Introduction
The incidence and mortality rates of gastric cancer
(GC), a common cancer threatening to human health, are the second
and third highest, respectively, in China in 2015 (1). Gastric carcinogenesis is a multi-gene,
multi-step complex process (2). It is
generally considered that Helicobacter pylori (H.
pylori) may serve a substantial function in the gastric
‘inflammation-cancer chain’ composed of chronic non-atrophic
gastritis (NAG), chronic atrophic gastritis (CAG), intestinal
metaplasia (IM), dysplasia (DYS) and GC (3). The risk of GC in individuals infected
with H. pylori was 3–6 times higher compared with uninfected
individuals (4), and animal
experiments confirmed that H. pylori may induce Mongolian
gerbil gastric adenocarcinoma (5,6). The
underlying mechanisms of H. pylori resulting in GC may have
a plurality of pathways, one of which may be the loss of the
connection between the cells. Gap junctions are the ubiquitous
connection between animal cells, mediating the gap junction
intercellular communication (GJIC) to participate in the exchange
of materials between cells, metabolic coupling and electrical
transmitting, performing the exchange of information and energy
substances and serving a notable regulatory function in
physiological processes including cell metabolism, homeostasis,
proliferation and differentiation (7).
Connexin 32 (Cx32) is the main member of gastric
epithelial cell gap junctions. In the development of GC, the
expression of Cx32 demonstrates a gradual downward trend from
normal gastric mucosa (NGM) to precancerous lesions to GC (8). The decrease of Cx32 expression is an
early molecular event in the process of GC. One previous study
revealed that the lowered expression of Cx32 in precancerous
lesions and GC is closely associated with H. pylori
infection, and the co-culturing of gastric epithelial cells and
H. pylori for 24 or 48 h significantly decreased the
expression of Cx32 (9), and the
eradication of H. pylori may increase the Cx32 expression in
gastric precancerous lesions (10).
In addition, clinical and in vitro experimental studies have
confirmed that H. pylori may significantly reduce gastric
epithelial cell GJIC function (11,12). These
studies indicated that H. pylori infection may reduce the
expression of Cx32 in gastric epithelial cells, thereby inhibiting
GJIC function, which may be the mechanism underlying H.
pylori-induced GC, but the specific mechanism of H.
pylori that results in Cx32 decline in gastric epithelial cells
remains unclear.
One previous study performed a high-throughput
transcription factor screening in NGM tissue without H.
pylori infection and in gastric mucosa tissues at five
different stages of the gastric ‘inflammation-cancer chain’ with
H. pylori infection (NAG→CAG→IM→DYS→GC), and revealed that
the GATA binding protein 3 (GATA-3) expression was low in normal
mucosa, comparatively increased significantly in NAG and
precancerous (CAG, IM and DYS) mucosa and highest in GC (13). Bioinformatics software analysis
revealed that there are multiple GATA-3 binding sites in the
promoter regions of Cx32 genes. Therefore, it was hypothesized that
H. pylori infection may downregulate Cx32 expression by
upregulating GATA-3 expression in gastric epithelial cells, thus
serving an important function in the gastric carcinogenesis caused
by H. pylori infection.
The present study intended to co-culture gastric
epithelial cells with H. pylori and collect the gastric
antrum tissues from H. pylori-gavaged animals in order to
observe the effects of H. pylori on GATA-3, Cx32 expression
and GJIC function, to silence GATA-3 by small interfering RNA
(siRNA) transfection to observe its effects on Cx32 expression and
to conduct dual luciferase reporter experiments by constructing a
Cx32 promoter vector. These experiments attempted to elucidate
whether H. pylori infection results in Cx32 downregulation
by upregulating GATA-3, and whether this is associated with GC.
Materials and methods
Co-culture of gastric epithelial cells
with H.pylori
H. pylori strains were isolated from five patients
(4 males and 1 female; mean age, 55 years; age range, 40–70 years)
diagnosed with GC subsequent to endoscopic and pathological
examinations in July 1–31st, 2012 at the Third Xiangya Hospital of
Central South University (Changsha, China), cultured and identified
as H. pylori by the bacteria colony morphology, and activity assays
of its enzymes, including urease (producing carbon dioxide and
ammonia), catalase (producing oxygen) and oxidase assays, and
identified as East Asian type cytotoxin-associated gene A
(CagA)+ H. pylori strains by CagA gene sequencing.
Written informed consent was obtained from all patients prior to
the study. Normal human gastric epithelial cell line GES-1 was
purchased from Shanghai Bogoo Biotechnology Co., Ltd. (Shanghai,
China) and the human GC cell line BGC823 was gifted from the Cancer
Research Institute, Central South University (Changsha, China).
Cells of logarithmic growth phase were digested with
trypsin to prepare a single cell suspension, counted using a
hemocytometer, seeded in 105/well in 6-well plates, and
cultured at 37°C and 5% CO2. Once the cells adhered
firmly by observation under a light microscope, Dulbecco's modified
Eagle's medium (DMEM) without fetal bovine serum (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used to culture the cells
for 24 h to synchronize the cells at the G0 phase. H. pylori
bacteria were collected using sterile phosphate buffered saline
(PBS), the concentrations measured using an ultraviolet
spectrophotometer (1 OD660=108/ml), and H.
pylori bacteria and GES-1 cells were seeded at a ratio of 50:1
in 6-well plates and co-cultured at 37°C for 12 and 24 h.
Collection of gastric tissues from
normal and H
pylori-gavaged animals. A total of 20 male Mongolian
gerbils (aged 6–8 weeks; weight, 51.9–66.8 g) purchased from the
Animal Experimental Center of Zhejiang Province (Hangzhou, China),
were housed and fed with food and water in individually ventilated
cages, with 12/12 h dark/light cycle, at 25°C temperature. They
were randomly allocated to the control (5 animals) and experimental
(15 animals) groups. The animal ethics protocol was approved by the
Ethics Committee of the Third Xiangya Hospital of Central South
University (Changsha, China). After all the gerbils fasted for 24
h, the experimental group were administered H. pylori gavage
(East-Asian type CagA+ from patients with GC, once a day
for 7 days total), and the controls were administered saline
gavage. After 48 weeks, the gerbils were anaesthetized with chloral
hydrate (400 mg/kg) and a chest and abdominal incision was
carefully made. Perfusion was performed using PBS, preventing the
heart from beating and resulting in euthanasia. The gastric antrum
tissues were collected and stored in liquid nitrogen immediately
for further detection of GATA-3 and Cx32 expression.
Western blot analysis
The GES-1 and BGC823 cells were washed with cool PBS
for 3 times, lyzed with radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Shanghai, China) on ice for
15–30 min, transferred to tubes, centrifuged at 12,000 × g at 4°C
for 5 min, and the supernatant transferred to a new tube, thus the
protein was extracted. The protein concentration was measured by
BCA protein assay kit (Beyotime Institute of Biotechnology). The
proteins were electrophoresed (20 µg/lane) via SDS-PAGE (10–12.5%
gel) and transferred to polyvinylidene fluoride membranes, washed
with Tris-buffered saline and Tween (TBST) for 5 min, blocked in 5%
skimmed-milk in TBST, and hybridized with primary antibodies. The
anti-human antibodies, including rabbit anti-Cx32 (cat. no.
10450-1-AP; dilution, 1:800), rabbit anti-GATA3 (cat. no.
10417-1-AP; dilution, 1:800), mouse anti-β-actin (cat. no.
