upregulation of
hypoxia-inducible factors (HIFs) and CA IX, which triggers the
development of ESRD or renal carcinogenesis (29–34).
Coincidentally, previous studies have documented that most of CCRCC
have VHL gene mutations, which can also lead to overexpression of
HIF pathway associated proteins, whereas CCPRCC lack these
characteristic chromosomal abnormalities (2,18,21). The strong expression of HIF-1α and CA
IX in all tumors, however, provide supporting evidence that CCPRCC
activate the HIF pathway independent of VHL gene mutation (2,3,9,18,35). Thus we reasonably speculate that
hypertension, obesity and smoking are the main causes of CCPRCC.
The results of laboratory tests were also analyzed, but they were
non-specific except for high serum uric acid in 6 patients and mild
abnormal glutamic-pyruvic transaminase in 2 patients. Further
research is required to find out whether there is a relationship
between diet and CCPRCC.
CCPRCC often presents as small masses with similar
morphological characteristics in both CCRCC and PRCC. However,
there are also many different histological and immunohistochemical
profiles among them. The CCPRCC has a combination of tubular,
papillary, cystic and occasionally solid structures. The papillae
in CCPRCC are short and aborted, which differs from PRCC, which has
longer papillae and often marked by foamy histiocytes. Meanwhile,
CCRCC rarely have papillary components, although the tumor cells of
CCPRCC and CCRCC all exhibit clear cytoplasm. In addition, the
Fuhrman nuclear grade of CCPRCC is low (1 or 2) and most of the
nuclei are characteristically situated away from the basement
membrane in a linear fashion. At least 25 kinds of antibodies are
used for immunohistochemical labeling of CCPRCC (22), but it is generally accepted that the
most important makers are CK7, AMACR, CD10 and CA IX (9,18,35,36). It is
helpful to identify CCPRCC by using these 4 kinds of antibodies if
there are too many overlapping features among the 3 subtypes of
RCC. CCPRCC typically is positive for CK7 and CA IX (sometimes
‘cup-like’ pattern), negative for AMACR and CD10 (sometimes focally
positive). CCRCC in contrast, usually exhibits positive for CD10
and CA IX (‘box-like’ pattern), while negative for CK7 and AMACR.
For PRCC, the tumor cells express strong positive immunostaining
for CK7, CD10 and AMACR, but negative for CA IX. Furthermore, to
our knowledge, we are the first to report the expression of Ki67
immunostaining in CCPRCC. Ki67 is a kind of nuclear-associated
antigen present in the G1, S, G2 and M-phase of all cycling human
cells (37). Many studies have
indicated that Ki67 is a prognostic marker in several neoplasms,
including kidney cancer (37,38). In this study, the Ki67 LI is
significantly lower than that in CCRCC (P<0.001) or that in PRCC
(P<0.001). This result is consistent with the low-grade nuclei
of CCPRCC, which indicates that patients with CCPRCC may have a
better prognosis.
Imaging characteristics of CCRCC and PRCC by
computed tomography scan have been described by several studies.
The typical enhancement pattern of CCRCC is heterogeneous. Their
attenuation values markedly increase in the CMP, approaching levels
in the adjacent renal cortex. Then they rapidly decrease in the NP
and EP (39,40). In contrast, the majority of PRCC cases
are homogenous and do not enhance markedly because of hypo-vascular
(40,41). However, no prior studies have
described the imaging of CCPRCC except Gill et al, who
briefly mentioned 6 cases (15). We
wonder whether there are some imaging features to identify CCPRCC
from other renal tumor subtypes. Based on the results, we draw the
following four conclusions. Firstly, most CCPRCC tumors were
smaller than 4 cm, which indicated a low stage (pT1a) according to
the 2010 TNM staging system. Secondly, the contours of CCPRCC
tumors were all smooth, and no calcification was observed in these
tumors. Thirdly, CCPRCC tumors often showed heterogeneity with
intravenous contrast because of the mixed organization structure.
The fourth and most important thing is that the multiphasic
attenuation curve for CCPRCC was like that for CCRCC. This might be
related to the activation of the HIF pathway in both CCPRCC and
CCRCC, which led to hyper vascularity in tumors. Nevertheless, not
all CCPRCC cases had such image features. We also observed 2 cases,
in which the enhancement magnitudes were relatively low and the
enhancement peaked in the NP, resembling typical of PRCC tumors. By
reviewing the 2 pathological sections, we found that they both
demonstrated significant cysts formation, covering 80–90% of the
tumor. The rest area of the tumors contained delicate papillae and
the stroma only had a small number of capillaries. We suspect that
this was due to insufficient tumor blood supply caused by the cyst
compression. But it still needs further verification.
