Introduction
Osteosarcoma is a bone cancer, which means it can
easily spread to other organs or tissues in the body. Osteosarcoma
is the most common malignant bone tumor among children, adolescents
and young adults and occurs most frequently in children and young
adults between the ages of 10 and 20 years and often during
adolescence. With a higher prevalence in boys compared with girls
(1). The incidence rate and 95%
confidence intervals for osteosarcoma is 4.0 (3.5–4.6) from 0–14
years of age, and 5.0 (4.6–5.6) for 0–19 years of age per year per
million persons (1). The surgical
procedures for OS primarily involve extensive resection or
amputation, and rendering patients disabled, thus having a major
impact on the quality of life of patients. Therefore, early
detection, diagnosis and treatment are important (2). MicroRNAs (miRNAs), which are a class of
21~25 nucleotides of non-coding single-stranded small RNAs, have
been demonstrated to serve as oncogenes or tumor suppressors in a
variety of tumor types, particularly OS (3).
Previous studies have demonstrated that miR-142 is
downregulated as a tumor suppressor gene in a variety of cancer
tissues and cell lines. Overexpression of miR-142-3p may cause cell
cycle arrest and exert its effect by targeting cell division cycle
25C (CDC25C) (4). In non-small cell
lung cancer, miRNA-142 exerts tumor suppressor effects and induces
apoptosis by targeting high mobility group box 1 (5,6). In
addition, miR-142 targets a number of different genes and inhibits
cancer cell proliferation, including targeting ASH1 like histone
lysine methyltransferase (ASH1L) and mixed-lineage leukemia 1
(MLL1), and inhibiting cell proliferation in thyroid follicular
carcinoma (7), and in colorectal
cancer (8), breast cancer (9,10). Similar
findings have been reported in previous studies on OS. miR-142 has
been demonstrated to inhibit the proliferation of OS cells by
targeting high mobility group AT-hook 1 (11), and subsequent studies have revealed
that miR-142 expression is reduced in OS tissues and cell lines
(12). miR-142 inhibits cell
proliferation and cell invasion by upregulating E-cadherin,
inhibiting matrix metalloproteinase-2 and −9, and affecting
Ras-related C3 botulinum toxin substrate 1 (Rac1) expression, and
arrests the cell cycle in the S phase (13). These results demonstrate that
overexpression of miR-142 in OS cell lines inhibits cell
proliferation.
Whether miR-142 may be useful in OS gene therapy
remains to be elucidated. The present study aimed to investigate
the effect of miR-142 on the apoptosis of OS cell lines and explore
the specific pathways underlying miR-142-induced OS cell apoptosis.
The findings in this study may lay the foundation for subsequent
studies into the therapeutic significance of miR-142 in OS.
Materials and methods
Materials
Human OS cell lines hFOB1.19, MG-63, U-2OS and
Saos-2 cells were purchased from the Cell Resource Center of
Shanghai Institutes for Biological Sciences (Shanghai, China). MTT,
DMSO, apoptosis detection kits and DAPI were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany), the miRNA-142
analogue was purchased from Shanghai GenePharma Co., Ltd.,
Shanghai, China with the following sequence: Fluorescein
(FAM)-5′-CAUAAAGUAGAAAGCACUACU-3′. Negative control (NC) miRNA was
synthesized and the sequence was FAM-5′-UUCUCCGAACGUGUCACGU-3′.
diethyl pyrocarbonate was dissolved in water and diluted into 10 µM
stock for cell transfection, which was added to the complete
culture medium when required. RPMI-1640 medium was obtained from
Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA), fetal
bovine serum (FBS) was purchased from (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The Cytoplasmic and
Mitochondrial Protein Extraction kit was obtained from Sangon
Biotech Co., Ltd. (Shanghai, China). The remaining reagents were of
analytical grade or molecular reagent grade. All primary and
secondary antibodies are listed in Table
I.
 | Table I.The antibodies used for western blot
analysis. |
Table I.
The antibodies used for western blot
analysis.
| Antibody name | Catalog number | Dilutions | Supplier |
|---|
| β-actin | ab5694 | 1:1,000 | Abcam |
| Caspase-3 | ab13847 | 1:500 | Abcam |
| Cleaved
Caspase-3 | ab136812 | 1:500 | Abcam |
| Anti-cytochrome
c | ab13575 | 1:5,000 | Abcam |
| p-Rb | ab184796 | 1:10,000 | Abcam |
| P-TEN | ab32199 | 1:10,000 | Abcam |
| Rb | ab181616 | 1:10,000 | Abcam |
| Goat anti-rabbit
IgG | ab6721 | 1:1,000 | Abcam |
Cell culture
Immortalized human fetal osteoblastic cell line
hFOB1.19 or OS cells (MG-63, U-2OS and Saos-2) were routinely
passaged. All cells were separately cultured in RPMI-1640 medium
with 10% FBS at 37°C in a humidified atmosphere with 5%
CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay for the expression of
miR-142 and phosphatase and tensin homolog (PTEN) gene
Cells in the logarithmic growth phase were collected
to detect the expression levels of miR-142 in hFOB1.19 cells or OS
cells. Cells in the logarithmic growth phase were digested with
0.25% trypsin and washed three times with PBS (pH 7.2). The final
concentration of the cells was adjusted to 8×104
cells/ml in RPMI-1640 medium containing 10% FBS and inoculated into
6-well plates (2×105 cells/well).
Transfection
Cells were transfected with 20 nM miR-142 20 nM,
miR-NC and control (adding the completed medium without mircoRNA)
using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Following transfection, the
cells were incubated for 48 h and then collected to determine the
expression of miR-142. Additionally, for quantitate the expression
of PTEN gene, cells in the logarithmic growth phase were
obtained, re-suspended and then seeded in 24-well plates at a
density of 5×104 cells/well. Cells were randomly divided
into 5 groups: Control group, treated with complete RPMI-1640
medium, miR-NC group and 3 miRNA-142 treatment groups, with final
concentrations of 20, 40 and 80 nM.