600008-1; dilution, 1:4,000) and the secondary antibodies
(horseradish peroxidase-labeled goat anti-rabbit or anti-mouse
antibodies; cat. nos. SA00001-2 and SA00001-1, respectively;
dilution, 1:3,000) were purchased from ProteinTech Group, Inc.
(Chicago, IL, USA). The hybridization for primary antibodies was
incubated at 4°C overnight, and that for secondary antibodies was
at room temperature for 45 min. ECL solutions (Thermo Fisher
Scientific, Inc.) were added to the membrane and images of the
protein bands were captured and analyzed by the Image Quant 350 Gel
Protein Imaging System (version 1.0.2; GE Healthcare) and Image
Analysis Software (version 7.0; GE Healthcare).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells or tissues by
grinding with liquid nitrogen, adding TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), followed by
chloroform, isopropanol, and 75% ethanol, each time centrifuging at
12,000 × g at 4°C for 15 min, and finally drying to be dissolved in
0.1% diethylpyrocarbonate water (Shanghai Sangon Biotechnology Co.,
Shanghai, China). RNA was reverse transcribed into cDNA using a
SuperScript Reverse Transcription kit (Thermo Fisher Scientific,
Inc.). Briefly, Oligo(dT)15 primer was added and heated
at 65°C for 10 min and cooling to 4°C on ice for 10 min, then the
buffer, dNTP mix and reverse transcriptase from the kit were added
for reaction at 42°C for 1 h, and finally the reaction was
inactivated by heating at 70°C for 15 min. The RT-qPCR reaction was
conducted using the SYBR®-Green Master Mix (Thermo
Fisher Scientific, Inc.) in the Realplex qPCR instrument
(Eppendorf, Hamburg, Germany) under the following conditions: 95°C
for 5 min, and then 95°C for 15 sec, 55°C for 15 sec and 72°C for
30 sec, for 40 cycles. Following this, the results were analyzed by
the 2−ΔΔCq method (14).
The primers were designed using Primer 5.0 software (Premier
Biosoft, Palo Alto, CA, USA) and synthesized from the Shanghai
Sangon Biological Engineering Co. The PCR primer sequences for
human cells are as follows: GATA-3 forward,
5′-GGAGTGTGTGAACTGTGGGG-3′ and reverse, 5′-TTCGCTTGGGCTTAATGAGG-3′;
Cx32 forward, 5′-GGATGCTCCGACAGCGTCTC-3′ and reverse,
5′-GCCCTCTGCTCCTCTTACCC-3′; β-actin forward,
5′-AGGGGCCGGACTCGTCATACT-3′ and reverse,
5′-GGCGGCACCACCATGTACCCT-3′. The PCR primer sequences for gerbil
gastric tissue were as follows: GATA-3 forward,
5′-CGGCAGGCAGATGAGAAAG-3′ and reverse, 5′-GGGCACATAGGGCGGATAG-3′;
Cx32 forward, 5′-CACCAACAGCACATAGAAAAG-3′ and reverse,
5′-GATGACATAGGTCCACCACAG-3′; β-actin forward,
5′-CCCATCTATGAGGGTTACGC-3′ and reverse,
5′-TTTAATGTCACGCACGATTTC-3′.
Detection of GJIC function in GES-1
cells
Scrape-loading and fluorescent dye transfer (SLDT)
technique was used to detect the GJIC function in GES-1 cells with
or without H. pylori infection. Briefly, GES-1 cells were
co-cultured in 6-well plates at 37°C with 95% air and 5%
CO2 to show monolayer cell concurrence under light
microscopy (magnification, ×40). The medium was removed, and each
well of cultured cells was washed with sterile PBS gently for 5 sec
at room temperature for 4 times. A total of 1 ml PBS containing
0.5% Lucifer Yellow (Thermo Fisher Scientific, Inc.), was added,
and the cell monolayer was gently scraped with a sharp blade to
form a scratch ~50-µm wide. Cells were kept still for 3 min, the
fluid was discarded, and the well was washed again with sterile PBS
gently for 5 sec at room temperature for 4 times to wash away the
residual fluorescent dye. Fluorescence and ordinary light images
were taken from the same vision field under an inverted
fluorescence microscope (magnification, ×40) (IX71; Olympus
Corporation, Tokyo, Japan). By observing the cells with fluorescent
dye next to the scrape line, the transfer distance or the number of
cells the dye passed, the functional status of GJIC was determined.
A greater number of cells to which the fluorescent dye had spread
to was considered to indicate a stronger GJIC function.
Transfection of GATA-3 siRNA into
BGC823 cells
BGC823 cells were cultured to 80–90% confluence,
digested with trypsin, carefully drawn off using a pipette into a
single cell suspension, and seeded into 100 mm-diameter cell
culture dishes (10 ml/dish) with a cell density of 2×105
cells/ml. The cells were cultured at 37°C and 5% CO2 in
an incubator for 24 h, then the DMEM medium (Thermo Fisher
Scientific, Inc.) was changed, and when the cells were 80%
confluent, transfection was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. They
were divided into three groups: i) No treatment as the control
group; ii) transfected with negative control as the NC group; and
iii) transfected with 10 µM GATA-3 siRNA as the siRNA group. A
total of 48 h after transfection, RNA or proteins were extracted.
siRNA was synthesized by Shanghai GenePharma Co., Ltd. (Shanghai,
China) with the following sequences: GATA-3 siRNA forward,
5′-CAUCGACGGUCAAGGCAACTT-3′ (the first 19 nucleotides corresponding
to the GATA-3 coding sequence nucleotides 156–174, and TT for
protection) and reverse. 5′-GUUGCCUUGACCGUCGAUGTT-3′; negative
control forward, 5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′.
Construction of pYr-PromDetect-Cx32P
vector
The fragment of Cx32 promoter −651 to +84
(transcription start site as +1, total 735 base pairs) was PCR
amplified from human genomic DNA by the following primers with
restriction enzyme BglII and NheI sites added (underlined
respectively): Forward, 5′-GAAGATCTAATGGTTAGCCTTTGCTTTC-3′ and
reverse, 5′-CTAGCTAGCTATGGTCTCATGTCTGTGCAGG-3′. The PCR reaction
was performed using PrimeSTAR-HS DNA polymerase in PCR buffer
containing 2.5 mM dNTP (Takara Bio, Inc., Otsu Japan), with the
thermocycling condition: 94°C for 2 min, then 94°C for 30 sec, 55°C
for 30 sec and 72°C for 90 sec, for 30 cycles, followed by 72°C for
3 min. The amplified fragment was inserted into the pYr-PromDetect
vector (pLuc; Changsha Yingrun Biotechnology Co., Ltd., Changsha,
China) and formed pYr-PromDetect-Cx32P vector (pLuc-Cx32P). The
sequencing result displayed an exact alignment with the Cx32
promoter sequence in the National Centre for Biotechnology
Information (NCBI; https://www.ncbi.nlm.nih.gov/pubmed).
Determination of potential GATA-3
binding sites in the Cx32 promoter
The DNA consensus sequence that the GATA-3
transcription factor binds to is 5′-A/T-GATA-A/G-3′, thus the
consensus sequence in the contrary strand is 5′-T/C-TATC-T/A-3′
(15,16). For the aforementioned cloned sequence
of the Cx32 promoter, a search was made for GATA or TATC, which
also satisfied the nucleotide requirement at the 5′ and 3′ ends,
and the potential binding sites of GATA3 was identified to be −623
to −618, −514 to −509 and −239 to −234.