The development and widespread use of radiologic
imaging techniques have increased the detection rate of incidental
small RCCs (42). Meanwhile, the
incidence of CCPRCC increased significantly in the RCCs with early
stage and low nuclear grade. Thus, predicting the biological
potential of CCPRCC is extremely helpful for choosing treatment.
The results of our study showed that neither cancer-specific death
nor tumor recurrence was observed at a median follow-up period of
50 months (range 12–121 months), whether in partial nephrectomy or
radical nephrectomy cases. Other CCPRCC studies have similarly
shown excellent oncologic outcomes (7,10,13,15). The
favorable prognosis of CCPRCC suggested that it was consistent with
the low expression of Ki67. Furthermore, the multivariate analysis
showed that only tumor size was an independent prognostic factor
whether it was in PFS or CSS, and the survival time of patients had
nothing to do with the operation methods. Thus in diagnosis and
treatment of RCC, preoperative CT examination is necessary. For
early stage tumors, especially those smaller than 4 cm and like the
radiological features of CCPRCC, we recommend renal tumor biopsy
before surgery in order to verify the diagnosis. Partial
nephrectomy is an appropriate surgical procedure for CCPRCC. In
patients of old age, ESRD, or with some comorbidities (such as
cardiac/pulmonary insufficiency), however, there is a high risk in
surgery. Whether these patients need close surveillance or
radiofrequency ablation requires further consideration.
Several limitations of our study should be
considered. Firstly, not all the 26 patients with CCPRCC underwent
CT because of the retrospective study. Only 14 patients were
evaluated for multiphasic enhancement, including 10 patients
underwent four-phase scanning. But this is the maximum number of
samples that have ever been reported and we believe that these
cases are enough for analyzing the image features of CCPRCC.
Secondly, this is a co-conducted study and the equipment models are
not the same in both hospitals. However, most of renal masses were
evaluated with a standardized protocol. Furthermore, to reduce
errors in measurement, the patients with CCRCC or PRCC were
selected according to the proportion of CCPRCC in the two
hospitals. We also believe that a robust identification method
should be as widely applicable as possible. Thirdly, the CT images
of patients with ESRD are not evaluated because samples are rare.
Whether their results are consistent with that of the patients with
normal renal function remains to be further studied. Fourthly,
though we have data on magnetic resonance and contrast-enhanced
ultrasound in patients with CCPRCC, a comparative study cannot be
carried out, because the data is little. We will pay more attention
to this part of the study. Finally, although the 10 year PFS and
CSS rates of CCPRCC were lower than those of CCRCC and PRCC in the
early stage of RCC with low nuclear grade, the differences were not
statistically significant. This result may be associated with small
sample size and lack of long-term follow-up time in our study. In
addition, there are no studies of metastases or recurrences of
CCPRCC published to date (1–3,7–10,26).
Therefore, we can not deny that CCPRCC has a better prognosis than
CCRCC or PRCC. In view of its lower incidence, maybe a
meta-analysis is necessary to answer this question.
In conclusion, the present study contributes to
further understanding of this unique renal tumor. We suggest that
urologists and oncologists should be aware of the image features of
CCPRCC and its favorable prognosis, apart from knowing its distinct
morphological features and diagnosing it with immunohistochemistry.
For early stage RCC cases, especially for those having a smaller
than 4 cm size, smooth contour and a high degree of enhancement in
CMP, the possibility of CCPRCC should be considered. If the
diagnosis is made by biopsy before operation, radical nephrectomy
requires careful consideration. Larger cohorts of patients will
provide more information for pathogenesis study, diagnosis and
treatment of this tumor type.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grants nos. 81570676, 81672531 and
81502089), the Priority Academic Program Development of Jiangsu
Higher Education Institutions ‘333 high level talents project’ in
Jiangsu province (grant no. BRA2015469), the six talents peak
project in Jiangsu Province (grants nos. WSN-011 and WSN-056) and
the Natural Science Foundation of Jiangsu Province (grant no.