RT-qPCR
Then, these cells were obtained to measure the
expression of PTEN gene following miR-142 transfection. Total RNA
was extracted using TRIzol reagent (Life Technologies; Thermo
Fisher Scientific, Inc.), and then was used as the template for
reverse transcription. cDNA was extracted from mRNA by TaKaRa
PrimeScript™ II 1st strand cDNA Synthesis kit (Clontech
Laboratories, Inc., Mountainview, CA, USA). The TaqMan real-time
PCR kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) and
the Eco real-time PCR system (Illumina, Inc., San Diego, CA, USA)
were used. GAPDH was used as an internal reference. The following
primers were used: Human miR-142-3p sequence:
FAM-5′-UUUCUACUUUAUG-3′. (PN4427975, assay ID: 000434) and U18
(PN4427975, assay ID: 001204; both from Life Technologies; Thermo
Fisher Scientific, Inc.); PTEN forward, 5′-AAAGCTGGAAAGGGACGAAC-3′
and reverse, 5′-CAGGTAACGGCTGAGGGAAC-3′; GAPDH forward,
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse, 5′-GAAGATGGTGATGGGATTTC-3′.
After the amplification reaction, The thermocycling conditions were
as follows: 35 cycles, 30 sec at 95°C, 30 sec at 56°C and extension
for 10 min at 72°C. The 2−ΔΔCq method was used for data
analysis (14).
MTT analysis for the inhibitory effect
of miR-142 on proliferation
Following transfection with different concentrations
of miR-142 (0, 20, 40, 80 nM) and control, MG-63, U-2OS and Saos-2
cell lines were trypsinized, re-suspended and seeded in 96-well
plates at a density of 1×104 cells/well. Cells were
incubated at 37°C for 48 h, and then 10 µl 5 mg/ml MTT reagent was
added to each well, the cells were incubated for another 4 h at
37°C. Then, the supernatant was discarded and 200 µl of DMSO was
added to each well. Finally, samples were measured on a multi-well
spectrophotometer, to measure the absorbance at 570 nm (OD value)
using a microplate reader. Cell inhibition rate
(%)=(1-ODtest/ODcontrol) ×100.
Fluorescence microscopy assay for
morphological observation of apoptotic cells
When MG-63 cells reached the logarithmic growth
phase, cells were treated with miR-NC and different concentrations
of miR-142 (20, 40 or 80 nM) for 48 h at 37°C, and then fixed with
cold 100% methanol and were incubated for 5 min at room
temperature, washed three times with PBS. Stained with DAPI for 5
min at room temperature, washed once with PBS. One side of the
cells was inverted onto a slide glass pre-incubated with a
fluorescent anti-quencher (Guangzhou RiboBio Co., Ltd., Guangzhou,
China) and observed under an inverted fluorescence microscope.
Flow cytometric analysis of the cell
cycle and apoptosis rate
The OS cell lines treated with control anddifferent
concentrations of miR-142 for 48 h at 37°C were collected and
treated according to the Annexin V/propidium iodide (PI) apoptosis
detection kit (BD Biosciences, Franklin Lakes, NJ, USA). Cells were
washed twice with PBS and then re-suspended with 1X binding buffer
to adjust the cell density to 1×108 cells/ml. Following
the addition of Annexin V and PI, the mixture was incubated at room
temperature for 15 min, and then transferred to a flow cytometer
tube. To avoid blockage of the flow cytometer channel by cell
masses, cells were passed through a 200-nm screen mesh filter prior
to analysis using the flow cytometer. The blank control and
single-stained cells were used to set gate and color compensation
in flow cytometry. A total of 1×104 cells were analyzed
each time. The experiment was repeated for three times, and
presented as the mean ± SD.
Western blot assay for the detection
of apoptosis- or cell cycle-associated protein expression
After washing twice by cold PBS, MG-63 cells treated
with control, miR-NC and different concentrations of miR-142 (20,
40 or 80 nM) for 48 h were collected. The cytosol and mitochondrial
protein was obtained using the Cytoplasmic and Mitochondrial
Protein Extraction kit. Protein concentration was determined by BCA
assay. Each sample (40 µg/lane) was then electrophoresed on 10%
SDS-PAGE and transferred to a polyvinylidene fluoride membrane. The
membrane was blocked with 5% skim milk for 1 h at room temperature,
then incubated with apoptosis-associated and cycle-specific
antibodies at 4°C overnight. Subsequently, the membrane was
incubated in milk with HRP-conjugated secondary antibodies at room
temperature for 1 h and washed three times with Tris buffered
saline with Tween-20. All antibodies used are listed in Table I. Membranes were developed in a dark
chamber following the addition of enhanced chemiluminescence
reagent (Beijing Leagene Biotechnology Co., Ltd.; cat. no.,
PW0111). Densitometric analysis of blotting bands was performed
using ImageJ software (version 1.41; National Institutes of Health,
Bethesda, MD, USA) and normalized to β-actin levels.
