Introduction
Osteosarcoma is the most common type of primary bone
malignancy and is frequently diagnosed in children and adolescents
(1). A large proportion of patients
diagnosed with osteosarcoma will develop distant metastasis
(2). Owing to the development of
multidisciplinary therapy, the mortality rate for patients with
osteosarcoma has been decreasing in recent years. Nevertheless,
patients continue to exhibit a high risk of metastasis and/or
recurrence (1,2). Previous studies have indicated that
several genetic aberrations are associated with tumor initiation
and osteosarcoma progression (3,4). Thus,
identifying the underlying mechanisms that lead to the development
and progression of osteosarcoma is crucial.
Disabled homolog 2-interacting protein (DAB2IP),
located at chromosome location 9q33.1-q33.3, is a member of the Ras
GTPase family (5–7). Downregulation of DAB2IP is frequently
detected in various types of human cancer, including
gastrointestinal cancer, breast tumor, prostate carcinoma,
pulmonary tumor, pancreatic cancer, hepatocellular cancer and
medulloblastoma (6,8–16). Loss of
DAB2IP expression may facilitate cell proliferation and restrain
cancer cell metastasis through several pathways, including
Ras-extracellular-related kinase, apoptosis signal-regulating
kinase 1 (ASK1)-c-Jun N-terminal kinase (JNK) and
phosphoinositide-3 kinase (PI3K)-Akt (6,7,17–19).
DAB2IP suppression may induce resistance to ionizing radiation and
chemoresistance in prostate cancer and nonmuscle invasive bladder
cancer (20–23). Furthermore, a role is hypothesized for
DAB2IP in normal brain development and vascular inflammation
(24–29), and the loss of DAB2IP may promote the
epithelial-mesenchymal transition (EMT) and induce cancer stem
cells to facilitate colorectal carcinoma progression and metastasis
(16,30).
Although its role as a potent tumor suppressor in
several carcinomas has been demonstrated (8–16), the
expression and biological function of DAB2IP in osteosarcoma remain
uncharacterized. In the present study, the endogenous expression of
DAB2IP and its role in osteosarcoma cell lines were assessed, as
was the effect of DAB2IP expression on cell progression and
motility in osteosarcoma cells.
Materials and methods
Cell lines and cell culture
The osteosarcoma MG-63 and HOS cell lines an
osteoblast hFOB 1.19 cell line and the human embryonic kidney 293T
cells were all purchased from the Cell Bank of Type Culture
Collection of Chinese Academy of Sciences (Shanghai, China). MG-63,
HOS and 293T cells were cultured in high-glucose Dulbecco's
modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). A DMEM/Ham's F12 (Thermo Fisher Scientific,
Inc.) mix was used to culture hFOB 1.19 cells. Media contained 10%
fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.) and
antibiotics, including 100 U/ml penicillin and 100 µg/ml
streptomycin. Cells were cultured at 37°C and 5% CO2 in
a humidified incubator.
Plasmid construction, lentivirus
infection and establishment of stably transfected cell lines
The pLEX DAB2IP, pLEX Control, DAB2IP short hairpin
(sh)RNA pLKO.3G and scrambled shRNA pLKO.3 G plasmids were gifts
from Professor Wang Min (Yale University, New Haven, CT, USA). The
DAB2IP shRNA sequence was as follows:
5′-TAAAAAAAGCCTTATTTACCTAGTGCAAACTCGAGTTTGCACTAGGTAAATAAGGC-3′. The
scrambled shRNA sequence was as follows:
5′-GACTATCATATGCTTACCGT-3′. The target vectors (1.2 µg) were mixed
with 1.2 µg lentivirus packaging helper plasmids pCMV-dR8.2
(Addgene, Cambridge, MA, USA) and 0.6 µg pCMV-VSVG plasmids
(Addgene), and the mixed vectors were diluted in 120 µl Opti-MEM
(Thermo Fisher Scientific, Inc.). Following this, 5 µl P3000
Reagent was added (Invitrogen; Thermo Fisher Scientific, Inc.) to
the final volume of 125 µl. Meantime, 7.5 µl
Lipofectamine® 3000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was diluted into Opti-MEM medium to a final
volume 125 µl. Then, the diluted vectors and diluted Lipofectamine
3000 reagent were mixed, and incubated for 15 min at room
temperature. The complex was added into ~70% confluence 293T cells
in the 6-wells plate. Viruses were harvested 48 h after
transfection and subsequently used to infect HOS and hFOB 1.19
cells, with puromycin (Thermo Fisher Scientific, Inc.) used to
select the positive clones. The stable cell lines obtained were
correspondingly designated as HOS pLEX DAB2IP, HOS pLEX Control;
hFOB 1.19 DAB2IP shRNA, hFOB 1.19 scrambled shRNA; hFOB 1.19 DAB2IP
shRNA-pLEX DAB2IP and hFOB 1.19 DAB2IP shRNA-pLEX. The effect of
the overexpression and knockdown on DAB2IP were assessed by western
blot analysis.
Western blot analysis
Cultured MG-63, HOS and hFOB 1.19 cells were
collected and washed twice with 1 ml of PBS. Following cell lysis
with protein lysis buffer (50 mM Tris (pH 7.4), 2 mM EDTA, 150 mM
NaCl, 1 mM Na3VO4, 1% Triton X-100, 20 mM
NaF, 10 mM Na4P2O7, 10 mg/ml
aprotinin), cells were collected and centrifuged at 4,000 × g for
15 min at 4°C. Protein concentration was quantified using the
Bradford assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Equivalent quantities of protein (20 µg/lane) were resolved using
SDS-PAGE on an 8% gel and transferred to polyvinylidene fluoride
membranes. Membranes were blocked using PBS with 0.1% Tween 20
(PBST) containing 5% skimmed milk powder for 2 h at room
temperature, and incubated with primary antibodies against DAB2IP
(cat. no. 48–7300; Invitrogen, Thermo Fisher Scientific, Inc.;
1:1,000) and GADPH (cat. no. 2118; Cell Signaling Technology,
Danvers, MA, USA; 1:1,000) overnight at 4°C. Subsequent to washing
with PBST, the membranes were incubated 2 h at room temperature
with goat anti-rabbit immunoglobulin G secondary antibodies
conjugated with horseradish peroxidase (cat. no. 7074; Cell
Signaling Technology; 1:1,000).
Cell proliferation assay
An MTS cell proliferation assay was performed using
the AQueous One solution Cell Proliferation Assay kit (Promega
Corporation, Madison, WI, USA) to assess cell proliferative
activity. HOS, hFOB 1.19 and hFOB 1.19 DAB2IP shRNA cells were
plated at density of 10,000 cells per well in 96-well plates. After
0, 24, 48, 72 or 96 h, 20 µl of MTS was added into each well, and
incubated at 37°C for 3 h. To measure the absorbance values, a
microplate reader was used, set at 490 nm. Three independent
experiments were performed.
