Introduction
Thyroid hormone receptors (TRs) are members of a
superfamily of nuclear receptors and ligand-dependent transcription
factors. TRs are encoded by two genes, TRα (THRA) and TRβ
(THRB) (1), and four
triiodothyronine (T3) and DNA-binding receptor isoforms, TRα1,
TRβ1, TRβ2 and TRβ3, that are only present in rats, are generated
by alternative mRNA splicing (2).
These isoforms are considered functional receptors and share a
common structure with several regions from the N- to the
C-terminus, including: A N-terminal activation domain (A and B
regions); a conserved DNA-binding domain (DBD) (C region); a hinge
domain (D region); and a ligand-binding domain (E and F regions)
(3). TRs can bind to thyroid hormone
response elements (TREs) as homodimers or heterodimers via the
retinoid X receptor (4). In addition
to the regulation of metabolism, growth and development, increasing
evidence has indicated that TRs have an important role in cell
proliferation and malignant transformation (5). Low or no expression of TRs and
alterations in TR genes, particularly TRβ, have been identified in
a number of cancer types (6,7). Studies have also demonstrated that
mutations in TRβ are closely associated with a number of cancer
types (8,9).
Cell-based studies and xenograft models have
confirmed that TRβ, particularly TRβ1, functions as a tumor
suppressor to suppress tumor cell proliferation, migration and
tumorigenesis (5,10). A novel rat TRβ isoform have confirmed
TRβΔ (GenBank number: DQ191165) was previously identified. rTRβΔ is
homologous to rTRβ1 and only contains an additional 108 nucleotides
or 36 amino acids (11). Structural
analysis has indicated that these additional 36 amino acids are
located in the DBD, which alters this highly conserved domain
(11). Nonetheless, previous studies
(12,13) have demonstrated that rTRβΔ is a
functional TR with transcription factor characteristics that
exhibit greater tumor-suppressive ability in vitro, compared
with rTRβ1. In the present study, the additional 36 amino acids
were introduced into the corresponding position of wild-type human
TRβ1 (wt-hTRβ1), which was termed modified human TRβ1 (m-hTRβ1);
then, the transcription factor characteristics and in vitro
anti-tumor effects of m-hTRβ1 were examined in human breast cancer
MDA-MB-468 cells without endogenous TRβ.
Materials and methods
Cells and reagents
MDA-MB-468 and MCF-7 human breast cancer cells were
obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). Chitosan (CS), with a molecular mass of
60,000–100,000 Da (degree of deacetylation ~85%) was purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). L-15 cell culture
medium, an Annexin V-fluorescein isothiocyanate (FITC) apoptosis
detection kit, NE-PER™ Nuclear and Cytoplasmic
Extraction Reagents and an anti-TRβ-1 antibody (cat. no. MA1-216;
dilution, 1:1,000) were purchased from Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). A Caspase-3 spectrophotometric assay kit
(cat. no. G007) was purchased from Nanjing KeyGen Biotech Co., Ltd.
(Nanjing, Jiangsu, China). Antibodies against Histone H3 (cat. no.
bs-0349R; dilution, 1:1,000), active Caspase-3 (cat. no.
bsm-33199M; dilution, 1:200) and Bak (cat. no. bs-1284R; dilution,
1:200) were purchased from BIOSS (Beijing, China). M-MLV reverse
transcriptase, TRIzol® total RNA extraction reagent and
quantitative real-time polymerase chain reaction (PCR) detection
kits (SYBR® Premix Ex Taq™ II; cat. no.
RR820B were obtained from Takara Bio, Inc. (Otsu, Japan).
KOD-Plus-Ver polymerase and the KOD-Plus-Mutagenesis kit were
obtained from Toyobo Life Science (Osaka, Japan).
Construction of pcDNA3.1-wt-hTRβ1 and
pcDNA3.1-m-hTRβ1 vectors
MCF-7 cells were cultured and collected. Total RNA
was extracted using TRIzol reagent and was used for reverse
transcription to generate cDNA. This ‘total cDNA’ was used as a
template for PCR to obtain the full-length wt-hTRβ1 cDNA. The
forward primer 5′-CCCAAGCTTATGACTCCCAACAGTATGAC-3′ contains a
restriction endonuclease site for HindIII (underlined), whilst the
reverse primer 5′-CCGGAATTCCTAATCCTCGAACACTTCC-3′ contains an EcoRI
site (underlined). The HindIII-EcoRI PCR product was subcloned into
pcDNA3.1 (Invitrogen; Thermo Fisher Scientific, Inc.) at the
corresponding sites. The constructed expression plasmid,
pcDNA3.1-wt-hTRβ1, was confirmed by sequence analysis.
Mutant constructs were generated by site-directed
mutagenesis. The following primers were used to generate mutations:
Primer 1
5′-TCTACCTCTCTGCATTCGGTCTGGATGTTGTCCTCAAGGCAGTGGGACCCTAGGTTTCTTTAGAAGAACCATTCAGAAAAAT-3′;
Primer 2
5′-ACTGGTGTCTGTATGGAACCAAATCCCTGTCTTCTCGTCTCTGGTGTGAGAAGGCTTGCAGCCTTCACACGTGATACAGCGGT-3′.
The underlined portion of the primers indicates the novel
introduced 108-bp exon, and the non-underlined portion indicates
the partial wt-hTRβ1 DBD sequence. Site-directed
mutagenesis was performed using a KOD-Plus-Mutagenesis kit. This
procedure involved PCR amplification using the
pcDNA3.1-wt-hTRβ1 plasmid as the template and
mutation primers were performed according to the manufacturer's
instructions. The desired mutations were confirmed by DNA
sequencing of the entire gene. The recombinant plasmid carrying the
108-bp exon was termed pcDNA3.1-m-hTRβ1.
Preparation of pDNA/nucleic
kinase substrate short peptide (NNS)CS complexes
The nuclear localization signal-linked NNS was fused
to CS to form the gene carrier NNSCS as previously
described (14). The empty plasmid
pcDNA3.1 and the plasmids pcDNA3.1-wt-hTRβ1, pcDNA3.1-m-hTRβ1 and
pGL3-TRE (the palindromic TRE is located upstream of the SV40
promoter in the pGL3-promoter vector, which contains a Luciferase
reporter gene) (11) were extracted,
and the pDNA/NNSCS complex (pcDNA3.1/NNSCS,
pcDNA3.1-wt-hTRβ1/NNSCS,
pcDNA3.1-m-hTRβ1/NNSCS and pGL3-TRE/NNSCS)
was prepared as previously described (14).