Statistical analysis
All statistical analysis was performed using IBM
SPSS software version 20 (IBM Corp., Armonk, NY, USA). Data are
expressed as the mean ± standard deviation. Statistical analysis
was performed using one-way analysis of variance followed by the
Dunnett's test for multiple group comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-142 suppresses the proliferation
of OS cells
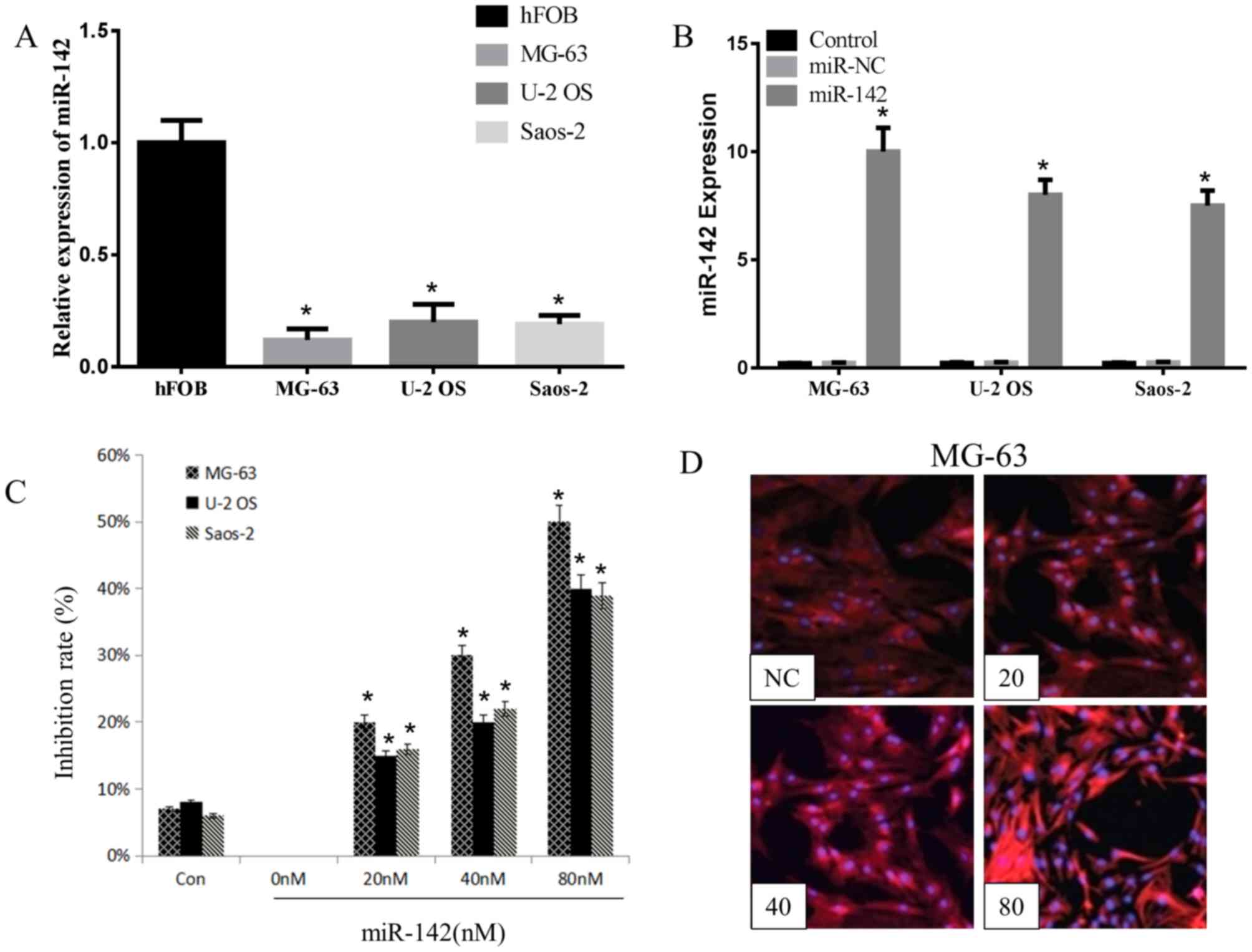
miR-142 has been demonstrated to be downregulated in
a variety of cancer tissues and cell lines, including OS cell lines
(14). The present study detected the
expression levels of miR-142 in hFOB1.19 cells or OS cells (MG-63,
U-2OS and Saos-2 cells) using RT-qPCR, and observed that miR-142
expression was significantly reduced in the three OS cell lines,
particularly in the MG-63 cell line, compared with that in hFOB1.19
cells (Fig. 1A). This suggests that
the overexpression of miR-142-3p serves a tumor suppressor
effect.
miR-142 or random miR-142 fragment was transfected
into MG-63, U-2OS and Saos-2 cells using Lipofectamine 2000 to
investigate the inhibitory effect of miR-142-3 on OS cell
proliferation. As indicated in Fig.
1B, a significantly higher expression of miR-142 was detected
in transfected groups compared with the miR-NC groups, particularly
in MG-63 cells. Wang and Youle (15)
reported that miR-142 serves a vital role in suppressing the
proliferation of colon cancer cells. In the MTT assay of the
present study, miR-142 significantly inhibited the growth of all
three OS cell lines post 48 h transfection. At 80 nM, the
inhibitory effect of miR-142 on MG-63 cells was 50±6% (Fig. 1C). The inhibitory rate was observed to
be concentration-dependent in all three cell lines. In addition,
the NC group exhibited a certain degree of inhibition, indicating
that the transfection caused toxicity to the cells. Of the three OS
cell lines; MG-63 was most sensitive to miR-142 (Fig. 1C). In the present study, compared with
all other OS cell lines, the MG-63 cell line exhibited the lowest
and highest expression of miR-142 prior to, and following
transfection with miR-142, respectively. Thus, MG-63 cells were
selected for apoptosis and western analysis. The fluorescence
intensity of Cy3 in MG-63 cells was detected by fluorescence
microscopy following miR-142 transfection. As presented in Fig. 1D, with increasing miR-142
concentrations, the distribution and intensity of red fluorescence
also increased. The fluorescence intensity of the control group was
similar to that of the 20 nM group.
