Introduction
Neuroblastoma (NB) is among the most common types of
solid tumor in children (1). NB has a
high degree of malignancy, a high mortality rate and a poor
prognosis, posing diagnostic and therapeutic challenges for
pediatricians (2,3). Spontaneous remission occurs in the
majority of infants with NB (3,4). However,
the disease may be disseminated to the liver, skin and bone marrow
at diagnosis (1). The survival rate
of infants with stage 4s NB >95% (2,3). Children
with high-risk NB are resistant to intensive therapy, and the
prognosis for recurrent or metastatic disease is poor, with a
5-year survival rate of <30% (5).
Therefore, the identification of effective and efficient targeted
therapies is required to improve the prognosis of patients with
NB.
It has been reported that MYCN gene amplification
occurs in 25–30% of NB cases, and that it is a predictive biomarker
of a poor prognosis and tumor aggressiveness (3). Eg5, also known as kinesin-like spindle
protein, is a member of the mitotic kinesin superfamily, which
modulates microtubule tracks for intracellular transport or cell
division (6). A number of Eg5
inhibitors have undergone clinical testing as antimitotic drugs
(7–11). Minstrel and its analogs, as well as
other chemically distinct small molecules, including S-trityl
L-cysteine (STLC), are allosteric inhibitors that bind to a unique
pocket in the Eg5 motor domain formed by secondary structural
elements (helix a2/loop L5/helix a3) (12,13).
In the present study, Eg5 expression was examined in
human NB SK-N-SH, SH-SY5Y and SK-N-BE2 cell lines, and tissue
specimens from patients with NB using immunofluorescence and
western blotting. It has been reported that the antitumor activity
of Eg5 inhibitors depends on cell cycle arrest and the promotion of
cell apoptosis (7). However, the
underlying molecular mechanisms remain to be elucidated.
Materials and methods
Materials
The human NB SH-SY5Y (SY5Y), SK-N-SH (SK) and
SK-N-BE2 (BE2) cell lines were purchased from The Cell Bank of Type
Culture Collection of Chinese Academy of Sciences (Shanghai,
China). The cells were maintained in a humidified atmosphere
containing 5% CO2 and 95% air at 37°C in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.). The anti-Eg5 (cat.
no. ab51976; 1:400) and anti-β-actin (ab6276 1;250) primary
antibodies were obtained from Abcam (Cambridge, UK). The goat
anti-mouse Alexa Fluor 647 (cat. no. ab150119; 1:250) was purchased
from Jackson ImmunoResearch Europe, Ltd. (Newmarket, UK). STLC was
obtained from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). The
apoptosis detection kit [BD Pharmingen-fluorescein isothiocyanate
(FITC) Annexin V Apoptosis Detection kit; cat no. 556547] and Cell
Cycle kit (BD Cycletest™ Plus; cat no. 340242) were purchased from
BD Biosciences (Franklin Lakes, NJ, USA).
Specimen information
The Institutional Review Board of Shanghai
Children's Hospital (Shanghai, China) approved the study protocol
and waived the requirement for informed consent (2015-02-11, no.
1). The NB and GNB tissue specimens were collected from 3 patients
with NB or GNB (16.7±6.43 months). In order to verify the
expression in different tissues, the experiments were also
performed in normal tissues without NB, including renal (collected
from patient with Wilms' tumor upon nephrectomy) and liver
(collected from patients with Choledochal cysts). The control
tissues were obtained from the Pathology Department of Shanghai
Children's Hospital (Shanghai China), and were retrospectively
analyzed. All diagnoses were confirmed pathologically. Clinical
information is reported in Table
I.
 | Table I.Clinical information of tissue
specimens. |
Table I.
Clinical information of tissue
specimens.
| Name | Age at the time of
diagnosis (months) | Sex | Diagnosis | Date of
collection | Pathology | Tissues |
|---|
| NB1 | 12 | Male | NB III | June, 2014 | NB | Tumor |
| NB2 | 14 | Female | NB III | August, 2015 | NB | Tumor |
| GNB | 24 | Male | GNB II | October, 2014 | GNB | Tumor |
| Liver
intestine | 22 | Male | Choledochal
cyst | October 2015 |
Choledochalcyst | Normal liver
intestine |
| Renal tubule
Glomerulus | 9 | Male | Wilms' tumor | November, 2015 | Wilms'tumor | Normal renal
tissue |
Immunofluorescence
Immunofluorescence staining was utilized to detect
the expression of Eg5 in NB cell lines. Cells were plated onto
glass coverslips and allowed to attach in order to obtain a
sufficient number of cells to perform immunofluorescence. SY5Y, SK
and BE2 cells were fixed with 3% paraformaldehyde and permeabilized
with 0.15% Triton X-100 for 12 h at 4°C. The primary antibodies
(1:200) were incubated with the slides at room temperature for 1 h.
An Alexa Fluor 488 secondary antibody was added for 45 min at room
temperature. DAPI (1 µg/ml; Beyotime Institute of Biotechnology,
Haimen, China) was used to stain the nuclei for 20 min at room
temperature.
Western blotting
SY5Y, SK or BE2 cells were lysed using
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.) containing protease and phosphatase inhibitors (Thermo Fisher
Scientific, Inc.). Protein concentration was measured using a
bicinchoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.), and equal amounts (30 µg) of protein from each cell lysate
were solubilized in 2X SDS loading buffer (Beyotime Institute of
Biotechnology, Haimen, China), then separated via 15% SDS-PAGE and
transferred into nitrocellulose membranes. The membranes were
blocked in 5% bovine serum albumin (Gibco; Thermo Fisher
Scientific, Inc.), and then probed with anti-Eg5 (1:400; cat no.
ab51976; Abcam, Cambridge, USA) and β-actin (1:5,000; AF003;
Beyotime Institute of Biotechnology) overnight at 4°C and
subsequent hybridization with a HRP-conjugated goat anti-mouse
secondary antibody (1:500; cat. no. 115035003; Jackson
ImmunoResearch Europe, Ltd.) for 1 h at room temperature. Following
3 washes with Tris-buffered saline with Tween, the membranes were
scanned using aLAS-4000 Mini system (FujiFilm, Tokyo, Japan).