Western blotting
MDA-MB-468 cells were seeded at a density of
1×106 cells/ml in the wells of a 6-well plate in L-15
medium supplemented with 10% fetal bovine serum (Thermo Fisher
Scientific, Inc.). When the cells had grown to 75% confluence,
transfection were performed as below, and
pcDNA3.1/NNSCS, pcDNA3.1-wt-hTRβ1/NNSCS and
pcDNA3.1-m-hTRβ1/NNSCS were added separately to the 6
wells to achieve DNA concentrations of 4 µg/well. Cells were
collected following 48 h of transfection, and nuclear proteins were
extracted using NE-PER® Nuclear and Cytoplasmic
Extraction Reagents (cat. no. 78833; Thermo Fisher Scientific,
Inc.). The nuclear protein levels were measured by BCA assay (cat.
no. CW0014; CWBIO, Beijing, China) and each sample containing 30 µg
nuclear protein were separated by 10% SDS-PAGE and transferred onto
nitrocellulose membranes. The membranes were blocked with TBS-T [25
mM Tris-Cl (pH 7.4), 150 mM NaCl and 0.05% Tween-20] containing 5%
skimmed milk for 2 h at room temperature, and then incubated with
primary antibodies against TRβ1 (cat. no. MA1-216; dilution,
1:1,000) and Histone H3 (cat. no. bs-0349R; dilution, 1:1,000).
TRβ1 recognizes an epitope in the A/B domain of hTRβ1, namely,
residues 1–101, which are common sequences of wt-hTβ1 and m-hTRβ1.
After being thoroughly washed in TBS-T, the membranes were
incubated with the secondary antibodies, HRP-conjugated mouse
anti-rabbit IgG (cat. no. bs-0295M-HRP; dilution, 1:5,000; BIOSS)
or Goat Anti-Mouse IgG, HRP Conjugated (cat. no. bs-0368G-HRP,
dilution 1:5,000, BIOSS) for 1.5 h at room temperature. After
thorough washing with TBS-T, the membranes were visualized using an
enhanced chemiluminescence kit (Merck KGaA) and Kodak films (Kodak,
Rochester, NY, USA).
Electrophoretic mobility shift assay
(EMSA)
The DNA-binding abilities of wt-hTRβ1 and m-hTRβ1
were first determined using a synthetic DR4 TRE; the
oligonucleotide sequences and labeling of the DR4 TRE probe were
reported previously (11). Following
the aforementioned transfection of MDA-MB-468 cells with
pcDNA3.1-wt-hTRβ1/NNSCS and
pcDNA3.1-m-hTRβ1/NNSCS for 72 h, nuclear proteins were
extracted and subjected to standard EMSA analysis, as previously
described (11).
Transcriptional activity analysis
pcDNA3.1/NNSCS,
pcDNA3.1-wt-hTRβ1/NNSCS and
pcDNA3.1-m-hTRβ1/NNSCS were co-transfected with
pGL3-TRE/NNSCS to determine the transcriptional activity
of wt-hTRβ1 and m-hTRβ1. A Renilla luciferase plasmid, pRL-TK
(Promega Corporation, Madison, WI, USA), was included to correct
for transfection efficiency. Following MDA-MB-468 cell transfection
for 6 h, the transfection solution was discarded and replaced with
serum-free medium, and 10 nM T3 (Sigma-Aldrich; Merck KGaA) was
added to each T3 intervention group. Following 48 h of transfection
(T3 exposure for 42 h), luciferase and Renilla activities were
assayed using a Dual-Glo® Luciferase Assay System
(Promega Corporation) according to the manufacturer's instructions.
Firefly luciferase activity was normalized to the Renilla controls.
At the same time, each group of nuclear proteins was extracted
using NE-PER® Nuclear and Cytoplasmic Extraction
Reagents (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions, and the overexpression of wt-hTRβ1 and
m-hTRβ1 protein was measured using a standard western blotting
assay as aforementioned. The results demonstrated that the
expression levels of wt-hTRβ1 and m-hTRβ1 for each condition are
identical.
Cell proliferation and apoptosis
assays
MDA-MB-468 cells were seeded at a density of
1×104 cells/ml in the wells of a 96-well plate. When the
cells had grown to 75% confluence, pcDNA3.1/NNSCS,
pcDNA3.1-wt-hTRβ1/NNSCS and
pcDNA3.1-m-hTRβ1/NNSCS were added to the wells.
Following 6 h of transfection, the transfection solution was
discarded and replaced with serum-free medium IL-15, and 10 nM T3
was added to the intervention groups. At 48 h following
transfection (T3 exposure for 42 h), MTT (Promega Corporation)
solution (20 µl, 5 mg/ml) was added to each well, and the plate was
incubated at 37°C for 4 h, following which the formazan crystals
were solubilized in dimethyl sulfoxide (200 µl/well). The
absorbance at 570 nm was recorded using a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA), and background
absorbance at 630 nm was subtracted.
MDA-MB-468 cells were seeded in wells of a 12-well
plate and transfected as aforementioned. At 48 h following
transfection (T3 exposure for 42 h), the cells were collected and
stained with FITC-conjugated Annexin V and propidium iodide for 10
min at room temperature. The stained cells were then detected by
flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
Bak, Bax and Caspase-3 expression
assays
For the expression assay, MDA-MB-468 cells were
transfected as aforementioned. At 48 h following transfection (T3
exposure for 42 h), the cells were collected and total RNA was
extracted using TRIzol reagent. mRNA (2 µg) was reverse transcribed
into total cDNA in a 20 µl reaction mixture using the PrimeScript™
RT reagent kit with gDNA Eraser (cat. no. RR047A; Takara Bio,
Inc.). Bak, Bax and Caspase-3 mRNA levels were analyzed by reverse
transcription-quantitative PCR (RT-qPCR) and were detected with
SYBR® Green using an iQ5TM system (Bio-Rad Laboratories,
Inc.) according to the manufacturer's protocol. The thermocycling
conditions were as follows: Initial denaturation at 95°C for 30
sec, followed by 35 cycles at 95°C for 5 sec, 56°C for 20 sec and
72°C for 20 sec. Primers for each gene were as follows: Bak,
forward 5′-TACCGCCATCAGCAGGAAC-3′ and reverse
5′-TCTGAGTCATAGCGTCGGTTG-3′; Bax, forward
5′-GGAGCTGCAGAGGATGATTG-3′ and reverse 5′-GGCCTTGAGCACCAGTTTG-3′;
Caspase-3, forward 5′-GGAAGCGAATCAATGGACTC-3′ and reverse
5′-TTCCCTGAGGTTTGCTGC-3′; and Gapdh, forward
5′-GCATCCTGGGCTACACTGAG-3′ and reverse 5-CCACCACCCTGTTGCTGTAG-3′.