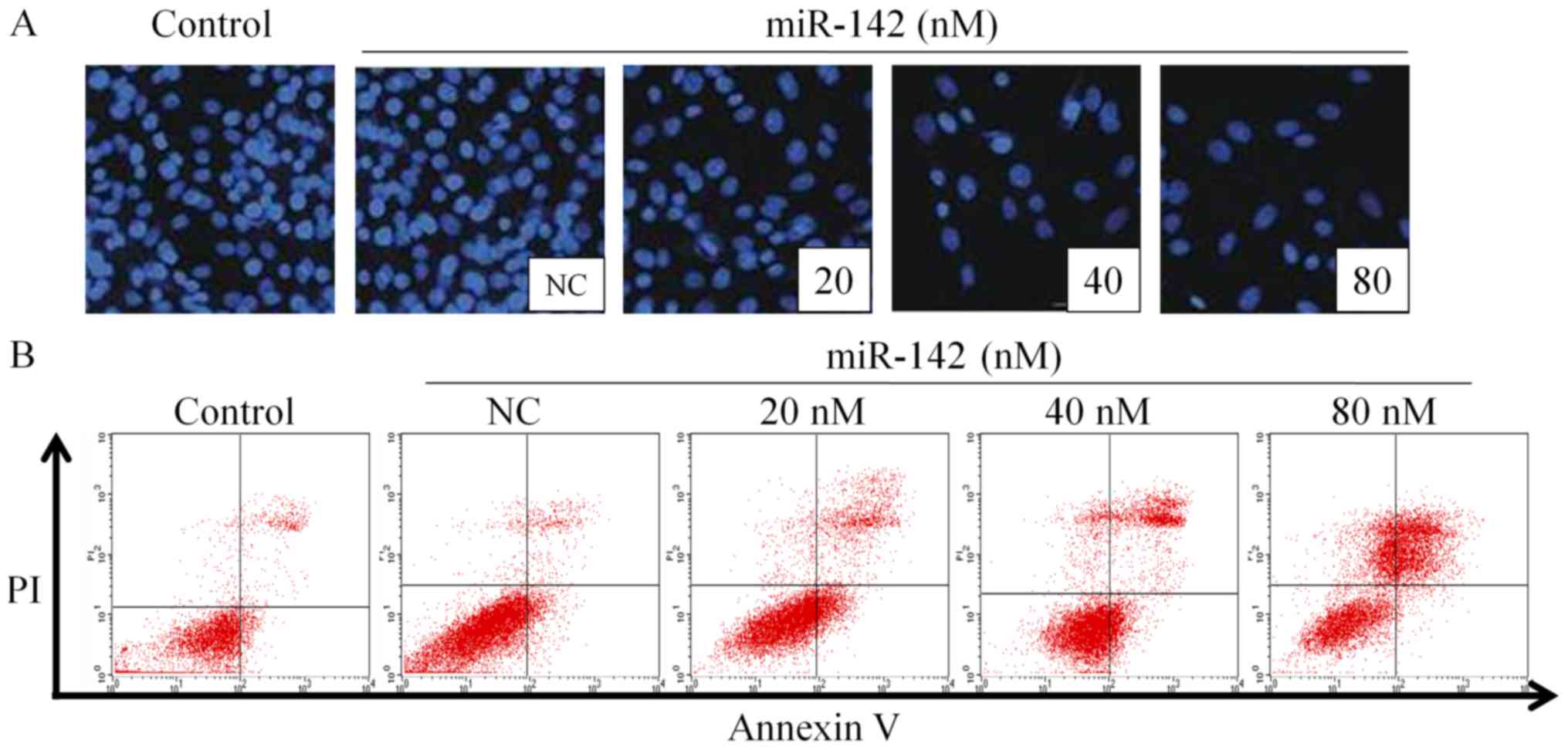
miR-142 promotes the apoptosis of
MG-63 cells
Post 48 h transfection with different concentrations
of miR-142, the nuclei of MG-63 cells was stained with DAPI, as
shown in Fig. 2A. As the miR-142
concentration increased, the percentage of apoptotic cells
increased gradually (Fig. 2B), which
is similar to a previous study whereby miR-142 was observed to
regulate apoptosis in cancer cells (16). This indicates that miR-142 exhibited
strong apoptosis-inducing effects on MG-63 cells.
MG-63 cells were harvested post 48 h transfection
and the percentage of apoptotic cells was detected by Annexin V/PI
double staining. The results in Table
II revealed that the population of normal cells significantly
decreased in miR-142-treated groups compared with the NC group
(P<0.05). Furthermore, the population of early apoptotic cells
was significantly increased in miR-142-transfected groups
(P<0.05), particularly in the 40 nM group. The percentage of
apoptotic cells overall, including early apoptosis and late
apoptosis, was significantly increased post miR-142 transfection
(80 nM) when compared with the NC group (3.28±0.72 vs. 52.91±8.79%;
P<0.05). In addition, miR-142 treatment induced the formation of
necrotic cells, particularly in the 80 nM group, which exhibited a
significant increase when compared with the NC group (1.28±0.01 vs.
10.02±5.25%; P<0.05). These results confirmed that miR-142
induced MG-63 cell apoptosis in a dose-dependent manner. Compared
with the blank control group, the proportion of early apoptotic
cells in the NC group was 3.24±0.51%, which difference was
significant (P<0.05), indicating that Lipofectamine 2000 was
mildly toxic to cells.
 | Table II.The effect of miR-142 on the
percentage of apoptotic osteosarcoma cells at different stages
(n=3). |
Table II.
The effect of miR-142 on the
percentage of apoptotic osteosarcoma cells at different stages
(n=3).
|
| Percentage of
cells, (%) |
|---|
|
|
|
|---|
|
|
|
| miR-142 |
|---|
|
|
|
|
|
|---|
| Groups | Blank control | Negative
control | 20 NM | 40 nM | 80 nM |
|---|
| Normal cells | 97.01±9.32 | 94.02±8.11 |
56.92±4.11a |
44.36±8.21a |
36.45±7.91a |
| Early
apoptosis | 1.24±0.01 |
3.24±0.51b |
23.12±1.21a |
36.02±1.11a |
7.89±2.31a |
| Late apoptosis | 0.04±0.01 | 0.04±0.21 |
14.02±3.64a |
13.81±7.41a |
45.02±6.48a |
| Necrotic cells | 0.98±0.01 | 1.28±0.01 | 6.21±4.11 | 9.52±6.33 |
10.02±5.25a |
miR-142 upregulates the expression of
apoptotic-associated proteins
It has reported that miR-142 aids in mediating
apoptosis signaling in OS (13,17–19). In
order to further explore the specific mechanism underlying
miR-142-induced OS cell apoptosis, caspase-3 and cytochrome
c (Cyto c) expression was detected. At 48 h post
transfection with different concentrations of miR-142-3p (20, 40
and 80 nM), the MG-63 cells were lysed and apoptosis-associated
protein expression was detected. The results indicated that
caspase-3, a key molecule that represents activation of apoptosis,
was significantly activated with increasing miR-142 concentrations
(40 and 80 nM), as indicated by a significant increase in cleaved
caspase-3 expression (Fig. 3A). It
has been proposed that activated caspases are involved in a
feedback mechanism, which disrupts mitochondrial function, thereby
amplifying the release of Cyto c (15). To further differentiate whether
apoptosis was an endogenous or exogenous pathway, and to determine
the effect of miR-142 on caspase activation and Cyto c
release, Cyto c expression was detected by western blot
analysis. As shown in Fig. 3A, the
expression of cytosol Cyto c was significantly increased
following miR-142 transfection (40 and 80 nM) when compared with
the NC group (P<0.05). It demonstrated that Cyto c, which
represents the endogenous pathway, was released from the
mitochondria into the cytoplasm under the action of miR-142,
indicating the primary pathway underlying miR-142-induced OS cell
apoptosis may be the endogenous mitochondrial pathway.