Colony formation assay
Approximately 1,000 HOS, hFOB 1.19 or hFOB 1.19
DAB2IP shRNA cells were seeded in a 35-mm dish and incubated for 10
days at 37°C. Formed colonies were then fixed with 100% methanol
for 20 min and stained with 0.1% crystal violet for 30 min at room
temperature. The number of colonies composed of >50 cells per
dish was counted under a light microscope. Each experiment was
repeated three times.
Cell apoptosis assay
Osteosarcoma cell apoptosis following lentiviral
transduction was examined using an Annexin V/propidium iodide (PI)
kit; osteoblast apoptosis was detected using an APC/PI kit (both
eBioscience; Thermo Fisher Scientific, Inc.). According to the
manufacturer's protocol, cells were collected and resuspended in
200 µl of 1X binding buffer at a concentration of 1×106
cells and stained with 5 µl annexin V-fluorescein isothiocitrate or
APC dye, followed by staining with 5 µl PI. The proportion of cells
undergoing apoptosis was analyzed by a flow cytometer (eBioscience;
Thermo Fisher Scientific, Inc.), and the results were analyzed
using FlowJo 7.6.2 software (Tree Star, Inc., Ashland, OR, USA).
Three independent experiments were performed.
Cell cycle assay
HOS, hFOB 1.19 and hFOB 1.19 DAB2IP shRNA cells
(1×106 cells/100-mm dishes) were plated in the
appropriate FBS-free medium and incubated for 24 h at 37°C.
Following a further 24 h of incubation at 37°C in medium containing
FBS, cells were collected and resuspended in 500 µl of cold PBS,
then stained with 25 µl of PI in the dark for 30 min at 4°C. Cells
were analyzed for cell cycle distribution using a flow cytometer
(BD Biosciences, Franklin Lakes, NJ, USA). ModFit LT3.1 software
(Verity Software House, Inc., Topsham, ME, USA) was used to analyze
the results. Each experiment was repeated three times.
Migration and invasion assays
Cell migration and invasion rates were determined as
described in previously published methods (31–33) in
HOS, hFOB 1.19 and hFOB 1.19 DAB2IP shRNA cells. To study
migration, 2×104 cells were seeded into each well of a
96-well plate. When cells had reached 80–90% confluence, a wound
was generated using the Cellplayer 96-well Woundmaker (Essen
BioScience, Inc., Ann Arbor, MI, USA). The images of cells were
automatically acquired every 2 h for 24 h using the Incucyte
LiveCell Imaging system (Essen BioScience, Inc.).
To study invasion, a scratch was made by using the
Cellplayer 96-well Woundmaker, when cells had reached 80–90%
confluence. Next, the wound was covered with 50 µl of Matrigel
solution (1 mg/ml Matrigel in PBS) that was allowed to set for 1 h
at 37°C. Appropriate growth medium (100 µl) was added and
representative images were recorded at 2 h intervals for the
duration of the experiment (24 h). All images were processed using
the IncuCyte™ software package version 20151.1 (Essen BioScience,
Inc.) to measure cell migration and invasion by obtaining the
relative wound density.
Statistical analysis
Data are presented as the mean ± standard deviation.
Comparisons between two groups were performed using unpaired
Student's t-tests. Multiple group comparison was performed using
one-way analysis of variance. And the post hoc test was Bonferroni
correction. P<0.05 was considered to indicate a statistically
significant difference. Statistical analyses were performed using
the commercially available packages PASW statistics 18.0 (SPSS Inc,
Chicago, IL, USA) and GraphPad Prism 6 (GraphPad Software, La
Jolla, CA, USA).
Results
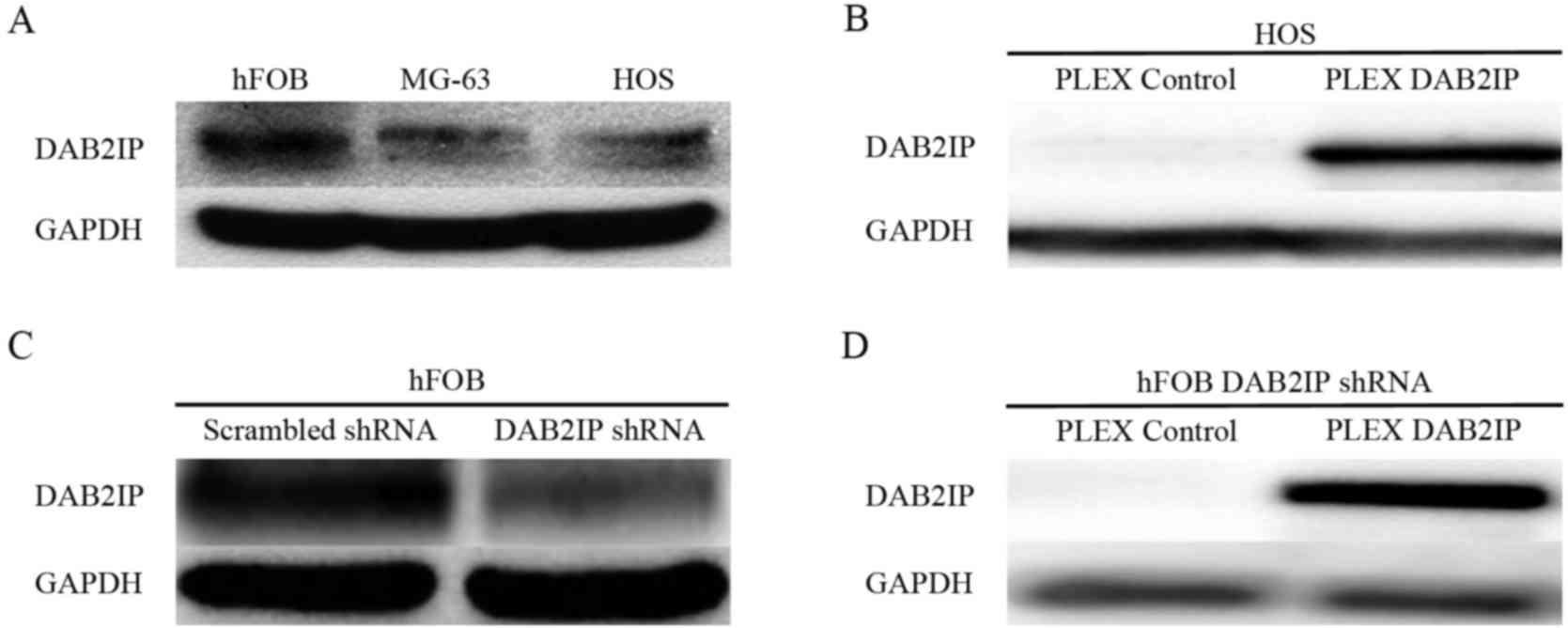
DAB2IP is downregulated in
osteosarcoma cell lines
Western blotting was performed to assess endogenous
DAB2IP expression levels in the normal human osteoblast cell line,
hFOB 1.19, and the human osteosarcoma MG63 and HOS cell lines.