The Cq values for all genes from different samples were collected.
Raw data were normalized to GAPDH expression, and the relative
expression level of each gene is represented as 2−ΔΔCq
(15).
The expression levels of active Caspase-3 and Bak
proteins were analyzed by western blot analysis as aforementioned.
Antibodies against active Caspase-3 (cat. no. bs-0081R; dilution,
1:300), Bak (cat. no. bs-1284R; dilution, 1:300) and GAPDH (cat.
no. sc-32233; dilution 1:1,000) (all Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) were used.
Caspase-3 activity assay
MDA-MB-468 cells were seeded in a 6-well plate and
transfected as aforementioned. At 48 h after transfection (T3
exposure for 42 h), the cells were lysed using the Lysis Buffer in
the Caspase-3 Spectrophotometric assay kits and the Caspase-3
activity was assessed using this kit according to the
manufacturer's instructions.
Statistical analysis
For all measurements, the data are expressed as the
mean ± standard deviation based on three independent experiments.
The results were analyzed using SPSS 17.0 (SPSS, Inc., Chicago, IL,
USA). One-way analysis of variance was used for multi-group
comparisons with the Student-Newman-Keuls as the post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
m-hTRβ1 construction, expression and
functional analysis
The amino acid sequences of the human and rTRβ1 DBDs
are highly conserved (16). rTRβ∆ has
an additional 108 bp sequence between exons 3 and 4, and the
segment containing the additional 36 amino acids was located on the
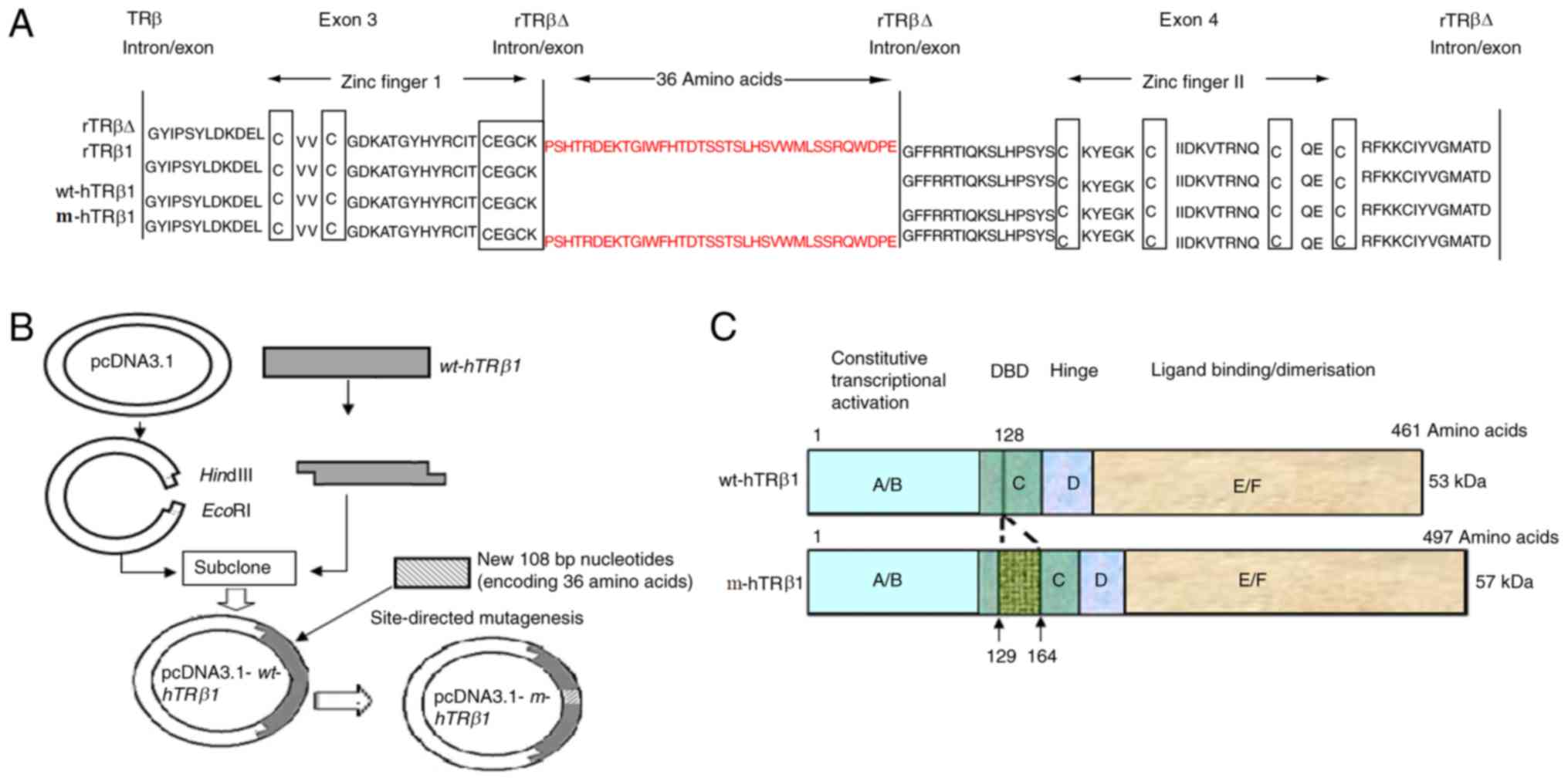
C-terminal side of the first two amino acids of the P-box (Fig. 1A) of the first zinc finger motif. This
increased the length of the linker between the two zinc fingers by
3.1-fold (17 to 53 amino acids). The full-length coding sequence of
wt-hTRβ1 (1368 bp) was successfully cloned into pcDNA3.1 to obtain
the expression plasmid pcDNA3.1-wt-hTRβ1 (Fig. 1B). The coding sequence of the
additional 36 residues (108 bp) was successfully introduced into a
similar position in wt-hTRβ1 by site-directed mutagenesis, and this
mutant was termed pcDNA3.1-m-hTRβ1 (Fig.