GATA-3 expression vector
The complete coding sequence of GATA-3 was PCR
amplified from human cDNA by using the following primers with
additional NheI and XhoI digestion sites added (underlined
respectively): Forward, 5′-CGCTAGCGATGGAGGTGACGGCGGA-3′ and
reverse, 5′-GCCTCGAGCTAACCCATGGCGGTGAC-3′. The PCR reaction was
performed using PrimeSTAR-HS DNA polymerase in PCR buffer
containing 2.5 mM dNTP (Takara Bio, Inc.), with the thermocycling
condition: 94°C for 2 min, then 94°C for 30 sec, 55°C for 30 sec
and 72°C for 90 sec, for 30 cycles, followed by 72°C for 3 min. The
amplified fragment was inserted into a GV230 vector (pGv) and
formed the pGATA3 vector. The sequencing result was matched against
the GATA-3 NCBI sequence.
Dual-luciferase reporter
experiment
The luciferase detection kit was purchased from
Changsha Yingrun Biotechnology Co., Ltd. and performed according to
the manufacturer's protocol. Briefly, GES-1 cells in the
logarithmic growth phase and growing in an active condition were
seeded in 24-well plates. When the cell density reached 70–80% in
the well, ~2×105 GES-1 cells were transfected with the
plasmids (pYr-PromDetect-Cx32P containing the test promoter
sequence Cx32P, or/and pGATA3 expressing the transcription factor
GATA3 to bind to the test promoter) by Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol, and cultured at 37°C and 5% CO2
in an incubator. After 48 h, the cells were washed with 1X PBS (100
µl/well, one time), cleaved by gentle shaking at room temperature
for 15 min, and the lysates were transferred to a detection tube.
When measuring in the luminometer (PerkinElmer, Inc., Waltham, MA,
USA), 100 µl Luciferase Assay Reagent-II and stop-Glo reagent were
automatically added and the results read using 1–2 sec and 5–10 sec
delays.
Using a dual luciferase reporter assay, the
Cx32 promoter sequence −651 to +84 (containing TATA box at
position −15 and transcription start site) was inserted into the 5′
upstream of Renilla luciferase (Rluc) coding sequence in the
reporter plasmid, immediately next to the Kozak sequence GCCACCATG,
starting the translation of Rluc. Furthermore, the Firefly
luciferase (Fluc) coding sequence was subsequent to the promoter
sequence derived from cytomegalovirus in the same plasmid producing
stable expression following transfection. The ratio of Rluc
fluorescence to Fluc fluorescence (R/F ratio) reflected the
activity of the inserted promoter sequence.
Statistical analysis
SPSS 19.0 statistical software (IBM Corp., Armonk,
NY, USA) and GraphPad Prism 7.00 software (GraphPad Software, Inc.,
La Jolla, CA, USA) were used for statistical analysis. The data
were calculated and presented as mean ± SD. Measurement data were
tested and distributed normally. Statistical significance of the
data was assessed by a Student's unpaired t-test between two
groups, or one-way analysis of variance followed by the post hoc
Bonferroni test among multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Changes of GATA-3 and Cx32 mRNA
expression subsequent to H. pylori infection
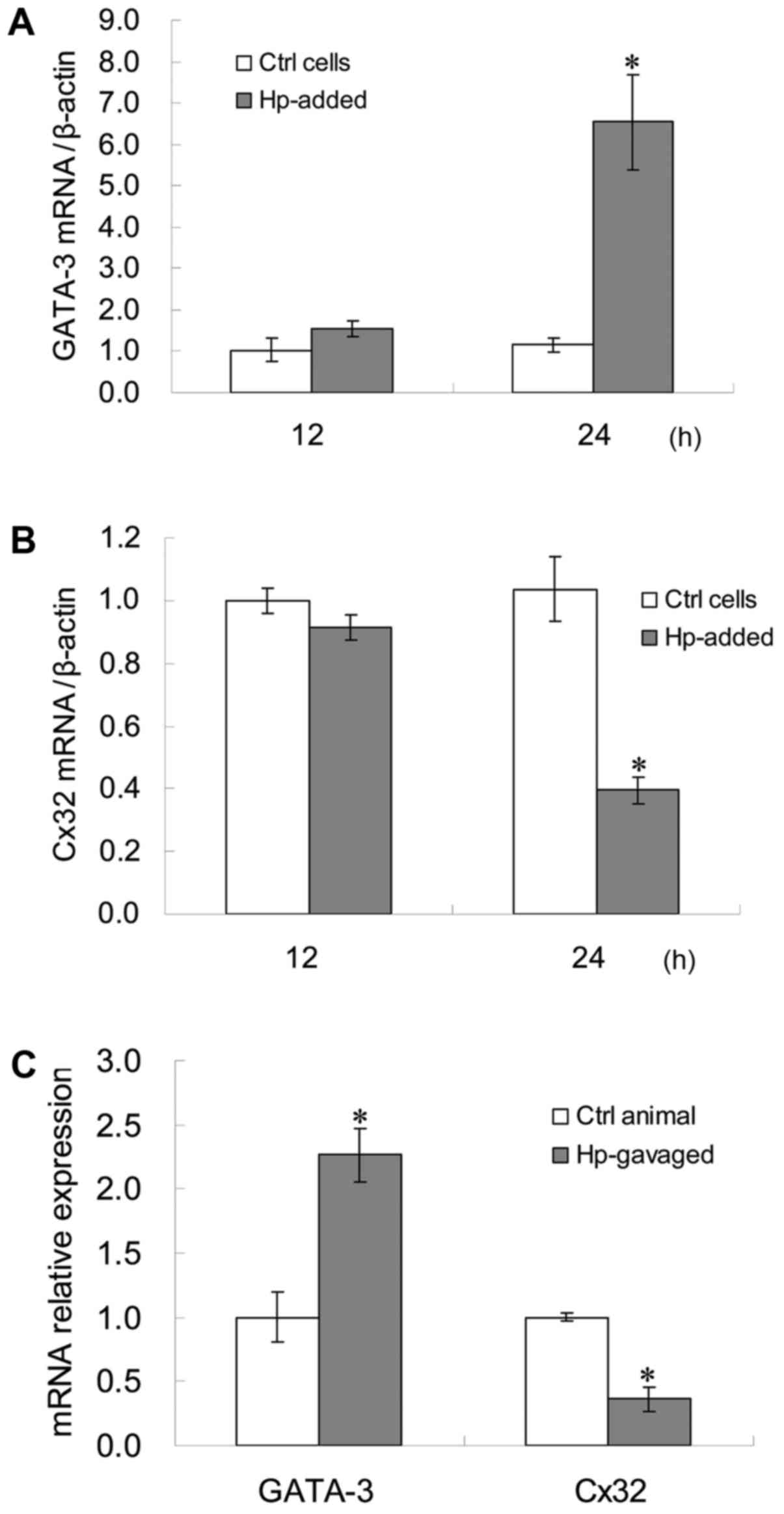
GATA-3 mRNA expression levels in GES-1 cells at 24 h
following H. pylori infection were significantly higher compared
with the control (P<0.05; Fig.
1A), while Cx32 mRNA expression levels in GES-1 cells at 24 h
following H. pylori infection were significantly lower compared
with the control (P<0.05; Fig.
