Introduction
Dermatofibrosarcoma protuberans (DFSP) is a
low-grade soft tissue tumor occurring in the dermis and
subcutaneous tissues, which accounts for ~1% of all soft tissue
sarcomas (1,2). It was initially characterized as a
keloid-like sarcoma (3), although
Hoffman gave its current name in 1925 (4).
DFSP usually occurs in young to middle-aged patients
but can present in all age groups (5). It is commonly found on the trunk,
however, it can also develop in the extremities, head or neck. DFSP
demonstrates local infiltrative growth but seldom metastasizes
distally (6). DFSP is divided
histopathologically into classical and non-classical types
(7). Classical-type DFSP typically
forms a radial or storiform pattern, with the cancer tissue
extending into the subcutaneous fat and forming a honeycomb-like
structure (8). Atypical DFSP
comprises at least 10 subtypes, of which the most common include
pigment type, mucus type, and sarcoma type (6). Fibrosarcomatous DFSP (FS-DFSP) is also
an atypical DFSP subtype, with high rates of recurrence and
metastasis.
The early clinical symptoms of DFSP are
non-specific, making diagnosis difficult and leading to a high
incidence of misdiagnosis. Pathological and immunohistochemical
examinations are thus currently the gold standard for diagnosing
DFSP, with surgical resection remaining the main treatment option.
In the present study, 70 cases of DFSP were retrospectively
analyzed and their clinical features, differential diagnosis, and
treatment were investigated.
Patients and methods
Patient information
The study group comprised 70 patients, including 41
primary cases at first diagnosis and 29 recurrent cases. There were
equal numbers of male and female patients. Patient ages ranged
between 5 and 76 years (mean age, 43 years). The tumor was located
on the trunk in 40 patients, the extremities in 19 patients, and
the head and neck in 11 patients. The disease course duration
ranged between 1 month and 40 years. The maximal tumor diameter
ranged between 0.5 and 15 cm.
Clinical manifestations and
diagnosis
The main clinical manifestations were pale red or
brown irregular indurations, showing slow growth. They appeared
either as solitary nodules or as multiple scattered confluent
masses. The skin lesions lacked typical characteristics. In total,
59 cases were diagnosed as DFSP and 11 cases were diagnosed as
FS-DFSP.
Therapy
All 70 patients underwent surgical treatment. In
cases with no preoperative pathological diagnosis, the tumors
underwent rapid intraoperative freezing to determine the nature of
the tumor. If the tumor was determined to be a low-grade malignant
spindle cell tumor, resection was extended to 3 cm, and
intraoperative frozen sections were obtained from all directions to
confirm that the margins were negative. A free skin graft or
partial flap was used to repair the wound following tumor
resection. In addition, five patients were administered with a
continuous course of radiotherapy if required, at a dose of
50.0–60.0 Gy.
Hematoxylin and eosin staining
Samples were fixed in 40% formaldehyde at 25°C for
12 h. The reagents used were hematoxylin, eosin, 0.5% hydrochloric
acid alcohol solution and 0.2% ammonia solution (pH between 7.5–8).
The sections were sliced 3 µm thick and heated in a microwave oven
for 1 h at 60°C. The dewaxing was performed by adding:
Dimethylbenzene for 10 min, three times; anhydrous ethanol for 5
min, two times; 95% ethanol for 5 min; 90% ethanol for 5 min; 80%
ethanol for 5 min; 75% ethanol for 5 min; and distilled water for 5
min. Staining was performed by adding: i) Hematoxylin for 10 min;
ii) distilled water, for 1 min; iii) 0.5% hydrochloric acid alcohol
solution for 20 sec; iv) distilled water for 2 min; v) 0.2% ammonia
for 40 sec; vi) distilled water for 2 min; vii) 0.5% eosin for 5
min; and viii) distilled water for 30 min. Dehydration was
performed by adding 80% ethanol for 3 min, 90% ethanol for 3 min,
95% ethanol for 3 min, anhydrous ethanol for 5 min and fresh
anhydrous ethanol for 5 min. Fresh xylene was added for 5 min, 3
times, to remove ethanol. Samples were sealed with neutral gum. The
sealed slices were placed incubated at 37°C. Following drying, the
sections were observed under a light microscope (magnifications,