1B). The DBD of m-hTRβ1 is only 36 amino acids longer than
wt-hTRβ1 (Fig. 1C).
To examine wt-hTRβ1 and m-hTRβ1 expression and
activity, pcDNA3.1 (control), pcDNA3.1-wt-hTRβ1 and
pcDNA3.1-m-hTRβ1 were transiently transfected into
MDA-MB-468 cells. Protein expression was assessed by western blot.
The two proteins (m-hTRβ1 and wt-hTRβ1) were ~57 and ~53 kDa,
respectively, (Fig. 2A, lanes 2 and
3), and no TRβ was detected in the control transfection group
(Fig. 2A, lane 1). The lower band
depicted in Fig. 2 is Histone H3,
which was used as a control. DNA binding was assessed by EMSA using
a DR4 TRE. Nuclear proteins containing TRβ (wt-hTRβ1 and m-hTRβ1)
can bind to DR4, as demonstrated by a shift in DNA electrophoretic
mobility. These DNA binding assays were sequence-specific, as the
receptors competed with a 100-fold excess of unlabeled DR4
(Fig. 2B). The EMSA results indicated
that TRβ (wt-hTRβ1 and m-hTRβ1) receptors may bind to DR4.
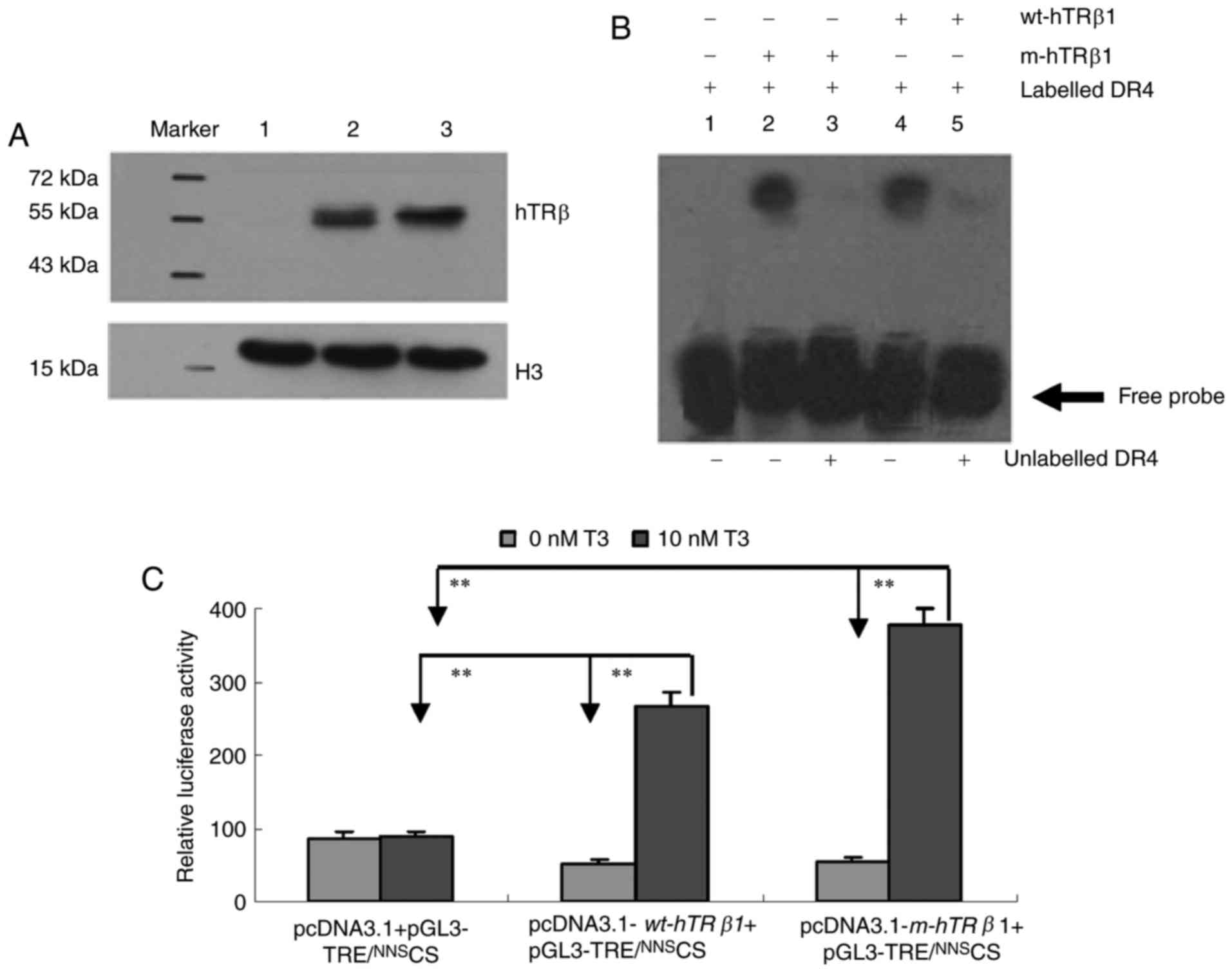 | Figure 2.Expression and properties of the
wt-hTRβ1 and m-hTRβ1 proteins. (A) Western blot detection of
wt-hTRβ1 and m-hTRβ1 expression in MDA-MB-468 cells. Lane 1,
pcDNA3.1/NNSCS; Lane 2,
pcDNA3.1-m-hTRβ1/NNSCS; and Lane 3,
pcDNA3.1-wt-hTRβ1/NNSCS. (B) DNA binding activity
was measured by EMSA. Lane 1, digoxin-labeled DR4 (17 fmol/µl);
lanes 2 and 4: digoxin-labeled DR4 (17 fmol/µl) incubated with
m-hTRβ1 and wt-hTRβ1 in the absence of competitor (100-fold excess
of unlabeled DR4); lanes 3 and 5: digoxin-labeled DR4 (17 fmol/µl)
incubated with m-hTRβ1 and wt-hTRβ1 in the presence of competitor
(100-fold excess of unlabeled DR4). (C) Luciferase activity in
MDA-MB-468 cell lysates. Firefly luciferase activity was normalized
to Renilla controls and data are presented as the mean ±
standard deviation. **P<0.01. wt-hTRβ1, wild-type human TRβ1;
m-hTRβ1, modified human TRβ1; TRE, thyroid hormone response
elements; NNS, nucleic kinase substrate short peptide; CS,
chitosan; T3, triiodothyronine. |
The transcriptional activities of wt-hTRβ1 and
m-hTRβ1 as T3 receptors were compared using a transient
transfection system. Relative luciferase activity levels were
assessed in each group. Transfection with
pcDNA3.1+pGL3-TRE/NNSCS resulted in basic luciferase
activity levels in the presence or absence of T3 (P>0.05). The
pcDNA3.1-wt-hTRβ1 + pGL3-TRE/NNSCS and
pcDNA3.1-m-hTRβ1 + pGL3-TRE/NNSCS
transfection group demonstrated a similar increase in luciferase
activity levels in the absence of T3 (P>0.05); these levels were
increased 5.4- and 6.9-fold, respectively, following treatment with
10 nM T3 (Fig. 2C).