DAB2IP expression was downregulated in the osteosarcoma cell lines
compared with the normal osteoblast cell line (Fig. 1A).
To examine the effects of DAB2IP expression in
osteosarcoma, DAB2IP was overexpressed or silenced in HOS cells.
Marked differences in DAPB2IP expression were observed between the
pLEX control and pLEX DAB2IP cells (Fig.
1B). The shRNA-mediated interference of DAB2IP (Fig. 1C) and its expression (Fig. 1D) through lentivirus infection were
confirmed.
DAB2IP inhibits cell proliferation and
colony formation
MTS and colony formation assays were performed to
determine the effects of ectopic DAB2IP expression on cell growth
in HOS pLEX DAB2IP cells and controls (Fig. 2). As demonstrated in Fig. 2A, the proliferation of HOS cells
transfected with pLEX DAB2IP was significantly reduced compared to
HOS cells transfected with a vector control. By contrast, it was
observed that the proliferation was significantly increased when
DAB2IP expression was knocked down in hFOB cells (Fig. 2B), and the restoration of DAB2IP
expression in DAB2IP-shRNA-treated hFOB cells suppresses osteoblast
proliferation (Fig. 2C). The number
of colonies formed by HOS cells transfected with pLEX DAB2IP was
decreased compared with the control group (Fig. 2D). Similar to the proliferation
results, the colony formation abilities of the DAB2IP-shRNA-treated
cells was significantly increased compared with those treated with
scrambled shRNA (Fig. 2E), and
restoring DAB2IP expression suppressed the colony formation ability
of the osteoblast cells (Fig.
2F).
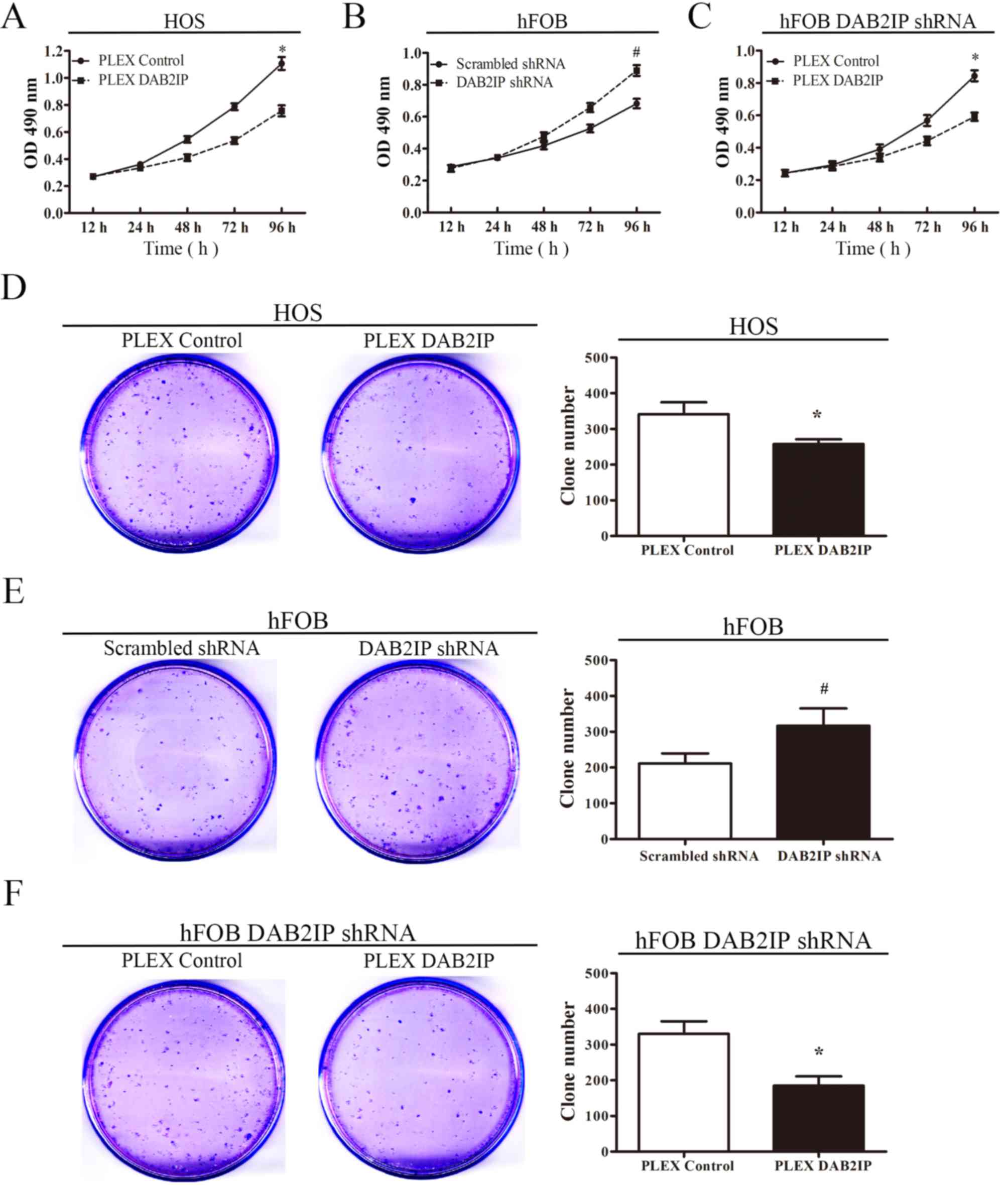 | Figure 2.Effect of DAB2IP overexpression and
silencing on the in vitro cell proliferation and colony
formation of osteosarcoma and osteoblast cells. Effect of DAB2IP on
the proliferation of (A) HOS, (B) hFOB 1.19 and (C) hFOB 1.19 pLEX
DAB2IP cells, as determined by an MTS cell proliferation assay.
Effect of DAB2IP on colony formation by (D) HOS, (E) hFOB 1.19 and
(F) hFOB 1.19 pLEX DAB2IP cells. *P<0.05, comparing HOS PLEX
control cells with HOS PLEX DAB2IP cells in A and D.