×40, ×100, ×200 and ×400). Unless specified, all step were
performed at room temperature.
Statistical analysis
Statistical analysis was performed using IBM SPSS
Statistics 21.0 software (IBM SPSS, Armonk, NY, USA). Descriptive
statistics were used to report patients' baseline characteristics.
The clinical features and outcomes of cases treated by pathological
type were compared using the χ2 test. All tests were
two-sided, with α=0.05.
Results
Among the 70 cases, 41 were first diagnosed in The
First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China), and 29 were referred to The First Affiliated Hospital of
Zhengzhou University for the treatment of recurrence, following
treatment in other hospitals. The skin flap or skin graft survived
well in all cases, with only one case of partial necrosis. The
follow-up period ranged between 3 and 36 months, with 18 cases of
recurrence (Tables I and II), and three cases of distant metastasis,
one of which was DFSP and two were FS-DFSP, with one of the latter
succumbing to mortality. Of the 41 primary patients (follow-up at
2.7 years), seven had recurrences (17.1%), compared with 11 (37.9%)
of the 29 recurrent patients (follow-up at 2.0 years, P=0.049). The
recurrence rate was significantly higher among the referred cases
compared with the newly diagnosed cases. In terms of the
pathological types, 12 of the 59 DFSP patients (follow-up 2.6
years) had recurrences (20.3%), compared with six (54.6%) of the 11
patients with FS-DFSP (follow-up 2.1 years, P=0.045) (Table III). The recurrence rate was
significantly higher among patients with FS-DFSP compared with
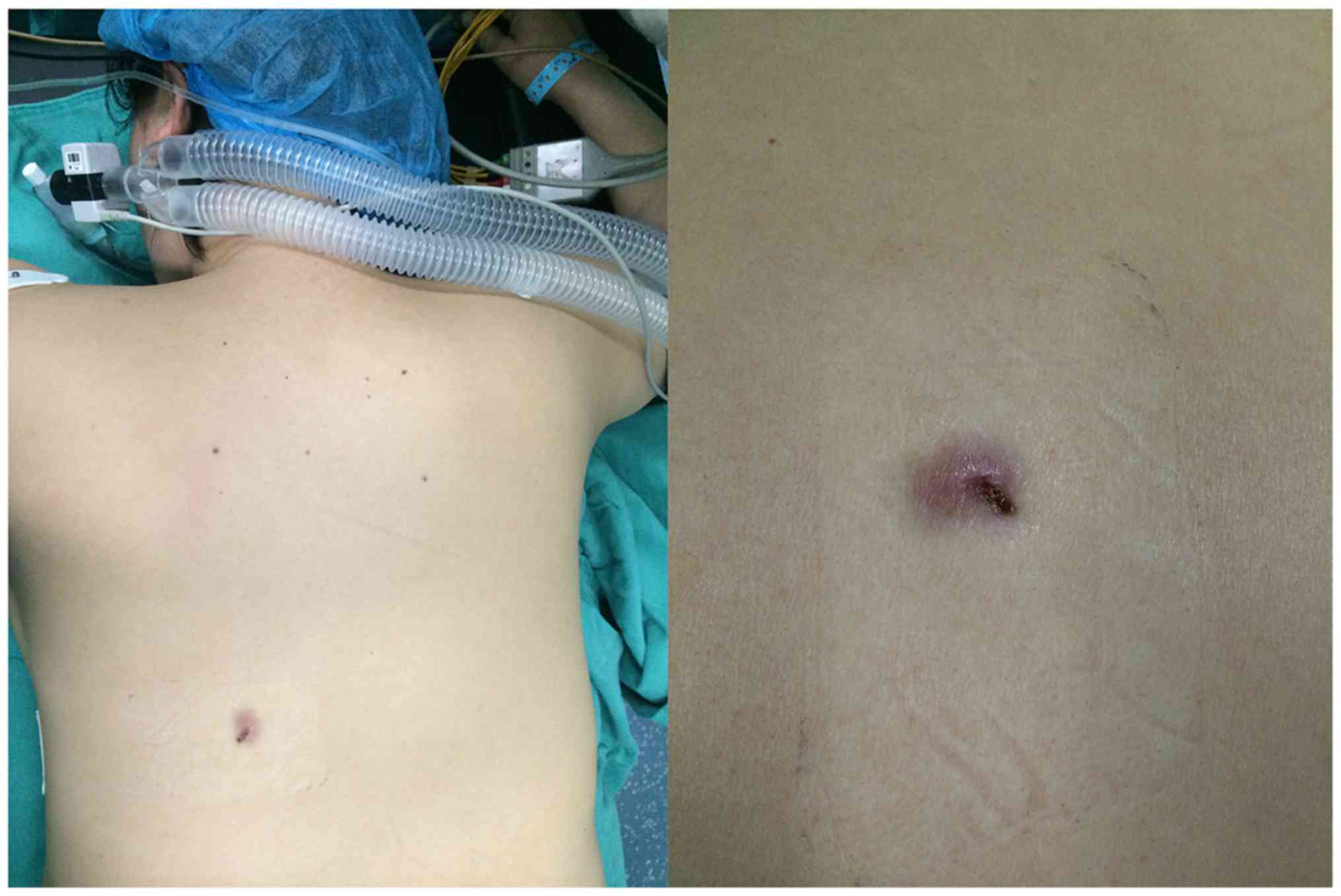
those with DFSP. The cases reported here include one patient with
an atypical skin lesion (Fig. 1), who
received a definitive diagnosis of DFSP following a biopsy at his
first visit, and who subsequently underwent extensive resection
with no recurrence in the following 3 years.
 | Table I.Baseline characteristics and
recurrence rates of primary and recurrent cases. |
Table I.
Baseline characteristics and
recurrence rates of primary and recurrent cases.
|
|
| Primary cases | Recurrent cases |
|---|
|
|
|
|
|
|---|
| Characteristic | n | n | % | n | % |
|---|
| Cases | 70 | 41 | 58.6 | 29 | 41.4 |
| Sex |
|
|
|
|
|
| Male | 35 | 20 | 48.8 | 15 | 51.7 |
|
Female | 35 | 21 | 51.2 | 14 | 48.3 |
| Site |
|
|
|
|
|
| Head and
neck | 11 | 6 | 14.7 | 5 | 17.2 |
|
Trunk | 40 | 24 | 58.5 | 16 | 55.2 |
|
Extremity | 19 | 11 | 26.8 | 8 | 27.6 |
| Age (years) |
|
|
|
|
|
|
<40 | 18 | 11 | 26.8 | 7 | 24.1 |
|
40–50 | 40 | 23 | 56.1 | 17 | 58.6 |
|
>50 | 12 | 7 | 17.1 | 5 | 17.3 |
| Tumor size
(cm)a |
|
|
|
|
|
|
<1 | 19 | 14 | 34.1 | 5 | 17.3 |
| 1–2 | 33 | 20 | 48.8 | 13 | 44.8 |
| 2+ | 18 | 7 | 17.1 | 11 | 37.9 |
| Recurrence |
|
|
|
|
|
| Yes | 18 | 7 | 17.1 | 11 | 37.9 |
| No | 52 | 34 | 82.9 | 18 | 62.1 |
 | Table II.Baseline characteristics and
recurrence rates of different pathological types. |
Table II.
Baseline characteristics and
recurrence rates of different pathological types.
|
|
| DFSP | FS-DFSP |
|---|
|
|
|
|
|
|---|
| Characteristic | n | n | % | n | % |
|---|
| Patients | 70 | 59 | 84.3 | 11 | 15.7 |
| Sex |
|
|
|
|
|
|
Male | 35 | 30 | 50.8 | 5 | 45.4 |
|
Female | 35 | 29 | 49.2 | 6 | 54.6 |
| Site |
|
|
|
|
|
| Head
and neck | 11 | 8 | 13.6 | 3 | 27.2 |
|
Trunk | 40 | 34 | 57.6 | 6 | 54.6 |
|
Extremity | 19 | 17 | 28.8 | 2 | 18.2 |
| Age (years) |
|
|
|
|
|
|
<40 | 18 | 16 | 27.1 | 2 | 18.2 |
|
40–50 | 40 | 33 | 55.9 | 7 | 63.6 |
|
>50 | 12 | 10 | 17.0 | 2 | 18.2 |
| Tumor size
(cm)a |
|
|
|
|
|
|
<1 | 19 | 15 | 25.4 | 4 | 36.4 |
|
1–2 | 33 | 29 | 49.2 | 4 | 36.4 |
| 2+ | 18 | 15 | 25.4 | 3 | 27.2 |
| Recurrence |
|
|
|
|
|
|
Yes | 18 | 12 | 20.3 | 6 | 54.6 |
| No | 52 | 47 | 79.7 | 5 | 45.4 |
 | Table III.Comparison of recurrence rates in
different pathological types. |
Table III.
Comparison of recurrence rates in
different pathological types.
| Type | Recurrence, n
(%) | No recurrence, n
(%) |
χ2-value | P-value |
|---|
| DFSP | 12 (20.33) | 47 (79.67) | 4.030 | 0.045 |
| FS-DFSP | 6 (54.55) | 5 (45.45) |
|
|
The follow three cases are representative: Case 1,
DFSP with multiple recurrences and metastasis; case 2, FS-DFSP;
case 3, classic DFSP, with pathogenesis being representative.