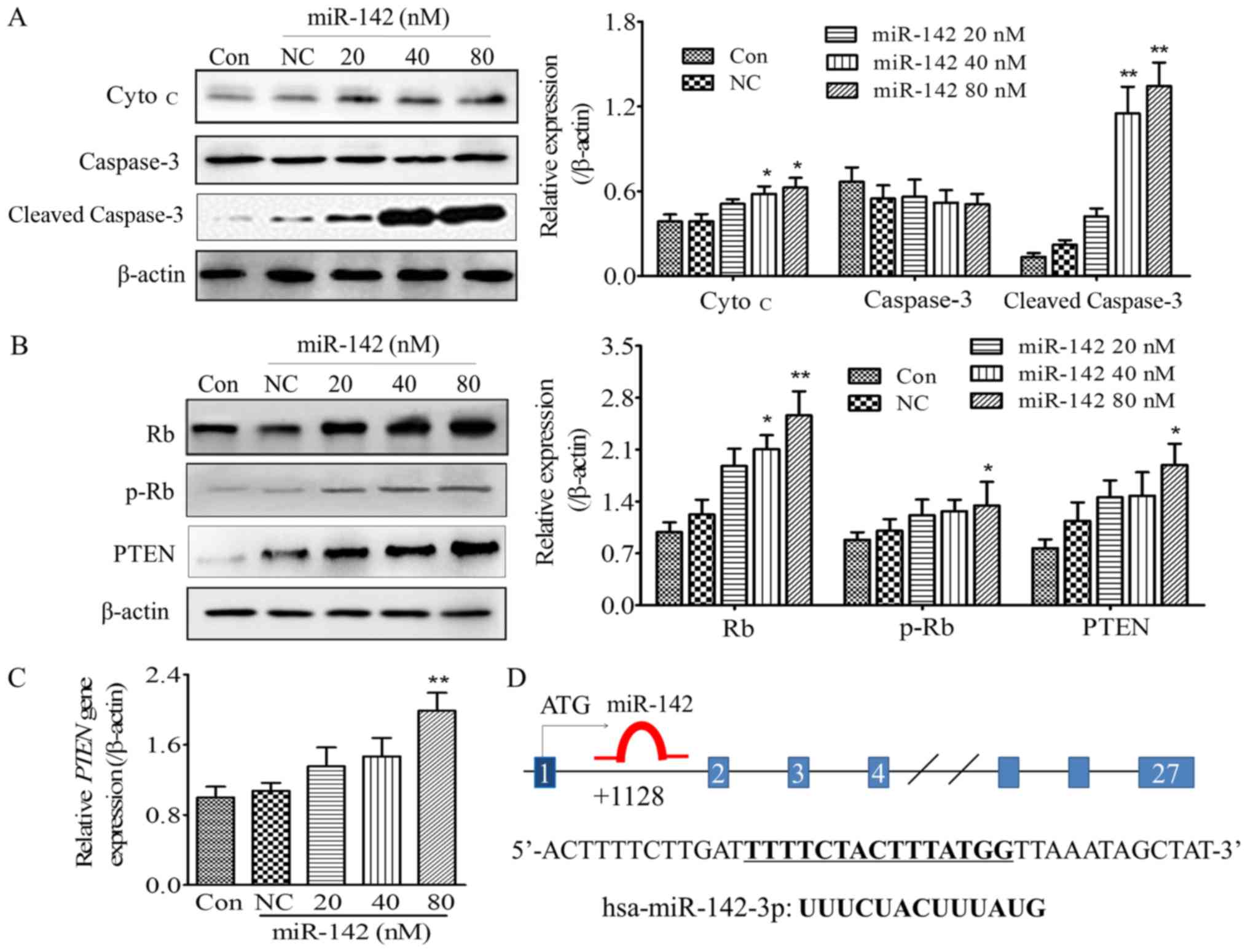 | Figure 3.Effect of miR-142 on
apoptosis-associated signaling molecules and tumor suppressors, Rb
and PTEN. (A) The protein expression of cytosol Cyto c and
caspase-3 cleaved. (B) The expression of PTEN and Rb and the
phosphorylation status of p-Rb in MG-63 cells post 48 h
transfection with different concentrations of miR-142. Left panel:
Representative images; Right panel: Quantification of the gray
scale of the bands in different groups using ImageJ software. (C)
The gene expression of PTEN measured by reverse
transcription-quantitative polymerase chain reaction. (D) The
potential binding pattern of mir-142 to the rb gene. *P<0.05,
**P<0.01, compared with Con and NC groups. miR, microRNA; NC,
negative control; p-, phosphorylated-, PTEN, phosphatase and tensin
homolog; Rb, Retinoblastoma-associated protein; Cyto c,
cytochrome c; Con, blank control. |
miR-142 increases PTEN expression and
Retinoblastoma-associated protein (Rb) phosphorylation
Zheng et al indicated that miR-142, acts as a
tumor suppressor in the OS cell lines, arresting cell cycle
progression in the S phase (13).
Phosphorylation of Rb was originally considered to drive cell cycle
progression, and influence cell proliferation and apoptosis
(16). The present study investigated
the influence of miR-142 upregulation of the tumor suppressor PTEN
and Rb phosphorylation on miR-142-regulated OS cell apoptosis. The
results demonstrated that miR-142-3p upregulated PTEN gene and
protein expression in a dose-dependent manner, with a significant
difference observed at a concentration of 80 nM (P<0.05 when
compared with the blank control or the NC group; Fig. 3B and C). Furthermore, phosphorylation
of Rb increased significantly with 80 nM miR-142 compared with the
control group, while the Rb expression also increased
significantly, suggesting that the upregulation of Rb serves an
important role in miR-142-induced apoptosis.