hTRβ1 inhibits MDA-MB-468 cell
proliferation and promotes MDA-MB-468 cell apoptosis
To examine the effect of wt-hTRβ1 and m-hTRβ1 on
cell proliferation, MDA-MB-468 cells were transiently transfected
with pcDNA3.1/NNSCS, pcDNA3.1-wt-hTRβ1/NNSCS
or pcDNA3.1-m-hTRβ1/NNSCS, and subjected to MTT assays.
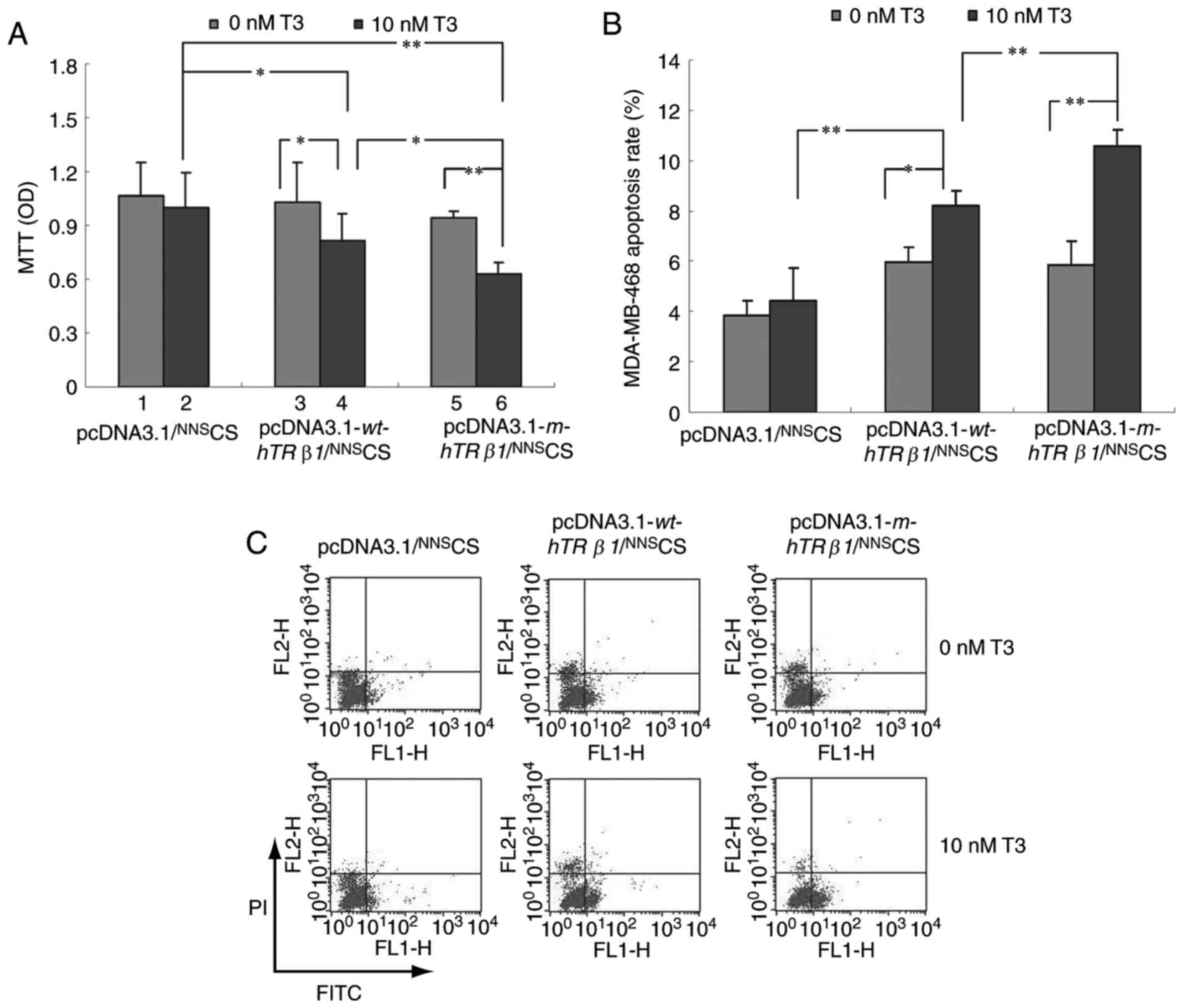
Fig. 3A depicted that T3 had no
significant effect on MDA-MB-468 cell growth. However, the
expression of wt-hTRβ1 and m-hTRβ1 inhibited cell proliferation in
the presence of T3. Furthermore, the effect of m-hTRβ1 on cell
proliferation inhibition was stronger, compared with wt-hTRβ1;
whereas, wt-hTRβ1 and m-hTRβ1 expression had no significant effect
on proliferation in the absence of T3.
To understand how wt-hTRβ1 and m-hTRβ1
overexpression inhibits cancer cell proliferation in vitro,
flow cytometry was conducted to evaluate apoptosis in MDA-MB-468
cells transfected with pcDNA3.1/NNSCS,
pcDNA3.1-wt-hTRβ1/NNSCS and
pcDNA3.1-m-hTRβ1/NNSCS. wt-hTRβ1 and
m-hTRβ1 enhanced apoptosis in the presence of 10 nM T3, and the
effects of m-hTRβ1 were stronger, compared with wt-hTRβ1. By
contrast, wt-hTRβ1 and m-hTRβ1 expression had no significant effect
on apoptosis in the absence of T3 (Fig.