Case 1
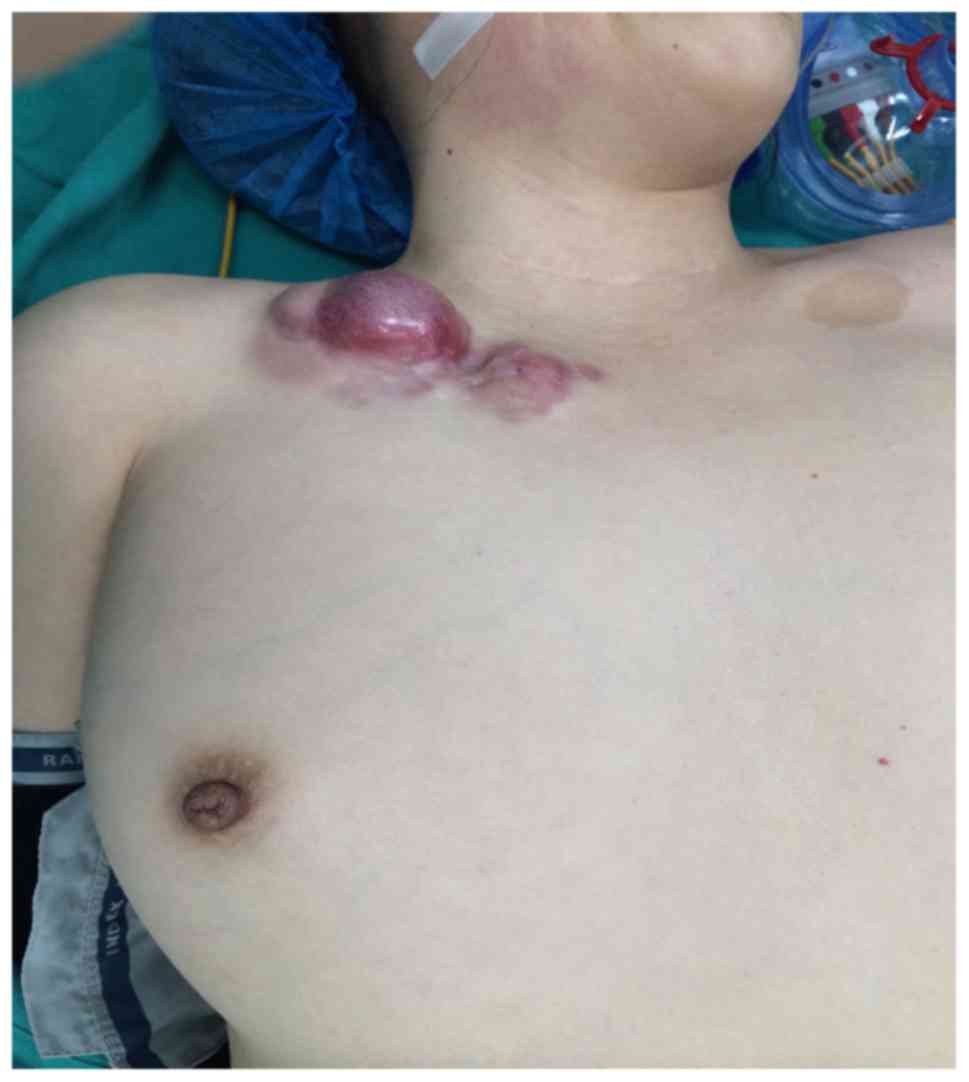
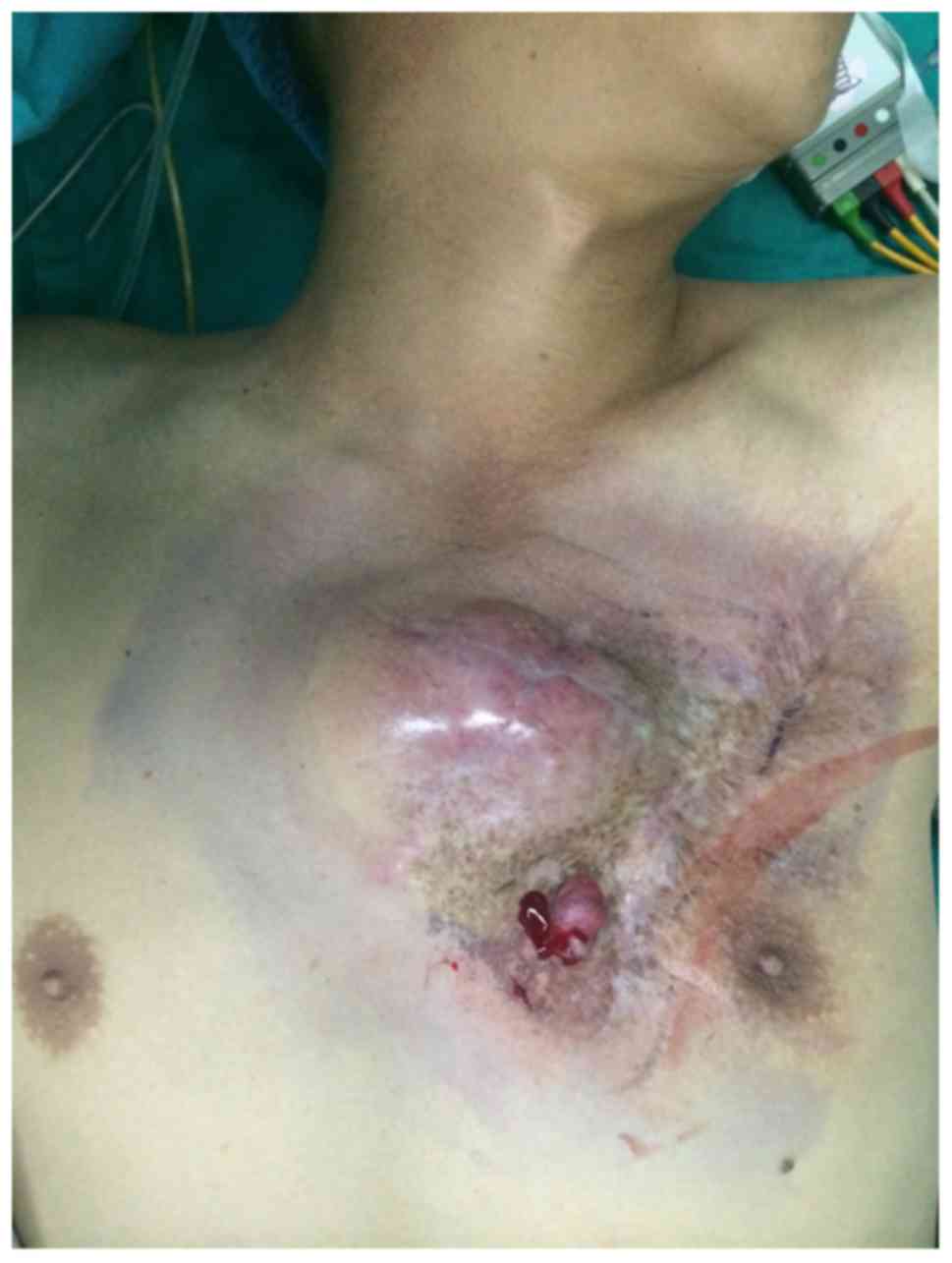
Case 1 was a 42-year-old woman with a history of
subtotal thyroidectomy for a thyroid tumor (unknown nature) in
2006, which left a 0.5×0.3 cm mass at the site of the right chest
wall drainage bag. The tumor gradually increased in size, and was
resected in 2012, without pathological examination of the right
chest wall. A red tumor recurred in the same place in 2014, which
was evident on the body surface. The tumor was hard, smooth,