Flow cytometric analysis of
apoptosis
Cells were seeded into a 12-well plate and treated
with 0, 1, 5, 10 or 20 µmol/l STLC for 72 h. The cells were then
harvested by trypsin and washed with PBS. The cells were stained
with 5 µl Annexin V and 5 µl 7-AAD1 h at 37°C using a BD
Pharmingen-FITC Annexin V Apoptosis Detection kit (cat. no. 556547;
BD Biosciences), according to the manufacturer's protocol.
Apoptotic cells were analyzed using a BD Fortessa flow cytometer
(BD Biosciences). The data were analyzed using FlowJo software
LLCv.10 (Ashland, OR, USA).
Flow cytometric analysis of cell
cycle
A total of 3×104 cells were seeded into a
12-well plate and treated with 1, 5, 10 or 20 µmol/l STLC for 72 h.
The cells were then harvested and washed with PBS. The cells were
fixed and treated with 10% propidium iodide at 37°C 2 h (BD
Biosciences), according to the manufacturer's protocol. Cells were
analyzed using a BD Fortessa flow cytometer (BD Biosciences). The
percentage of cells in the G1, S and G2-M phases was calculated.
All experiments were performed in triplicate. The data were
analyzed using FlowJo software LLCv.10 (Ashland, OR, SA).
Fluorescence in situ hybridization
(FISH)
A total of 3×104 cells (SK, SY5Y and BE2)
were seeded into a 6-well plate. The cells were then harvested by
trypsin and washed with PBS. BE2 were seeded into a 6-well plate
treated with 1 µmol/l or 5 µmol/l STLC for 48 h. Vysis LIS N-MYC SO
Probe (Abbott Pharmaceutical Co., Ltd., Lake Bluff, IL, USA) with
its corresponding hybridization buffer was removed, 1 µl probe, 2
µl ddH2O and 37 µl hybridization buffer were added to
make up a final volume of 40 µl. The solution was microcentrifuged
for 2 sec at 37°C at 550 × g, 10 µl probe was added onto the slide
and a square glass coverslip was added for 2 min at 72°C. The
solution was placed into a humidified chamber at 37°C overnight. A
coplin jar containing 40 ml 0.4X SSC was placed in a 75°C water
bath for 30 min. A total of 40 ml 2X SSC/0.1% NP40 was added to
another coplin jar at room temperature. Slides were removed from
the incubation chamber and rubber cement and coverslips from the
first 4 slides were removed. The slides were placed directly into
2X SSC/0.1% NP40 for 1 min at 37°C. Slides were removed and dried
off using a paper towel. Slides were air dried in the dark. A total
of 10 µl DAPI II was applied at room temperature for 1 h, prior to
being transferred to a 24×50 mm coverslip. Epi-fluorescence
microscopy was then used at ×1,000 magnification.
mRNA microarray analysis
SY5Y were seeded into a 6-well plate treated with 0
or 5 µmol/l STLC for 72 h at 37°C, the cells were then harvested by
trypsin and washed twice with PBS. Total RNA was isolated using
TRIzol® reagent (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocols, and purified using an
RNeasy Mini kit (Qiagen GmbH, Hilden, Germany). RNA samples were
processed for array hybridization using the GeneChip one-cycle
target labeling kit (Affymetrix, Inc, Santa Clara, CA, USA) for
amplification and labeling of total RNA. Target synthesis was
performed following the Affymetrix GeneChip Expression Analysis
Technical Manual, rev. 5 (http://www.affymetrix.com/support/technical/manual/expression_manual.affx)
with minor modification. Using 2–4 µg total RNA as an input, mRNA
was converted to double-stranded cDNA and purified by
phenol-chloroform-isoamyl alcohol extraction and ethanol
precipitation to generate biotinylated cRNA targets and then
biotinylated cRNA targets for the GeneChip®
PrimeView™/U133 plus 2.0 Human Gene Expression Array. The
biotinylated cRNA targets were then hybridized with the microarray.
Following hybridization, arrays were stained with Cy5-dCTP in the
Fluidics Station 450 at 45°C for 16 h and scanned on the Affymetrix
Scanner 3000. The microarray experiments were performed according
to the protocol of Affymetrix (Thermo Fisher Scientific, Inc.). The
raw data were normalized with the MAS 5.0/RMA algorithm to the
control group using the Gene Spring Software 11.0 (Agilent
Technologies, Inc., Santa Clara, CA, USA). Genes with a fold change
of >2 were selected for further analysis.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA,
USA). Data are expressed as the mean ± standard deviation.
Comparisons of the means of 2 groups were conducted using Student's
t-test. Comparisons of the means of ≥3 groups were conducted using
one-way analysis of variance. Comparisons between the groups was
made by analyzing data with Student-Newman-Keuls post hoc method.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression level of MYC oncogene
(MYCN) in NB cells
Previous studies have demonstrated that
amplification and overexpression of MYCN is associated with a poor
prognosis in NB (2,3). To investigate and confirm the expression
of MYCN in SK, SY5Y and BE2 cell lines, MYCN gene amplification was
detected by fluorescence in situ hybridization (FISH). MYCN
gene amplification was identified in BE2 cells (Fig. 1).