BK20151024).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YQW, MG and CQ conceived and designed the
experiments. YD, ZJW, CHH, HXL, XL, PFW and GCL collaborated in
assessing the patient data. YD, JW, ZJW, CHH, HXL, XL, PFW and GCL
performed analysis and interpretation of the data. YQW wrote the
paper. MG and CQ checked and edited the paper. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the First Affiliated Hospital of Nanjing Medical University
(approval no. 2016-SRFA-011) and the Ethics Committee of the
Affiliated Xuzhou Hospital of Medical College of Southeast
University (approval no. XZXY-LJ-20160111-008). Written informed
consent was obtained from all patients and/or their guardians.
Consent for publication
Informed consent for the publication of any
associated data and accompanying images was obtained from all
individuals.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CCPRCC
|
clear cell papillary renal cell
carcinoma
|
|
CCRCC
|
clear cell renal cell carcinoma
|
|
PRCC
|
papillary renal cell carcinoma
|
|
ESRD
|
end-stage renal disease
|
|
RCC
|
renal cell carcinoma
|
|
ISUP
|
International Society of Urological
Pathology
|
|
WHO
|
World Health Organization
|
|
CRCC
|
chromophobe renal cell carcinoma
|
|
CT
|
computed tomography
|
|
Ki67 LI
|
Ki67 labeling index
|
|
CMP
|
corticomedullary phase
|
|
NP
|
nephrographic phase
|
|
EP
|
excretory phase
|
|
ROI
|
region of interest
|
|
CSS
|
cancer-specific survival
|
|
PFS
|
progression-free survival
|
|
SD
|
standard deviation
|
|
MRC
|
multilocular renal cyst
|
|
BMI
|
body mass index
|
|
RN
|
radical nephrectomy
|
|
PN
|
partial nephrectomy
|
|
HIFs
|
hypoxia-inducible factors
|
References
|
1
|
Tickoo SK, de Peralta-Venturina MN, Harik
LR, Worcester HD, Salama ME, Young AN, Moch H and Amin MB: Spectrum
of epithelial neoplasms in end-stage renal disease: An experience
from 66 tumor-bearing kidneys with emphasis on histologic patterns
distinct from those in sporadic adult renal neoplasia. Am J Surg
Pathol. 30:141–153. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gobbo S, Eble JN, Grignon DJ, Martignoni
G, Maclennan GT, Shah RB, Zhang S, Brunelli M and Cheng L: Clear
cell papillary renal cell carcinoma: A distinct histopathologic and
molecular genetic entity. Am J Surg Pathol. 32:1239–1245. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aydin H, Chen L, Cheng L, Vaziri S, He H,
Ganapathi R, Delahunt B, Maqi-Galluzzi C and Zhou M: Clear cell
tubulopapillary renal cell carcinoma: A study of 36 distinctive
low-grade epithelial tumors of the kidney. Am J Surg Pathol.
34:1608–1621. 2010.PubMed/NCBI
|
|
4
|
Adam J, Couturier J, Molinié V,
Vieillefond A and Sibony M: Clear-cell papillary renal cell
carcinoma: 24 cases of a distinct low-grade renal tumour and a
comparative genomic hybridization array study of seven cases.
Histopathology. 58:1064–1071. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Srigley JR, Delahunt B, Eble JN, Egevad L,
Epstein JI, Grignon D, Hes O, Moch H, Montironi R, Tickoo SK, et
al: The International Society of Urological Pathology (ISUP)
Vancouver Classification of renal neoplasia. Am J Surg Pathol.
37:1469–1489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE
and Ulbright TM: The 2016 WHO Classification of Tumours of the
urinary system and male genital organs-part A: Renal, penile, and
testicular tumours. Eur Urol. 70:93–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou H, Zheng S, Truong LD, Ro JY, Ayala
AG and Shen SS: Clear cell papillary renal cell carcinoma is the
fourth most common histologic type of renal cell carcinoma in 290
consecutive nephrectomies for renal cell carcinoma. Hum Pathol.
45:59–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park JH, Lee C, Suh JH and Moon KC: Clear
cell papillary renal cell carcinoma: A report of 15 cases including
three cases of concurrent other-type renal cell carcinomas. Korean
J Pathol. 46:541–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Williamson SR, Eble JN, Cheng L and
Grignon DJ: Clear cell papillary renal cell carcinoma: Differential
diagnosis and extended immunohistochemical profile. Mod Pathol.
26:697–708. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alexiev BA and Drachenberg CB: Clear cell
papillary renal cell carcinoma: Incidence, morphological features,
immunohistochemical profile, and biologic behavior: A single
institution. Pathol Res Pract. 210:234–241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhatnagar R and Alexiev BA: Renal-cell
carcinomas in end-stage kidneys: A clinicopathological study with
emphasis on clear-cell papillary renal-cell carcinoma and acquired
cystic kidney disease-associated carcinoma. Int J Surg Pathol.