irregular in shape, and measured 5.2×2.5×3.0 cm, with no pain to
the touch, no bleeding, and no rupture or ulceration. The
surrounding skin was reddish in color and uneven, resembling a
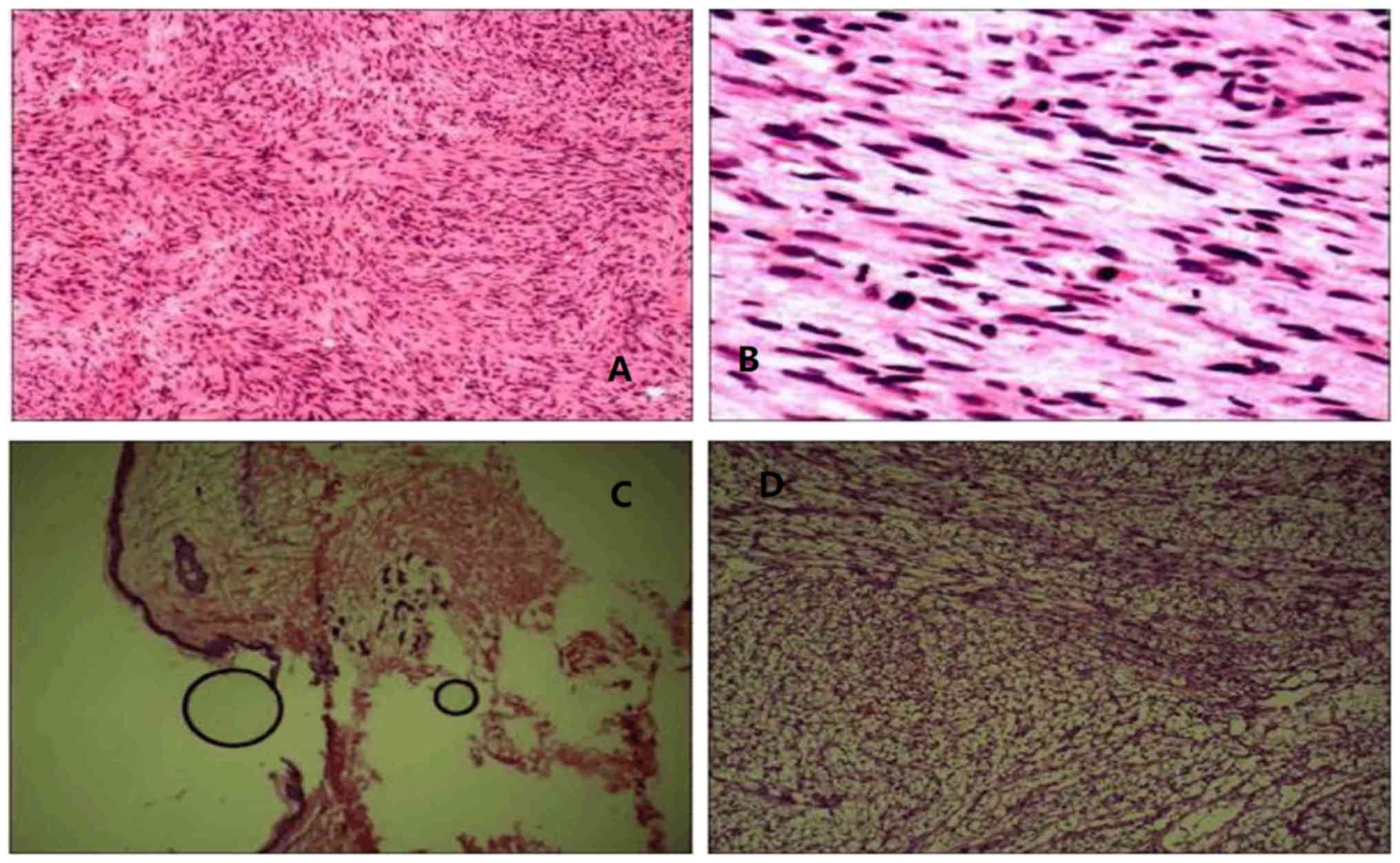
keloid. Telangiectasia was evident on the surface (Fig. 2). During surgery, the resection was
expanded 0.2 cm from the tumor rim. However, examination of
rapid-freeze biopsy showed a low-degree of malignant chest wall
spindle cell tumor, therefore, the surgeon expanded the resection