Discussion
miRNAs have been identified to serve as oncogenes or
tumor supressors in a variety of tumors (17–19), also
in OS (20). miR-142 expression is
significantly dysregulated in numerous diseases, particularly in
cancer (21). The target genes of
miR-142 in tumor research have been confirmed, including: CDC25C
(4), Rac1 (13), high mobility group protein A1 (HMGA1)
(11), high mobility group box-1
protein (HMGB1) (5), Wiskott-Aldrich
syndrome like, Integrin αV, and additional cytoskeletal elements
(9), suppressor of cytokine signaling
6 (SOCS6) (22), ASH1L and MLL1
(7), ATP binding cassette subfamily G
member 2 (Junior blood group), and leucine rich repeat containing G
protein-coupled receptor 5 (8), CD133
(23), interleukin 6 signal
transducer (24) and glucocorticoid
receptor α (25). A previous study
reported that miR-142 expression is reduced in a variety of cancer
cells, such as breast cancer stem cells (10) and pancreatic cancer cells (26). Overexpression of miR-142 exhibits a
tumor suppressor effect (11).
Therefore, it is considered to be an anti-oncomirs, despite other
studies reporting that miR-142 may serve an oncomir role in certain
tumors like human T-cell acute lymphoblastic leukemia (T-ALL)
(25). In the current study, the
expression levels of miR-142 were detected in hFOB1.19 cells or OS
cells (MG-63, U-2OS and Saos-2 cells), whereby a lower expression
of miR-142 was observed in OS cells, particularly in the MG-63 cell
line. The subsequent experiments in the present study all suggested
that miR-142 serves a tumor suppressor role in OS.
miR-142, as a marker of prognosis in a variety of
cancer types, including lung cancer (27), colon cancer (28) and leukemia (29), may affect cell proliferation, and
induce apoptosis. Here, miR-142-overexpressed cells were obtained
by liposomal transfection and miR-142 was observed to inhibit the
growth of three types of OS cell lines post 48 h transfection in a
dose-dependent manner. Previous studies have reported that the
transfection of miR-142-3p for 48 h induces apoptosis in lung
cancer cell lines NCI-H23 (5) and
glioma-related murine M2 macrophage (30). In the present study, to investigate
whether miR-142-3p was able to induce MG-63 cell apoptosis,
fluorescence microscopy assays and flow cytometric analysis were
performed. The results indicated that miR-142 enhanced the
population of early apoptotic cells, promoted MG-63 cell apoptosis
in a dose-dependent manner and induced the formation of necrotic
cells.
The associations between miR-142-mediated
proliferation and apoptosis signaling have been previously reported
in OS. As previous study reported, miR-142 can mediates cancer cell
proliferation and apoptosis in OS (11,13,31,32).
Ding et al (31) reported that
the expression level of miR-142 was significantly lower in OS
tissues and cells due to hypermethylation, suggesting that miR-142
served an important role in the inhibition of the proliferation,
and invasiveness of OS cell lines induced by demethylation agents.
Xu et al (11) has identified
that overexpression of miR-142-3p in OS cells suppressed cell
proliferation, migration and invasion by directly targeting HMGA1.
Zheng et al (13) reported
that the expression of miR-142 was significantly reduced, but Rac1
was increased in OS tissues and cell lines, as miR-142 directly
targeted to the 3′-untranslated region (UTR) of the Rac1 gene,
which then regulates Rac1 expression at the transcriptional and
translational levels. Liu et al (32) confirmed that miR-142-3p inhibits OS
cell growth and promotes apoptosis by directly binding to the HMGB1
3′-UTR, negatively regulating its expression, and thus decreasing
HMGB1 expression. However, to the best of our knowledge, there are
no reports on the interaction between miR-142-3p and the
upregulation of tumor suppressors (Rb and PTEN) or activation of
the caspase signaling pathway. Therefore, the roles of miR-142 in
proliferation and apoptosis of OS cells were further elucidated by
investigating the expression of apoptosis-associated proteins and
PTEN, to decipher the molecular pathway underlying the effects of
miR-142-3p on OS.
The present study determined the expression of PTEN
and caspase-3, and the phosphorylation levels of Rb following
miR-142 transfection. PTEN, a tumor suppressor gene with active
phosphatase, promotes cell apoptosis through the PI3K/Akt signaling
pathway (33). It was reported that
miRNAs enhance tumor formation and metastasis by directly targeting
the 3′-UTR of the PTEN gene, and promote the proliferation of human
cancer cells through direct suppression of PTEN expression
(34). In the present study, miR-142
overexpression resulted in upregulation of PTEN gene and protein
expression, thus suppressing cell proliferation. In the caspase
family, caspase-3 is the most critical downstream protease in the
caspase cascade. Upregulating the expression of caspase-3 promotes
the apoptosis of tumor cells. In the current study, the detection
of the apoptosis of tumor cells following the upregulation of
caspase-3 expression was not only verified by annexin V/PI double
staining, but also demonstrated by the expression of
apoptosis-associated proteins at a molecular level. In the present
study, miR-142-3p significantly upregulated the protein expression
of cleaved caspase-3, suggesting it activated the caspase signaling
pathway and regulated caspase-3-induced apoptosis. Schwartzbauer
and Robbins (35) reported that the
activity of caspase-3 and cell apoptosis rate were reduced
following PTEN inhibition by PTEN cDNA in rat primary
cardiomyocytes, which indicates that PTEN is able to increase
caspase-3 expression and further promotes the occurrence of
apoptosis. Similarly, in the present study, miR-142 transfection
significantly upregulated PTEN gene and protein expression, leading
to the activation of caspase-3. These data suggest that PTEN may
influence the level of apoptosis of OS cells through adjusting the
expression of caspase-3.
Rb is a tumor suppressor in OS cell lines (36). Notably, phosphorylation of Rb, which
is an important gene in cell cycle regulation, is considered to
drive cell cycle progression, and influence cell proliferation and
apoptosis (16). Therefore, the
influence of miR-142 on Rb activation was detected in the present
study, and the western blotting results revealed that miR-142
significantly promoted the phosphorylation of Rb in MG-63 cells,
which may be associated with miR-142-induced OS cell apoptosis. In
patients with OS, the mutation of the Rb gene inhibits Rb from
activating its downstream signaling molecules, thus causing a
dysregulation of the cell cycle, and subsequently, uncontrolled
proliferation. The Saos-2 cell line is resistant to methotrexate, a
therapeutic drug for OS, due to lack of an active Rb gene (37), compared with Saos-2 cells, the
activity of RB1 in MG-63 cells is unaffected. This may be the
reason why the proliferation-inhibitory activity against MG-63 was
superior to that of SaOS-2 following miR-142 transfection. In the
present study, miR-142 was suggested to induce MG-63 cell apoptosis
by regulating Rb activity.