#P<0.05, comparing hFOB Scrambled shRNA cells with
hFOB DAB2IP shRNA cells in B and E. *P<0.05, comparing hFOB
DAB2IP shRNA PLEX control cells with hFOB DAB2IP shRNA PLEX DAB2IP
cells in C and F. DAB2IP, disabled homolog 2 interactive protein;
shRNA, short hairpin RNA; OD, optical density; pLEX DAB2IP, plasmid
containing DAB2IP. |
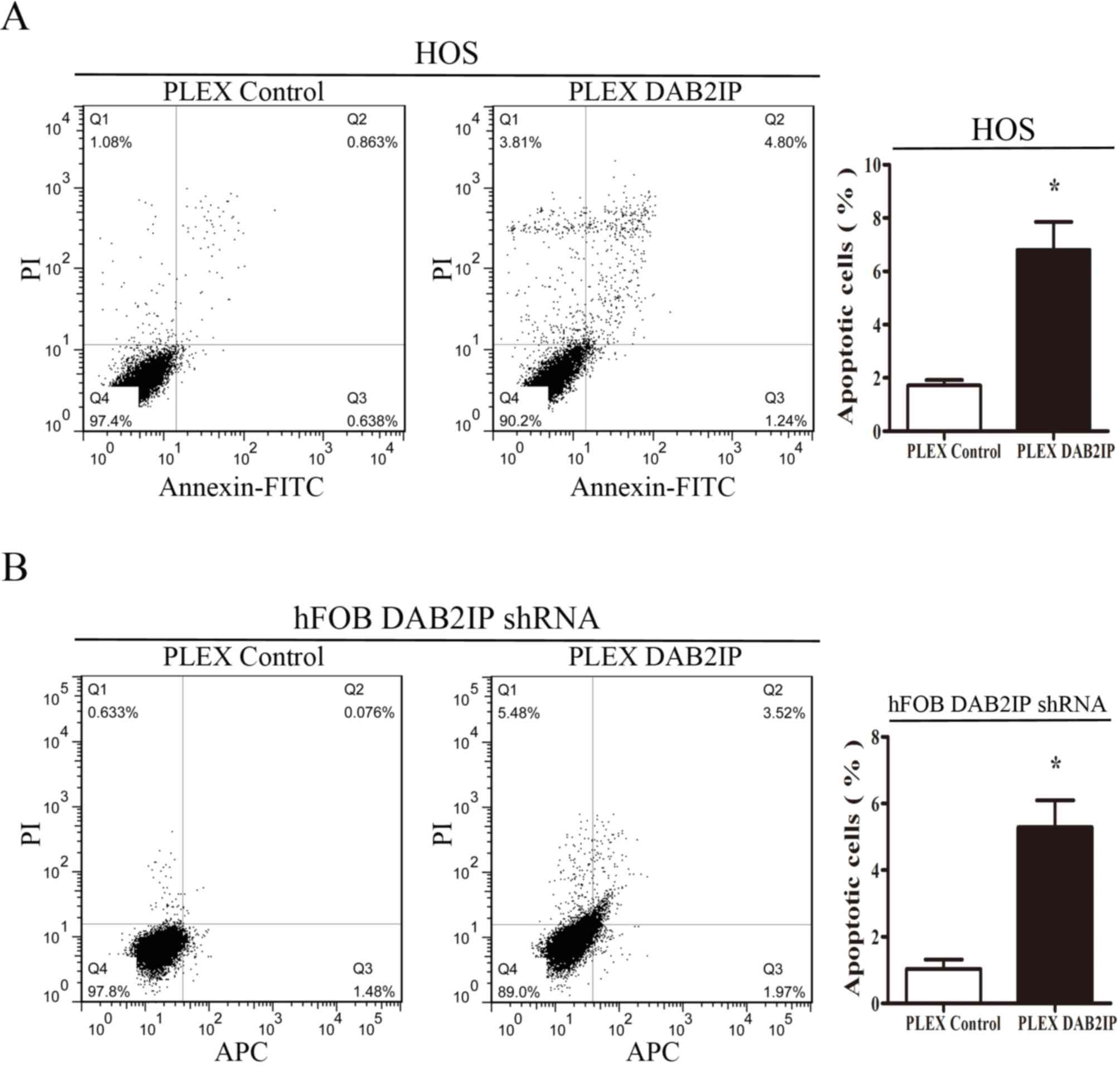
DAB2IP promotes bone cell apoptosis
and increases G0/G1 phase distribution
To determine whether DAB2IP could induce apoptosis
in osteosarcoma cells, the cell cycle distribution of HOS pLEX and
HOS pLEX DAB2IP cells was evaluated by flow cytometry. As presented
in Fig. 3A, the rate of apoptosis in
HOS pLEX DAB2IP cells was significantly higher than control HOS
pLEX cells (6.804±1.051% vs. 1.717±0.208%, respectively).
Similarly, upregulation of DAB2IP induced apoptosis in pLEX-DAB2IP
cells compared with control cells (5.291±0.808% vs. 1.038±0.277%,
respectively; Fig. 3B). These
findings suggested that DAB2IP induces cell apoptosis in
osteosarcoma cells and osteoblasts.
In order to further assess the mechanism by which
DAB2IP affects cell proliferation, cell cycle progression was
determined using flow cytometry. As shown in Fig. 3B, a significantly greater proportion
of HOS pLEX DAB2IP cells were in the G0/G1 phase compared with the
control cells (47.543±1.810% vs. 39.940±1.628%, respectively;
Fig. 4A). By contrast, downregulation
of DAB2IP expression in hFOB 1.19 cells resulted in a greater
proportion of cells in S-phase than the hFOB 1.19 Scrambled shRNA
cells (47.893±2.409% vs. 37.277±2.247%, respectively; Fig. 4B). However, the G0/G1 phase
distribution of pLEX DAB2IP cells was higher than pLEX control
cells (43.097±5.997% vs. 31.450±3.922%), with fewer cells in S
phase (39.613±3.964%; Fig. 4C).