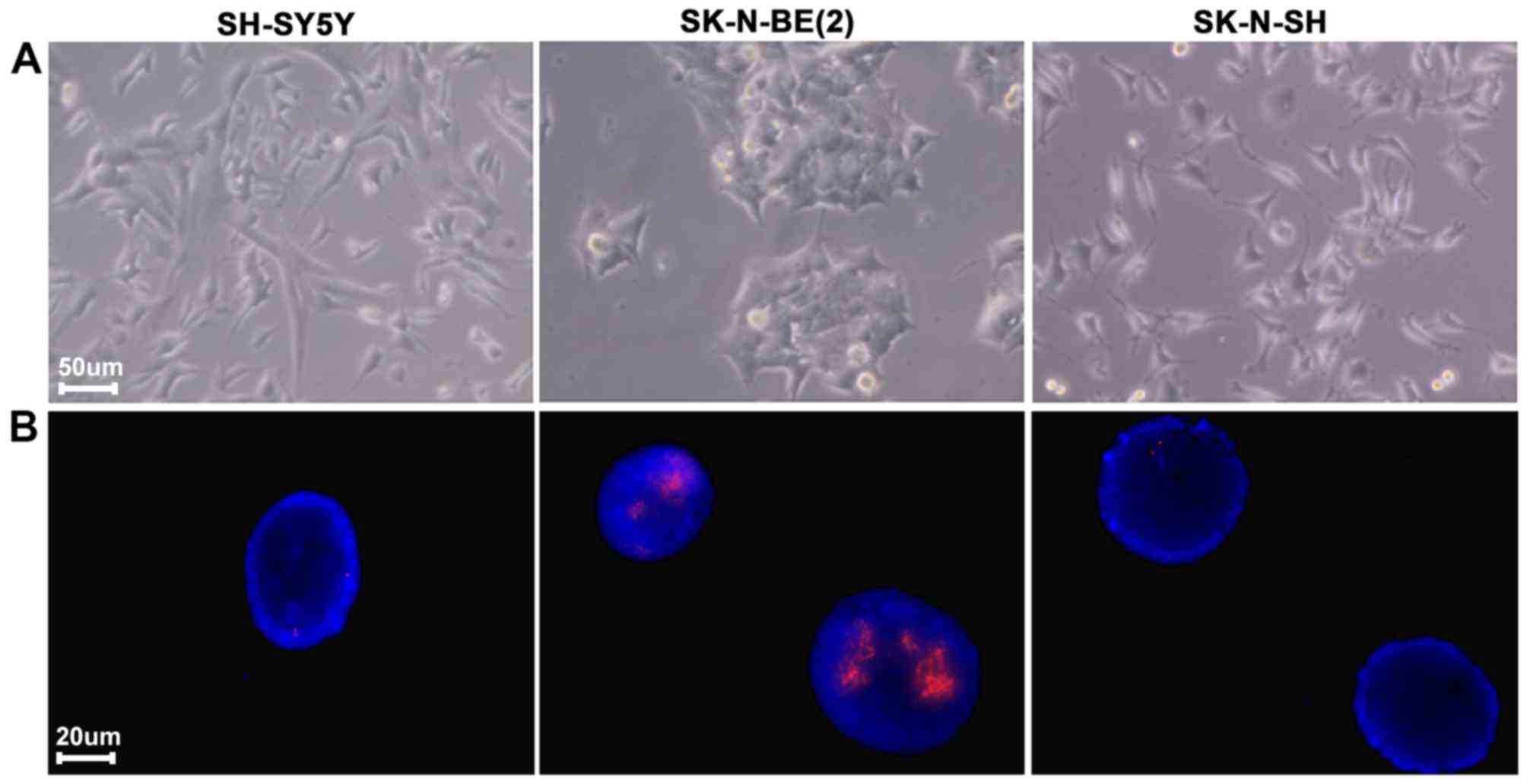
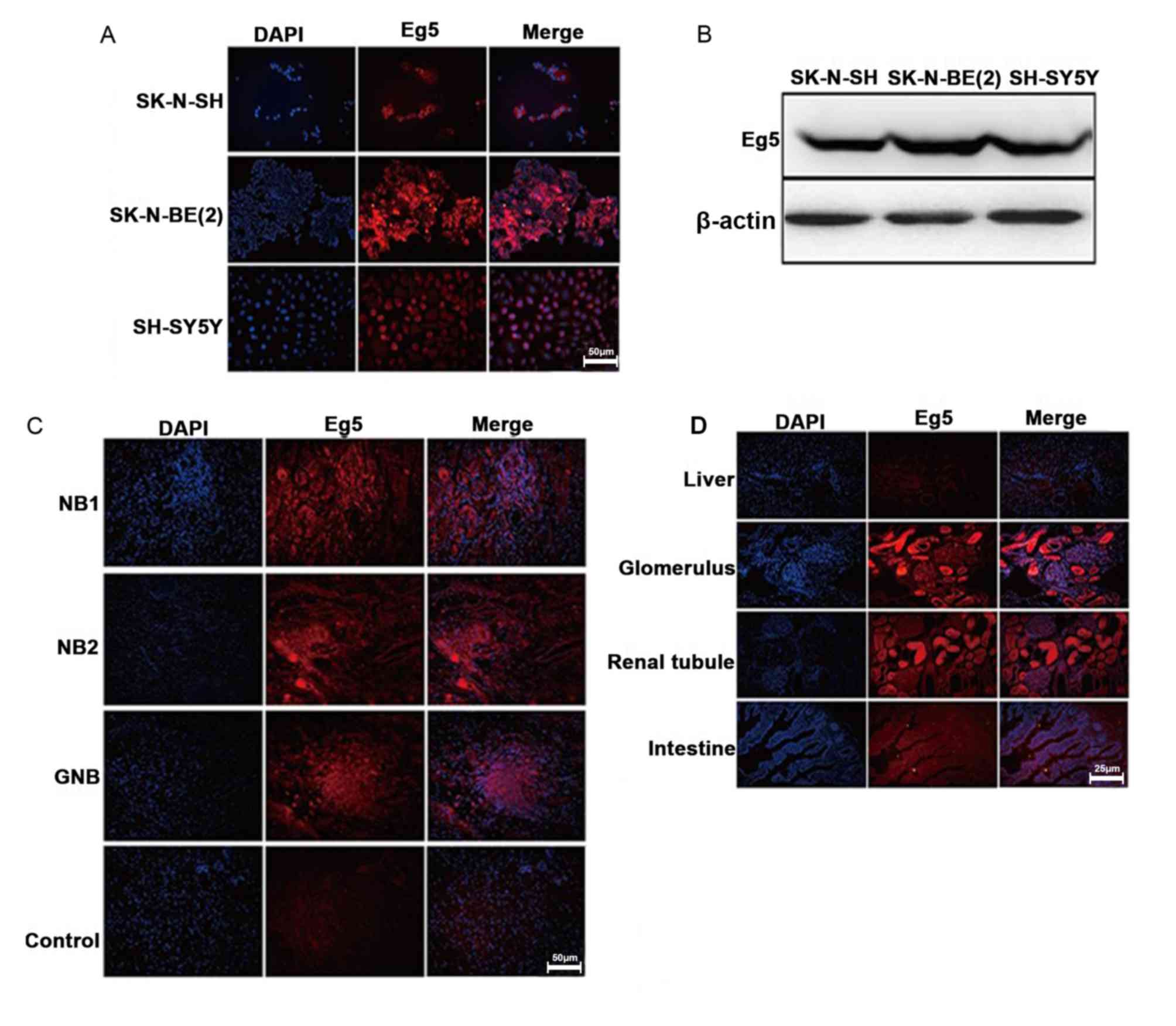
Eg5 in NB tissues specimens and cell
lines
Eg5 is expressed in the testis, thymus, tonsils and
bone marrow and is absent from the adult human central nervous
system. Eg5 is overexpressed in breast, lung, ovarian, bladder and
pancreatic cancer. Previous studies have demonstrated that Eg5 is
of central importance in driving the assembly of the mitotic
spindle, and is also a promising chemotherapeutic target.
Inhibition of Eg5 by small molecule inhibitors results in monopolar
spindles and mitotic arrest, which may lead to cell death (14,15).
However, to the best of our knowledge, no previous studies have
reported whether Eg5 is expressed in NB. Therefore, in the present
study, the expression level of Eg5 was determined in SK, SY5Y and
BE2 cell lines (Fig. 2A and B). Eg5
protein expression in NB and NGB tissues was observed using
immunofluorescence (Fig. 2C). Eg5
staining was also observed in the glomerulus but not in liver,
renal tubule or intestine tissues (Fig.