margin to 3 cm. The postoperative pathological diagnosis was
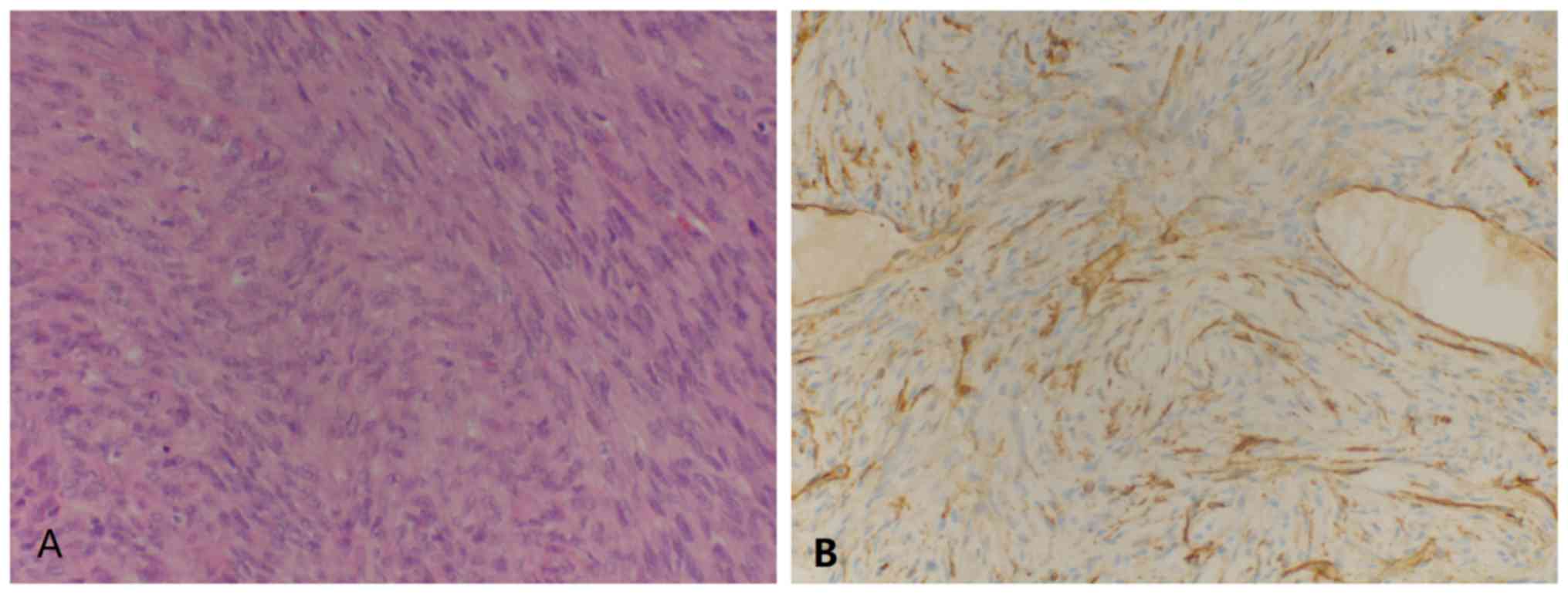
FS-DFSP of the chest wall. Immunohistochemistry demonstrated
CD34(+) (Fig. 3) and Ki-67(20%+)
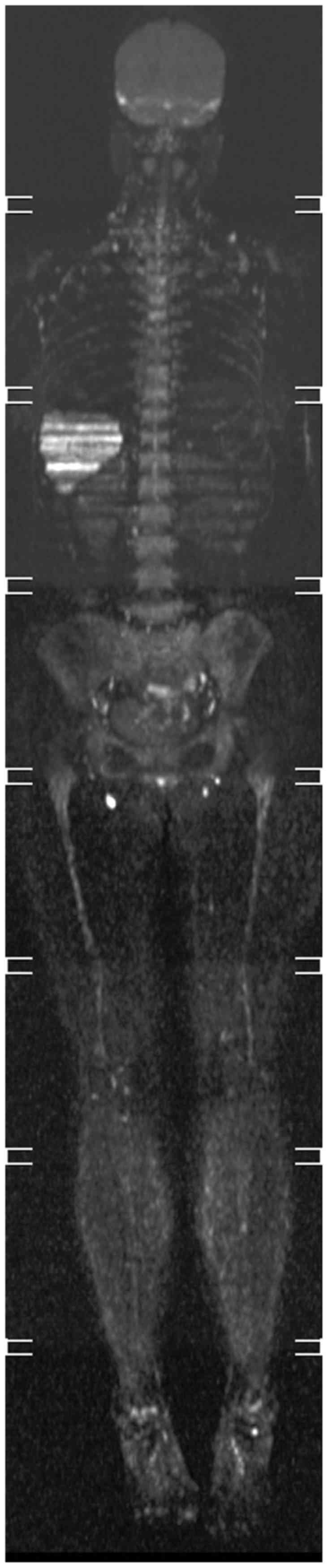
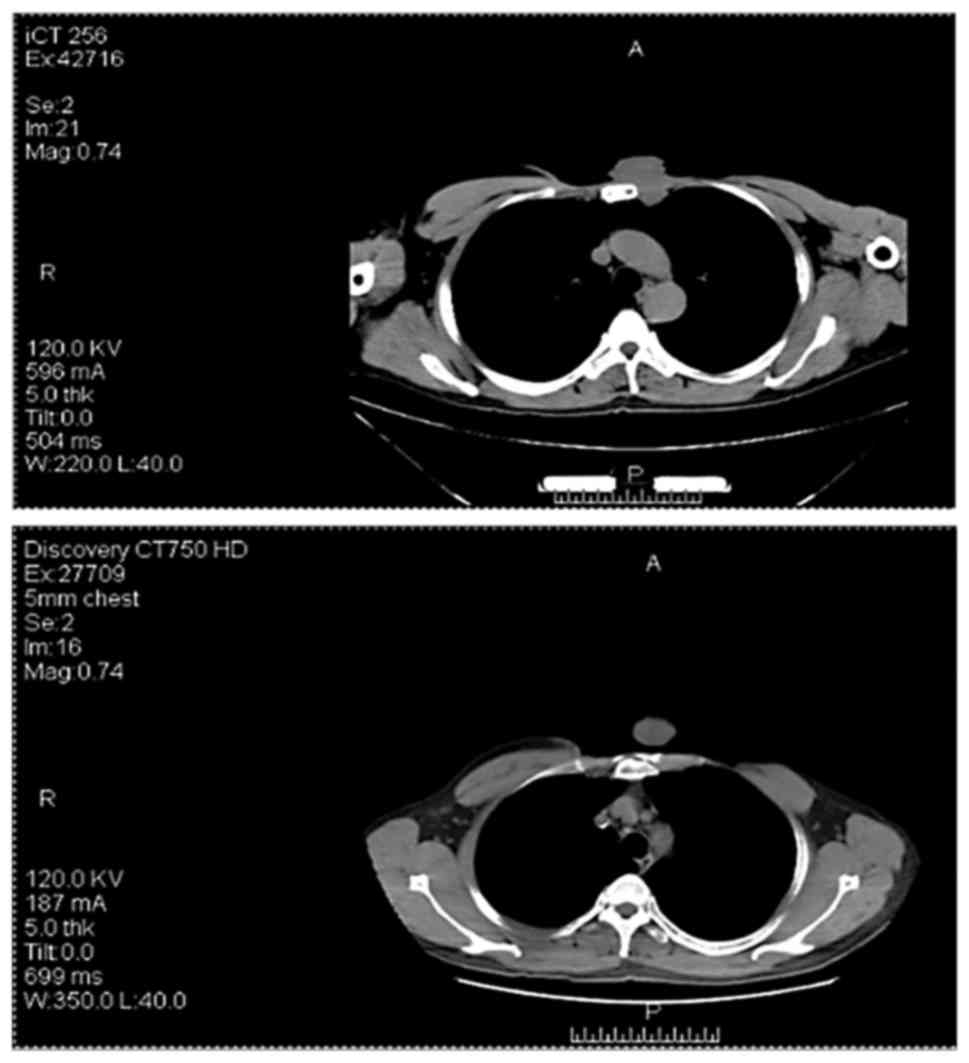
results. The tumor recurred in 2016, and preoperative positron
emission tomography-computed tomography (CT) revealed distant bone
metastases (Fig. 4). The patient
received no further surgical treatment and succumbed to mortality
in 2017.