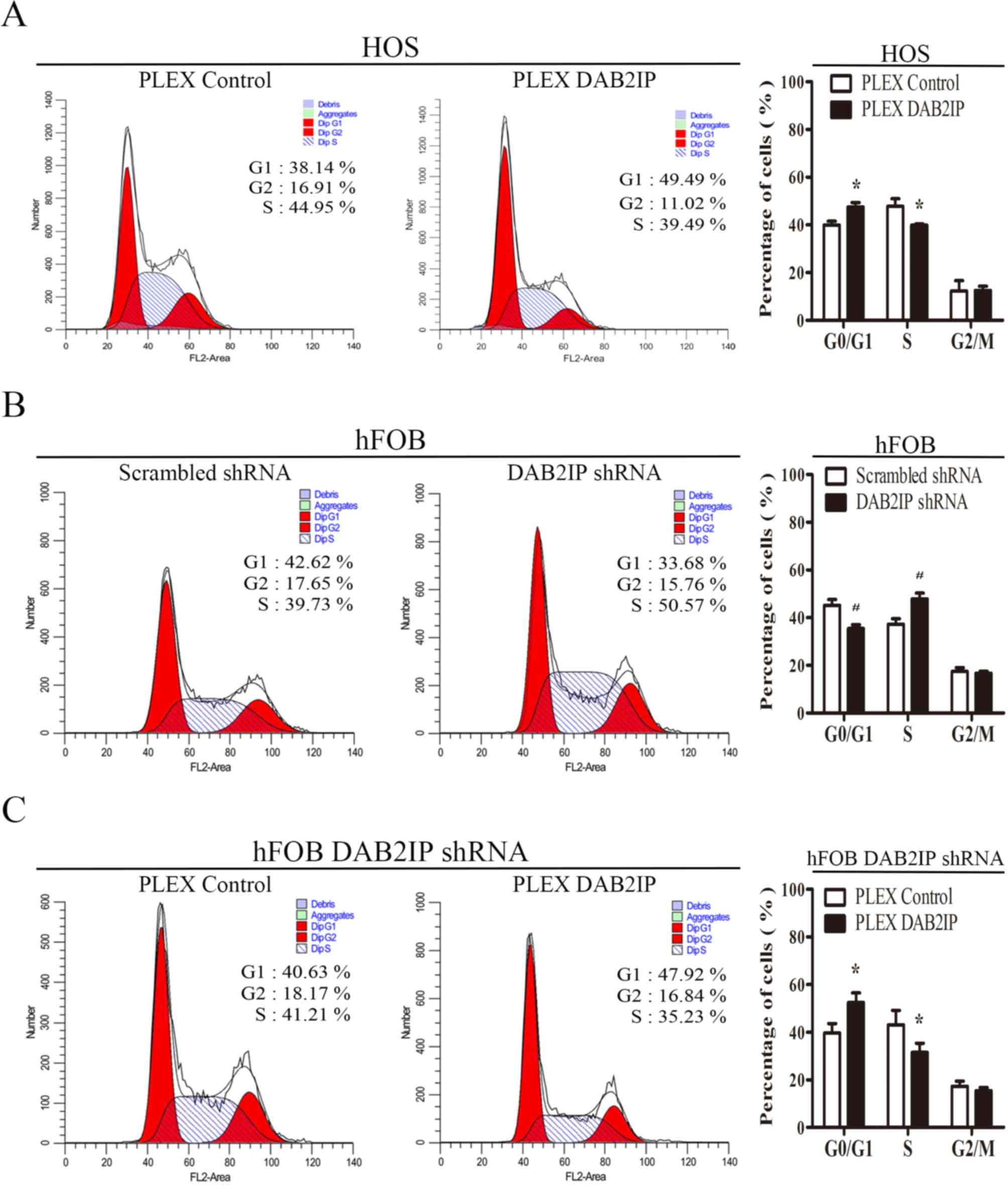 | Figure 4.Effects of DAB2IP expression on the
cell cycle distribution of osteosarcoma cells and normal
osteoblasts. (A) Effect of DAB2IP expression on cell-cycle phase
distribution of HOS osteosarcoma cells, as determined by flow
cytometry. (B and C) Effect of DAB2IP expression on the cell-cycle
phase distribution of hFOB 1.19 osteoblasts, as determined by flow
cytometry. Representative images are on the left, and
quantification on the right. All results are representative of
three independent experiments. *P<0.05, comparing HOS PLEX
control cells with HOS PLEX DAB2IP cells. *P<0.05, comparing
hFOB Scrambled shRNA cells with hFOB DAB2IP shRNA cells.
*P<0.05, comparing hFOB DAB2IP shRNA PLEX control cells with
hFOB DAB2IP shRNA PLEX DAB2IP cells. DAB2IP, disabled homolog 2
interactive protein; shRNA, short hairpin RNA; shRNA, short hairpin
RNA; pLEX DAB2IP, cells transfected with plasmid expressing
DAB2IP. |
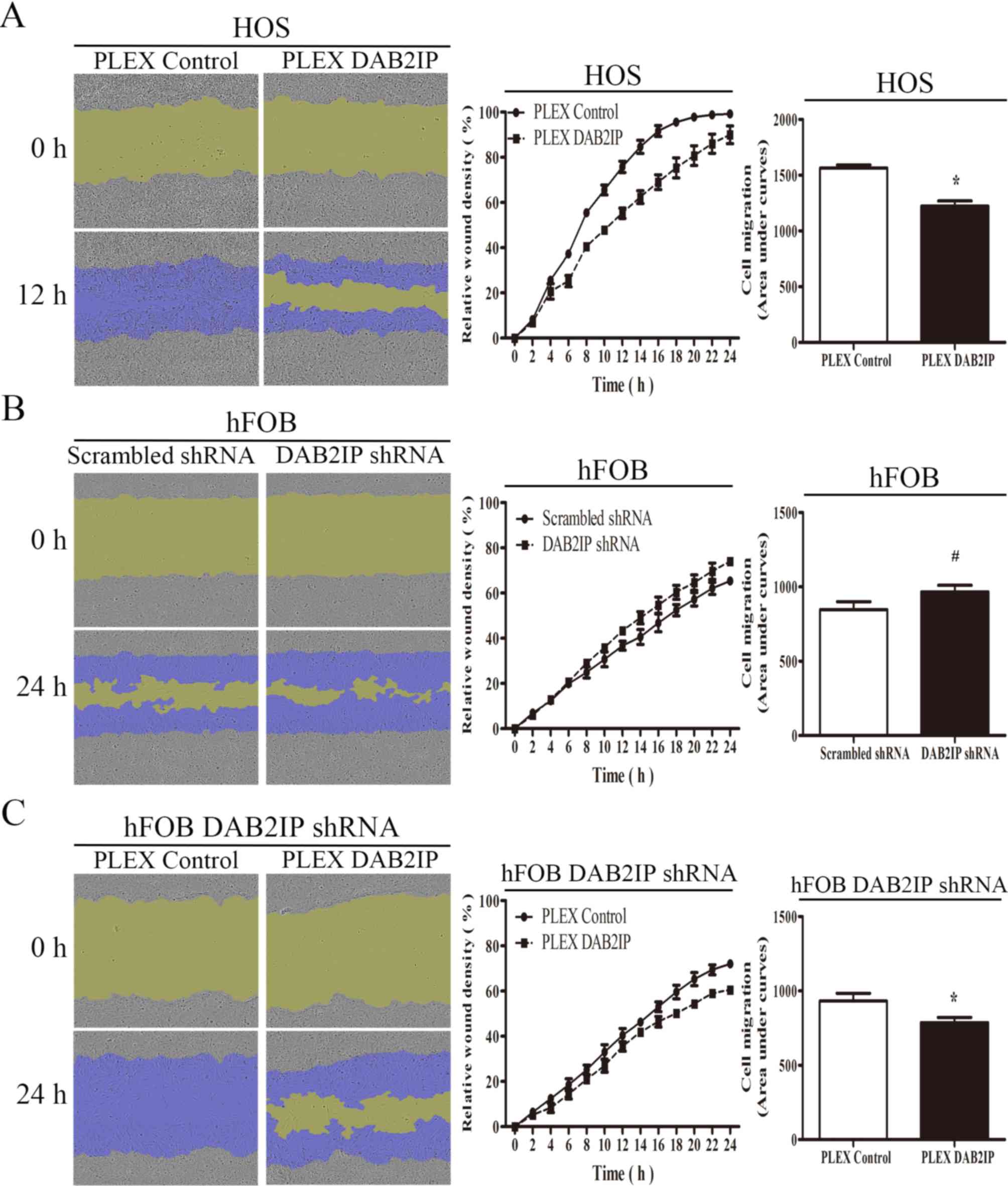
DAB2IP suppresses cell migration and
invasiveness
Given that DAB2IP can inhibit the proliferation of
osteosarcoma and normal osteoblasts, its function on cell motility
was assessed. The effect of DAB2IP on cell migration was examined
using a scratch wound-healing assay, with cell migration measured
by determining the relative wound density. The migration rate of
HOS pLEX DAB2IP cells was significantly lower than that observed
for HOS pLEX control cells (Fig. 5A).