20:19–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aron M, Chang E, Herrera L, Hes O, Hirsch
MS, Comperat E, Camparo P, Rao P, Picken M, Michal M, et al: Clear
cell-papillary renal cell carcinoma of the kidney not associated
with end-stage renal disease: Clinicopathologic correlation with
expanded immunophenotypic and molecular characterization of a large
cohort with emphasis on relationship with renal angiomyoadenomatous
tumor. Am J Surg Pathol. 39:873–888. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leroy X, Camparo P, Gnemmi V, Aubert S,
Flamand V, Roupret M, Fantoni JC and Compérat E: Clear cell
papillary renal cell carcinoma is an indolent and low-grade
neoplasm with overexpression of cyclin-D1. Histopathology.
64:1032–1036. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rao P, Monzon F, Jonasch E, Matin SF and
Tamboli P: Clear cell papillary renal cell carcinoma in patients
with von Hippel-Lindau syndrome-clinicopathological features and
comparative genomic analysis of 3 cases. Hum Pathol. 45:1966–1972.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gill S, Kauffman EC, Kandel S, George S,
Schwaab T and Xu B: Incidence of clear cell papillary renal cell
carcinoma in low-grade renal cell carcinoma cases: A 12-year
retrospective clinicopathologic study from a single cancer center.
Int J Surg Pathol. 24:207–212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sahni VA, Hirsch MS and Silverman SG:
Renal angiomyoadenomatous tumour: Imaging features. Can Urol Assoc
J. 6:E140–E143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shao T, Yousef P, Shipilova I, Saleeb R,
Lee JY and Krizova A: Clear cell papillary renal cell carcinoma as
part of histologically discordant multifocal renal cell carcinoma:
A case report and review of literature. Pathol Res Pract.
212:229–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi SS, Shen Q, Xia QY, Tu P, Shi QL, Zhou
XJ and Rao Q: Clear cell papillary renal cell carcinoma: A
clinicopathological study emphasizing ultrastructural features and
cytogenetic heterogeneity. Int J Clin Exp Pathol. 6:2936–2942.
2013.PubMed/NCBI
|
|
19
|
Deml KF, Schildhaus HU, Compérat E, von
Teichman A, Storz M, Schraml P, Bonventre JV, Fend F, Fleige B,
Nerlich A, et al: Clear cell papillary renal cell carcinoma and
renal angiomyoadenomatous tumor: Two variants of a morphologic,
immunohistochemical, and genetic distinct entity of renal cell
carcinoma. Am J Surg Pathol. 39:889–901. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan WX, Cao WR, Zhao J, Zhang W, Wang XL,
Yuan Q and Dang SQ: Clear cell papillary renal cell carcinoma: A
clinicopathologic analysis of 6 cases. Int J Clin Exp Pathol.
8:4595–4599. 2015.PubMed/NCBI
|
|
21
|
Lawrie CH, Larrea E, Larrinaga G,
Goicoechea I, Arestin M, Fernandez-Mercado M, Hes O, Cáceres F,
Manterola L and López JI: Targeted next-generation sequencing and
non-coding RNA expression analysis of clear cell papillary renal
cell carcinoma suggests distinct pathological mechanisms from other
renal tumour subtypes. J Pathol. 232:32–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Diolombi ML, Cheng L, Argani P and Epstein
JI: Do clear cell papillary renal cell carcinomas have malignant
potential? Am J Surg Pathol. 39:1621–1634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alexiev BA and Zou YS: Clear cell
papillary renal cell carcinoma: A chromosomal microarray analysis
of two cases using a novel Molecular Inversion Probe (MIP)
technology. Pathol Res Pract. 210:1049–1053. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fisher KE, Yin-Goen Q, Alexis D,
Sirintrapun JS, Harrison W, Benjamin Isett R, Rossi MR, Moreno CS,
Young AN and Osunkoya AO: Gene expression profiling of clear cell
papillary renal cell carcinoma: Comparison with clear cell renal
cell carcinoma and papillary renal cell carcinoma. Mod Pathol.
27:222–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Munari E, Marchionni L, Chitre A, Hayashi
M, Marrtignoni G, Brunelli M, Gobbo S, Arqani P, Allaf M, Hoque MO
and Netto GJ: Clear cell papillary renal cell carcinoma: micro-RNA
expression profiling and comparison with clear cell renal cell
carcinoma and papillary renal cell carcinoma. Hum Pathol.