Case 2
Case 2 was of a 57-year-old woman who had undergone
surgical resection for a tumor at the top of her head at a local
hospital in 2007, without pathological examination. The tumor
recurred twice in the same site in 2013 and 2015, and the patient
underwent surgery on both occasions, without pathological
examination. The patient attended the Department of Plastic
Surgery, The First Affiliated Hospital of Zhengzhou University for
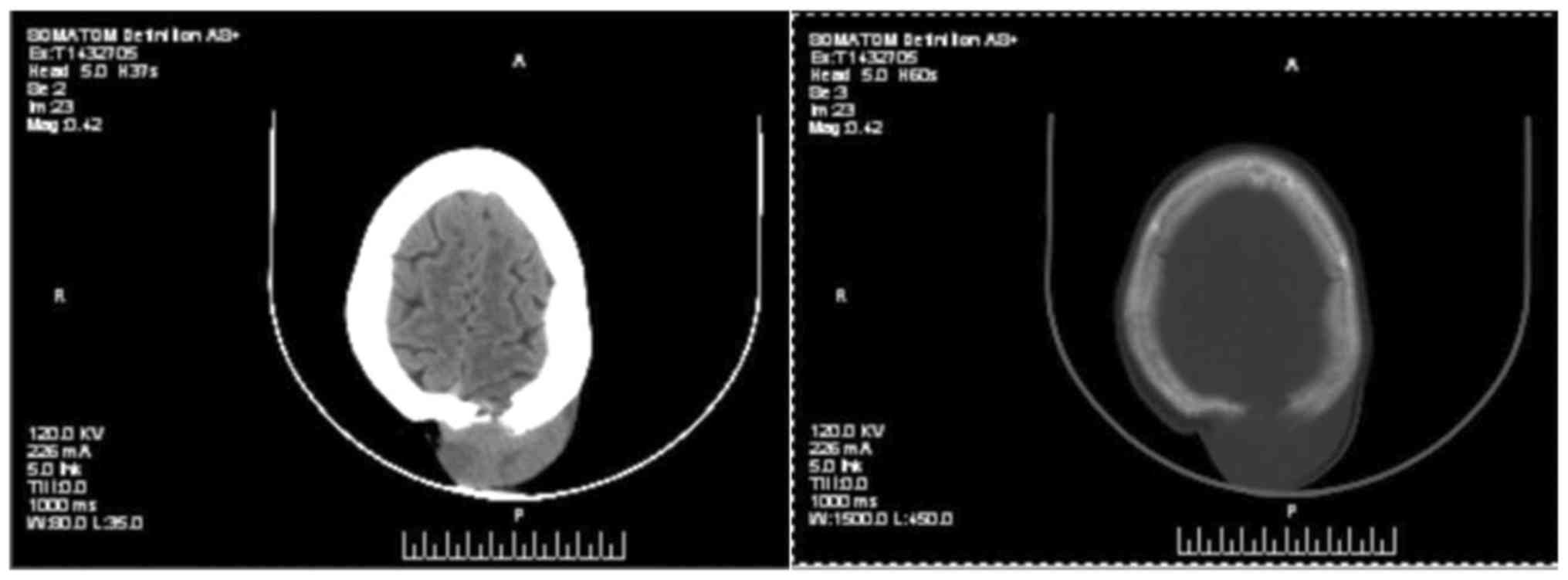
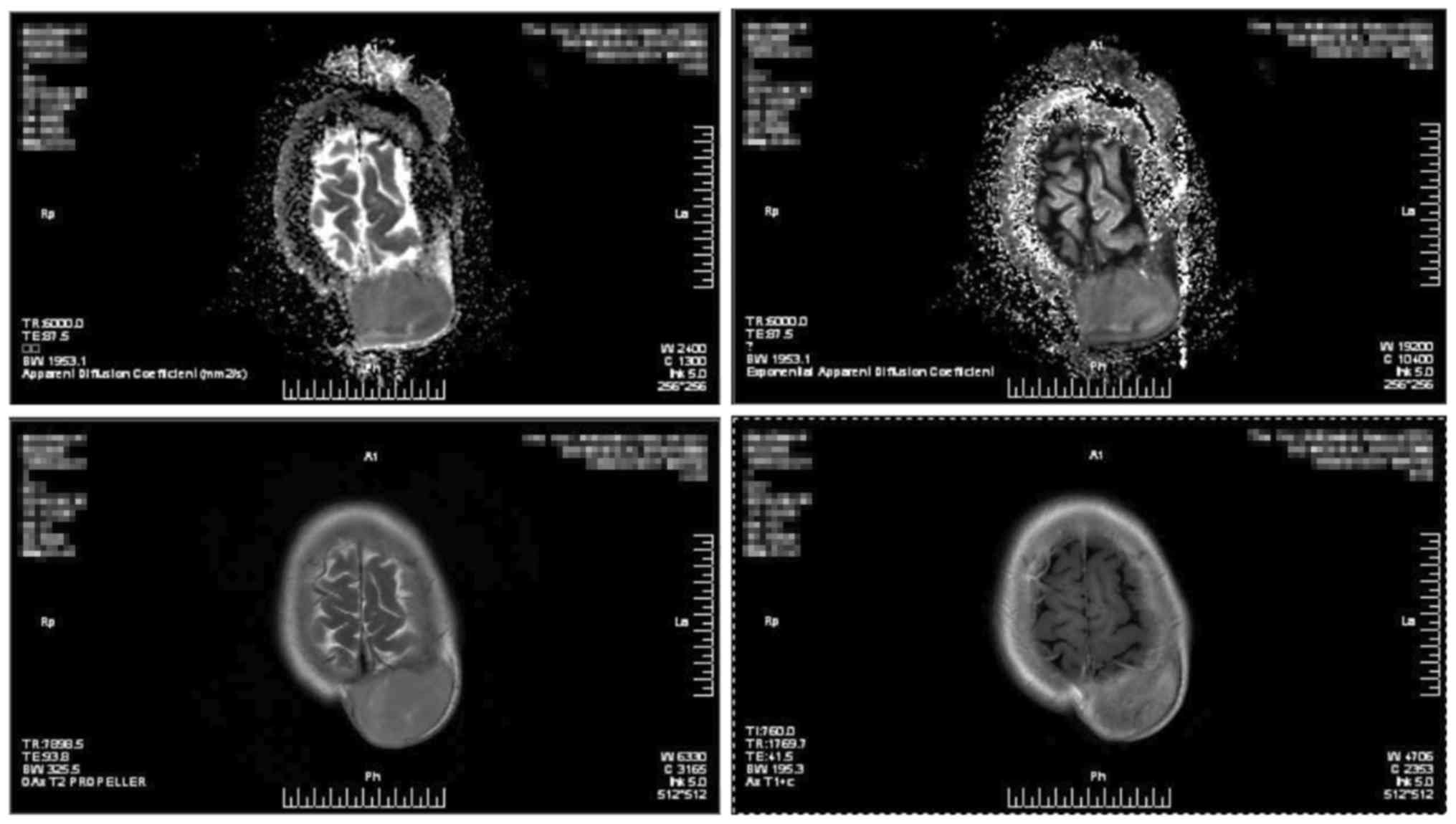
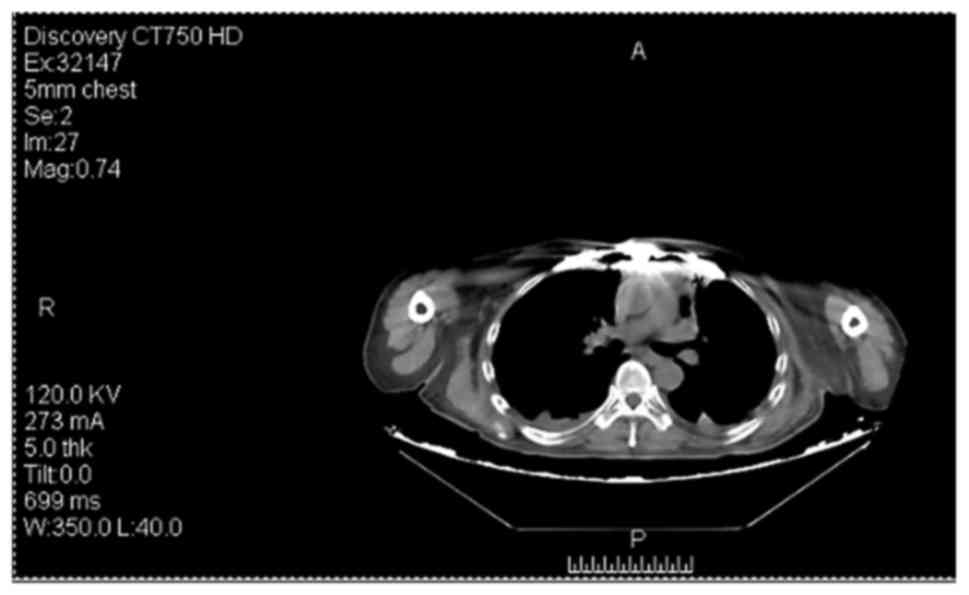
treatment of the fourth recurrence in 2016. Preoperative CT
examination revealed a subcutaneous tumor on the left side of the
top of her head, and magnetic resonance imaging suggested
involvement of the adjacent skull (Figs.
5 and 6). To avoid bleeding
during surgery, internal vascular embolization of the tumor was
initially performed, and the resection was then expanded to 3 cm,
with confirmation that the margin was negative. The wound was
closed using pedicled flaps. Postoperative pathological examination
suggested that the tumor was FS-DFSP. Immunohistochemistry revealed
CD34(+) and Ki-67(15%+). The postoperative blood supply to the
flaps was poor, and flap necrosis was observed 2 weeks following
surgery. However, the wound healed, and there was no sign of tumor
recurrence for 1.5 years.
Case 3
Case 3 was of a 46-year-old man, who developed a
2.3×2.5 cm lump in 1976, following a chest-wall injury. The lump
was not pruritic or painful, and the patient sought no attention
for it. However, the tumor had grown to adult-fist size by 2008,
and the patient underwent chest wall tumor resection at another
hospital. The intraoperative and postoperative pathological
diagnoses were of DFSP. In 2014, the tumor reappeared in the
original position. It was red and cauliflower-like in appearance,
and increased gradually to 10.0×8.0 cm. A red, cauliflower-shaped
mass of about 1.0×1.0 cm was found in the lower right of the tumor.
The hard surface was free of rupture and bleeding (Fig. 7). The resection was expanded to 3 cm
and the wound was closed using a skin graft, followed by vacuum
sealing negative pressure drainage for 9 days following surgery.
The skin graft survived well. The results of intraoperative frozen
biopsies showed negative margins. Immunohistochemistry revealed
CD34(+) and Ki-67(30%+) (Fig. 8).
However, the tumor recurred twice in 2014 and 2015 (Fig. 9), respectively, and invaded the chest
wall. An expanded resection was performed without removal of the
ribs, however, the tumor recurred in 2016 at the original site. A
chest CT indicated that the tumor had invaded the ribs, and the
majority of the ribs on one side were missing (Fig. 10). Another resection was performed,