However, downregulation of DAB2IP expression in hFOB 1.19
immortalized human osteoblasts induced migration (Fig. 5B). hFOB 1.19 DAB2IP shRNA cells
exhibited significantly reduced cell migration compared with
control transfected cells (Fig.
5C).
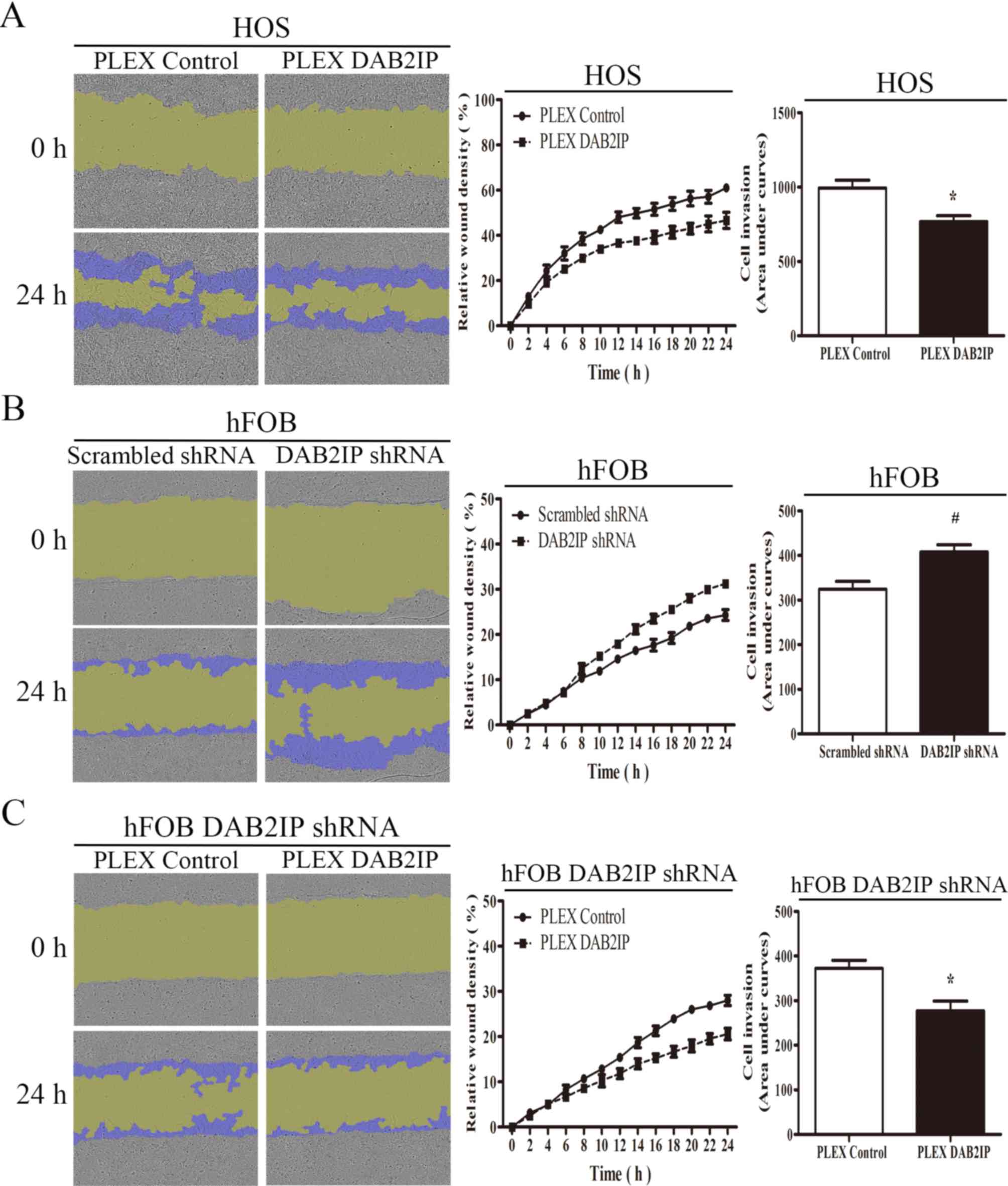
The effect of DAB2IP on the invasive potential of
osteosarcoma and normal osteoblasts was then investigated. As
presented in Fig. 6A, the
upregulation of DAB2IP could inhibit the osteosarcoma HOS cell
invasion into Matrigel, whereas the downregulation of DAB2IP in
hFOB 1.19 cells promoted cell invasion through the Matrigel
barrier, with restoration of DAB2IP expression suppressing cell
invasion when compared with hFOB 1.19 DAB2IP shRNA cells (Fig. 6B and C, respectively). Collectively,
these results demonstrated that DAB2IP inhibits cell migration and
invasion in osteosarcoma cell lines.
Discussion
In the present study, a lentivirus
transfection-mediated approach was used to investigate the role of
DAB2IP in osteosarcoma cells and osteoblasts. Overexpression of
DAB2IP led to a significant change in proliferation, apoptosis,
cell cycle distribution, migration and invasion in the HOS cell
line. Downregulation of DAB2IP in hFOB 1.19 cells confirmed that it
promoted proliferation, with restoration of its expression
resulting in the suppression of cell growth.
DAB2IP is a member of the Ras GTPase family that was
originally identified in prostate cancer (5). To date, its downregulation has been
observed in a range of types of human malignancy, including in
cancer of the prostate, breast, liver, pancreas, gastrointestinal
tract and bladder (6,8–16). In
addition, downregulation of DAB2IP gene expression, predominantly
due to epigenetic modification, was associated with unfavorable
tumor characteristics and outcomes in cancer of the prostate,
bladder, gastrointestinal tract and liver (8–11,34,35). Two
genome-wide association studies of aggressive prostate cancer
suggested that DAB2IP is a putative prostate tumor-suppressor gene
(36). In the present study,
differential DAB2IP expression was confirmed through western blot
analysis, suggesting that DAB2IP may function as a tumor suppressor
gene.