2D).
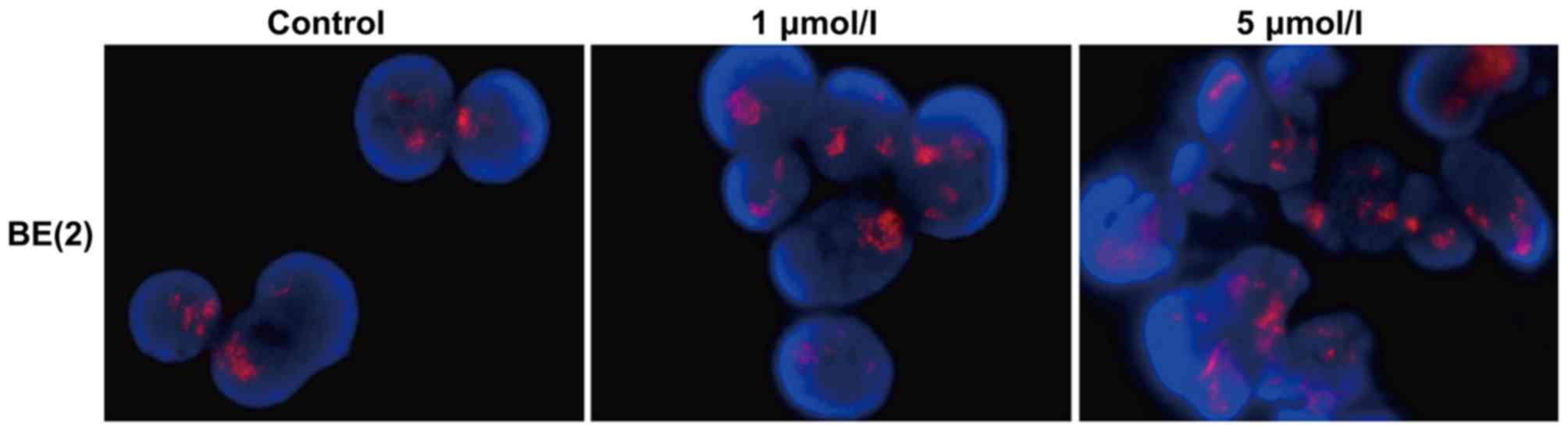
STLC has no effect on MYCN gene
amplification and expression
As a selective allosteric inhibitor of Eg5, STLC
blocked the bipolar spindle formation that causes mitotic arrest
and ultimately leads to apoptotic cell death (7). In order to investigate whether STLC
affects MYCN expression, the BE2 cells were treated in the absence
or presence of the inhibitor (1 and 5 µmol/l STLC for 48 h due to
the differences observed at these concentrations). STLC treatment
did not affect MYCN amplification (Fig.
3), suggesting that the antitumor activity of STLC was not
dependent upon MYCN amplification.
STLC inhibits the expression of Eg5
protein and promotes cell apoptosis and cell cycle arrest
STLC is known to cause cell death and inhibit cell
proliferation in solid tumors (11).
The present study aimed to determine whether STLC may induce
apoptosis and cell cycle arrest in NB cells. The percentage of
apoptotic cells in the SY5Y and BE2 cell lines increased with
increased STLC concentrations (Fig.