and chest surgery was arranged to repair the ribs.
Discussion
Clinically, DFSP often masquerades as a benign,
indolent tumor. Microscopically, it extends far beyond the assessed
clinical margins, spreading in the dermis and subcutaneous tissue
(3). DFSP has a distinctive
histologic appearance but can mimic other diseases. Histologically,
several variants of DFSP have been described and it is important
they are well characterized to avoid misdiagnosis with other types
of tumor. These variants include pigmented, myxoid, myoid, granular
cell, sclerotic, atrophic DFSP and giant cell fibroblastoma
(2). At this point, it is necessary
to distinguish the tumor from other tumors. The high incidence of
misdiagnosis of DFSP highlights the importance for pathological
examination of early skin lesions in order to clarify the diagnosis
(9,10). Histology with immunohistochemistry
remain the gold standard for ensuring an accurate diagnosis
(11,12). DFSP may be a more aggressive tumor,
its behavior can be affected by surgical treatment (13). The standard treatment is wide local
resection with at least a 2-cm margin (2). According to the literature, the
recurrence rate of DFSP following expansion to 2 cm remains high,
whereas the recurrence rate was significantly reduced following
expansion to >3 cm (14–17). Therefore, the resection was expanded
to 3 cm in all later cases, and negative margins were confirmed by
pathological examination of intraoperative frozen specimens.
The total postoperative recurrence rate in the
present study was 25.7%. Based on available retrospective data, the
risk of metastasis and recurrence is elevated in FS-DFSP, compared
with that in DFSP. In 2015, Hoesly et al (9) also reported that DFSP-FS exhibited more
aggressive behavior than DFSP, with lower recurrence-free survival
rate and increased metastatic potential. Furthermore, recurrent
cases are more likely to relapse following treatment than primary
cases. Five patients (7%) received postoperative radiotherapy for
positive margins following maximal excision. These five patients
showed no recurrence at the time of reporting. DuBay et al
(1) reported that wide local excision
with careful pathologic analysis of margins had a low recurrence
rate and was used for the majority of patients with DFSP lesions.
Mohs micrographic surgery (MMS) entails a more elaborate technique
and is particularly suitable for cases of DFSP where an extended
excision is difficult (3). A study by
Paradisi et al (18) showed
that the recurrence rates of MMS were significantly lower than
those of WLE. Although this technique is frequently used in certain
countries, it was not available at The First Affiliated Hospital of
Zhengzhou University due to technical constraints.