DAB2IP can facilitate the dissociation of 14–3-3
proteins from ASK1, leading to enhanced ASK1-JNK activation in
response to tumor necrosis factor (TNF) (7). DAB2IP also associates with TNF
receptor-associated factor 2 (TRAF2) and mediates TNF/TRAF2-induced
ASK1-JNK activation while inhibiting IκB kinase/nuclear factor κB
(NF-κB) signaling (24). Furthermore,
DAB2IP induces G0/G1 cell cycle arrest and promotes apoptosis
through suppression of the PI3K-Akt pathway accompanied with
activating ASK-JNK signaling in prostate cancer cells (18). In the present study, DAB2IP expression
was downregulated in osteosarcoma cell lines compared with normal
human osteoblasts. Gain- and loss-of-function approaches were
employed to investigate the effect of DAB2IP expression on
cell-cycle distribution and apoptosis. Overexpression of DAB2IP led
to an increased rate of apoptosis and induced G0/G1 cell cycle
arrest, as reported in a previous study (18). Therefore, DAB2IP may inhibit cell
growth by inducing apoptosis and inhibiting cell cycle
progression.
The majority of osteosarcoma patients develop
metastasis, and outcomes for these patients remain poor (37–39).
Previous studies suggest that DAB2IP may regulate cancer cell
metastasis (13,15,16,19,40).
A previous study revealed that loss of DAB2IP facilitated EMT,
leading to prostate cancer metastasis via the GSK-3β-β-catenin
signaling pathway (19). Min et
al (41) identified that the
epigenetic silencing of DAB2IP by EZH2 promoted tumorigenesis and
distant metastasis by Ras and NF-κB activation. Further previous
studies revealed that DAB2IP could restrain tumor growth and
metastasis by suppressing tumor angiogenesis, pre-metastatic niche
formation and tumor EMT initiation via inhibition of vascular
endothelial growth factor receptor 2-dependent signaling in the
tumor niche (42). In the present
study, an increase in DAB2IP expression in osteosarcoma cells
significantly reduced cancer cell migration and invasion. The
decreased expression of DAB2IP in osteoblasts enhanced their
migration and invasion. Furthermore, the restoration of DAB2IP
expression may suppress cell migration and invasion.
DAB2IP acts as a tumor suppressor in osteosarcoma,
inhibiting cell proliferation and survival in osteosarcoma cells
and osteoblasts by inducing apoptosis and a greater G0/G1-phase
distribution. Additionally, the expression of DAB2IP may inhibit
the migration and invasion of osteosarcoma cells and normal
osteoblasts. The identification of these roles for DAB2IP in
osteosarcoma may be beneficial, enriching the understanding of the
mechanism of osteosarcoma progression, and possibly providing an
approach for the early detection of osteosarcoma and novel
therapeutic modalities.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81472999 and
81272350), the Key Natural Science Foundation of Guangdong (grant
no. 2015A030311038) and the Science and Technology Planning Project
of Guangdong Province (grant no. 2014A020212064).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH conducted the experiments and participated in
analyzing the experiment data, and also was a major contributor in
writing the manuscript. SH participated in analyzing the experiment
data particularly the cell migration and invasion assay, and also
participated in writing the manuscript. ZL, JZ and JS participated
in performing the experiment and analyzing the experiment data. WJ
and XL designed the experiment and participated in analyzing the
experiment data particularly the cell cycle assay and the cell
apoptosis assay, and also participated in writing the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan ML, Choong PF and Dass CR:
Osteosarcoma: Conventional treatment vs. Gene therapy. Cancer Biol
Ther. 8:106–117. 2009. View Article : Google Scholar
|
|
3
|
Broadhead ML, Clark JC, Myers DE, Dass CR
and Choong PF: The molecular pathogenesis of osteosarcoma: A
review. Sarcoma. 2011:9592482011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He JP, Hao Y, Wang XL, Yang XJ, Shao JF,
Guo FJ and Feng JX: Review of the molecular pathogenesis of
osteosarcoma. Asian Pac J Cancer Prev. 15:5967–5976. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen H, Pong RC, Wang Z and Hsieh JT:
Differential regulation of the human gene DAB2IP in normal and
malignant prostatic epithelia: Cloning and characterization.
Genomics. 79:573–581. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Z, Tseng CP, Pong RC, Chen H,
McConnell JD, Navone N and Hsieh JT: The mechanism of
growth-inhibitory effect of DOC-2/DAB2 in prostate cancer.
Characterization of a novel GTPase-activating protein associated
with N-terminal domain of DOC-2/DAB2. J Biol Chem. 277:12622–12631.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang R, He X, Liu W, Lu M, Hsieh JT and
Min W: AIP1 mediates TNF-alpha-induced ASK1 activation by
facilitating dissociation of ASK1 from its inhibitor 14-3-3. J Clin
Invest. 111:1933–1943. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dote H, Toyooka S, Tsukuda K, Yano M,
Ouchida M, Doihara H, Suzuki M, Chen H, Hsieh JT, Gazdar AF and
Shimizu N: Aberrant promoter methylation in human DAB2 interactive
protein (hDAB2IP) gene in breast cancer. Clin Cancer Res.
10:2082–2089. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dote H, Toyooka S, Tsukuda K, Yano M, Ota
T, Murakami M, Naito M, Toyota M, Gazdar AF and Shimizu N: Aberrant
promoter methylation in human DAB2 interactive protein (hDAB2IP)
gene in gastrointestinal tumour. Br J Cancer. 92:1117–1125. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yano M, Toyooka S, Tsukuda K, Dote H,
Ouchida M, Hanabata T, Aoe M, Date H, Gazdar AF and Shimizu N:
Aberrant promoter methylation of human DAB2 interactive protein
(hDAB2IP) gene in lung cancers. Int J Cancer. 113:59–66. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiu GH, Xie H, Wheelhouse N, Harrison D,
Chen GG, Salto-Tellez M, Lai P, Ross JA and Hooi SC: Differential
expression of hDAB2IPA and hDAB2IPB in normal tissues and promoter
methylation of hDAB2IPA in hepatocellular carcinoma. J Hepatol.
46:655–663. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smits M, van Rijn S, Hulleman E, Biesmans
D, van Vuurden DG, Kool M, Haberler C, Aronica E, Vandertop WP,
Noske DP and Würdinger T: EZH2-regulated DAB2IP is a
medulloblastoma tumor suppressor and a positive marker for
survival. Clin Cancer Res. 18:4048–4058. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang X, Li N, Li X, Zhao W, Qiao Y, Liang
L and Ding Y: Low expression of DAB2IP contributes to malignant
development and poor prognosis in hepatocellular carcinoma. J
Gastroenterol Hepatol. 27:1117–1125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duan YF, Li DF, Liu YH, Mei P, Qin YX, Li
LF, Lin QX and Li ZJ: Decreased expression of DAB2IP in pancreatic
cancer with wild-type KRAS. Hepatobiliary Pancreat Dis Int.
12:204–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen YJ, Kong ZL, Wan FN, Wang HK, Bian
XJ, Gan HL, Wang CF and Ye DW: Downregulation of DAB2IP results in
cell proliferation and invasion and contributes to unfavorable
outcomes in bladder cancer. Cancer Sci. 105:704–712. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Min J, Liu L, Li X, Jiang J, Wang J, Zhang
B, Cao D, Yu D, Tao D, Hu J, et al: Absence of DAB2IP promotes
cancer stem cell like signatures and indicates poor survival
outcome in colorectal cancer. Sci Rep. 5:165782015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Min W, Lin Y, Tang S, Yu L, Zhang H, Wan
T, Luhn T, Fu H and Chen H: AIP1 recruits phosphatase PP2A to ASK1
in tumor necrosis factor-induced ASK1-JNK activation. Circ Res.