Apoptosis pathways may be divided into endogenous
and exogenous pathways. The former is also known as the
mitochondrial pathway, primarily because it involves the release of
mitochondrial-associated proteins, including Cyto c and a
change in the mitochondrial membrane potential. The present study
reported that miR-142 significantly activated caspase-3. The
activated caspase may feedback, disrupting mitochondrial function,
and amplifying further Cyto c release (38). To further differentiate whether
apoptosis was an endogenous or exogenous pathway, the cytosol and
mitochondrial proteins were obtained using Cytoplasmic and
Mitochondrial Protein Extraction kits, respectively. As the results
indicated, significantly increased protein expression of cytosol
Cyto c in the miR-142-treated groups (40 and 80 nM) was
observed, suggesting that miR-142 induces apoptosis through the
mitochondrial pathway, due to miR-142 enhancing the release of Cyto
c from mitochondria to the cytosol. Furthermore, Rb and PTEN
expression was significantly activated, as well as Cyto c,
indicating that multiple signaling pathways were involved with
crosstalk occurring between themselves. miR-142 has been revealed
to be aberrantly expressed in AML, and may be used as a novel
diagnostic marker (29). Furthermore,
expression of miR-142 could be considered a prognostic indicator in
ESCC (39). Therefore, further in
vivo studies on the targets and effects of miRNAs should be
performed to provide a basis for early diagnosis or prognosis of OS
using the abnormal expression of miRNAs as biomarkers. In our
future studies, the influence of PTEN upregulation, Rb
phosphorylation and caspase signaling pathway activation on
miR-142-regulated cell apoptosis requires further investigation,
with comparisons using multiple OS cell lines, including Saos-2 and
U-2OS cells.
In summary, it was confirmed that the overexpression
miR-142 significantly inhibited the proliferation and induced the
apoptosis of MG-63 cells, thus suppressing OS. These observations
may be associated with the upregulation of tumor suppressors, Rb
and PTEN, and activation of the caspase signaling pathway. These
results have laid a foundation for the application of miR-142 in
the prevention and treatment of OS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YG performed the cell culture, qPCR and transfection
experiments. QZ was responsible for fluorescent staining and flow
cytometry, ZY perform the western blot analysis, SL collected data
and statistics. JL designed the study and wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Cyto c
|
cytochrome c
|
|
PTEN
|
phosphatase and tensin homolog
|
|
HMGB1
|
high mobility group box-1 protein
|
|
HMGA1
|
high mobility group protein A1
|
|
MLL1
|
myeloid/lymphoid or mixed-lineage
leukemia 1
|
|
MMP-2
|
matrix metalloproteinase-2
|
|
Rac1
|
ras-related C3 botulinum toxin
substrate 1
|
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berindan-Neagoe I, Monroig PC, Pasculli B
and Calin GA: MicroRNAome genome: A treasure for cancer diagnosis
and therapy. CA Cancer J Clin. 64:311–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao XC, Yu Y, Hou LK, Sun XH, Ge J, Zhang
B and Wang X: miR-142-3p inhibits cancer cell proliferation by
targeting CDC25C. Cell Prolif. 49:58–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiao P and Liu WL: MiR-142-3p functions as
a potential tumor suppressor directly targeting HMGB1 in
non-small-cell lung carcinoma. Int J Clin Exp Pathol.
8:10800–10807. 2015.PubMed/NCBI
|
|
6
|
Su YH, Zhou Z, Yang KP, Wang XG, Zhu Y and
Fa XE: MIR-142-5p and miR-9 may be involved in squamous lung cancer
by regulating cell cycle related genes. Eur Rev Med Pharmacol Sci.
17:3213–3220. 2013.PubMed/NCBI
|
|
7
|
Colamaio M, Puca F, Ragozzino E, Gemei M,
Decaussin-Petrucci M, Aiello C, Bastos AU, Federico A, Chiappetta
G, Del Vecchio L, et al: miR-142-3p down-regulation contributes to
thyroid follicular tumorigenesis by targeting ASH1L and MLL1. J
Clin Endocrinol Metab. 100:E59–E69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shen WW, Zeng Z, Zhu WX and Fu GH:
MiR-142-3p functions as a tumor suppressor by targeting CD133,
ABCG2, and Lgr5 in colon cancer cells. J Mol Med (Berl).
91:989–1000. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwickert A, Weghake E, Brüggemann K,
Engbers A, Brinkmann BF, Kemper B, Seggewiß J, Stock C, Ebnet K,
Kiesel L, et al: microRNA miR-142-3p inhibits breast cancer cell
invasiveness by synchronous targeting of WASL, integrin Alpha V,
and additional cytoskeletal elements. PLoS One. 10:e01439932015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Isobe T, Hisamori S, Hogan DJ, Zabala M,
Hendrickson DG, Dalerba P, Cai S, Scheeren F, Kuo AH, Sikandar SS,
et al: miR-142 regulates the tumorigenicity of human breast cancer
stem cells through the canonical WNT signaling pathway. Elife.
3:2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu G, Wang J, Jia Y, Shen F, Han W and
Kang Y: MiR-142-3p functions as a potential tumor suppressor in
human osteosarcoma by targeting HMGA1. Cell Physiol Biochem.
33:1329–1339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Namløs HM, Meza-Zepeda LA, Barøy T,
Østensen IH, Kresse SH, Kuijjer ML, Serra M, Bürger H,
Cleton-Jansen AM and Myklebost O: Modulation of the osteosarcoma
expression phenotype by microRNAs. PLoS One. 7:e480862012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng Z, Ding M, Ni J, Song D, Huang J and
Wang J: MiR-142 acts as a tumor suppressor in osteosarcoma cell
lines by targeting Rac1. Oncol Rep. 33:1291–1299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang C and Youle RJ: The role of
mitochondria in apoptosis*. Annu Rev Genet. 43:95–118. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Young AP and Longmore GD: Differential
regulation of apoptotic genes by Rb in human versus mouse cells.