4A). Compared with the untreated control group, the change in
the percentage of apoptotic cells was notable at 5 µmol/l STLC.
Flow cytometric analysis indicated that STLC treatment induced cell
cycle arrest at G2/M phase (Fig. 4B).
In accordance with the cell apoptosis results, the population shift
in cell cycle arrest from G1 phase to G2/M phase was notable at 5
µmol/l STLC. The expression of Eg5 decreased in a dose-dependent
manner in response to STLC treatment (Fig. 4C) with no marked differences between
the 48 and 72 h time points.
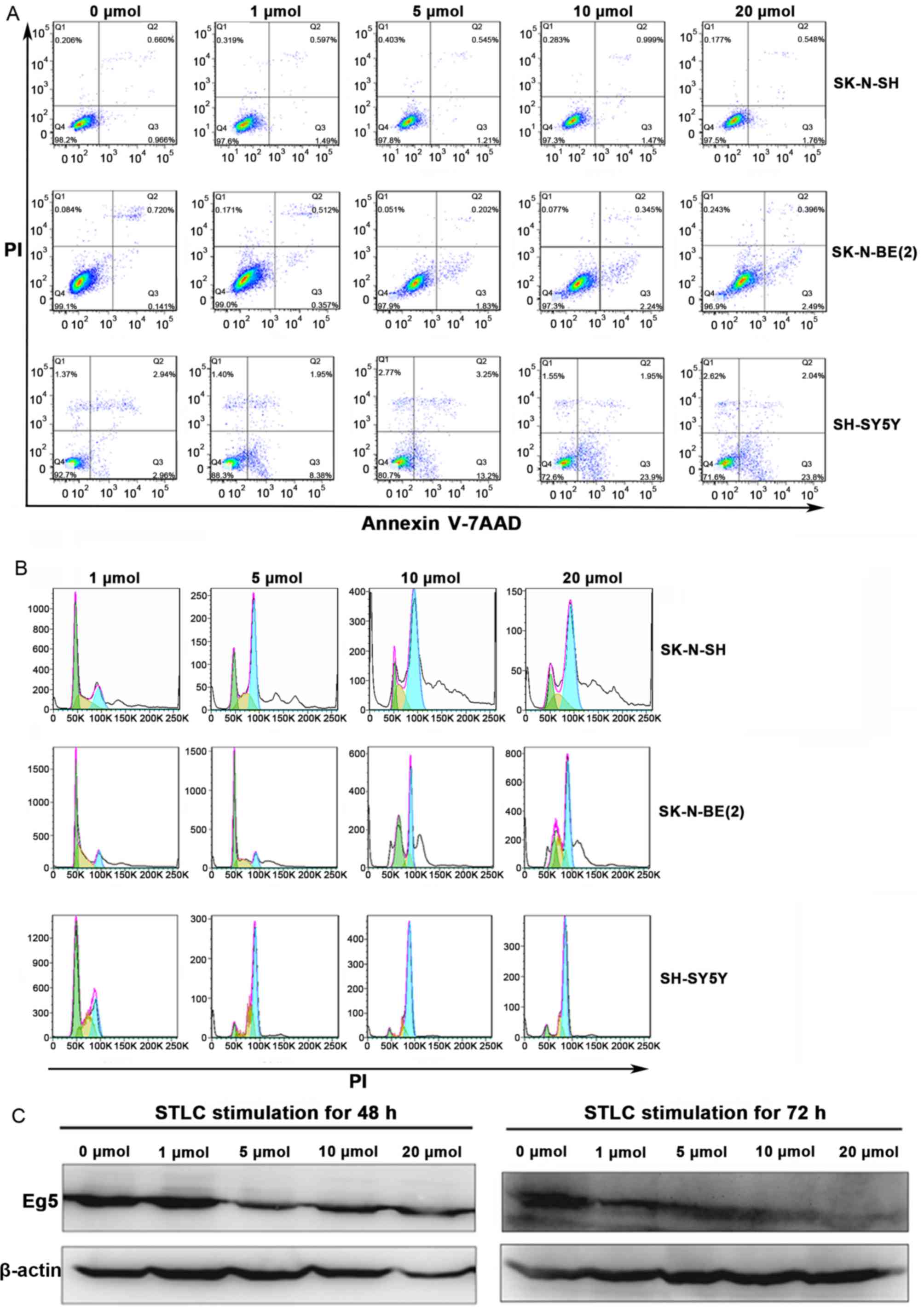 | Figure 4.The effect of STLC treatment on the
expression of Eg5, cell apoptosis and cell cycle progression in NB.
SK, SY5Y and BE2 cells were treated with 0, 1, 5, 10 and 20 mmol
STLC for 72 h. (A) Flow cytometric analysis of the cell apoptosis.