1B). The correlation between GATA-3 and Cx32 mRNA expression
levels in GES-1 cells at 24 h after H. pylori infection was
statistically significant (r=−0.6713; P=0.0477) (data not shown).
GATA-3 mRNA expression levels in the gerbil gastric antrum tissues
subsequent to H. pylori infection were significantly higher
compared with the control, while Cx32 mRNA expression levels in the
gerbil gastric antrum tissues subsequent to H. pylori infection
were significantly lower compared with the control (P<0.05;
Fig. 1C).
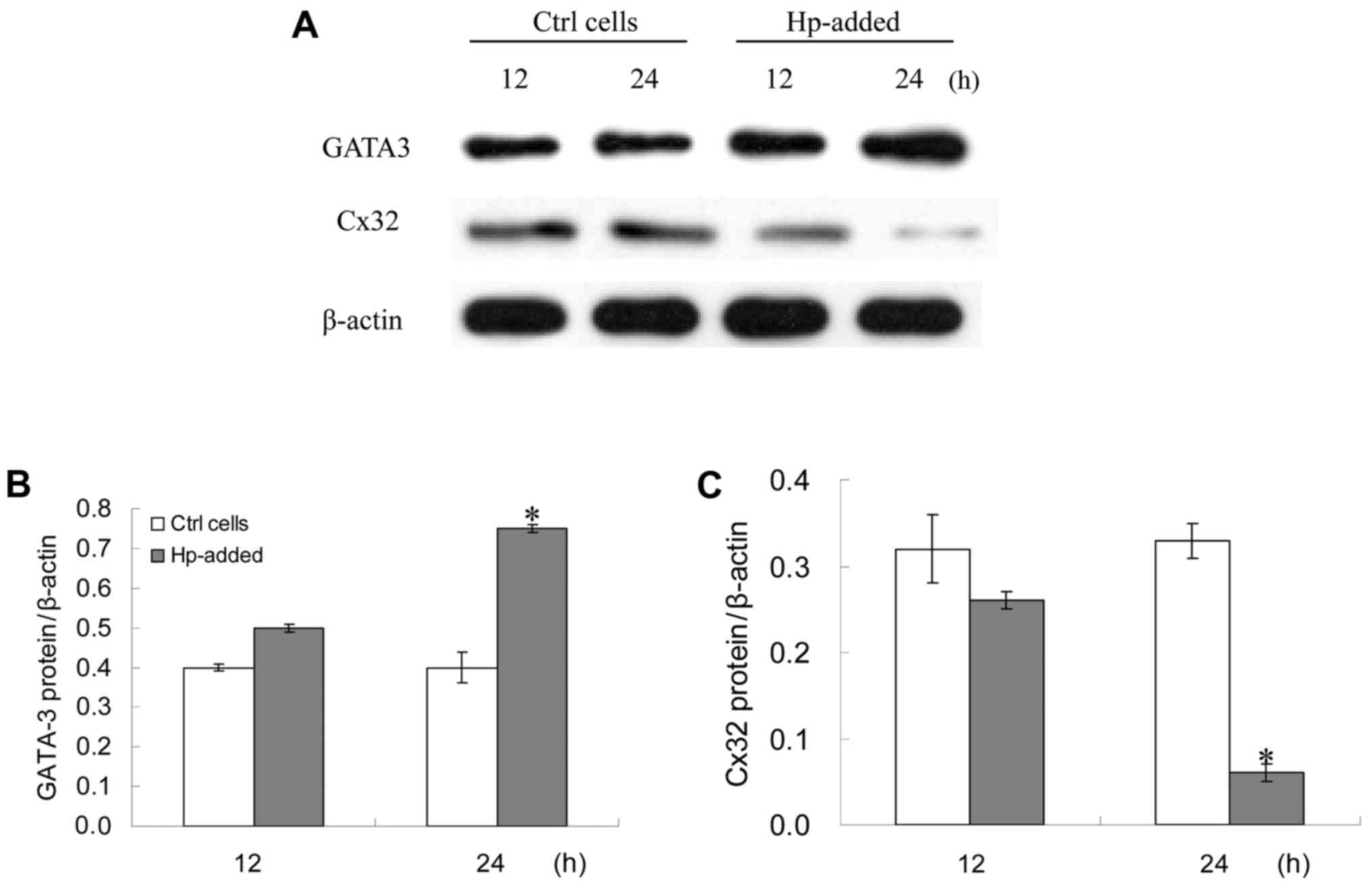
Changes of GATA-3 and Cx32 protein
expression levels in GES-1 cells subsequent to H. pylori
infection
GATA-3 protein expression levels in GES-1 cells at
24 h following H. pylori infection were significantly higher
compared with the control, while Cx32 protein expression levels in
GES-1 cells at 24 h following H. pylori infection were
significantly lower compared with the control (P<0.05; Fig. 2). The correlation between GATA-3 and
Cx32 protein expression levels in GES-1 cells at 24 h after H.
pylori infection was statistically significant (r=−0.7888;
P=0.0115) (data not shown).
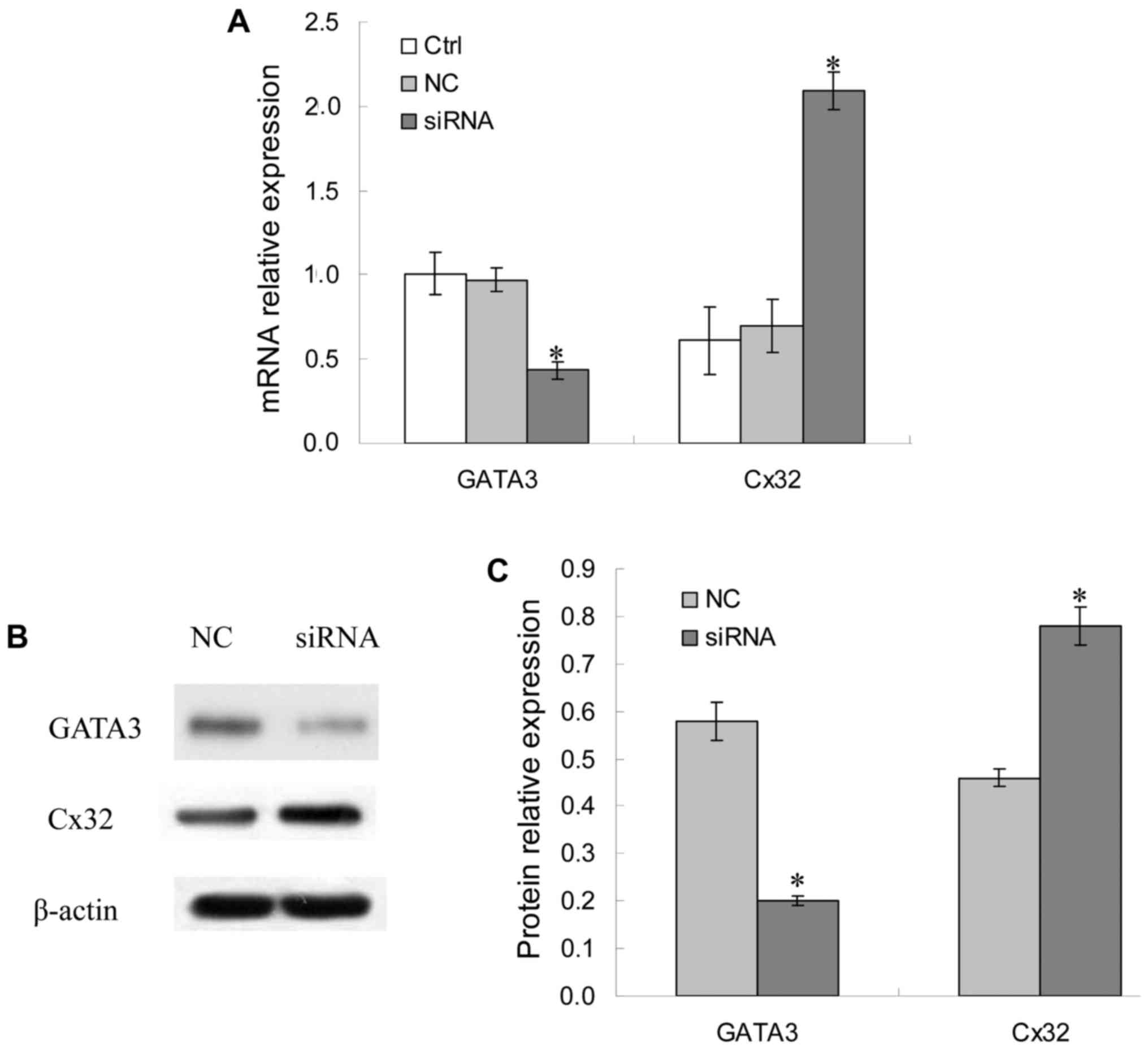
Cx32 mRNA and protein expression
levels following GATA-3 siRNA transfection in BGC823 cells
As presented in Fig.