3B and C).
Effects of hTRβ1 on Bak, Bax and
Caspase-3 expression in MDA-MB-468 cells
wt-hTRβ1 and m-hTRβ1 reduced cell proliferation
mainly through the promotion of apoptosis (Fig. 3). To determine the possible pathways
that wt-hTRβ1 and m-hTRβ1 act to promote apoptosis, the relative
expression levels of key apoptotic regulators, including Bak, Bax
and Caspase-3, were detected in the different transfection groups.
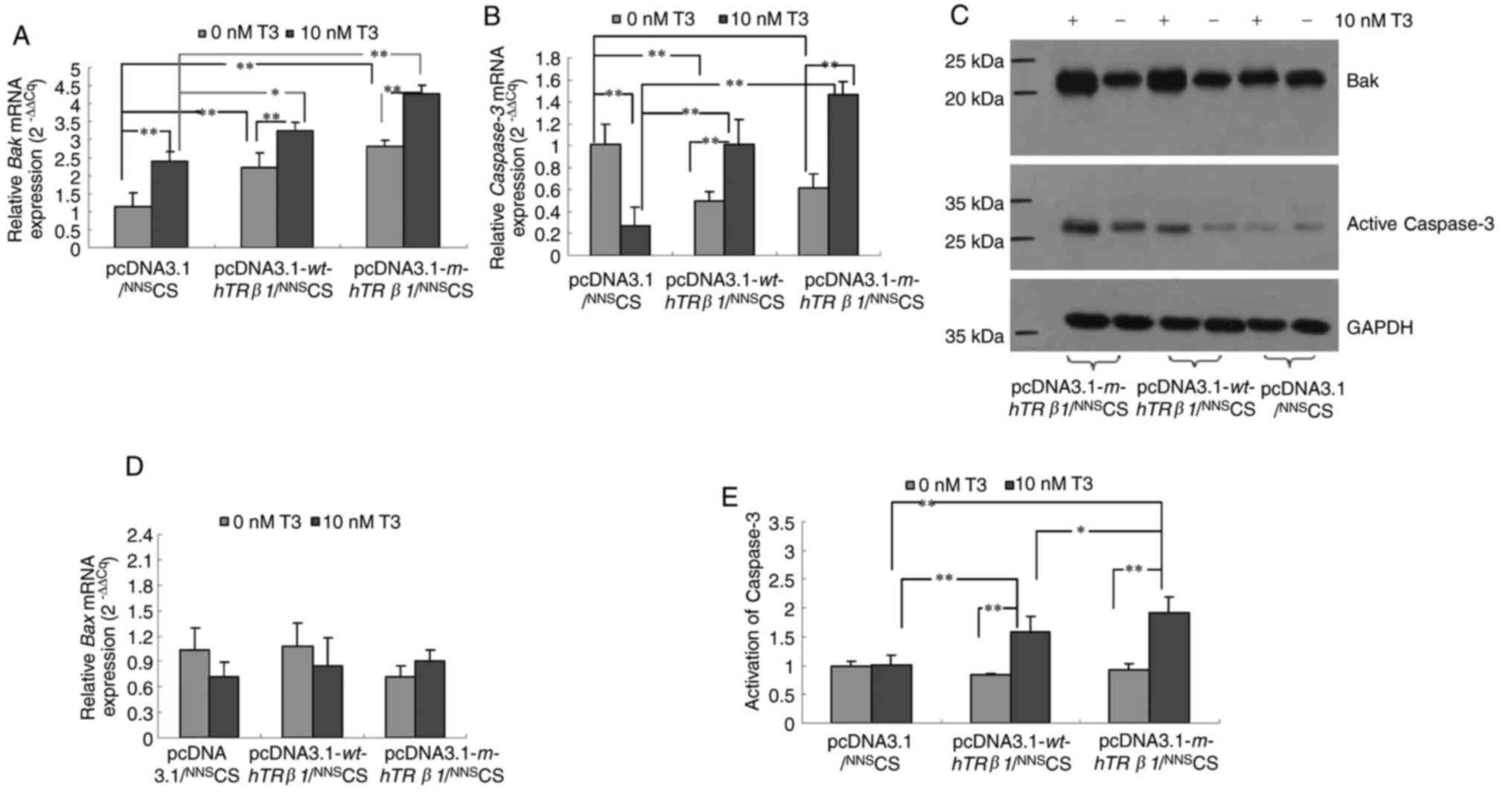
Expression of wt-hTRβ1 and m-hTRβ1 resulted in a gradual but
significant upregulation of Bak and Caspase-3 mRNA and protein
expression in the presence of T3. However, wt-hTRβ1 and m-hTRβ1
expression downregulated Caspase-3 gene expression in the absence
of T3 (Fig. 4A-C). Expression of
wt-hTRβ1 and m-hTRβ1 had no effect on Bax mRNA expression
regardless of T3 presence or absence (Fig. 4D).
hTRβ1 increases the activity of
Caspase-3 in MDA-MB-468 cells
As the expression of wt-hTRβ1 and m-hTRβ1
upregulated Caspase-3 expression in MDA-MB-468 cells in the
presence of T3 (Fig. 4B and D),
Caspase-3 activity was evaluated in these cells. Fig. 4E depicted that the expression of
wt-hTRβ1 and m-hTRβ1 also increased the activity of Caspase-3 in
the presence of T3. Increased Caspase-3 activity is indicative of
elevated apoptotic activity (17),
and elevated Bak promotes apoptosis via the activation of caspases
(18). These data indicated that
wt-hTRβ1 and m-hTRβ1 can increase the expression of Bak to promote
apoptosis through the activation of Caspase-3. In addition, the
effect of m-hTRβ1 is stronger, compared with wt-hTRβ1.
Discussion
TRs can function as a tumor suppressor (5,10).
Evidence from in vivo and in vitro studies supports
the tumor-suppressive function of TRβ1 in breast cancer (1,19,20). Nonetheless, controversial conclusions
are abundant regarding the role of thyroid hormones and their
receptor TRβ1 in the occurrence and development of breast cancer
(21). Thyroid hormones and breast
cancer and normal mammary glands (1).