The percentage of apoptotic cells was dose-dependent in the NB
cells. (B) The effect of STLC on the progression of the cell cycle
in NB cells. The amplitude of curves corresponds to the cell
number, the peak on the left (green) represents cells in the G1
phase of the cell cycle, while the peak on the right (blue)
represents cells in the G2/M phase. Compared with cells treated
with1 mmol STLC, the percentage of G2/M phase was significantly
increased in higher concentrations. (C) Western blotting revealed
that STLC treatment of SY5Y cells for 48 and 72 h resulted in a
decrease of Eg5 expression. STLC, S-trityl-L-cysteine; NB,
neuroblastoma; PI, propidium iodine. |
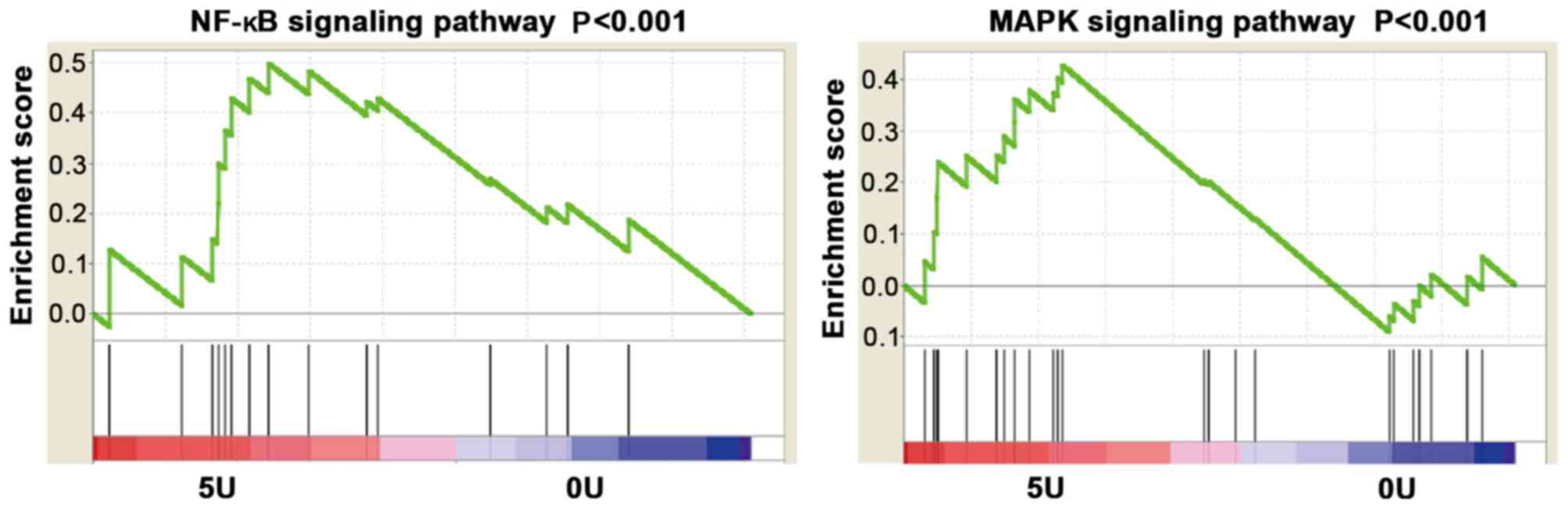
mRNA microarray analysis
GSEA analysis demonstrated that STLC regulates MAPK
and NF-κB signaling pathways. The results demonstrated that the
MAPK and NF-κB signaling pathways were activated in cells treated
with 5 µmol/l STLC compared with the control group (Fig. 5).
Discussion
NB is a pediatric disease, which arises from
sympatho-adrenergic neural crest progenitor cells of the peripheral
nervous system (16,17). Due to the high risk and recurrence
rate of advanced NB, and the lack of specific biomarkers, there are
currently no efficient treatment approaches for neuroblastoma
(18). Methods for predicting patient
response to chemotherapy are being developed (3). The present study aimed to identify the
signaling pathways associated with STLC.
Previous research has suggested that promoting cell
apoptosis and cell cycle progression arrest may be an important
molecular mechanism of antitumor activity (17,18). In
the present study, three NB cell lines (SY5Y, SK and BE2) were used
to examine STLC-induced cell apoptosis and cell cycle
progression.
As a kinesin spindle protein, Eg5 is involved in
mitosis and its inhibition promotes mitotic arrest (19,20). Eg5
inhibitors are effective compared with anti-tubulin drugs, which
have dose-limiting side effects (21). Eg5 does not serve a role in resting
and non-dividing cells (13).
Beneficial effects of suppression of Eg5 function/expression have
been suggested in breast, lung, colon, ovary and prostate cancer
in vitro (22–24).
Numerous inhibitors of Eg5 have been discovered,
including monastrol, S-trityl-L-cysteine (STLC) and ispinesib
(7). These inhibitors bind an
allosteric site located between a helix 3 and loop 5 of the Eg5
domain (13). Although Eg5 inhibitors
have reached pre-clinical dose-limiting toxicity trials, the
molecular mechanism underlying the antitumor activity has not been
elucidated (25). EMD 534085, a
potent, reversible Eg5 inhibitor, has demonstrated significant
preclinical antitumor activity (10).
Previous studies reported that a selective inhibitor, LY2523355,
arrests cancer cells in mitosis and causes rapid cell death
(8,10,26).
Previous research has evaluated the function of STLC in prostate
cancer, indicating that docetaxel-resistant prostate cancer cells
remain responsive to Eg5 inhibition in response to STLC treatment
(12,27). STLC has been demonstrated to exhibit
higher potency compared with monastrol or terpendole E in inducing
mitotic arrest (28).
In the present study, the anticancer activity of
STLC was characterized in NB. Eg5 was demonstrated to be
overexpressed in NB tissue specimens and cell lines. It was also
observed that the expression level of Eg5 was increased in BE2
cells exhibiting MYCN amplification compared with SY5Y and SK
cells. Therefore, it was hypothesized that the expression of MYCN,
an essential regulator of cancer progression (3), is associated with the antitumor activity
of STLC. However, immunofluorescence analysis revealed that STLC
did not affect the expression of MYCN. To further investigate
whether STLC exhibits antitumor activity in NB, cell apoptosis and
cell cycle progression was analyzed. Cell cycle analysis
demonstrated that STLC treatment resulted in a dose-dependent
increase in the number of cells with polyploidy DNA content, and
that the cell cycle was arrested at G2/M phase. Cell apoptosis
analysis revealed that STLC induced cell apoptosis in a
dose-dependent manner.
Centrosome separation is stringently controlled by
mitotic kinases, including cyclin-dependent kinase 1 (Cdk1),
polo-like kinase 1, Aurora A and mitosis gene-A-related kinase 2.