102:840–848. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie D, Gore C, Zhou J, Pong RC, Zhang H,
Yu L, Vessella RL, Min W and Hsieh JT: DAB2IP coordinates both
PI3K-Akt and ASK1 pathways for cell survival and apoptosis. Proc
Natl Acad Sci USA. 106:19878–8383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie D, Gore C, Liu J, Pong RC, Mason R,
Hao G, Long M, Kabbani W, Yu L, Zhang H, et al: Role of DAB2IP in
modulating epithelial-to-mesenchymal transition and prostate cancer
metastasis. Proc Natl Acad Sci USA. 107:2485–2490. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kong Z, Xie D, Boike T, Raghavan P, Burma
S, Chen DJ, Habib AA, Chakraborty A, Hsieh JT and Saha D:
Downregulation of human DAB2IP gene expression in prostate cancer
cells results in resistance to ionizing radiation. Cancer Res.
70:2829–2839. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu K, Xie D, Zou Y, Zhang T, Pong RC, Xiao
G, Fazli L, Gleave M, He D, Boothman DA and Hsieh JT: The mechanism
of DAB2IP in chemoresistance of prostate cancer cells. Clin Cancer
Res. 19:4740–4749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu K, Wang B, Chen Y, Zhou J, Huang J, Hui
K, Zeng J, Zhu J, Zhang K, Li L, et al: DAB2IP regulates the
chemoresistance to pirarubicin and tumor recurrence of non-muscle
invasive bladder cancer through STAT3/Twist1/P-glycoprotein
signaling. Cell Signal. 27:2515–2523. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang T, Shen Y, Chen Y, Hsieh JT and Kong
Z: The ATM inhibitor KU55933 sensitizes radioresistant bladder
cancer cells with DAB2IP gene defect. Int J Radiat Biol.
91:368–378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang H, Zhang R, Luo Y, D'Alessio A,
Pober JS and Min W: AIP1/DAB2IP, a novel member of the Ras-GAP
family, transduces TRAF2-induced ASK1-JNK activation. J Biol Chem.
279:44955–49965. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee GH, Kim SH, Homayouni R and
D'Arcangelo G: Dab2ip regulates neuronal migration and neurite
outgrowth in the developing neocortex. PLoS One. 7:e465922012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harrison SC, Cooper JA, Li K, Talmud PJ,
Sofat R, Stephens JW and Hamsten A; HIFMECH Consortium; Sanders J,
Montgomery H, et al: Association of a sequence variant in DAB2IP
with coronary heart disease. Eur Heart J. 33:881–888. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Zhou HJ, Ji W and Min W:
AIP1-mediated stress signaling in atherosclerosis and
arteriosclerosis. Curr Atheroscler Rep. 17:5032015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang H, He Y, Dai S, Xu Z, Luo Y, Wan T,
Luo D, Jones D, Tang S, Chen H, et al: AIP1 functions as an
endogenous inhibitor of VEGFR2-mediated signaling and inflammatory
angiogenesis in mice. J Clin Invest. 118:3904–3916. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qiao S, Kim SH, Heck D, Goldowitz D,
LeDoux MS and Homayouni R: Dab2IP GTPase activating protein
regulates dendrite development and synapse number in cerebellum.
PLoS One. 8:e536352013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yun EJ, Baek ST, Xie D, Tseng SF, Dobin T,
Hernandez E, Zhou J, Zhang L, Yang J, Sun H, et al: DAB2IP
regulates cancer stem cell phenotypes through modulating stem cell
factor receptor and ZEB1. Oncogene. 34:2741–2752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thery C, Amigorena S, Raposo G and Clayton
A: Isolation and characterization of exosomes from cell culture
supernatants and biological fluids. Curr Protoc Cell Biol Chapter.
3:Unit 3.222006.
|
|
32
|
Salomon C, Ryan J, Sobrevia L, Kobayashi
M, Ashman K, Mitchell M and Rice GE: Exosomal signaling during
hypoxia mediates microvascular endothelial cell migration and
vasculogenesis. PLoS One. 8:e684512013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kobayashi M, Salomon C, Tapia J, Illanes
SE, Mitchell MD and Rice GE: Ovarian cancer cell invasiveness is
associated with discordant exosomal sequestration of Let-7 miRNA
and miR-200. J Transl Med. 12:42014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen H, Toyooka S, Gazdar AF and Hsieh JT:
Epigenetic regulation of a novel tumor suppressor gene (hDAB2IP) in
prostate cancer cell lines. J Biol Chem. 278:3121–3130. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen H, Tu SW and Hsieh JT:
Down-regulation of human DAB2IP gene expression mediated by
polycomb Ezh2 compLEX and histone deacetylase in prostate cancer. J
Biol Chem. 280:22437–22444. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Duggan D, Zheng SL, Knowlton M, Benitez D,
Dimitrov L, Wiklund F, Robbins C, Isaacs SD, Cheng Y, Li G, et al:
Two genome-wide association studies of aggressive prostate cancer
implicate putative prostate tumor suppressor gene DAB2IP. J Natl
Cancer Inst. 99:1836–1844. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu K, He Q, Liao G and Han J:
Identification of critical genes and gene interaction networks that
mediate osteosarcoma metastasis to the lungs. Exp Ther Med.
10:1796–1806. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi R, Li J, Tang F, Luo YI and Tu CQ:
Identification and functional study of osteosarcoma metastasis
marker genes. Oncol Lett. 10:1848–1852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang L, Liu ZM, Rao YW, Cui SQ, Wang H and
Jia XJ: Downregulation of microRNA-586 inhibits proliferation,
invasion and metastasis and promotes apoptosis in human
osteosarcoma U2-OS cell line. Cytogenet Genome Res. 146:268–278.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu J, Wan P, Wang M, Zhang J, Gao X, Hu B,
Han J, Chen L, Sun K, Wu J, et al: AIP1-mediated actin disassembly
is required for postnatal germ cell migration and spermatogonial
stem cell niche establishment. Cell Death Dis. 6:e18182015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Min J, Zaslavsky A, Fedele G, McLaughlin
SK, Reczek EE, de Raedt T, Guney I, Strochlic DE, Macconaill LE,
Beroukhim R, et al: An oncogene-tumor suppressor cascade drives
metastatic prostate cancer by coordinately activating Ras and
nuclear factor-kappaB. Nat Med. 16:286–294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ji W, Li Y, He Y, Yin M, Zhou HJ, Boggon
TJ, Zhang H and Min W: AIP1 expression in tumor niche suppresses
tumor progression and metastasis. Cancer Res. 75:3492–3504. 2015.
View Article : Google Scholar : PubMed/NCBI
|