Decreased expression, promoter hypermethylation or expression of
truncated TRβ1 has been observed in human breast cancer (22), and truncated TRβ1 has been associated
with cancer occurrence (23). rTRβΔ
is an extended receptor (11), and to
explore the association between the extended receptor rTRβΔ and
cancer, TRβΔ was overexpressed in rat breast cancer SHZ-88 cells
in vitro. The results demonstrated that rTRβΔ clearly
inhibited proliferation of SHZ-88 cells. Although rTRβΔ is highly
homologous to rTRβ1, the former contains a larger DBD (Fig. 1A); thus, the strong inhibitory effect
of rTRβΔ on breast cancer cells may be closely associated with this
additional 108 bp. Sequence analysis has revealed high conservation
between the DBD region of rats and humans (Fig. 1A). Accordingly, this extra 108-bp exon
from the DBD of rTRβΔ was introduced into the corresponding
location of wt-hTRβ1 by site-directed mutagenesis,
but did not change the original reading frame of
wt-hTRβ1 (Fig. 1B and
C).
wt-hTRβ1 contains an ‘EG…G’ P-box sequence in the
DBD (24), and studies have indicated
that the first two amino acids (EG) of the P-box are the most
crucial for TRE binding specificity (25,26). If
the first two amino acids are mutated, TRβ binding ability to TRE
is significantly reduced or completely lost, though the
requirements of the third amino acid are not strict (24,27). The
additional 36 amino acids were introduced to the C-terminal EG of
the P-box, and the third amino acid was changed from G to P
(Fig. 1A). In the present study, the
aim was to clarify whether artificial m-hTRβ1 is a functional
receptor and to evaluate the effect of m-hTRβ1 on MDA-MB-468
cells.
Overall, the results of the EMSA and transcriptional
activity analysis demonstrated that m-hTRβ1 can bind TREs (DR4)
with transactivation by T3. An in vitro analysis
demonstrated that the overexpression of m-hTRβ1 in MDA-MB-468 cells
could inhibit their proliferation via the promotion of apoptosis in
the presence of T3; the effect of m-hTRβ1 was stronger, compared
with wt-hTRβ1. One study has demonstrated that the levels of the
thyroid hormone T3 are elevated in the serum of patients with
breast cancer (28), where this
hormone promotes the growth and motility of breast cancer cells
(18,29,30).
However, since Beatson (31)
described the treatment of metastatic breast tumors with thyroid
extracts, a number of in vitro and in vivo studies
have demonstrated that T3 can inhibit breast tumor cell
proliferation and metastasis (1,32). These
data indicated that thyroid hormones may have a bidirectional
effect on breast cancer cells, particularly among different breast
cancer cell lines. The results indicated that T3 inhibits the
proliferation of MDA-MB-468 cells that overexpress m-hTRβ1.
Previous studies have demonstrated that TRβ
expression reduces MCF-7 tumor growth through the activation of
apoptosis in vivo (20) and
the induction of apoptosis in human breast cancer MCF-7 cells in
the presence of T3 in vitro (1). To explore the underlying mechanism that
m-hTRβ1 promotes MDA-MB-468 cell apoptosis, the expression of
pro-apoptotic Caspase-3, Bax and Bak, was detected
and the Caspase-3 activity was examined. TRβ expression induces
apoptosis in breast cancer cells via an increase in cleaved PARP
and Caspase-3 (20,33). In the present study, it was determined
that in the presence of T3, the overexpression m-hTRβ1 promoted
MDA-MB-468 cell apoptosis via the upregulation of Caspase-3
and Bak mRNA and protein expression and via an increase in
Caspase-3 activity. In addition, the pro-apoptotic effect of
m-hTRβ1 was stronger, compared with wt-hTRβ1. These results
indicated that modification of the DBD region may alter the TRβ
activation intensity of target genes.
To conclude, an artificial m-hTRβ1 was
constructed through the introduction of a novel 108-bp exon into
the DBD of wt-hTRβ1. m-hTRβ1 was functional in
MDA-MB-468 cells and inhibited MDA-MB-468 cell proliferation, as it
promoted apoptosis in the presence of T3. In the future, the role
of m-hTRβ1 in other cancer cell lines in vitro and its role
in tumorigenesis in vivo, using xenograft models, will be
ascertained. This information may provide a novel possibility of
gene therapy for TR-deficient breast cancer. Therefore, inhibition
of the growth of cancer cells (TR-deficient) and improvements in
patient survival may be possible via TR transgenes.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81770915 and
81301737) and the Shandong Provincial Natural Science Foundation,
China (grant nos. ZR2012HQ034 and ZR2015HL025).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
RLZ designed the project, and contributed to all
experiments, analysis of data and to writing the manuscript. XXP
contributed to all experiments and to writing the manuscript. YYZ,
YLS, LJW, WS and QL conducted the experiments. All authors read and
provided their approval for the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sar P, Peter R, Rath B, Das Mohapatra A
and Mishra SK: 3,3′5 Triiodo L thyronine induces apoptosis in human
breast cancer MCF-7 cells, repressing SMP30 expression through
negative thyroid response elements. PLoS One. 6:e208612011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng SY, Leonard JL and Davis PJ:
Molecular aspects of thyroid hormone actions. Endocr Rev.
31:139–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bassett JH, Harvey CB and Williams GR:
Mechanisms of thyroid hormone receptor-specific nuclear and extra
nuclearactions. Mol Cell Endocrinol. 213:1–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paquette MA, Atlas E, Wade MG and Yauk CL:
Thyroid hormone response element half-site organization and its
effect on thyroid hormone mediated transcription. PLoS One.
9:e1011552014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu L, Tian G, Yang Q, De G, Zhang Z, Wang
Y, Nie H, Zhang Y, Yang X and Li J: Thyroid hormone receptor β1
suppresses proliferation and migration by inhibiting PI3K/Akt
signaling in human colorectal cancer cells. Oncol Rep.
36:1419–1426. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ling Y, Shi X, Wang Y, Ling X and Li Q:
Down-regulation of thyroid hormone receptor β1 gene expression in
gastric cancer involves promoter methylation. Biochem Biophys Res
Commun. 444:147–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosignolo F, Maggisano V, Sponziello M,
Celano M, Di Gioia CR, D'Agostino M, Giacomelli L, Verrienti A,
Dima M, Pecce V and Durante C: Reduced expression of THRβ in
papillary thyroid carcinomas: Relationship with BRAF mutation,
aggressiveness and miR expression. J Endocrinol Invest.