However, the molecular mechanism by which these kinases contribute
toward the activity of Eg5 inhibitors remains unknown (29,30).
Previous studies have identified that Cdk1 triggers centrosome
separation in the late G2 phase by phosphorylating the motor
protein Eg5 at Thr927 (31,32). In the present study, a robust increase
in the percentage of apoptotic and G2/M-phase DNA content was
observed in response to 5 µmol/l STLC compared with control cells.
At the transcriptional level, a significant activation of the NF-κB
and MAPK pathways was identified at 5 µmol/l STLC compared with
control cells. Neuronal differentiation in early development
involves neuronal fate determination and a series of morphological
changes, including neurite initiation, extension, and maturation of
axons and dendrites (33). During
neurite growth, multiple signaling pathways are activated,
including the MAPK and PI3K-Akt pathways (34). Whether or not the activation of NF-κB
and MAPK pathways in response to the STLC treatment was associated
with neuronal differentiation remains to be elucidated.
To investigate the possible signaling pathways
associated with STLC-induced apoptosis, the activity, expression
and localization of the caspase 3/8 were analyzed. It has been
previously investigated how anti-mitotic drugs cause apoptosis. A
number of studies have suggested that the effectiveness of the
spindle checkpoint is the primary determinant in response to
taxanes and other anti-mitotic drugs (35–37).
Future studies may involve determining the apoptotic signaling
pathway by mRNA expression profiling in order to confirm which
genes or targets from these signaling pathways significantly affect
cell cycle progression.
To the best of our knowledge, the present study was
the first to demonstrate cell apoptosis and cell cycle arrest in NB
cell lines induced by STLC. The investigation of the NF-κB and MAPK
signaling pathways provides a basis for elucidating the molecular
mechanism of STLC antitumor, as well as for the development of
therapeutic strategies targeting specific biomarkers in NB.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Shanghai Science and Technology Committee (grant no.
12DZ2295006).
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
WW and SJ conducted experiments, acquired and
analysed the datasets. WX and JL were responsible for tissue
collection. QS and YH contributed toward analysing and interpreting
datasets. ZL conceived and designed the study, secured the funding
and gave final approval of the version to be published. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The Institutional Review Board of Shanghai
Children's Hospital (Shanghai, China) approved the study protocol
and waived the requirement for informed consent (2015-02-11,
approval no. 1).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hoy SM: Dinutuximab: A review in high-risk
neuroblastoma. Target Oncol. 11:247–253. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brodeur GM: Neuroblastoma: Biological
insights into a clinical enigma. Nat Rev Cancer. 3:203–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sano H, Bonadio J, Gerbing RB, London WB,
Matthay KK, Lukens JN and Shimada H: International neuroblastoma
pathology classification adds independent prognostic information
beyond the prognostic contribution of age. Eur J Cancer.
42:1113–1119. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kraal K, Blom T, Tytgat L, van Santen H,
van Noesel M, Smets A, Bramer J, Caron H, Kremer L and van der Pal
H: Neuroblastoma with intraspinal extension: Health problems in
long-tterm survivors. Pediatr Blood Cancer. 63:990–996. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gigliotti AR, de Ioris MA, de Grandis E,
Podda M, Cellini M, Sorrentino S, de Bernardi B, Paladini D and
Gandolfo C: Congenital neuroblastoma with symptoms of epidural
compression at birth. Pediatr Hematol Oncol. 33:94–101. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yokoyama H, Sawada J, Katoh S, Matsuno K,
Ogo N, Ishikawa Y, Hashimoto H, Fujii S and Asai A: Structural
basis of new allosteric inhibition in kinesin spindle protein Eg5.
ACS Chem Boil. 10:1128–1136. 2015. View Article : Google Scholar
|
|
7
|
El-Nassan HB: Advances in the discovery of
kinesin spindle protein (Eg5) inhibitors as antitumor agents. Eur J
Med Chem. 62:614–631. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wakui H, Yamamoto N, Nakamichi S, Tamura
Y, Nokihara H, Yamada Y and Tamura T: Phase 1 and dose-finding
study of patritumab (U3-1287), a human monoclonal antibody
targeting HER3, in Japanese patients with advanced solid tumors.
Cancer Chemother Pharmacol. 73:511–516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gerecitano JF, Stephenson JJ, Lewis NL,
Osmukhina A, Li J, Wu K, You Z, Huszar D, Skolnik JM and Schwartz
GK: A Phase I trial of the kinesin spindle protein (Eg5) inhibitor
AZD4877 in patients with solid and lymphoid malignancies. Invest
New Drugs. 31:355–362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hollebecque A, Deutsch E, Massard C,
Gomez-Roca C, Bahleda R, Ribrag V, Bourgier C, Lazar V, Lacroix L,
Gazzah A, et al: A phase I, dose-escalation study of the
Eg5-inhibitor EMD 534085 in patients with advanced solid tumors or
lymphoma. Invest New Drugs. 31:1530–1538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishikawa K, Tohyama K, Mitsuhashi S and
Maruta S: Photocontrol of the mitotic kinesin Eg5 using a novel
S-trityl-l-cysteine analogue as a photochromic inhibitor. J
Biochem. 155:257–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaan HY, Weiss J, Menger D, Ulaganathan V,
Tkocz K, Laggner C, Popowycz F, Joseph B and Kozielski F:
Structure-activity relationship and multidrug resistance study of
new S-trityl-L-cysteine derivatives as inhibitors of Eg5. J Med
Chem. 54:1576–1586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishikawa K, Tamura Y and Maruta S:
Photocontrol of mitotic kinesin Eg5 facilitated by thiol-reactive
photochromic molecules incorporated into the loop L5 functional
loop. J Biochem. 155:195–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun D, Lu J, Ding K, Bi D, Niu Z, Cao Q,
Zhang J and Ding S: The expression of Eg5 predicts a poor outcome
for patients with renal cell carcinoma. Med Oncol. 30:4762013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Muretta JM, Jun Y, Gross SP, Major J,
Thomas DD and Rosenfeld SS: The structural kinetics of switch-1 and
the neck linker explain the functions of kinesin-1 and Eg5. Proc
Natl Acad Sci USA. 112:E6606–E6613. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ngan ES: Heterogeneity of neuroblastoma.