3A, compared with the control group, GATA-3 mRNA expression
levels in the GATA-3 siRNA group decreased significantly
(P<0.05), while that in the NC group presented no notable
difference, indicating that GATA-3 siRNA specifically and
effectively interfered with the expression of GATA-3 mRNA. However,
Cx32 mRNA expression levels in the siRNA group were significantly
increased (P<0.05), while that in the NC group had no
significant difference (P>0.05) compared with the control group.
The correlation between GATA3 and Cx32 mRNA expression in BGC803
cells in the NC group and GATA-3 siRNA group was statistically
significant (r=−0.751, P=0.015; r=−0.880, P=0.001, respectively)
(data not shown).
As there were no significant differences in GATA-3
and Cx32 mRNA expression levels between the control group and NC
group (P>0.05), the protein expression levels of GATA-3 and Cx32
in the NC group and siRNA group were detected. The results revealed
that the GATA-3 protein expression levels in the siRNA group
decreased significantly compared with the NC group (P<0.05),
consistent with the RT-qPCR results, suggesting an effective
interference effect. Additionally, Cx32 protein expression levels
in the GATA-3 siRNA group were significantly higher compared with
the NC group (P<0.05; Fig. 3B and
C). The correlation between GATA3 and Cx32 protein expression
in BGC803 cells in the NC group and GATA-3 siRNA group was
statistically significant (r=−0.867, P=0.024; r=−0.759, P=0.018,
respectively) (data not shown).
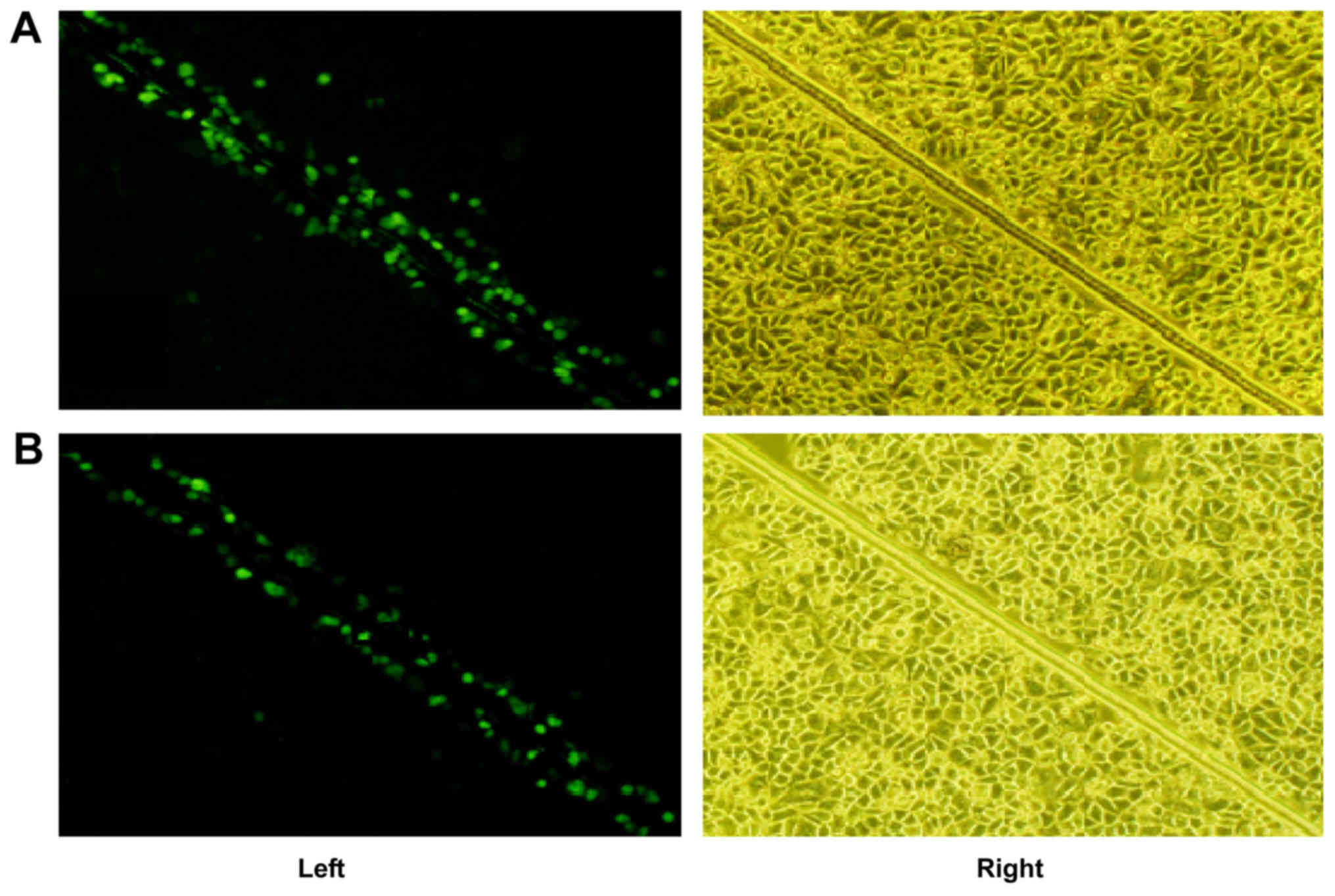
Changes of GJIC function in GES-1
cells subsequent to H
pylori infection. In the control group, the
fluorescent dye transferred from the GES-1 cells along the scrape
to cells spanning 3–4 rows either side of the scrape, indicating a
strong function of GJIC; while in the H. pylori group, the
fluorescent dye was mostly limited to a single row of cells next to
the scrape, with a limited amount of dye penetrating through to the
second row of cells, indicating that the GJIC was weakened or
absent, as presented in Fig. 4.