38:1283–1289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guigon CJ, Kim DW, Willingham MC and Cheng
SY: Mutation of thyroid hormone receptor-β in mice predisposes to
the development of mammary tumors. Oncogene. 30:3381–3390. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JW, Zhao L, Willingham M and Cheng
SY: Oncogenic mutations of thyroid hormone receptor β. Oncotarget.
6:8115–8131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim WG, Zhao L, Kim DW, Willingham MC and
Cheng SY: Inhibition of tumorigenesis by the thyroid hormone
receptor β in xenograft models. Thyroid. 24:260–269. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao RL, Sun B, Liu Y, Li JH, Xiong WL,
Liang DC, Guo G, Zuo AJ and Zhang JY: Cloning and identification of
a novel thyroid hormone receptor β isoform expressed in the
pituitary gland. Mol Cell Biochem. 389:141–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao RL, Song W, Sun YL, Li Q, Li M, Chu
HR and Peng XX: Effects of thyroid hormone receptor βΔ on apoptosis
and proliferation of hepatoma RH-35 cells. Chin J Endocrinol Metab.
32:691–695. 2016.
|
|
13
|
Zhang YY, Peng XX, Sun YL, Song W and Zhao
RL: Effect of thyroid hormone receptor βΔ on proliferation and
apoptosis of rat breast cancer cells SHZ-88. Cancer Res Prev Treat.
43:1018–1022. 2016.
|
|
14
|
Zhao R, Sun B, Liu T, Liu Y, Zhou S, Zuo A
and Liang D: Optimize nuclear localization and intra-nucleus
disassociation of the exogenefor facilitating transfection efficacy
of the chitosan. Int J Pharm. 413:254–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jones I, Srinivas M, Ng L and Forrest D:
The thyroid hormone receptor beta gene: Structure and functions in
the brain and sensory systems. Thyroid. 13:1057–1068. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cryns V and Yuan J: Proteases to die for.
Genes Dev. 12:1551–1570. 1988. View Article : Google Scholar
|
|
18
|
Guo W, Zhang Y, Ling Z, Liu X, Zhao X,
Yuan Z, Nie C and Wei Y: Caspase-3 feedback loop enhances
Bid-induced AIF/endoG and Bak activationin Bax and p53-independent
manner. Cell Death Dis. 6:e19192015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ruiz-Llorente L, Ardila-González S, Fanjul
LF, Martínez-Iglesias O and Aranda A: microRNAs 424 and 503 are
mediators of the anti-proliferative and anti-invasive action of the
thyroid hormone receptor beta. Oncotarget. 5:2918–2933. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park JW, Zhao L and Cheng SY: Inhibition
of estrogen-dependent tumorigenesis by the thyroid hormonereceptor
β in xenograft models. Am J Cancer Res. 3:302–311. 2013.PubMed/NCBI
|
|
21
|
de Sibio MT, de Oliveira M, Moretto FC,
Olimpio RM, Conde SJ, Luvizon AC and Nogueira CR: Triiodothyronine
and breast cancer. World J Clin Oncol. 5:503–508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Z, Meng ZH, Chandrasekaran R, Kuo WL,
Collins CC, Gray JW and Dairkee SH: Biallelic inactivation of the
thyroid hormone receptor beta1 gene in earlystage breast cancer.
Cancer Res. 62:1939–1943. 2002.PubMed/NCBI
|
|
23
|
Jazdzewski K, Boguslawska J, Jendrzejewski
J, Liyanarachchi S, Pachucki J, Wardyn KA, Nauman A and de la
Chapelle A: Thyroid hormone receptor beta (THRB) is a major target
gene for microRNAs deregulated in papillary thyroid carcinoma
(PTC). J Clin Endocrinol Metab. 96:E546–E553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nelson CC, Hendy SC, Faris JS and Romaniuk
PJ: Retinoid X receptor alters the Determination of DNA binding
specificity by the P-box amino acids of the thyroid hormone
receptor. J Biol Chem. 271:19464–19474. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Figueira AC, Lima LM, Lima LH, Ranzani AT,
Mule Gdos S and Polikarpov I: Recognition by the thyroid hormone
receptor of canonical DNA response elements. Biochemistry.
49:893–904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Berglund H, Wolf-Watz M, Lundbäck T, van
den Berg S and Härd T: Structure and dynamics of the glucocorticoid
receptor DNA-binding domain: Comparison of wild type and a mutant
with altered specificity. Biochemistry. 36:11188–11197. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shibusawa N, Hashimoto K, Nikrodhanond AA,
Liberman MC, Applebury ML, Liao XH, Robbins JT, Refetoff S, Cohen
RN and Wondisford FE: Thyroid hormone action in the absence of
thyroid hormone receptor DNA-binding in vivo. J Clin Invest.
112:88–97. 2003. View Article : Google Scholar
|
|
28
|
Shi XZ, Jin X, Xu P and Shen HM:
Relationship between breast cancer and levels of serum thyroid
hormones and antibodies: A meta-analysis. Asian Pac J Cancer Prev.
15:6643–6647. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rasool M, Naseer MI, Zaigham K, Malik A,
Riaz N, Alam R, Manan A, Sheikh IA and Asif M: Comparative study of
alterations in Tri-iodothyronine (T3) and Thyroxine (T4) hormone
levels in breast and ovarian cancer. Pak J Med Sci. 30:1356–1360.
2014.PubMed/NCBI
|
|
30
|
Flamini MI, Uzair ID, Pennacchio GE, Neira
FJ, Mondaca JM, Cuello-Carrión FD, Jahn GA, Simoncini T and Sanchez
AM: Thyroid hormone controls breast cancer cell movement via
integrin αV/β3/SRC/FAK/PI3-Kinases. Horm Cancer. 8:16–27. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beatson GT: On the etiology of cancer,
with a note of some experiments. Br Med J. 1:399–400. 1899.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ruiz-Llorente L, Martínez-Iglesias O,
García-Silva S, Tenbaum S, Regadera J and Aranda A: The thyroid
hormone receptors as tumor suppressors. Horm Mol Biol Clin
Investig. 5:79–89. 2011.PubMed/NCBI
|
|
33
|
Gu G, Gelsomino L, Covington KR, Beyer AR,
Wang J, Rechoum Y, Huffman K, Carstens R, Andò S and Fuqua SA:
Targeting thyroid hormone receptor beta in triple-negative breast
cancer. Breast Cancer Res Treat. 150:535–545. 2015. View Article : Google Scholar : PubMed/NCBI
|