The main limitation of the present study is that was
a retrospective analysis, and the method of surgery was not the
most advanced. Compared with other studies, the follow-up time was
also short and, with time, there may be different results.
Therefore, a large prospective randomized trial (stratified random
sampling) and multicenter study is necessary to demonstrate
possible differences.
In conclusion, DFSP is a rare disease and an
improved awareness and understanding of this condition is required
by surgeons and pathologists to allow its early diagnosis and
treatment. Pathological examinations are required in patients with
suspected DFSP, with the aim of minimizing the misdiagnosis rate.
Once diagnosed, DFSP requires prompt treatment by extended tumor
resection, followed by an increased follow-up frequency. Combined
treatment requires consideration to reduce the recurrence rate in
unresectable cases or in patients with repeated recurrence
following resection. When surgery is insufficient or disease is
metastatic, imatinib may be more important in treatment in the
future (7,19,20). These
data may be useful to assist clinicians when they treat patients
with DFSP. In subsequent investigations, combining MMS with
radiation or chemotherapy is worth consideration.
Acknowledgements
The authors would like to thank The First Affiliated
Hospital of Zhengzhou University for providing clinical information
and Mr. Erwei Xu (Zhengzhou University) for assistance with the
photographs.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QW was responsible for the conception and design of
the present study and revised the manuscript. AL performed data
analysis and drafted the manuscript. Both authors interpreted
results, and read and approved the final version of the paper. The
authors confirm that the content has not been published elsewhere
and does not overlap their published work.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First Affiliated Hospital of Zhengzhou University. Information
was collected from the medical records of patients, with no
influence on diagnosis or treatment. Prior to the study, patients
were fully informed to clarify the purpose and significance of the
study.
Consent for publication
The patient or guardian provided written informed
consent for the publication of any associated data and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DFSP
|
dermatofibrosarcoma protuberans
|
|
FS-DFSP
|
fibrosarcomatous DFSP
|
|
MMS
|
Mohs micrographic surgery
|
References
|
1
|
DuBay D, Cimmino V, Lowe L, Johnson TM and
Sondak VK: Low recurrence rate after surgery for
dermatofibrosarcoma protuberans: A multidisciplinary approach from
a single institution. Cancer. 100:1008–1016. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llombart B, Serra-Guillén C, Monteagudo C,
López Guerrero JA and Sanmartin O: Dermatofibrosarcoma protuberans:
A comprehensive review and update on diagnosis and management.
Semin Diagn Patho. 30:13–28. 2013. View Article : Google Scholar
|
|
3
|
Snow SN, Gordon EM, Larson PO, Bagheri MM,
Bentz ML and Sable DB: Dermatofibrosarcoma protuberans: A report on
29 patients treated by Mohs micrographic surgery with long-term
follow-up and review of the literature. Cancer. 101:28–38. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoffman E: Über das knollentreibende
fibrosarkom der haut (Dermatofibrosarkoma protuberans). Dermat
Ztschr. 43:1–28. 1925.(In German). View Article : Google Scholar
|
|
5
|
Al-Rahbi S, Al-Lawati T, Al-Kharusi S,
Thomas S and Al-Harrasi K: Dermatofibrosarcoma Protuberans: A rare
malignancy of the breast. Oman Med J. 30:378–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bogucki B, Neuhaus I and Hurst EA:
Dermatofibrosarcoma protuberans: A review of the literature.
Dermatol Surg. 38:537–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fletcher CD: The evolving classification
of soft tissue tumours-an update based on the new 2013 WHO
classification. Histopathology. 64:2–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sigel JE, Bergfeld WF and Goldblum JR: A
morphologic study of dermatofibrosarcoma protuberans: Expansion of
a histologic profile. J Cutan Pathol. 27:159–163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hoesly PM, Lowe GC, Lohse CM, Brewer JD
and Lehman JS: Prognostic impact of fibrosarcomatous transformation
in dermatofibrosarcoma protuberans: A cohort study. J Am Acad
Dermatol. 72:419–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iorizzo LJ III and Brown MD: Atypical
fibroxanthoma: A review of the literature. Dermatol Surg.
37:146–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karanian M, Perot G, Coindre JM, Chibon F,
Pedeutour F and Neuville A: Fluorescence in situ hybridization
analysis is a helpful test for the diagnosis of dermatofibrosarcoma
protuberans. Mod Pathol. 28:230–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saiag P, Grob JJ, Lebbe C, Malvehy J, del
Marmol V, Pehamberger H, Peris K, Stratigos A, Middelton M, Basholt
L, et al: Diagnosis and treatment of dermatofibrosarcoma
protuberans. European consensus-based interdisciplinary guideline.
Eur J Cancer. 51:2604–2608. 2015.
|
|
13
|
Szollosi Z and Nemes Z: Transformed
dermatofibrosarcoma protuberans: A clinicopathological study of
eight cases. J Clin Pathol. 58:751–756. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Popov P, Bohling T, Asko-Seljavaara S and
Tukiainen E: Microscopic margins and results of surgery for
dermatofibrosarcoma protuberans. Plast Reconstr Surg.
119:1779–1784. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tom WD, Hybarger CP and Rasgon BM:
Dermatofibrosarcoma protuberans of the head and neck: Treatment
with Mohs surgery using inverted horizontal paraffin sections.
Laryngoscope. 113:1289–1293. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pallure V, Dupin N and Guillot B;
Association for Recommendations in Dermatology: Surgical treatment
of Darier-Ferrand dermatofibrosarcoma: A systematic review.
Dermatol Surg. 39:1417–1433. 2013.PubMed/NCBI
|
|
17
|
Kurlander DE, Martires KJ, Chen Y,
Barnholtz-Sloan JS and Bordeaux JS: Risk of subsequent primary
malignancies after dermatofibrosarcoma protuberans diagnosis: A
national study. J Am Acad Dermatol. 68:790–796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paradisi A, Abeni D, Rusciani A, Cigna E,
Wolter M, Scuderi N, Rusciani L, Kaufmann R and Podda M:
Dermatofibrosarcoma protuberans: Wide local excision vs. Mohs
micrographic surgery. Cancer Treat Rev. 34:728–736. 2008.
View Article : Google Scholar
|
|
19
|
Mendenhall WM, Zlotecki RA and Scarborough
MT: Dermatofibrosarcoma protuberans. Cancer. 101:2503–2508. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tazzari M, Indio V, Vergani B, de Cecco L,
Rini F, Negri T, Camisaschi C, Fiore M, Stacchiotti S, Dagrada GP,
et al: Adaptive immunity in fibrosarcomatous dermatofibrosarcoma
protuberans and response to imatinib treatment. J Invest Dermatol.
137:484–493. 2017. View Article : Google Scholar : PubMed/NCBI
|