GATA-3 negatively regulates Cx32 by
directly binding to its promoter
In the Cx32 promoter sequence −651 to +84 as
presented in Fig. 5A, the
6-nucleotide sequences at positions −623, −514 and −239 are in
accordance with the consensus sequence and may be presumed to be
GATA-3 binding sites. The 9-nucleotide sequences at positions −224,
−187 and −101 are the consensus sequences of the transcription
factor early growth response 2 (EGR2), and the 6-nucleotide
sequence at positions −44 and −33 is the consensus sequence of
transcription factor SRY-box 10 (SOX10).
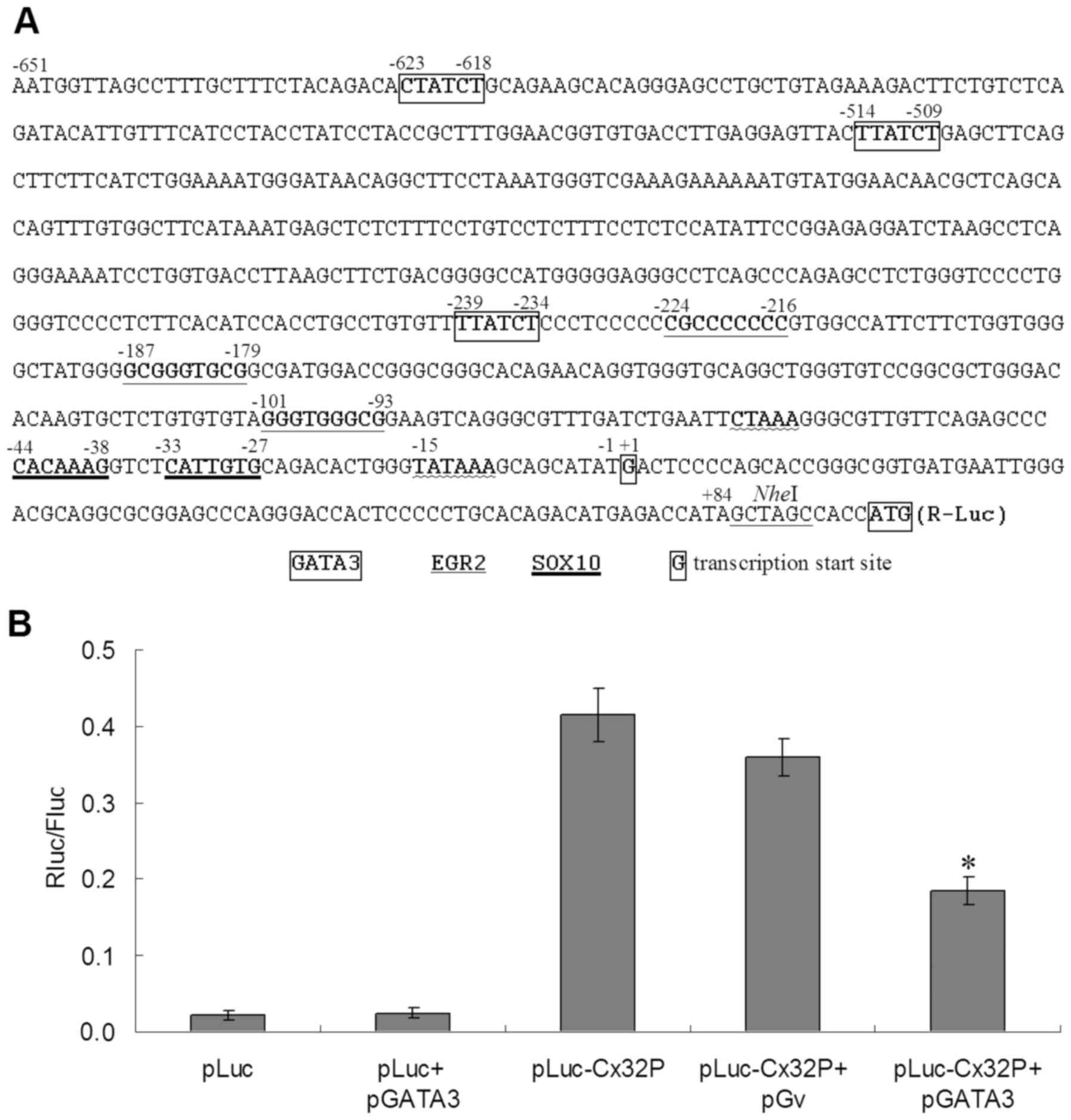 | Figure 5.Result of the dual luciferase
reporter assay. (A) The Cx32 promoter sequence −651 to +84.
Potential transcription factor binding sites: positions −623, −514
and −239 for GATA-3; positions −224, −187 and −101 for EGR2; and
positions −44 and −33 for SOX10. Position −15 for TATA box.
Position +1G for transcription start site. Boxed ATG for
translation start codon. (B) The Rluc/Fluc ratios of different
plasmid transfection from the dual luciferase reporter experiment.
The mean Rluc/Fluc ratio was 0.022 for pLuc transfection, 0.029 for
pLuc+pGATA3, 0.415 for pLuc-Cx32P, 0.359 for pLuc-Cx32P+pGv and
0.185 for pLuc-Cx32P+pGATA3. *P<0.05 vs. pLuc-Cx32P or
pLuc-Cx32P+pGv groups. Cx32P, connexin 32 promoter; pLuc, plasmid
of dual luciferases; pGv, plasmid GV230 blank vector; pGATA3,
plasmid of GATA binding protein 3; Rluc, Renilla luciferase;
Fluc, firefly luciferase; EGR2, early growth response 2; SOX10,
SRY-Box 10. |
In the dual-luciferase reporter experiment, the
ratio of Renilla luciferase (Rluc) fluorescence to Firefly
luciferase (Fluc) fluorescence (R/F ratio) reflected the activity
of inserted promoter sequence. As presented in Fig. 5B, the mean R/F ratio of pLuc
transfection was 0.022, and that of pLuc+pGATA3 was 0.029,
indicating that GATA3 overexpression has no effect on the reporter
plasmid without a Cx32 promoter. The mean R/F ratio of
pLuc-Cx32P transfection was 0.415, and that of pLuc-Cx32P+pGv was
0.359, suggesting that Cx32 promoter is activated in GES-1
cells, while co-transfection with another plasmid may decrease the
activity of the promoter, but the difference in promoter activity
with or without another plasmid was not significant (P>0.05).
The mean R/F ratio of pLuc-Cx32P+pGATA3 transfection was 0.185,
which is significantly different compared with pLuc-Cx32P and
pLuc-Cx32P+pGv (P<0.05), indicating that GATA-3 inhibits
expression activity by direct binding to the promoter.
Discussion
In the present study, the expression levels of
GATA-3 mRNA and protein in H. pylori-infected GES-1 cells
increased with increasing incubation time, and also in the gerbil
gastric antrum tissues following H. pylori infection. Whilst
the mechanism of how H. pylori bacteria may activate the
expression of GATA-3 remains unclear, the mechanism underlying
GATA-3 overexpression promoting carcinogenesis was investigated in
the present study. H. pylori may interact with the
epithelial cells and infiltrate natural killer (NK) cells in the
gastrointestinal tract. According to Lindgren et al
(17), co-culture with H.
pylori lysate caused the GATA-3 overexpression, which may
inhibit the transforming growth factor-β expression, thereby
inhibiting NK cell activity, resulting in the decreased
interferon-γ secretion by NK cells, thus increasing the risk of
carcinogenesis in gastric epithelial cells. This is the potential
mechanism by which the high expression of GATA-3 participates in
the regulation of tumor immunity resulting in tumor immune
escape.