Oncogene. 23:2587–2599. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kent OA and Mendell JT: A small piece in
the cancer puzzle: microRNAs as tumor suppressors and oncogenes.
Oncogene. 25:6188–6196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee YS and Dutta A: MicroRNAs: Small but
potent oncogenes or tumor suppressors. Curr Opin Investig Drugs.
7:560–564. 2006.PubMed/NCBI
|
|
20
|
Sampson VB, Yoo S, Kumar A, Vetter NS and
Kolb EA: Micrornas and potential targets in osteosarcoma: Review.
Front Pediatr. 3:692015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shrestha A, Mukhametshina RT, Taghizadeh
S, Vásquez-Pacheco E, Cabrera-Fuentes H, Rizvanov A, Mari B,
Carraro G and Bellusci S: MicroRNA-142 is a multifaceted regulator
in organogenesis, homeostasis, and disease. Dev Dyn. 246:285–290.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qi X, Li J, Zhou C, Lv C and Tian M:
Mir-142-3p suppresses socs6 expression and promotes cell
proliferation in nasopharyngeal carcinoma. Cell Physiol Biochem.
36:1743–1752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chai S, Tong M, Ng KY, Kwan PS, Chan YP,
Fung TM, Lee TK, Wong N, Xie D, Yuan YF, et al: Regulatory role of
miR-142-3p on the functional hepatic cancer stem cell marker CD133.
Oncotarget. 5:5725–5735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sonda N, Simonato F, Peranzoni E, Calì B,
Bortoluzzi S, Bisognin A, Wang E, Marincola FM, Naldini L, Gentner
B, et al: miR-142-3p prevents macrophage differentiation during
cancer-induced myelopoiesis. Immunity. 38:1236–1249. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lv M, Zhang X, Jia H, Li D, Zhang B, Zhang
H, Hong M, Jiang T, Jiang Q, Lu J, et al: An oncogenic role of
miR-142-3p in human T-cell acute lymphoblastic leukemia (T-ALL) by
targeting glucocorticoid receptor-α and cAMP/PKA pathways.
Leukemia. 26:769–777. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu Y, Ji N, Wei W, Sun W, Gong X and Wang
X: MiR-142 modulates human pancreatic cancer proliferation and
invasion by targeting hypoxia-inducible factor 1 (HIF-1α) in the
tumor microenvironments. Biol Open. 6:252–259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaduthanam S, Gade S, Meister M, Brase JC,
Johannes M, Dienemann H, Warth A, Schnabel PA, Herth FJ, Sültmann
H, et al: Serum miR-142-3p is associated with early relapse in
operable lung adenocarcinoma patients. Lung Cancer. 80:223–227.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ghanbari R, Mosakhani N, Asadi J, Nouraee
N, Mowla SJ, Yazdani Y, Mohamadkhani A, Poustchi H, Knuutila S and
Malekzadeh R: Downregulation of plasma mir-142-3p and mir-26a-5p in
patients with colorectal carcinoma. Iran J Cancer Prev.
8:e23292015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang F, Wang XS, Yang GH, Zhai PF, Xiao Z,
Xia LY, Chen LR, Wang Y, Wang XZ, Bi LX, et al: miR-29a and
miR-142-3p downregulation and diagnostic implication in human acute
myeloid leukemia. Mol Biol Rep. 39:2713–2722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu S, Wei J, Wang F, Kong LY, Ling XY,
Nduom E, Gabrusiewicz K, Doucette T, Yang Y, Yaghi NK, et al:
Effect of miR-142-3p on the M2 macrophage and therapeutic efficacy
against murine glioblastoma. J Natl Cancer Inst. 106:2014.
View Article : Google Scholar
|
|
31
|
Ding M, Hu J, Ni J, Zheng Z, Song D and
Wang J: Demethylation of microRNA-142 induced by demethylation
agents plays a suppressive role in osteosarcoma cells. Oncol Lett.
9:2261–2267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu K, Huang J, Ni J, Song D, Ding M, Wang
J, Huang X and Li W: MALAT1 promotes osteosarcoma development by
regulation of HMGB1 via miR-142-3p and miR-129-5p. Cell Cycle.
16:578–587. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu XX, Cao LY, Chen X, Xiao J, Zou Y and
Chen Q: PTEN inhibits cell proliferation, promotes cell apoptosis,
and induces cell cycle arrest via downregulating the
pi3K/akt/htertpathway in lung adenocarcinoma a549 Cells. Biomed Res
Int. 2016:24768422016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bermúdez BM, Goulielmaki E and
Papakonstanti EA: Focus on PTEN regulation. Front Oncol.
5:1662015.PubMed/NCBI
|
|
35
|
Schwartzbauer G and Robbins J: The tumor
suppressor gene PTEN can regulate cardiac hypertrophy and survival.
J Biol Chem. 276:35786–35793. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hellwinkel OJ, Müller J, Pollmann A and
Kabisch H: Osteosarcoma cell lines display variable individual
reactions on wildtype p53 and Rb tumour-suppressor transgenes. J
Gene Med. 7:407–419. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Iida K, Nobori T, Matsumine A, Isaka A,
Seto M, Shiraishi T and Uchida A: Effect of retinoblastoma tumor
suppressor gene expression on chemosensitivity of human
osteosarcoma cell lines. Oncol Rep. 10:1961–1965. 2003.PubMed/NCBI
|
|
38
|
Zhu Y, Li M, Wang X, Jin H, Liu S, Xu J
and Chen Q: Caspase cleavage of cytochrome c1 disrupts
mitochondrial function and enhances cytochrome c release. Cell Res.
22:127–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin RJ, Xiao DW, Liao LD, Chen T, Xie ZF,
Huang WZ, Wang WS, Jiang TF, Wu BL, Li EM and Xu LY: MiR-142-3p as
a potential prognostic biomarker for esophageal squamous cell
carcinoma. J Surg Oncol. 105:175–182. 2012. View Article : Google Scholar : PubMed/NCBI
|