Oncoscience. 2:837–838. 2015.PubMed/NCBI
|
|
17
|
Mei H, Lin ZY and Tong QS: Risk
stratification and therapeutics of neuroblastoma: The challenges
remain. World J Pediatr. 12:5–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alderton GK: Neuroblastoma: Enhancing
risk. Nat Rev Cancer. 16:52016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Exertier P, Javerzat S, Wang B, Franco M,
Herbert J, Platonova N, Winandy M, Pujol N, Nivelles O, Ormenese S,
et al: Impaired angiogenesis and tumor development by inhibition of
the mitotic kinesinEg5. Oncotarget. 4:2302–2316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salmela AL and Kallio MJ: Mitosis as an
anti-cancer drug target. Chromosoma. 122:431–449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun L, Lu J, Niu Z, Ding K, Bi D, Liu S,
Li J, Wu F, Zhang H, Zhao Z and Ding S: A potent chemotherapeutic
strategy with Eg5 inhibitor against gemcitabine resistant bladder
cancer. PLoS One. 10:e01444842015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Good JA, Wang F, Rath O, Kaan HY,
Talapatra SK, Podgórski D, MacKay SP and Kozielski F: Optimized
S-trityl-L-cysteine-based inhibitors of kinesin spindle protein
with potent in vivo antitumor activity in lung cancer
xenograftmodels. J Med Chem. 56:1878–1893. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu G, Xu Z and Hao D: MicroRNA-451
inhibits neuroblastoma proliferation, invasion and migration by
targeting macrophage migration inhibitory factor. Mol Med Rep.
13:2253–2260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stafman LL and Beierle EA: Cell
proliferation in neuroblastoma. Cancers (Basel). 8:E132016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abualhasan MN, Good JA, Wittayanarakul K,
Anthony NG, Berretta G, Rath O, Kozielski F, Sutcliffe OB and
Mackay SP: Doing the methylene shuffle-Further insights into the
inhibition of mitotic kinesin Eg5 with S-trityl l-cysteine. Eur J
Med Chem. 54:483–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ye XS, Fan L, van Horn RD, Nakai R, Ohta
Y, Akinaga S, Murakata C, Yamashita Y, Yin T, Credille KM, et al: A
novel Eg5 Inhibitor (LY2523355) causes mitotic arrest and apoptosis
in cancer cells and shows potent antitumor activity in xenograft
tumor models. Mol Cancer Ther. 14:2463–2472. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Johnson K, Moriarty C, Tania N, Ortman A,
DiPietrantonio K, Edens B, Eisenman J, Ok D, Krikorian S, Barragan
J, et al: Kif11 dependent cell cycle progression in radial glial
cells is required for proper neurogenesis in the zebrafish neural
tube. Dev Boil. 387:73–92. 2014. View Article : Google Scholar
|
|
28
|
Wiltshire C, Singh BL, Stockley J, Fleming
J, Doyle B, Barnetson R, Robson CN, Kozielski F and Leung HY:
Docetaxel-resistant prostate cancer cells remain sensitive to
S-trityl-l-cysteine-Mediated Eg5 inhibition. Mol Cancer Ther.
9:1730–1739. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen C, Tian F, Lu L, Wang Y, Xiao Z, Yu C
and Yu X: Characterization of Cep85-a new antagonist of Nek2A that
is involved in the regulation of centrosome disjunction. J Cell
Sci. 128:3290–3303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bruinsma W, Aprelia M, Kool J, Macurek L,
Lindqvist A and Medema RH: Spatial separation of Plk1
phosphorylation and activity. Front Oncol. 5:1322015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Smith E, Hégarat N, Vesely C, Roseboom I,
Larch C, Streicher H, Straatman K, Flynn H, Skehel M, Hirota T, et
al: Differential control of Eg5-dependent centrosome separation by
Plk1 and Cdk1. EMBO J. 30:2233–2245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cahu J, Olichon A, Hentrich C, Schek H,
Drinjakovic J, Zhang C, Doherty-Kirby A, Lajoie G and Surrey T:
Phosphorylation by Cdk1 increases the binding of Eg5 to
microtubules in vitro and in Xenopus egg extract spindles. PLoS
One. 3:e39362008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baas PW and Matamoros AJ: Inhibition of
kinesin-5 improves regeneration of injured axons by a novel
microtubule-based mechanism. Neural Regen Res. 10:8452015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ou XH, Li S, Xu BZ, Wang ZB, Quan S, Li M,
Zhang QH, Ouyang YC, Schatten H, Xing FQ and Sun QY: p38α MAPK is a
MTOC-associated protein regulating spindle assembly, spindle length
and accurate chromosome segregation during mouse oocyte meiotic
maturation. Cell Cycle. 9:4130–4143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Srivastava V and Lee H: Synthesis and
bio-evaluation of novel quinolino-stilbene derivatives as potential
anticancer agents. Bioorg Med Chem. 23:7629–7640. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gavriilidis P, Giakoustidis A and
Giakoustidis D: Aurora kinases and potential medical applications
of Aurora kinase inhibitors: A review. J Clin Med Res. 7:7422015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Marques S, Fonseca J, Silva PM and Bousbaa
H: Targeting the spindle assembly checkpoint for breast cancer
treatment. Curr Cancer Drug Targets. 15:272–281. 2015. View Article : Google Scholar : PubMed/NCBI
|