In the present study, once the gastric epithelial
cells were infected with H. pylori, the Cx32 expression at
the transcriptional and protein levels were decreased with
increasing incubation time, and GJIC function was inhibited. This
is consistent with previous results in gastric precancerous lesions
(10). The loss of GJIC function in
tumor cells has been associated with the downregulated
tissue-specific expression of connexin genes (18). Cx32 is the principal protein
constituting the gap junction in gastric epithelial cells (19). It was additionally revealed that in
gastric carcinogenesis, the expression of Cx32 was decreased in
chronic non-atrophic gastritis, gastric precancerous lesions and
GC, with the lowest expression in GC (10). In the present study, it was
hypothesized that GATA-3 may downregulate the expression of Cx32,
which then promotes the tumor incidence by decreasing the
intercellular connection and communication.
In the present study, the association between the
expression of GATA-3 and expression of Cx32 was examined. By siRNA
transfection, the expression of GATA-3 was negatively associated
with Cx32 expression in GES-1 cells. The fluorescent dyes of the
H. pylori-infection group were mostly limited to the single
cell line surrounding the scratch, with only a limited amount of
dye penetrating through to the second cell line, indicating that
the GJIC function was substantially reduced or absent, while in the
control group, the fluorescence dye transferred to the neighboring
3–4 column cells, demonstrating a stronger GJIC function. Using a
dual luciferase reporter assay, GATA-3 inhibited the expression of
the luciferase reporter downstream to the Cx32 promoter, suggesting
that GATA-3 inhibits the expression of Cx32 by binding to the Cx32
promoter sites. These experiments revealed that GATA-3 inhibits the
Cx32 expression activity by direct binding to the Cx32
promoter.
Cx32 is positively regulated by the transcription
factors SOX10 and EGR2 functioning at the Cx32 promoter −44
to −38 and −33 to −27 sites, and −101 to −93, −187 to −179 and −224
to −216 sites (20,21). In the present study, the potential
binding sites of GATA3 were identified to be −239 to −234, −514 to
−509 and −623 to −618, all upstream of the SOX10 and EGR2 binding
sites. As a novel high-mobility group-box-containing tumor
suppressor, SOX10 may additionally inhibit the growth and
metastasis of digestive tract cancer types by suppressing the
Wnt/β-catenin pathway (22). The EGR2
signaling pathway also attenuated the carcinogenesis of GC
(23). These indicate that in the
Cx32 promoter, SOX10 and EGR2 function as activating factors whilst
GATA3 functions as an inhibiting factor, which is consistent with
their general roles in GC development.
Certain relevant questions should be mentioned.
Firstly, whether the expression of GATA-3 in different tumor types
or tumor tissues is upregulated or downregulated varies between
studies (24). The result of the
present study indicates that GATA-3 is highly expressed in the GC
cell line BGC823, which is consistent with markedly higher GATA-3
expression in GC tissues (13).
Similar results have been demonstrated in pancreatic cancer and
pancreatic carcinoma cell lines (25). GATA3 is expressed in a wide variety of
benign and malignant cutaneous epithelial neoplasms (26). But in breast cancer, GATA3 expression
has been associated with estrogen receptor positive (ER+/luminal)
phenotypes, accounting for ~2/3 of all breast cancer cases, while
loss of GATA3 expression is associated with ER-, less
differentiated, invasive breast cancer (24). As a mechanism, progestin-activated
progesterone receptor (PR) reduces GATA3 expression through
regulation at the transcriptional and post-translational levels,
promoting tumor growth (27).
Therefore, in order to provide novel therapeutic targets, further
investigation of GATA3-associated pathways will be necessary to
further understanding of different cancer incidence and
dissemination.
Secondly, the association between upregulated GATA-3
and carcinogenesis is multifaceted. GATA-3 is additionally a
specific transcription factor for Th2 type cytokines, its
abnormally high expression not only is able to promote the
development of T helper (Th)2 cells, but may also inhibit cell
differentiation in the Thl direction, resulting in a Thl/Th2 type
cytokine imbalance to exhibit immunosuppression (28). Tumor formation is closely associated
with the immune escape of tumor antigens, and the body's immune
tolerance and immune suppression promote tumor growth and
metastasis (29).
Finally, the results suggest that H. pylori
infection causes upregulation of GATA-3 and decreases the
expression of Cx32 and GJIC function. It is possible to intervene
with this regulating course to improve the intercellular
communication, benefiting the prevention and therapy of gastric
cancer. Clinical and experimental studies in vitro confirmed
that H. pylori may significantly reduce GJIC function in
gastric epithelial cells (11,12). One
previous study demonstrated that the eradication of H.
pylori may increase Cx32 expression in precancerous lesions,
thereby maintaining GJIC function (10). The expression of Cx32 in BGC823 cells
was upregulated by GATA-3 siRNA transfection in the present study,
yet the application of GATA-3 siRNA in the gastric antrum tissues
remains questionable. Cx32 may also inhibit cell proliferation by
cell cycle arresting effects and cell cycle regulatory proteins,
and thus serves an important function in the inhibition of GC
development (30). Therefore,
enhancement of Cx32 expression by demethylation of the Cx32
gene promoter may also provide ways to prevent gastric
carcinogenesis (31). Together with
the consistent methods and results in a previous study on Cx43
expression in gastric carcinogenesis (32), potential methods in enhancing GJIC
function may be effective in preventing and treating GC.
To conclude, the present study revealed that there
is a negative association between GATA-3 and Cx32 expression,
suggesting that H. pylori infection may upregulate GATA-3
expression, which negatively regulates the expression of its
downstream target gene Cx32, resulting in GJIC dysfunction,
therefore serving an important function in the incidence and
development of GC. The detailed mechanisms, general meaning and
therapeutic application need to be further explored.
Acknowledgements
Not applicable.
Funding
The present study is supported by the National
Natural Science Foundation of China (grant nos. 81172301 and
81570509), National Innovative Training Program of China (grant no.
201310533059) and the Science and Technology Project of Changsha
(grant no. k1406048-31).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LH arranged the experiments, analyzed the data and
wrote the manuscript. CX designed the experiments and revised the
manuscript. DC, YG, XL, KC, TH and YQ performed the experiments. LZ
analyzed the data.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Third Xiangya Hospital of Central South University, China. The
patients provided their written informed consent to participate in
this study.
Consent for publication
The patients provided their written informed consent
for their data to be published.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CagA
|
cytotoxin-associated gene A
|
|
GATA-3
|
GATA binding protein 3
|
|
Cx32
|
connexin 32
|
|
GJIC
|
gap junction intercellular
communication
|
|
NAG
|
non-atrophic gastritis
|
|
CAG
|
chronic atrophic gastritis
|
|
IM
|
intestinal metaplasia
|
|
DYS
|
dysplasia
|
|
NGM
|
normal gastric mucosa
|
|
GC
|
gastric cancer
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grabsch HI and Tan P: Gastric cancer
pathology and underlying molecular mechanisms. Dig Surg.
30:150–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahn HJ and Lee DS: Helicobacter pylori in
gastric carcinogenesis. World J Gastrointest Oncol. 7:455–465.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim SS, Ruiz VE, Carroll JD and Moss SF:
Helicobacter pylori in the pathogenesis of gastric cancer and
gastric lymphoma. Cancer Lett. 305:228–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsubara S, Takasu S, Tsukamoto T, Mutoh
M, Masuda S, Sugimura T, Wakabayashi K and Totsuka Y: Induction of
glandular stomach cancers in Helicobacter pylori-infected Mongolian
gerbils by 1-nitrosoindole-3-acetonitrile. Int J Cancer.