45:1130–1138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alexiev BA, Thomas C and Zou YS: Clear
cell papillary renal cell carcinoma with angiomyomatous stroma: A
histological, immunohistochemical, and fluorescence in situ
hybridization study. Virchows Arch. 464:709–716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dhakal HP, Mckenney JK, Khor LY, Reynolds
JP, Magi-Galluzzi C and Przybycin CG: Renal neoplasms with
overlapping features of clear cell renal cell carcinoma and clear
cell papillary renal cell carcinoma: A clinicopathologic study of
37 cases from a single institution. Am J Surg Pathol. 40:141–154.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Delahunt B, Bethwaite PB, Thornton A and
Ribas JL: Proliferation of renal cell carcinoma assessed by
fixation-resistant polyclonal Ki-67 antibody labeling. Correlation
with clinical outcome. Cancer. 75:2714–2719. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chow WH, Gridley G, Fraumeni JF Jr and
Järvholm B: Obesity, hypertension, and the risk of kidney cancer in
men. New Engl J Med. 343:1305–1311. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sanfilippo KM, Mctigue KM, Fidler CJ,
Neaton JD, Chang Y, Fried LF, Liu S and Kuller LH: Hypertension and
obesity and the risk of kidney cancer in 2 large cohorts of US men
and women. Hypertension. 63:934–941. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khandekar MJ, Cohen P and Spiegelman BM:
Molecular mechanisms of cancer development in obesity. Nat Rev
Cancer. 11:886–895. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shoji K, Tanaka T and Nangaku M: Role of
hypoxia in progressive chronic kidney disease and implications for
therapy. Curr Opin Nephrol Hypertens. 23:161–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kabaria R, Klaassen Z and Terris MK: Renal
cell carcinoma: Links and risks. Int J Nephrol Renovasc Dis.
9:45–52. 2016.PubMed/NCBI
|
|
34
|
Weikert S, Boeing H, Pischon T, Weikert C,
Olsen A, Tjonneland A, Overvad K, Becker N, Linseisen J,
Trichopoulou A, et al: Blood pressure and risk of renal cell
carcinoma in the European prospective investigation into cancer and
nutrition. Am J Epidemiol. 167:438–446. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kuroda N, Ohe C, Kawakami F, Mikami S,
Furuya M, Matsuura K, Moriyama M, Nagashima Y, Zhou M, Petersson F,
et al: Clear cell papillary renal cell carcinoma: A review. Int J
Clin Exp Pathol. 7:7312–7318. 2014.PubMed/NCBI
|
|
36
|
Pramick M, Ziober A and Bing Z: Useful
immunohistochemical panel for differentiating clear cell papillary
renal cell carcinoma from its mimics. Ann Diagn Pathol. 17:437–440.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gayed BA, Youssef RF, Bagrodia A, Darwish
OM, Kapur P, Sagalowsky A, Lotan Y and Margulis V: Ki67 is an
independent predictor of oncological outcomes in patients with
localized clear-cell renal cell carcinoma. BJU Int. 113:668–673.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gerdes J: Ki-67 and other proliferation
markers useful for immunohistological diagnostic and prognostic
evaluations in human malignancies. Semin Cancer Biol. 1:199–206.
1990.PubMed/NCBI
|
|
39
|
Young JR, Margolis D, Sauk S, Pantuck AJ,
Sayre J and Raman SS: Clear cell renal cell carcinoma:
Discrimination from other renal cell carcinoma subtypes and
oncocytoma at multiphasic multidetector CT. Radiology. 267:444–453.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang J, Lefkowitz RA, Ishill NM, Wang L,
Moskowitz CS, Russo P, Eisenberg H and Hricak H: Solid renal
cortical tumors: Differentiation with CT. Radiology. 244:494–504.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tsuda K, Kinouchi T, Tanikawa G, Yasuhara
Y, Yanagawa M, Kakimoto K, Ono Y, Meguro N, Maeda O, Arisawa J and
Usami M: Imaging characteristics of papillary renal cell carcinoma
by computed tomography scan and magnetic resonance imaging. Int J
Urol. 12:795–800. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim JM, Song PH, Kim HT and Park TC: The
prognostic factors for patients with pT1a renal cell carcinoma.
Korean J Urol. 51:233–238. 2010. View Article : Google Scholar : PubMed/NCBI
|