130:259–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng Q, Chen XY, Shi Y and Xiao SD:
Development of gastric adenocarcinoma in Mongolian gerbils after
long-term infection with Helicobacter pylori. J Gastroenterol
Hepatol. 9:1192–1198. 2004. View Article : Google Scholar
|
|
7
|
Mesnil M, Crespin S, Avanzo JL and
Zaidan-Dagli ML: Defective gap junctional intercellular
communication in the carcinogenic process. Biochim Biophys Acta.
1719:125–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu J, Zhou HF, Wang CH, Zhang B, Liu D,
Wang W and Sui GJ: Decreased expression of Cx32 and Cx43 and their
function of gap junction intercellular communication in gastric
cancer. Zhonghua Zhong Liu Za Zhi. 29:742–747. 2007.(In Chinese).
PubMed/NCBI
|
|
9
|
Xu CX, Qi YM, Yang WB, Wang F, Zhou JD and
Shen SR: Effect of CagA+helicobacter pylori strain on
the expression of connexin 43 and cell proliferation in BGC2823
cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 32:288–294. 2007.(In
Chinese). PubMed/NCBI
|
|
10
|
Xu CX, Jia Y, Yang WB, Wang F and Shen SR:
Relationship between Helicobacter pylori infection and expression
of connexin (Cx) 32 and Cx43 genes in gastric cancer and gastric
precancerous lesions. Zhonghua Yi Xue Za Zhi. 88:1523–1527.
2008.(In Chinese). PubMed/NCBI
|
|
11
|
Jia Y, Xu CX and Yang WB: Expressions of
connexin 32 and connexin 43 in patients with gastric precancerous
lesion after eradication of Helicobacter pylori. Zhong Nan Da Xue
Xue Bao Yi Xue Ban. 33:628–633. 2008.(In Chinese). PubMed/NCBI
|
|
12
|
Xu CX, Jia Y, Yang WB, Zou HF, Wang F and
Shen SR: Helicobacter pylori infection and changes of cell gap
junction of gastric epithelial cells in patients with gastric
cancer and precancerous lesion. Zhong Nan Da Xue Xue Bao Yi Xue
Ban. 33:338–343. 2008.(In Chinese). PubMed/NCBI
|
|
13
|
Hu TZ, Huang LH, Xu CX, Liu XM, Wang Y,
Xiao J, Zhou L, Luo L and Jiang XX: Expressional profiles of
transcription factors in the progression of Helicobacter
pylori-associated gastric carcinoma based on protein/DNA array
analysis. Med Oncol. 32:2652015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sadat MA, Kumatori A, Suzuki S, Yamaguchi
Y, Tsuji Y and Nakamura M: GATA-3 represses gp91phox gene
expression in eosinophil-committed HL-60-C15 cells. FEBS Lett.
436:390–394. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shield PW, Papadimos DJ and Walsh MD:
GATA3: A promising marker for metastatic breast carcinoma in serous
effusion specimens. Cancer Cytopathol. 122:307–312. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lindgren Å, Yun CH, Sjöling Å, Berggren C,
Sun JB, Jonsson E, Holmgren J, Svennerholm AM and Lundin SB:
Impaired IFN-γ production after stimulation with bacterial
components by natural killer cells from gastric cancer patients.
Exp Cell Res. 317:849–858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saito T, Nishimura M, Kudo R and Yamasaki
H: Suppressed gap junctional intercellular communication in
carcinogenesis of endometrium. Int J Cancer. 93:317–323. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uchida Y, Matsuda K, Sasahara K, Kawabata
H and Nishioka M: Immunohistochemistry of gap junctions in normal
and diseased gastric mucosa of humans. Gastroenterology.
109:1492–1496. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bondurand N, Girard M, Pingault V, Lemort
N, Dubourg O and Goossens M: Human connexin 32, a gap junction
protein altered in the X-linked form of Charcot-Marie-Tooth
disease, is directly regulated by the transcription factor SOX10.
Hum Mol Genet. 10:2783–2795. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Houlden H, Girard M, Cockerell C, Ingram
D, Wood NW, Goossens M, Walker RW and Reilly MM: Connexin 32
promoter P2 mutations: A mechanism of peripheral nerve dysfunction.
Ann Neurol. 56:730–734. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tong X, Li L, Li X, Heng L, Zhong L, Su X,
Rong R, Hu S, Liu W, Jia B, et al: SOX10, a novel
HMG-box-containing tumor suppressor, inhibits growth and metastasis
of digestive cancers by suppressing the Wnt/β-catenin pathway.
Oncotarget. 5:10571–10583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Zhang Z, Yu M, Li L, Du G, Xiao W
and Yang H: Involvement of miR-20a in promoting gastric cancer
progression by targeting early growth response 2 (EGR2). Int J Mol
Sci. 14:16226–16239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cohen H, Ben-Hamo R, Gidoni M, Yitzhaki I,
Kozol R, Zilberberg A and Efroni S: Shift in GATA3 functions, and
GATA3 mutations, control progression and clinical presentation in
breast cancer. Breast Cancer Res. 16:4642014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gulbinas A, Berberat PO, Dambrauskas Z,
Giese T, Giese N, Autschbach F, Kleeff J, Meuer S, Büchler MW and
Friess H: Aberrant GATA-3 expression in human pancreatic cancer. J
Histochem Cytochem. 54:161–169. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mertens RB, de Peralta-Venturina MN,
Balzer BL and Frishberg DP: GATA3 Expression in normal skin and in
benign and malignant epidermal and cutaneous adnexal neoplasms. Am
J Dermatopathol. 37:885–891. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Izzo F, Mercogliano F, Venturutti L, Tkach
M, Inurrigarro G, Schillaci R, Cerchietti L, Elizalde PV and
Proietti CJ: Progesterone receptor activation downregulates GATA3
by transcriptional repression and increased protein turnover
promoting breast tumor growth. Breast Cancer Res. 16:4912014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chou J, Provot S and Werb Z: GATA-3 in
development and cancer differentiation: Cells GATA Have It! J Cell
Physiol. 222:42–49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tashireva LA, Perelmuter VM, Manskikh VN,
Denisov EV, Savelieva OE, Kaygorodova EV and Zavyalova MV: Types of
immune-inflammatory responses as a reflection of cell-cell
interactions under conditions of tissue regeneration and tumor
growth. Biochemistry (Mosc). 82:542–555. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jee H, Lee SH, Park JW, Lee BR, Nam KT and
Kim DY: Connexin32 inhibits gastric carcinogenesis through cell
cycle arrest and altered expression of p21Cip1 and p27Kip1. BMB
Rep. 46:25–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Huang LH, Xu CX, Xiao J, Zhou L,
Cao D, Liu XM and Qi Y: Cx32 and Cx43 promoter methylation in
Helicobacter pylori-associated gastric tumorigenesis. World J
Gastroenterol. 20:11770–11779. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu X, Cao K, Xu C, Hu T, Zhou L, Cao D,
Xiao J, Luo L, Guo Y and Qi Y: GATA-3 augmentation down-regulates
Connexin43 in Helicobacter pylori associated gastric
carcinogenesis. Cancer Biol Ther. 16:987–996. 2015. View Article : Google Scholar : PubMed/NCBI
|